Abstract
Pupil diameter was monitored during picture viewing to assess effects of hedonic valence and emotional arousal on pupillary responses. Autonomic activity (heart rate and skin conductance) was concurrently measured to determine whether pupillary changes are mediated by parasympathetic or sympathetic activation. Following an initial light reflex, pupillary changes were larger when viewing emotionally arousing pictures, regardless of whether these were pleasant or unpleasant. Pupillary changes during picture viewing covaried with skin conductance change, supporting the interpretation that sympathetic nervous systemactivity modulates these changes in the context of affective picture viewing. Taken together, the data provide strong support for the hypothesis that the pupil’s response during affective picture viewing reflects emotional arousal associated with increased sympathetic activity.
Descriptors: Pupil, Arousal, Emotion, Pleasure, Sympathetic, Skin conductance
Hess (e.g., Hess & Polt, 1960) famously reported bi-directional effects of emotion on pupil change, reporting that the pupil constricted (shut down) when people viewed unpleasant pictures and dilated when they viewed pleasant pictures. These results proved difficult to replicate, however, and this early research suffered from numerous methodological difficulties, including the use of very few pictures (e.g., five), very small number of subjects (e.g., five), the method of assessing pupil change, and no statistical analysis. Libby, Lacey, and Lacey (1973) later conducted amore extensive investigation of pupillary changes during affective picture viewing, presenting 30 pictures to 34 participants while both pupil diameter and heart rate were measured. In their study, the pupil was photographed two times a second during a 15-s exposure for each picture. Then, “six clerks” scored the resulting 81,600 measurements on the basis of a wall chart onto which the negative was projected. The reported results were somewhat confusing: Although “attention-getting” pictures, a factor onto which emotionality loaded, was associated with greater pupil dilation, a second “pleasure” factor suggested that neutral pictures prompted larger pupil dilation than emotional pictures.
More recent data have suggested that emotional arousal is a key element inmodulating the pupil’s response. For instance, in a poster abstract, Steinhauer, Boller, Zubin, and Pearlman (1983) describe data in which pupil diameter increased when people viewed pleasant and unpleasant pictures, and, more recently, Aboyoun and Dabbs (1998) presented pictures of clothed and naked individuals to men and women, finding that pupil dilation reflected general, rather than gender-specific, arousal. Nonetheless, a systematic comparison of pupillary reactions as a function of picture emotionality, and the role of emotional arousal in modulating pupillary changes, does not yet exist. One goal of the current study was to reassess the effects of hedonic valence and arousal on pupillary responses during picture viewing using a modern infrared eye-tracking system and a large set of well-validated pictures from the International Affective Picture System (IAPS; Lang, Bradley & Cuthbert, 2005), which allowed experimental control of both rated pleasure and arousal.
A second goal of the current study was to assess the contribution of sympathetic and parasympathetic activity to pupil change in the affective picture viewing context. As discussed more fully by Steinhauer, Siegle, Condray, and Pless (2004), changes in pupil diameter are controlled by two muscles—the dilator and the sphincter—that are differentially influenced by activity in the sympathetic and parasympathetic branches of the nervous system. Increased sympathetic activity increases the activity of the dilator muscle, prompting dilation, whereas inhibition of parasympathetic activity lessens constriction of the sphinctermuscle, which also results in dilation. Thus, increases in pupillary diameter can be mediated by activity in either division of the autonomic nervous system.
In the picture viewing context, previous studies have consistently found that skin conductance changes are larger when viewing pleasant and unpleasant, compared to neutral, pictures, indicating that this sympathetically mediated response covaries with emotional arousal (Lang, Greenwald, Bradley, & Hamm, 1993). On the other hand, cardiac deceleration is generally greater when viewing unpleasant, compared to either pleasant or neutral, pictures (Bradley, Codispoti, Cuthbert, & Lang, 2001), and pharmacological blockade studies of fear bradycardia in animals suggest that this deceleratory activity is mediated primarily by changes in parasympathetic activity (Berntson, Boysen, Bauer, & Torello, 1989).
