Abstract
Emotion influences the perception of respiratory sensations, although the specific mechanism underlying this modulation is not yet clear. We examined the impact of viewing pleasant, neutral, and unpleasant affective pictures on the respiratory-related evoked potential (RREP) elicited by a short inspiratory occlusion in healthy volunteers. Reduced P3 amplitude of the RREP was found for respiratory probes presented when viewing pleasant or unpleasant series, when compared to those presented during the neutral series. Earlier RREP components, such as Nf, P1, N1, and P2, showed no modulation by emotion. The results suggest that emotion impacts the perception of respiratory sensations by reducing the attentional resources available for processing afferent respiratory sensory signals.
Descriptors: Dyspnea, EEG, Emotion, Perception, Respiration, Respiratory-related evoked potential
Respiration is a continuous process which, under normal conditions, is not consciously perceived. In some circumstances, however, we become aware of respiratory sensations, e.g., during breath-holding, strong physical exercise, some forms of meditation, or in disease-related conditions (Altose, Cherniack, & Fishman, 1985; Davenport & Vovk, 2009). In respiratory diseases such as asthma or chronic obstructive pulmonary disease (COPD), although the perception of respiratory sensations such as dyspnea (breathlessness) may be a troublesome and frightening experience (American Thoracic Society, 1999; GINA, 2008; GOLD, 2008), adequate perception of respiratory sensations is important for motivating patients to seek timely medical treatment (Banzett, Dempsey, O’Donnell, & Wamboldt, 2000). Reduced perception of initial bronchoconstriction and dyspnea in asthma patients, for example, might lead to increased morbidity due to delayed or inadequate medication use, delayed visits to the physician or emergency department and might even include near-fatal attacks (Barnes, 1994; Barreiro, Gea, Sanjuas, Marcos, Broquetas, & Milic-Emili, 2004; Fritz et al., 2007; Kifle, Seng, & Davenport, 1997; Kikuchi et al., 1994; Magadle, Berar-Yanay, & Weiner, 2002). However, over-perception of respiratory sensations can also be a problem, leading to overuse of medication and related negative health effects (Main, Moss-Morris, Booth, Kaptein, & Kolbe, 2003).
A growing body of literature suggests that psychological factors such as emotion, attention, and learning can strongly impact reports of respiratory perception, often independent of ventilatory changes (Chetta, Foresi, Marangio, & Olivieri, 2005; De Peuter, Van Diest, Lemaigre, Verleden, Demedts, & Van den Bergh, 2004; Lehrer, Feldman, Giardino, Song, & Schmaling, 2002; von Leupoldt & Dahme, 2007). Several studies in healthy volunteers and patients with asthma or COPD have demonstrated that individuals with a personality characterized by high negative emotionality report more dyspnea or respiratory sensations than those with low negative emotionality, regardless of their pulmonary status (De Peuter, Lemaigre, Van Diest, & Van den Bergh, 2008; Han et al., 2004; Li et al., 2006; Put, Van den Bergh, Van Ongeval, De Peuter, Demedts, & Verleden, 2004; Vögele & von Leupoldt, 2008). For example, patients with COPD and comorbid panic disorder and/or panic symptoms report greater resistive load induced dyspnea than matched patients with COPD without panic comorbidity, despite similar limitations in their respiratory function (Livermore, Butler, Sharpe, McBain, Gandevia, & McKenzie, 2008).
Similarly, experimental induction of negative emotion in patients and healthy individuals resulted in increased reports of respiratory sensations (Bogaerts, Notebaert, Van Diest, Devriese, De Peuter, & Van den Bergh, 2005; Rietveld & Prins, 1998; von Leupoldt, Mertz, Kegat, Burmester, & Dahme, 2006; von Leupoldt, Riedel, & Dahme, 2006; von Leupoldt et al., 2008a). However, whether these reports of respiratory sensation are accompanied by measurable changes in the neural processing of respiratory signals remains unknown. Because either over- or under-perception of respiratory sensations has critical implications for both clinical assessment and treatment, it is essential to determine effects of emotion on neural correlates of respiratory perception.
One technique for studying the neural processing of respiratory perception is the respiratory-related evoked potential (RREP) recorded from the electroencephalogram (EEG) (Davenport, Friedman, Thompson,&Franzen, 1986). The RREP is a measure of cerebral cortical activity elicited by short inspiratory occlusion or breathing against inspiratory resistive loads (Bloch-Salisbury, Harver, & Squires, 1998; Chou & Davenport, 2007; Davenport, Colrain, & Hill, 1996; Davenport et al., 1986; Logie, Colrain, & Webster, 1998; Redolfi et al., 2005; Webster & Colrain, 2000a). The RREP quantifies the initial arrival and processing of sensory afferent respiratory information in the sensorimotor cortex by the early components (Nf, P1, N1) and subsequent cognitive processing in other associative cortical areas by the later components (P2, P3) (Davenport & Vovk, 2009). In particular, the later RREP components such as the P3 seem to be vulnerable to higher order cognitive processes not related to respiration per se as previous studies demonstrated an attenuation or absence of the P3 under distraction conditions compared to conditions where attention was focused on the respiratory stimulus (Davenport, Chan, Zhang, & Chou, 2007; Harver, Squires, Bloch-Salisbury, & Katkin, 1995; Webster & Colrain, 2000b).
In the present study, we measured RREPs to study the influence of emotional engagement on the neural processing of respiratory perception in healthy volunteers. Emotional states were induced by presenting series of pleasant, neutral, and unpleasant pictures. To assess emotional engagement, we collected reports of hedonic valence and arousal, as well as skin conductance activity, event-related potentials measured at picture onset (commonly referred to as late positive potential), and indices of respiratory motor drive. Previous studies measuring these responses during affective picture viewing have reliably found enhanced responding when viewing emotional (pleasant or unpleasant) compared to neutral pictures (e.g., Bradley & Lang, 2007; Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Van Diest, Janssens, Bogaerts, Fannes, Davenport, & Van Den Bergh, 2009), and we expected similar patterns of modulation as evidence of emotional engagement in the current study.
The RREP was measured using a probe which briefly occluded respiration immediately following the onset of inspiration. When external startling (e.g., Cuthbert, Schupp, Bradley, McManis, & Lang, 1998; Keil, Bradley, Junghoefer, Russmann, Lowenthal, & Lang, 2007) or painful (Kenntner-Mabiala, Andreatta, Wieser, Mühlberger, & Pauli, 2008) probes are presented during affective picture viewing, previous studies have found that the resulting amplitude of the P3 component is reduced, suggesting less attention is available for processing the probe when processing emotionally salient cues. Assuming that internal processing is similarly modulated by enhanced motivated attention in emotion, we hypothesized similar affective modulation for respiratory probes, with reduced P3 amplitudes for the RREP for probes presented during pleasant or unpleasant affective picture series compared to non-arousing neutral series.
Method
Participants
Fourteen healthy adults (6 female, mean age = 19.7, SD = 2.2) participated after providing informed written consent. Normal baseline lung function was confirmed by spirometry (Discovery, Futuremed America Inc., Granada Hills, CA) according to standards published by the European Respiratory Society (Quanjer, Tammeling, Cotes, Pedersen, Peslin, & Yernault, 1993). All participants underwent a questionnaire screening to exclude those with symptoms of depression (Beck Depression Inventory-II; Beck, Steer, & Brown, 1996) or anxiety (State-Trait Anxiety Inventory, STAI; Spielberger, Gorsuch, & Lushene, 1970). Baseline characteristics of the participants are listed in Table 1. The study protocol was approved by the Institutional Review Board of the University of Florida.
Table 1.
Baseline Characteristics of Participants (Means, SD)
| Characteristics | Data |
|---|---|
| Age (yrs) | 19.7 (2.2) |
| Sex (female/male) | 6/8 |
| Weight (kg) | 69.7 (13.1) |
| Height (cm) | 177.3 (13.4) |
| Forced expiratory volume in 1 s (L) | 3.75 (.67) |
| Forced expiratory volume in 1 s (% of predicted value) | 95.3 (10.6) |
| Forced vital capacity (L) | 4.43 (.96) |
| Forced vital capacity (% of predicted value) | 97.1 (13.4) |
| Forced expiratory volume in 1 s/Forced vital capacity (%) | 83.9 (8.13) |
| Depression | 6.0 (4.3) |
| State anxiety | 34.5 (7.4) |
| Trait anxiety | 35.4 (6.9) |
Affective Picture Series
Four hundred and thirty-two pictures were selected from the International Affective Picture System (IAPS; Lang, Bradley, & Cuthbert, 2008), based upon normative ratings. Pictures were grouped into pleasant, neutral, and unpleasant categories, each consisting of 144 pictures. For each category, 4 series of 36 pictures each were selected, which were matched for picture content and mean ratings of hedonic valence and arousal. In addition, each of the 4 pleasant and 4 unpleasant series was equated for rated arousal. Each picture in the resulting 12 affective picture series was presented on a monitor for 10 s, without an inter-stimulus interval, resulting in a total presentation time of 6 min for each series. The picture order within each series was randomized for each volunteer using experimental stimulus software (Presentation, Neurobehavioral Systems Inc., Albany, NY).
Evaluative Ratings
Evaluative ratings of hedonic valence and arousal were obtained after each 6-min picture series using a paper and pencil version of the Self-Assessment Manikin (SAM, Bradley & Lang, 1994), which acquires ratings of valence and arousal using a 9-point scale.
Respiratory Sensation
Participants rated the experienced intensity of the inspiratory occlusions after each picture series on a horizontal visual analog scale (100 mm), ranging from 0 ( = not noticeable) to 100 ( = maximally imaginable intensity).
Physiological Data Collection and Reduction
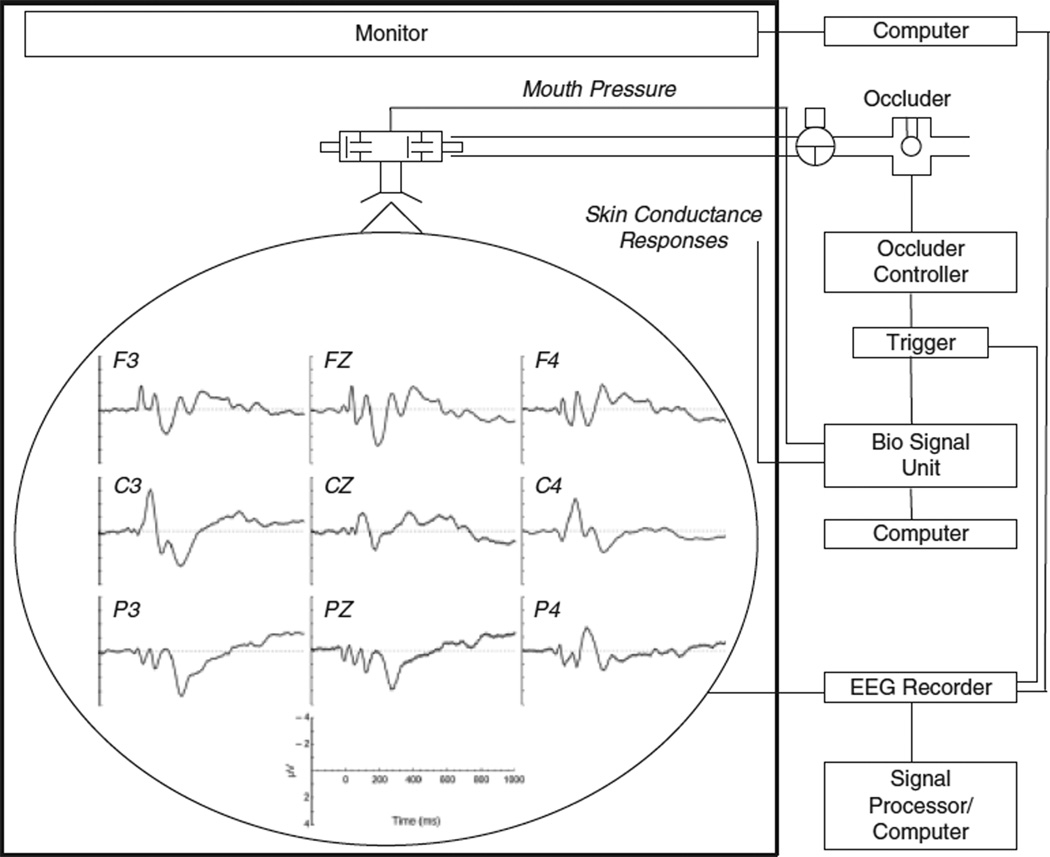
As illustrated in Figure 1, participants breathed, via a mouthpiece, through a breathing circuit consisting of a non-rebreathing two-way valve (Hans Rudolph Inc., Kansas City, MO) with the nose occluded by a clip. The inspiratory port of the valve was connected to a pressure-activated occluder. Mouth pressure (Pm, in cmH2O) was continuously recorded from a port in the center of the non-rebreathing valve by a differential-pressure transducer (model MP-45, Validyne Engineering, Northridge, CA), connected to a PowerLab biosignal recording unit (ADInstruments, Bella Vista, Australia), and displayed on a monitor by the biosignal software package Labview (ADInstruments). Inspiration was interrupted by manual activation of the occluder after the onset of inspiration as indicated by the Pm signal on the monitor with a parallel marker signal being sent to the EEG recorder. Because of the great interindividual variability in breathing patterns and durations of end expiratory pauses, manual occluder activations have the advantage of a more precise presentation of an occlusion directly after the onset of inspiratory flow, which is hardly achievable with time-locked activation routines. Occlusions (500 ms duration) were presented every two to six breaths during picture viewing, resulting in 107, 105, and 108 occlusions (means across participants) for the pleasant, neutral, and unpleasant picture series, respectively.