If pupillary changes during picture viewing are mediated by differences in parasympathetic activity, we expected that the pattern of pupillary changes would covary with the pattern of cardiac deceleration and be most pronounced for unpleasant pictures. If, on the other hand, pupillary changes during picture viewing are initiated by sympathetically mediated changes that increase activity of the dilator muscle, we expected that papillary responses would covary with skin conductance activity, with increases in both measures when viewing emotionally arousing compared to neutral pictures, regardless of hedonic valence.
The most important natural function of the pupil is to dynamically respond to changes in environmental illumination, and, in humans, the pupil reflexively responds to such changes with an initial constriction (i.e., the light reflex) that is related to stimulus luminosity (Beatty & Lucero-Wagoner, 2000). Because the human eye is differentially sensitive to light in the green, red, and blue spectrums (making it more difficult to accurately estimate luminance for color photographs), we presented pictures in grayscale. Moreover, we controlled luminance by equating both the average luminance across images in each set of pictures (i.e., pleasant, neutral, and unpleasant) as well as by equating the distribution of luminance values in each picture set. We expected that, although the magnitude of the initial light reflex would covary with luminance, it would not systematically differ as a function of hedonic picture content, allowing an accurate assessment of pupil change that is specifically related to picture emotionality.
Method
Participants
Twenty-seven (11 female) 18–22-year-old students from a University of Florida General Psychology course participated for course credit. Of these, 85% (n = 23) reported they were white/Caucasian, 7% (n = 2) Arab/Middle Eastern, and 7% biracial/multiethnic.
Materials and Design
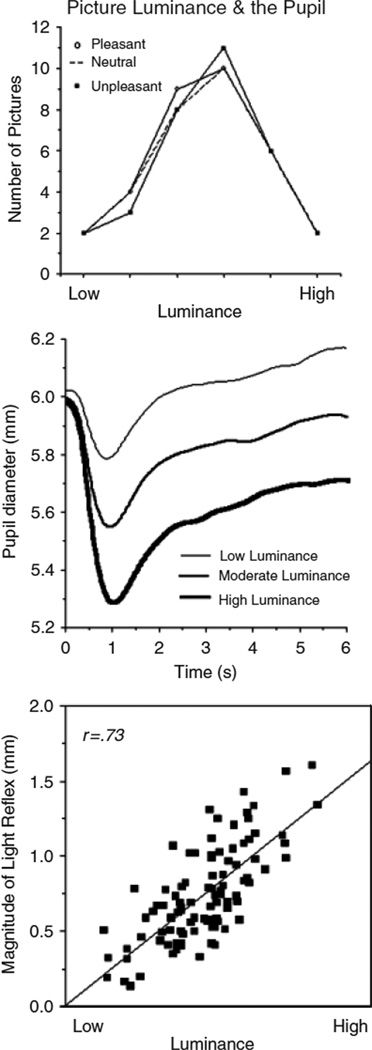
Stimuli were 96 pictures1 selected from the International Affective Picture System (IAPS: Lang et al., 2005), consisting of 32 pleasant (mean pleasure/arousal = 7.0, 5.5), 32 neutral (mean pleasure/arousal = 4.9, 3.4), and 32 unpleasant (mean pleasure/arousal = 2.4, 5.9) pictures. Rated arousal was equivalent for pleasant and unpleasant stimuli. All pictures portrayed people, were balanced for stimulus complexity, were landscape (1024 × 768) in orientation, and were displayed in 16-bit grayscale. Using Adobe Photoshop (version 7.01; Adobe Systems Inc., San Jose, CA), the mean luminosity of the selected pictures was modified such that themean and distribution of luminosity values for each of the pictures sets (pleasant, neutral, unpleasant) did not differ, as depicted in Figure 1 (top).
Figure 1.
Top panel: The mean and distribution of luminance values for pleasant, neutral, and unpleasant pictures were matched. Middle panel: Pupillary response following picture onset begins with an initial light reflex (decrease in pupillary diameter) that is strongly modulated by luminance. Bottom panel: The magnitude of the initial light reflex varies with picture luminance on a picture-by-picture basis (each square is a picture).
Pictures were displayed for 6 s each, with an intertrial interval of 10 s. A grayscale image with the mean luminosity computed across all pictures was displayed 2 s before picture presentation on each trial to control level of illumination prior to picture onset. An acoustic startle probe was presented between 3 and 5 s after picture onset on half (n = 16) of the trials for each picture content; the startle reflex data are not presented here.2 Pictures were arranged in blocks of six, with two pictures of each hedonic content (pleasant, neutral, and unpleasant) in each block; pictures were viewed in one of two different orders across participants, with a specific picture viewed in either the first half or the second half of the study, across orders.