Figure 1.
Schematic representation of the experimental set-up. The graphs superimposed on the head demonstrate the group mean scalp topography of the respiratory-related evoked potential averaged across all picture series according to standard sensor placements of the International 10/20 System.
To characterize the effects of affective content on the respiratory motor drive, the P0.1 (in cmH2O) was measured every 60 s during picture viewing. P0.1 is the negative inspiratory occlusion pressure at the mouth (Pm) 100 ms after the onset of an inspiratory effort against a closed airway and reflects the summed motor output of the central respiratory controller (Whitelaw & Derenne, 1993). P0.1 was derived offline from the continuous Pm signal with the biosignal software package Labview. To avoid biases in the EEG signal averaging, inspiratory occlusions for the measurement of P0.1 were not included in the RREP.
Skin conductance activity was measured as an autonomic marker of affective arousal using Ag/AgCl standard electrodes, filled with 0.05-m NaCl Unibase paste and attached to the distal phalanx of the left ring and small finger. A signal in the range of 0–40 µS was acquired with a GSR Amp (ADInstruments) and conveyed to the PowerLab biosignal recording unit (ADInstruments), connected to the biosignal software package Labview. Using a scoring algorithm in Matlab (The MathWorks, Natick, MA), the number of skin conductance responses was calculated offline for each series using the common minimal response amplitude of >0.05 µS (Dawson, Schell, & Filion, 2000).
EEG data were recorded from the scalp using a 129-channel system (Electrical Geodesics Inc., Eugene, OR). Scalp impedance for each sensor was kept below 70 kΩ. The EEG was recorded continuously with a sampling rate of 250 Hz, with the vertex sensor as reference electrode, and on-line bandpass filtered from 0.01 to 100 Hz. All further processing was performed offline, using functions built into BESA 5.1. First, continuous EEG data were low-pass filtered at 30 Hz using a digital filter. Segments with occlusions for P0.1 measurements as well as incomplete inspiratory occlusions (e.g., due to swallowing or delayed triggering) were removed based on visual off-line inspection of the Pm signal. Raw EEG data were visually inspected and were corrected for ocular artifacts (blinks and eye movements) using the algorithm implemented in BESA (see Ille, Berg, & Scherg, 2002). Single channels that were flat or showed bad signal throughout were interpolated, with a maximum of twelve channels per subject that were not located at adjacent scalp areas. Epochs were then extracted, including a segment ranging from 200 ms before until 1000 ms after stimulus onset. Using a maximum of 200 µVas the cutoff amplitude, 82, 83, and 85 occlusion epochs were retained on average for the pleasant, neutral, and unpleasant picture series, respectively.
Based on previous RREP reports (Bloch-Salisbury et al., 1998; Chou & Davenport, 2007; Davenport et al., 1986, 1996, 2007; Harver et al., 1995; Logie et al., 1998; Redolfi et al., 2005; Webster & Colrain, 2000a, 2000b), the RREP components were identified as follows: Nf was the negative peak in the frontal region (latency: 25–45 ms), P1 was the positive peak in the centro-parietal region (latency: 45–65 ms), N1 was the negative peak in the centro-lateral region (latency: 85–125 ms), P2 was the positive peak in the centro-parietal region (latency: 180–230 ms), and P3 was the positive peak in the centro-parietal region (latency: 250–350 ms).
The late positive potential (LPP) was defined by averaging over a set of centro-parietal sensors in a window from 400–700 ms after the onset of each picture (Keil, Bradley, Hauk, Rockstroh, Elbert, & Lang, 2002).
Procedure
After positioning of the EEG sensor net, skin conductance electrodes, and nose clip, participants were seated in a recliner and breathed through the breathing circuit. The experimental protocol was divided into 4 blocks. In each block, participants were presented 3 picture series (1 pleasant, 1 neutral, 1 unpleasant) in randomized order while the late positive potential, RREP, P0.1, and skin conductance were measured. Each series was preceded by a 1-min habituation epoch to allow adaptation to the mouthpiece breathing, during which no inspiratory occlusions were presented. In order to focus attention on the respiratory stimulus in all picture series, participants were instructed to press a button every time they perceived an inspiratory occlusion, and button presses were recorded.
Immediately after each picture series, participants rated hedonic valence and arousal using SAM and the perceived intensity of the respiratory stimulus on a visual analog scale, followed by a 2-min rest period. After each block of 3 picture series, participants were allowed a longer rest period of 5 min. The presentation order of the 4-picture series for each valence category (pleasant, neutral, unpleasant) across the first, second, third, or fourth block was randomized across participants. The consent form informed participants that they might view ‘‘sexually explicit pictures or violent pictures,’’ but apart from that, participants received no prior information regarding the emotional content of the picture series.