Apparatus
Picture presentation was controlled by an IBM-compatible computer running Presentation software (Neurobehavioral Systems, San Francisco, CA). Pictures were displayed on a 19-in. monitor (Samsung SyncMaster 191T) located in the experimental room, at a distance of 99.06 cm (39 in.) from where the participant was seated.
Pupil diameter was recorded using an ASL model 504 eye-tracker system (Applied Science Laboratories, Bedford, MA), which allows free movement of the head and consists of a video camera and an infrared light source pointed at the participant’s right eye. A magnetic sensor, attached to a headband, tracked and adjusted for head movement. The recording video camera was located in a wood box in front of the subject, and a red translucent screen obscured it from view. Pupil diameter was sampled at 60 Hz for 2 s prior to picture onset, for 6 s during picture onset, and 3 s following picture offset.
Skin conductance and heart rate were measured using VPM software (Cook, 2001) running on an IBM-compatible computer. Skin conductance was recorded using two large sensors placed adjacently on the hypothenar eminence of the left palmar surface after being filled with 0.05 M NaCl paste. A Coulbourn S71-22 skin conductance coupler (Coulbourn Instruments, Allentown, PA) sampled electrodermal activity at 20 Hz. The electrocardiogram was recorded from the left and right forearm using standard Ag/AgCl electrodes, filled with electrolyte paste. The signal was filtered using a Coulbourn S75-01 bioamplifier, and a Schmitt trigger interrupted the computer each time it detected a cardiac R-wave. Interbeat intervals were recorded to the nearest millisecond and reduced off-line into heart rate in beats per minute, in half-second bins.
Procedure
Upon arrival at the laboratory, each participant signed a consent form and was seated in a recliner in a small, sound-attenuated, dimly lit room. The headband for tracking head movement and sensors for measuring heart rate and skin conductance were then attached. Each participant was instructed that a series of pictures would be displayed and that each picture should be viewed the entire time it was on the screen. Following three practice trials, the set of 96 pictures was presented. Then, the sensors were removed and the participant was asked to fill out a postexperimental questionnaire. The experimenter subsequently debriefed, paid, and thanked the participant.
Data Reduction
Samples where the pupil was obscured due to blinking were identified, and linear interpolation was used to estimate pupil size. For each trial, a 1-s prepicture baseline average was subtracted from each of the following pupil samples. Based on the average waveform during picture viewing (see below), the initial light reflex during picture viewing was scored as the maximum extent of pupil constriction in a window from 0.6 to 1.6 s after picture onset. The pupil response to the picture content following the initial light reflex was calculated as the mean change (from baseline) in a window from 2 to 6 s after picture onset. For both skin conductance and heart rate, a 1-s prepicture baseline was subtracted from the values at each half-second during picture viewing. For skin conductance, the maximum change between 1 and 4 s after picture onset was computed and a log tranform(log [SCR + 1]) done prior to analysis of variance in order to normalize the data. For heart rate, the average change score across the 6-s viewing interval was analyzed.
Multivariate statistics are reported for all analyses.
Results
Figure 1 (middle) illustrates the mean pupil diameter across the picture viewing interval. Anotable feature is the initial light reflex immediately following picture onset, in which pupil diameter decreases due to the increase in illumination. As expected, the magnitude of the initial light reflex was modulated by picture luminosity, F(2,25) = 60.6, p < .0001, with significant differences in the magnitude of the light reflex for pictures that were lower, moderate, or relatively higher in luminosity, as illustrated in Figure 1 (middle), Fs(1,26) > 42, ps < .0001. Moreover, the light reflex was highly correlated with the mean luminosity on a picture-by-picture basis, as illustrated in Figure 1 (bottom), F(1,94) = 107, p < .0001.
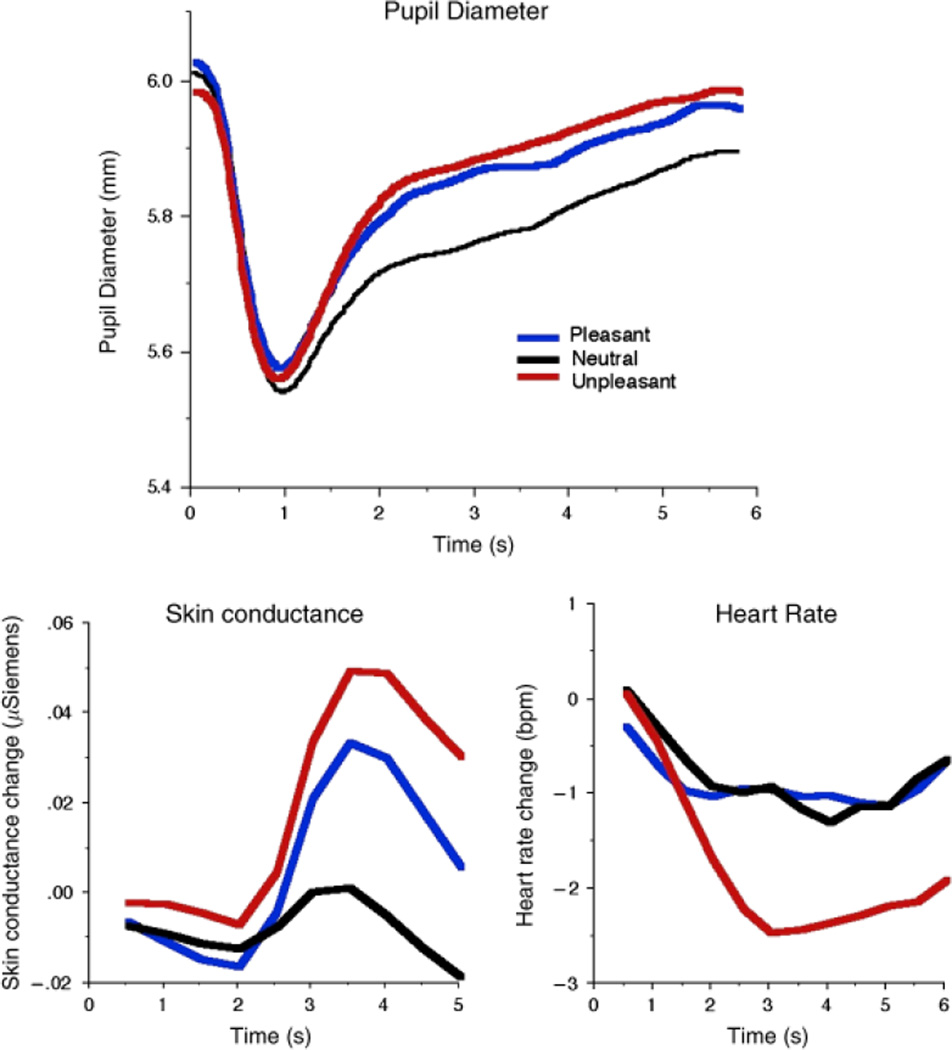
Figure 2 (top panel) illustrates mean pupil diameter as a function of picture emotionality. Consistent with our efforts to control luminosity, there were no significant differences in the amplitude of the initial light reflex as a function of picture emotionality. On the other hand, pupil diameter following the initial light reaction was significantly affected by picture emotionality, with a sustained difference in pupil diameter when participants viewed emotional, compared to neutral, pictures from about 2 s until the end of the viewing interval, as illustrated in Figure 2 (top). When pupillary changes were averaged from 2 to 6 s after picture onset, picture content resulted in a main effect, F(2,25) = 10.25, p < .001, with both pleasant and unpleasant pictures prompting relative increases in pupil diameter that were larger than those elicited when viewing neutral pictures, Fs(1,26) = 7.21 and 15.39, respectively, ps < .001. The difference in pupil size between pleasant and unpleasant pictures did not reach significance, F(1,26) = 3.04, p = .09.
Figure 2.
Top panel: Pupillary response when viewing affective pictures shows greater pupillary increases following the initial light reflex for pleasant and unpleasant, compared to neutral, pictures, indicating modulation by emotional arousal. Bottom left: Skin conductance changes during picture viewing are similar to pupillary responses, showing larger increases when pleasant or unpleasant, compared to neutral, pictures are viewed. Bottom Right: Heart rate change during picture viewing shows greater cardiac deceleration when unpleasant pictures are viewed.