Statistical Analysis
Accuracy of occlusion detection was calculated as the proportion (%) of correct responses (i.e., number correct of the total number of occlusions presented within one picture series). The late positive potential, skin conductance responses, P0.1, button press, and ratings of hedonic valence, arousal, and intensity of inspiratory occlusions were averaged across the 4-picture series for each of the three picture contents and analyzed in separate one-way ANOVAs with picture content (pleasant, neutral, unpleasant) as a repeated measure.
RREP components were analyzed in separate two-way repeated measures ANOVAs with 3(hedonic content) × 2(hemisphere: left vs. right) levels, followed up by separate on-way ANOVAs (hedonic content) for each hemisphere. Hemisphere was included as a factor because of earlier findings suggesting hemispheric differences in RREP amplitudes (Revelette & Davenport, 1990). A Greenhouse-Geisser correction was applied in case of violated sphericity assumptions with reported significance levels referring to corrected degrees of freedom. All analyses were calculated with SPSS 15.0 software (SPSS Inc., Chicago, IL) using a .05 significance level.
Results
Manipulation Check
Evaluative reports
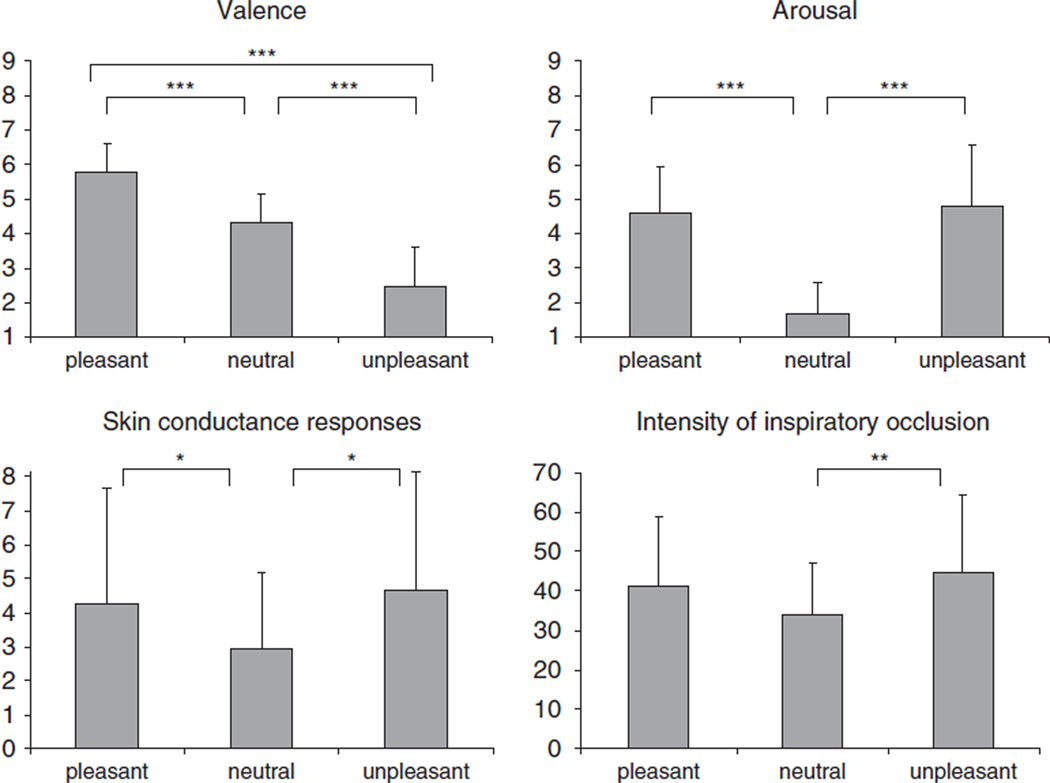
As illustrated in Figure 2, evaluative ratings differed significantly following viewing of pleasant, neutral or unpleasant picture series, in both rated hedonic valence F(2,26) = 56.52, p < .001, η2 = 0.81 and rated arousal, F(2,26) = 30.27, p < .001, η2 = 0.70. Hedonic valence ratings showed the expected increase from unpleasant to neutral to pleasant series with significant differences between all series (unpleasant vs. neutral: t(13) = 7.41, p < .001; pleasant vs. neutral: t(13) = 5.82, p < .001; unpleasant vs. pleasant: t(13) = 8.49, p < .001). Ratings of affective arousal demonstrated the expected higher arousal during the pleasant and unpleasant series compared to the neutral series (pleasant vs. neutral: t(13) = 7.09, p < .001; unpleasant vs. neutral: t(13) = 5.59, p < .001). No difference in arousal reports was found between the pleasant and unpleasant series (t(13) = −0.51, n.s.).
Figure 2.
Mean ratings of hedonic valence (left upper panel), arousal (right upper panel), intensity of respiratory occlusion (right lower panel) and mean skin conductance responses (left lower panel) during pleasant, neutral, and unpleasant affective picture series. Error bars represent standard deviations of the mean. *p < .05, **p < .01, ***p < .001.
Respiratory motor drive
P0.1 differed significantly between the three categories of affective pictures, F(2,26) = 11.77, p < .001, η2 = 0.48. Replicating previous data (Van Diest et al., 2009), higher negative P0.1 values were found during the pleasant (−1.43 ± 0.67 cmH2O) and unpleasant series (−1.56 ± 0.59 cmH2O), compared to the neutral series (−1.28 ± 0.69 cmH2O) (pleasant vs. neutral: t(13) = −2.83, p < .01; unpleasant vs. neutral: t(13) = 4.69, p < .001). In addition, P0.1 values were slightly more negative during the unpleasant compared to the pleasant series (t(13) = 2.12, p < .05).
Skin conductance responses
The number of skin conductance responses (Figure 2) differed marginally between the three categories of affective pictures, F(2,26) = 2.82, p < .08, η2 = 0.18. More skin conductance responses were observed during the pleasant and unpleasant series compared to the neutral series (pleasant vs. neutral: t(13) = 1.83, p < .05; unpleasant vs. neutral: t(13) = −2.40, p < .05) without differences between the pleasant and unpleasant series (t(13) = −0.51, n.s.).
Late positive potential
The late positive potential differed significantly when viewing pleasant, neutral, and unpleasant picture series, F(2,26) = 5.14, p < .05, η2 = 0.28. Follow-up tests indicated larger late positive potentials when viewing pleasant (0.73 ± 1.08 µV) or unpleasant series (0.67 ± 0.76 µV) compared to the neutral series (0.07 ± 1.02 µV) (pleasant vs. neutral: t(13) = 2.55, p < .05; unpleasant vs. neutral: t(13) = −3.05, p < .01). No difference in the late positive potential was found between the pleasant and unpleasant series (t(13) = 0.25, n.s.).
Respiratory-Related Evoked Potential
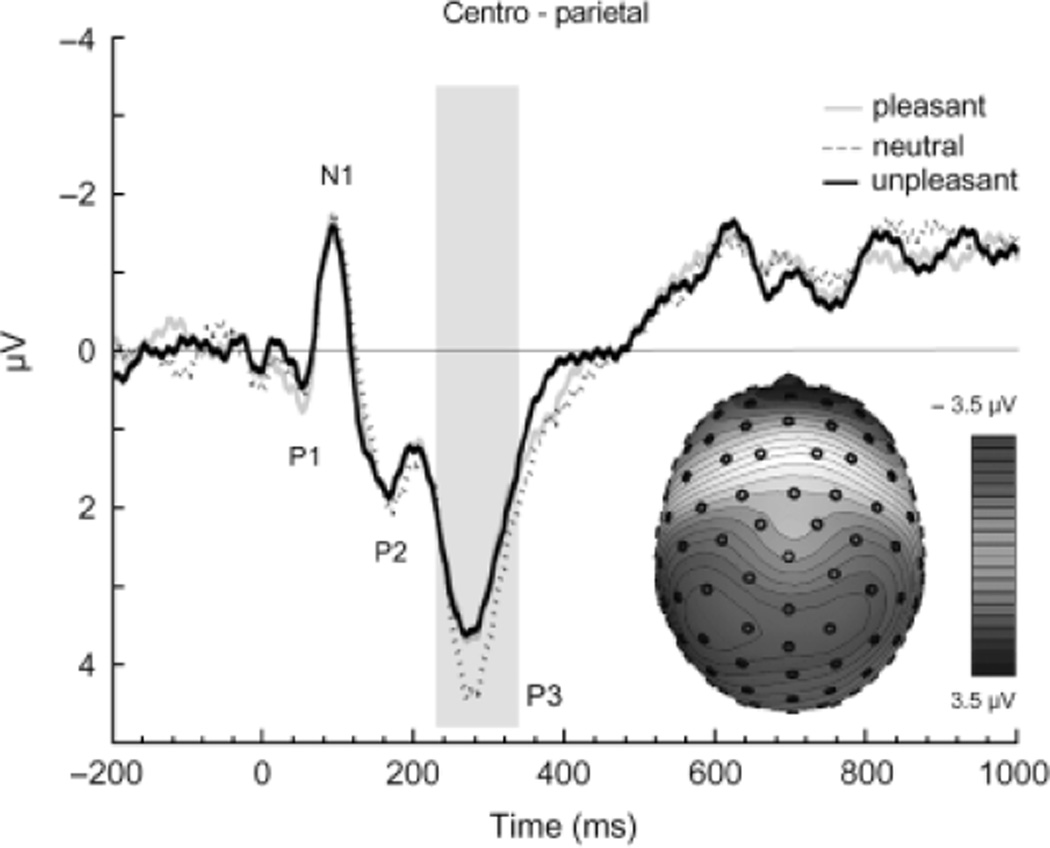
As illustrated in Figure 1, the commonly observed RREP components Nf, P1, N1, P2, and P3 were obtained (see Table 2). The ANOVA for the P3 component indicated a significant interaction of hedonic content and hemisphere F(2,26) = 3.56, p < .05, η2 = 0.22. Follow-up analyses indicated a significant difference in P3 magnitude for inspiratory occlusions as a function of picture content in the left hemisphere, F(2,26) = 3.33, p < .05, η2 = 0.20. As shown in Figure 3, P3 magnitude was reduced in the left hemisphere for inspiratory occlusions presented when participants were viewing either pleasant or unpleasant pictures, compared to when they were viewing neutral pictures (pleasant vs. neutral: t(13) = −2.68, p < .01; unpleasant vs. neutral: t(13)=1.96, p < .05). No difference in P3 magnitude was found between the pleasant and unpleasant series (t(13) = 0.17, n.s.). The pattern of P3 modulation was similar in the right hemisphere (see Table 2), but did not reach statistical significance. No differences as a function of picture content were observed for the other RREP components (e.g., Nf, P1, N1, and P2).
Table 2.
Mean (SD) Amplitudes (µV) of the Respiratory-Related Evoked Potential (RREP) during Pleasant, Neutral and Unpleasant Picture Series for Electrodes on the Left and Right Hemisphere
| Pleasant |
Neutral |
Unpleasant |
||||
|---|---|---|---|---|---|---|
| RREP | Left | Right | Left | Right | Left | Right |
| Nf (frontal) | −1.67 (1.05) | −1.59 (1.28) | −1.33 (1.17) | −1.13 (1.09) | −1.58 (0.93) | −1.10 (0.81) |
| P1 (centro-parietal) | 1.13 (1.58) | 1.04 (1.06) | 0.60 (1.43) | 0.83 (1.54) | 0.70 (1.13) | 0.79 (1.04) |
| N1 (centro-lateral) | −2.49 (2.13) | −2.21 (1.59) | −2.65 (2.35) | −2.29 (1.56) | −2.37 (2.43) | −2.10 (1.70) |
| P2 (centro-parietal) | 3.40 (3.51) | 2.80 (3.64) | 3.58 (3.84) | 2.83 (4.01) | 3.45 (3.62) | 2.69 (3.85) |
| P3 (centro-parietal) | 3.50 (2.71)** | 2.65 (2.26) | 4.22 (2.60) | 2.85 (1.80) | 3.44 (2.51)* | 2.66 (2.05) |
p<.05;
p<.01 (compared to left hemisphere during neutral series).
Figure 3.
Group mean respiratory-related evoked potential over the left hemispheric centro-parietal region elicited by inspiratory occlusions during pleasant, neutral, and unpleasant affective picture series. In addition, the group mean scalp topography for the P3 is shown, averaged across the pleasant, neutral, and unpleasant series.