Skin Conductance
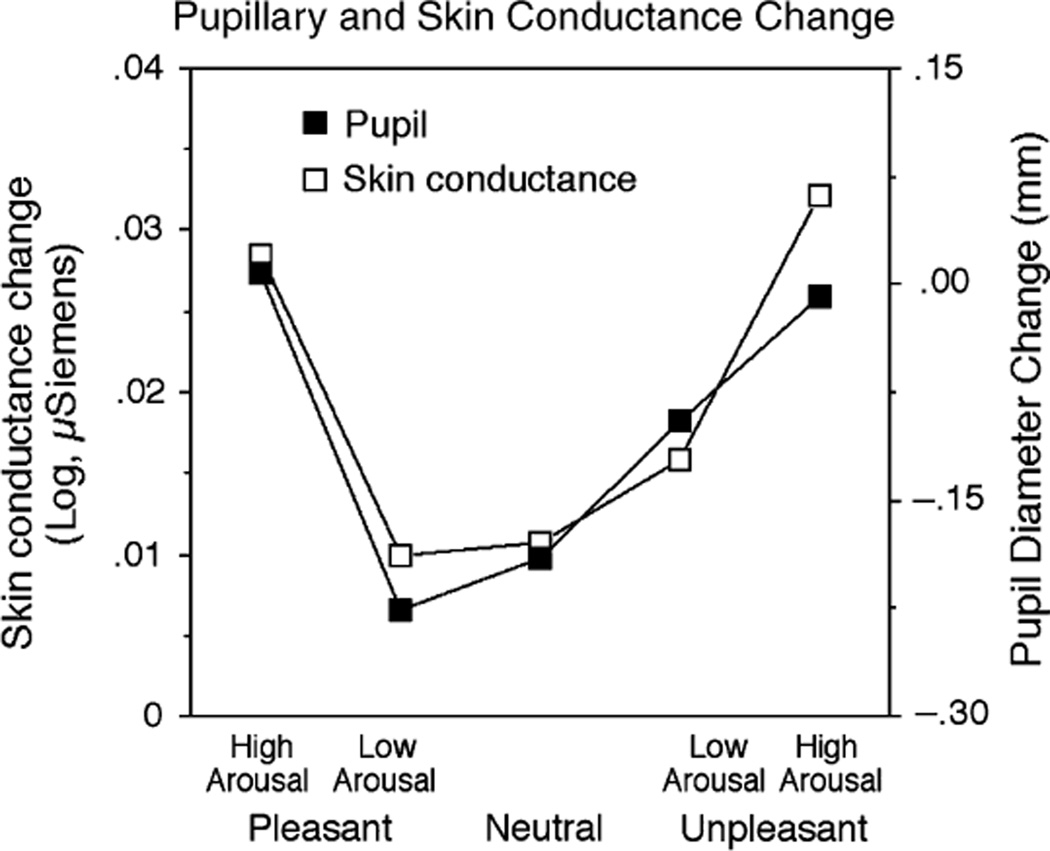
Skin conductance change paralleled the pattern of papillary changes, as illustrated in Figure 2 (bottom left). Replicating many previous studies, hedonic content affected skin conductance change, F(2,24) = 3.62, p < .05, with larger changes when participants viewed unpleasant, F(1,25) = 6.79, p < .02, or pleasant, F(1,25) = 3.69, p = .03 one-tailed, compared to neutral, pictures. To the extent that emotionality is the factor driving changes in the pupil and in skin conductance, we expected that both responses would be most pronounced for pictures rated as highest in emotional arousal. To assess this relationship, pleasant and unpleasant pictures were each divided into high and low arousal pictures on the basis of the median arousal rating in each picture set. As illustrated in Figure 3, pictures rated higher in emotional arousal—pleasant or unpleasant—prompted equivalent changes in electrodermal activity (F < 1), with both showing significantly larger changes compared to neutral pictures, Fs(1,25) = 8.75 and 7.28, respectively, ps < .02. Similarly, for pupil diameter, highly arousing pleasant pictures and unpleasant pictures prompted equivalent pupillary changes (F < 1), with both types of picture associated with larger changes than when participants viewed neutral pictures, Fs(1,26) = 34.39 and 18.56, respectively, ps < .01. Pupil change when participants viewed pictures rated lower in emotional arousal did not differ from when they viewed neutral pictures in either measure, except for unpleasant pictures rated lower in arousal in the pupillary analysis, F(1,26) = 6.01, p < .05.