Respiratory Sensation
No difference in the accuracy of detecting respiratory occlusions was observed as a function of pleasant, neutral, and unpleasant picture content (98.2, 97.8, and 98.8, respectively), suggesting a similar attentional focus to the inspiratory occlusions across the three picture categories. As illustrated in Figure 2, ratings of the perceived intensity of inspiratory occlusions differed significantly as a function of picture content, F(2,26) = 4.14, p < .05, η2 = 0.24. Higher intensity ratings were found following unpleasant picture series, compared to neutral pictures, t(13) = −2.84, p < .01, whereas a similar trend to report higher respiratory sensation following the pleasant picture series did not reach statistical significance, t(13) = 1.83, n.s. No difference in intensity ratings was observed between the pleasant and unpleasant series (t(13) = −0.89, n.s.).
Discussion
When viewing emotional pictures, enhanced arousal ratings, autonomic skin conductance activity and centro-parietal late positive potentials were observed, compared to when viewing neutral pictures. These results replicate previous studies finding that this pattern of modulation is associated with emotional engagement (e.g., Bradley, Cuthbert, & Lang, 1996; Cuthbert et al., 2000; Lang, Greenwald, Bradley, & Hamm, 1993; Smith, Bradley, & Lang, 2005; for review, see Bradley & Lang, 2007). Similarly, the respiratory motor drive showed modulation by emotional arousal, with higher P0.1 during pleasant and unpleasant series compared to neutral series, which also replicates previous findings demonstrating increases in P0.1 (Van Diest et al., 2009) or other respiratory measures (Boiten, Frijda, & Wientjes, 1994; Gomez, Shafy, & Danuser, 2008; Kreibig, Wilhelm, Roth, & Gross, 2007; Ritz, George, & Dahme, 2000) during arousing emotional states.
Most importantly, the later RREP component P3 measured to brief inspiratory occlusions presented when viewing emotional (pleasant or unpleasant) picture series was reduced compared to when the same respiratory probe was presented in the context of a neutral picture series. No influence of emotion was found for the earlier components of the RREP (e.g., Nf, P1, N1, or P2). These findings suggest that emotional processing impacts the perception of internal respiratory sensations in a manner very similar to that found for external sensory probes (e.g., Cuthbert et al., 1998; Keil et al., 2007; Kenntner-Mabiala et al., 2008). This effect can be related to the theoretical concept of motivated attention, in which emotional stimuli are held to naturally engage attentional resources, reducing the amount available for processing other cues (Bradley & Lang, 2007; Lang, Bradley, & Cuthbert, 1997). That is, the processing of affectively arousing and motivationally relevant stimuli demands resources, reducing those available even for processing afferent sensory respiratory signals such as those presented here.
Attenuation of the P3 component of RREPs during emotional processing suggests that the attenuated processing of sensory signals by emotion (as indexed by probe P3 amplitude) is a salient feature across different sensory modalities, including internal respiratory stimulation. Thus, processing of either external or internal sensory stimuli seems similarly modulated when affective cues capture attention. In line with this assumption are previous findings demonstrating that perception-related brain structures such as the insular cortex are commonly activated during externally applied noxious thermal stimulation and internal rectal balloon distension (Dunckley et al., 2005) as well as during externally applied heat pain and internally generated dyspnea due to increased respiratory muscle effort (von Leupoldt et al., 2008b). Similarly, reduced perceptual sensitivity for both externally applied pain and internally generated dyspnea due to increased respiratory muscle effort has been observed following stroke-related reductions in insular cortex activity (Schön et al., 2008). Taken together, the data suggest that the neural processing of respiratory signals is attenuated in the context of high emotional arousal.
Stronger attenuation of the P3 component of the RREP during emotional processing was found in the left hemisphere, although the pattern was similar in the right hemisphere as well. It might be speculated that the general tendency across all picture categories for smaller P2 and P3 amplitude of the RREP in the right hemisphere might have resulted in a floor effect, preventing strong emotional modulation of the P3 component in this hemisphere.
Whereas the P3 amplitude of the RREP was attenuated during affective processing, reports of respiratory sensation were higher following the arousing unpleasant picture series, with a similar trend toward reports of higher sensation following the arousing pleasant series as well. One interpretation centers on the fact that reports of respiratory experience were retrospective and occurred concurrently with ratings of the hedonic valence and arousal of the picture series that were just viewed. In this case, reports of respiratory sensation may primarily reflect what participants might have expected to occur when emotion is elicited (e.g., more intense respiratory experience). If reports of the intensity of the inspiratory occlusion were obtained coincident with each probe, this hypothesis would predict similar affective modulation as the ERP, and can be pursued in future studies.
A second interpretation is that experienced emotional arousal enhances the evaluative judgments of respiratory sensations (Janssens, Verleden, De Peuter, Van Diest, & Van den Bergh, 2009; Rietveld, 2003). This is consistent with previous studies that found increased ratings of respiratory sensations in individuals characterized by high negative emotionality (Bogaerts et al., 2005; De Peuter et al., 2008; Han et al., 2004; Li et al., 2006; Livermore et al., 2008; Put et al., 2004; Vögele & von Leupoldt, 2008) and increased reports of respiratory sensations elicited by CO2 inhalation, exercise, or resistive-loaded breathing following experimental induction of negative emotional states using unpleasant odours, films, or affective picture series (Bogaerts et al., 2005; Rietveld & Prins, 1998; von Leupoldt, Mertz et al., 2006; von Leupoldt, Riedel, & Dahme, 2006; von Leupoldt et al., 2008a). This could lead to less efficient disease management in some individuals, as patients with respiratory diseases with a comorbid depression or anxiety disorder show inadequate medication use (Main et al., 2003), frequent visits to the physician (Feldman, Lehrer, Borson, Hallstrand, & Siddique, 2005), and higher rehospitalization rates (Dahlen & Janson, 2002). From a clinical perspective, the detection of symptoms of negative emotionality in respiratory patients would therefore appear to be highly important for successful disease management (Carrieri-Kohlman et al., 2001), including psychotherapeutic interventions that can successfully reduce the symptoms (Chetta et al., 2005; Kaplan & Ries, 2002; Kunik et al., 2008).