Figure 3.
Changes in pupillary diameter and skin conductance covary, with the largest reactions for pleasant and unpleasant pictures rated highest in emotional arousal for both psychophysiological measures.
Heart Rate
For heart rate, a different pattern of modulation was found, as illustrated in Figure 2 (bottom right). A main effect of picture content, F(2,25) = 8.33 p < .001, indicated that unpleasant pictures prompted a larger cardiac deceleration (mean = −1.76 beats per minute [bpm]), which was significantly different from the heart rate changes elicited when participants viewed pleasant (−0.87 bpm) or neutral (−.81 bpm) pictures, Fs(1,26) = 11.62 and 14.19, ps < .001, respectively. Heart rate change when participants viewed pleasant and neutral pictures did not differ. When heart rate change was computed as a function of both pleasure and arousal, unpleasant arousing pictures continued to prompt greater deceleration compared to any other picture content, Fs(1,26) = 16.94, 7.74, 21.66, and 10.29, p < .01, for pleasant low, pleasant high, neutral, and unpleasant low, respectively.
Picture Analysis
For each of the 96 pictures, skin conductance change, heart rate change, and picture luminance were entered into a hierarchical multiple regression analysis predicting the mean pupil change (2 to 6 s after picture onset) during picture viewing. In this analysis, both luminance, F(1,92) = 65, p < .0001, and skin conductance changes, F(1,92) = 10.7, p < .005, were independent predictors of pupil diameter during picture viewing. When effects due to luminance were removed, skin conductance change remained a highly reliable predictor of the residual pupillary changes, F(1,92) = 10.5, p < .005. Heart rate change was not related to pupillary changes during picture viewing.
Discussion
An initial decrease in pupil diameter following picture onset was strongly related to luminance as expected, and experimental control insured that this initial light reflex did not differ as a function of picture content, allowing an assessment of emotion on pupillary changes during picture perception. The data clearly indicated that, following the initial light reflex, pupillary increases were larger when participants viewed pleasant or unpleasant, compared to neutral, pictures. These data disconfirm earlier hypotheses regarding differential pupillary changes as a function of hedonic valence (Hess & Polt, 1960) and support the more recent consensus that pupil diameter increases when people process emotionally engaging stimuli, regardless of hedonic valence. In a recent study, Partala, Jokiniemi, and Surakka (2000) reported increased pupil dilation even when participants listened to affectively engaging, compared to neutral, sounds, suggesting that emotional arousal prompts pupillary increases even when the perceptual context is not visual.
Whereas Libby et al. (1973) originally reported the largest pupillary changes when unpleasant pictures were viewed, together with an association between pupillary change and cardiac deceleration, these effects were not replicated in the present study and may reflect differences in picture selection. Thus, whereas in the present study, pleasant and unpleasant pictures were matched for emotional arousal on the basis of IAPS ratings (Lang et al., 2005), level of arousal was not considered by Libby et al. (1973). Although their picture set apparently included pleasant stimuli typically rated as highly arousing (e.g. “nude on grass,” “beautiful female model”), some pleasant pictures are more difficult to characterize in terms of arousal (e.g., “piece of sandstone shaped like nude,” “several light bulbs, sexually suggestive”), and others do not appear, on the surface, to be pleasant (e.g., “locomotive, view of wheels”). Because greater cardiac deceleration is typically found when unpleasant pictures are viewed (Bradley et al., 2001), an association between cardiac and pupillary changes is likely when pleasant, arousing pictures are not well represented in the stimulus set. Supporting this interpretation, when pleasant pictures were removed from the current analysis, the rank correlation between cardiac deceleration and pupillary change across pictures was −.33, which is almost identical to the −.35 correlation reported previously by Libby et al.
Rather than varying with cardiac deceleration, the current study found that pupillary changes covaried with skin conductance reactions, providing collateral support for the hypothesis that pupil diameter during picture viewing predominantly reflects sympathetic nervous system activity. During picture viewing, both skin conductance and pupillary changes were greatest for emotional, compared to neutral, pictures and these effects were accentuated for the pictures rated as most highly arousing. Furthermore, when the picture was used as the unit of analysis, the relationship between skin conductance and pupil changes persisted, even when specific effects due to luminosity were removed using hierarchical multiple regression.
In studies that have explored effects of mental load and sustained cognitive processing on pupil change, the observed papillary dilation appeared to be mediated by parasympathetic inhibition of the sphincter muscle (Steinhauer et al., 2004). The close covariation of pupil dilation with skin conductance in the current study suggests that, for emotional processing, the mechanism may be different and involve direct sympathetic innervations of the dilator muscle. Taken together, the current data are consistent with the hypothesis that pupillary changes during affective picture viewing are mediated by increased sympathetic activity, and strongly suggest that pupil dilation is determined by emotional arousal, independent of whether pictures are pleasant or unpleasant in hedonic valence.
Acknowledgments
This research was supported in part by a grant from the National Institute ofMental Health (P50MH72850) to the Center for the Study of Emotion and Attention (CSEA) at the University of Florida. Laura Miccoli and Miguel Escrig are now at the Jaume I University of Castellón, Spain.
Footnotes
The library numbers for IAPS pictures (Lang et al., 2005) used in this study are: pleasant: 2208, 2250, 2260, 2501, 2560, 2650, 4611, 4617, 4640, 4653, 4659, 4666, 4687, 4694, 5621, 8041, 8080, 8090, 8116, 8120, 8161, 8180, 8200, 8280, 8300, 8320, 8330, 8370, 8380, 8400, 8420, 8465; neutral: 2020, 2190, 2200, 2210, 2214, 2215, 2220, 2221, 2235, 2240, 2270, 2272, 2278, 2383, 2393, 2410, 2441, 2491, 2493, 2514, 2579, 2745.1, 2749, 2752, 2810, 2850, 2870, 2890, 3210, 5455, 7550, 9210; unpleasant: 2120, 2205, 2520, 2590, 2691, 2730, 2750, 2800, 3015, 3030, 3053, 3100, 3170, 3180, 3181, 3400, 3500, 3530, 3550, 6210, 6211, 6212, 6821, 6834, 6838, 9041, 9250, 9254, 9341, 9405, 9800, 9921.
The data analysis included both trials on which a startle probe was delivered and no-probe trials. When this factor was included in the analysis of the pupillary data, effects of picture emotionality were identical for both trial types.
REFERENCES
- Aboyoun DC, Dabbs JM. The Hess pupil dilation findings: Sex or novelty? Social Behavior and Personality. 1998;26:415–420. [Google Scholar]
- Beatty J, Lucero-Wagoner B. The pupillary system. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. Cambridge, UK: Cambridge University Press; 2000. pp. 14–162. [Google Scholar]
- Berntson GG, Boysen ST, Bauer HR, Torello MS. Conspecific screams and laughter: Cardiac and behavioral reactions of infant chimpanzees. Developmental Psychobiology. 1989;22:771–787. doi: 10.1002/dev.420220803. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Cook EW., III . VPM reference manual. Birmingham, Alabama: Author; 2001. [Google Scholar]
- Hess EH, Polt JM. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-6. Gainesville, FL: University of Florida; 2005. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Libby WL, Lacey BC, Lacey JI. Pupillary and cardiac activity during visual attention. Psychophysiology. 1973;10:270–294. doi: 10.1111/j.1469-8986.1973.tb00526.x. [DOI] [PubMed] [Google Scholar]
- Partala T, Jokiniemi M, Surakka V. Eye tracking research & application: Proceedings of the 2000 symposium on eye tracking research and applications. New York: ACM Press; 2000. Pupillary responses to emotionally provocative stimuli; pp. 123–129. [Google Scholar]
- Steinhauer SR, Boller F, Zubin J, Pearlman S. Pupillary dilation to emotional visual stimuli revisited. Psychophysiology. 1983;20:S472. [Abstract] [Google Scholar]
- Steinhauer SR, Siegle GJ, Condray J, Pless M. Sympathetic and parasympathetic innervation of pupillary dilation during sustained processing. International Journal of Psychophysiology. 2004;53:77–86. doi: 10.1016/j.ijpsycho.2003.12.005. [DOI] [PubMed] [Google Scholar]