In summary, the results of this study demonstrate that emotion impacts the perception of respiratory sensations in healthy individuals as evidenced by an attenuation of the P3 component of the respiratory-related evoked potential elicited by short inspiratory occlusions. No emotional impact on earlier components such as Nf, P1, N1, and P2 was observed. Following emotional exposure, retrospective reports of respiratory sensation were, however, enhanced. Future studies that focus on respiratory patient groups will be important in determining whether emotional attenuation of the neural processing of respiratory sensory signals and evaluative reports are comparable in individuals suffering from affective symptoms and/or respiratory disorders.
Acknowledgments
The authors wish to thank Bethany Wangelin, Marie Karlsson, Vincent Costa, Joshua R. Shumen, Mark Hotchkiss, Nicole Wedell, Katja Wedell, and A. Daniel Martin for assistance and support. This study was supported in part by a grant from the National Institute of Mental Health (P50 MH 72850) to Peter J. Lang, and by a stipend (Heisenberg-Stipendium, DFG LE 1843/9-1) from the German Research Society (Deutsche Forschungsgemeinschaft, DFG) to Andreas von Leupoldt.
REFERENCES
- Altose M, Cherniack N, Fishman AP. Respiratory sensations and dyspnea. Journal of Applied Physiology. 1985;58:1051–1054. doi: 10.1152/jappl.1985.58.4.1051. [DOI] [PubMed] [Google Scholar]
- American Thoracic Society. Dyspnea: Mechanisms, assessment, and management: A consensus statement. American Journal of Respiratory and Critical Care Medicine. 1999;159:321–340. doi: 10.1164/ajrccm.159.1.ats898. [DOI] [PubMed] [Google Scholar]
- Banzett RB, Dempsey JA, O’Donnell DE, Wamboldt MZ. Symptom perception and respiratory sensation in asthma. American Journal of Respiratory and Critical Care Medicine. 2000;162:1178–1182. doi: 10.1164/ajrccm.162.3.9909112. [DOI] [PubMed] [Google Scholar]
- Barnes PJ. Blunted perception and death from asthma. The New England Journal of Medicine. 1994;330:1383–1384. doi: 10.1056/NEJM199405123301910. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Gea J, Sanjuas C, Marcos R, Broquetas J, Milic-Emili J. Dyspnoea at rest and at the end of different exercises in patients with near-fatal asthma. The European Respiratory Journal. 2004;24:219–225. doi: 10.1183/09031936.04.00074703. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- Bloch-Salisbury E, Harver A, Squires NK. Event-related potentials to inspiratory flow-resistive loads in young adults: Stimulus magnitude effects. Biological Psychology. 1998;49:165–186. doi: 10.1016/s0301-0511(98)00034-9. [DOI] [PubMed] [Google Scholar]
- Bogaerts K, Notebaert K, Van Diest I, Devriese S, De Peuter S, Van den Bergh O. Accuracy of respiratory symptom perception in different affective contexts. Journal of Psychosomatic Research. 2005;58:537–543. doi: 10.1016/j.jpsychores.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Boiten FA, Frijda NH, Wientjes CJE. Emotions and respiratory patterns: Review and critical analysis. International Journal of Psychophysiology. 1994;17:103–128. doi: 10.1016/0167-8760(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: SAM and the semantic differential. Journal of Experimental Psychiatry & Behavior Therapy. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Cuthbert BN, Lang PJ. Picture media and emotion: Effects of a sustained affective context. Psychophysiology. 1996;33:662–670. doi: 10.1111/j.1469-8986.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. The International Affective Picture System (IAPS) in the study of emotion and attention. In: Coan JA, Allen, John JB, editors. Handbook of emotion elicitation and assessment. New York: Oxford University Press; 2007. pp. 29–46. [Google Scholar]
- Carrieri-Kohlman G, Gormley JM, Eiser S, Demir-Deviren S, Nguyen H, Paul SM, Stulbarg MS. Dyspnea and the affective response during exercise training in obstructive pulmonary disease. Nursing Research. 2001;50:136–146. doi: 10.1097/00006199-200105000-00002. [DOI] [PubMed] [Google Scholar]
- Chetta A, Foresi A, Marangio E, Olivieri D. Psychological implications of respiratory health and disease. Respiration. 2005;72:210–215. doi: 10.1159/000084056. [DOI] [PubMed] [Google Scholar]
- Chou YL, Davenport PW. The effect of increased background resistance on the resistive load threshold for eliciting the respiratory-related evoked potential. Journal of Applied Physiology. 2007;103:2012–2017. doi: 10.1152/japplphysiol.01232.2006. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley M, McManis M, Lang PJ. Probing affective pictures: Attended startle and tone probes. Psychophysiology. 1998;35:344–347. doi: 10.1017/s0048577298970536. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Dahlen I, Janson C. Anxiety and depression are related to the outcome of emergency treatment in patients with obstructive pulmonary disease. Chest. 2002;122:1633–1637. doi: 10.1378/chest.122.5.1633. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Chan PY, Zhang W, Chou YL. Detection threshold for inspiratory resistive loads and respiratory-related evoked potentials. Journal of Applied Physiology. 2007;102:276–285. doi: 10.1152/japplphysiol.01436.2005. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Colrain IM, Hill PM. Scalp topography of the short-latency components of the respiratory-related evoked potential in children. Journal of Applied Physiology. 1996;80:1785–1791. doi: 10.1152/jappl.1996.80.5.1785. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Friedman WA, Thompson FJ, Franzen O. Respiratory-related cortical potentials evoked by inspiratory occlusion in humans. Journal of Applied Physiology. 1986;60:1843–1848. doi: 10.1152/jappl.1986.60.6.1843. [DOI] [PubMed] [Google Scholar]
- Davenport PW, Vovk A. Cortical and subcortical central neural pathways in respiratory sensations. Respiratory Physiology & Neurobiology. 2009;167:72–86. doi: 10.1016/j.resp.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo JT, Tassinary LG, Berntson GB, editors. Handbook of psychophysiology. Cambridge: University Press; 2000. pp. 200–223. [Google Scholar]
- De Peuter S, Van Diest I, Lemaigre V, Verleden G, Demedts M, Van den Bergh O. Dyspnea: The role of psychological processes. Clinical Psychology Review. 2004;24:557–581. doi: 10.1016/j.cpr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- De Peuter S, Lemaigre V, Van Diest I, Van den Bergh O. Illness-specific catastrophic thinking and overperception in asthma. Health Psychology. 2008;27:93–99. doi: 10.1037/0278-6133.27.1.93. [DOI] [PubMed] [Google Scholar]
- Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: Differentiating pain intensity from unpleasantness. Neuroscience. 2005;133:533–542. doi: 10.1016/j.neuroscience.2005.02.041. [DOI] [PubMed] [Google Scholar]
- Feldman JM, Lehrer PM, Borson S, Hallstrand TS, Siddique MI. Health care use and quality of life among patients with asthma and panic disorder. The Journal of Asthma. 2005;42:179–184. doi: 10.1081/JAS-200054633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz GK, Adams SK, McQuaid EL, Klein R, Kopel S, Nassau J, Mansell A. Symptom perception in pediatric asthma: Resistive loading and in vivo assessment compared. Chest. 2007;132:884–889. doi: 10.1378/chest.06-2140. [DOI] [PubMed] [Google Scholar]
- Global Initiative for Asthma (GINA) Global Strategy for Asthma Management and Prevention. 2008 Retrieved from: http://www.ginasthma.org.
- Global Initiative for Chronic Obstructive Lung Disease (GOLD) Global Strategy for Diagnosis, Management, and Prevention of COPD. 2008 Retrieved from: http://www.goldcopd.com.
- Gomez P, Shafy S, Danuser B. Respiration, metabolic balance, and attention in affective picture processing. Biological Psychology. 2008;78:138–149. doi: 10.1016/j.biopsycho.2008.01.013. [DOI] [PubMed] [Google Scholar]
- Han JN, Zhu YJ, Li SW, Luo DM, Hu Z, Van Diest I, et al. Medically unexplained dyspnea: Psychophysiological characteristics and role of breathing therapy. Chinese Medical Journal. 2004;117:6–13. [PubMed] [Google Scholar]
- Harver A, Squires N, Bloch-Salisbury E, Katkin E. Event-related potentials to airway occlusion in young and old subjects. Psychophysiology. 1995;32:121–129. doi: 10.1111/j.1469-8986.1995.tb03303.x. [DOI] [PubMed] [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neurophysiology. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Janssens T, Verleden G, De Peuter S, Van Diest I, Van den Bergh O. Inaccurate perception of asthma symptoms: A cognitive-affective framework and implications for asthma treatment. Clinical Psychology Review. 2009;29:317–327. doi: 10.1016/j.cpr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Kaplan RM, Ries AL. Chronic obstructive pulmonary disease: Behavioural assessment and treatment. In: Kaptein AA, Creer TL, editors. Respiratory Disorders and Behavioral Medicine. London: Martin Dunitz Ltd.; 2002. pp. 85–116. [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Keil A, Bradley MM, Junghoefer M, Russmann T, Lowenthal W, Lang PJ. Cross-modal attention capture by affective stimuli: Evidence from event-related potentials. Cognitive, Affective, & Behavioral Neuroscience. 2007;7:18–24. doi: 10.3758/cabn.7.1.18. [DOI] [PubMed] [Google Scholar]
- Kenntner-Mabiala R, Andreatta M, Wieser MJ, Mühlberger A, Pauli P. Distinct effects of attention and affect on pain perception and somatosensory evoked potentials. Biological Psychology. 2008;78:114–122. doi: 10.1016/j.biopsycho.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Kifle Y, Seng V, Davenport PW. Magnitude estimation of inspiratory resistive loads in children with life-threatening asthma. American Journal of Respiratory and Critical Care Medicine. 1997;156:1530–1535. doi: 10.1164/ajrccm.156.5.9703011. [DOI] [PubMed] [Google Scholar]
- Kikuchi Y, Okabe S, Tamura G, Hida W, Homma M, Shirato K, Takishima T. Chemosensitivity and perception of dyspnea in patients with a history of near-fatal asthma. The New England Journal of Medicine. 1994;330:1329–1334. doi: 10.1056/NEJM199405123301901. [DOI] [PubMed] [Google Scholar]
- Kreibig SD, Wilhelm FH, Roth WT, Gross JJ. Cardiovascular, electrodermal, and respiratory response patterns to fear- and sadness-inducing films. Psychophysiology. 2007;44:787–806. doi: 10.1111/j.1469-8986.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- Kunik ME, Veazey C, Cully JA, Souchek J, Graham DP, Hopko D, et al. COPD education and cognitive behavioral therapy group treatment for clinically significant symptoms of depression and anxiety in COPD patients: A randomized controlled trial. Psychological Medicine. 2008;38:385–396. doi: 10.1017/S0033291707001687. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation, and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Hillsdale, NJ: Lawrence Erlbaum Associates; 1997. pp. 97–135. [Google Scholar]
- Lehrer P, Feldman J, Giardino N, Song HS, Schmaling K. Psychological aspects of asthma. Journal of Consulting and Clinical Psychology. 2002;70:691–711. doi: 10.1037//0022-006x.70.3.691. [DOI] [PubMed] [Google Scholar]
- Li W, Daems E, Van deWoestijne KP, Van Diest I, Gallego J, De Peuter S, et al. Air hunger and ventilation in response to hypercapnia: Effects of repetition and anxiety. Physiology and Behaviour. 2006;88:47–54. doi: 10.1016/j.physbeh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Livermore N, Butler JE, Sharpe L, McBain RA, Gandevia SC, McKenzie DK. Panic attacks and perception of inspiratory resistive loads in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2008;178:7–12. doi: 10.1164/rccm.200711-1700OC. [DOI] [PubMed] [Google Scholar]
- Logie SL, Colrain IM, Webster KE. Source localisation of the early components of the respiratory-related evoked potential. Brain Topography. 1998;11:153–164. doi: 10.1023/a:1022210723257. [DOI] [PubMed] [Google Scholar]
- Magadle R, Berar-Yanay N, Weiner P. The risk of hospitalization and near-fatal and fatal asthma in relation to the perception of dyspnea. Chest. 2002;121:329–333. doi: 10.1378/chest.121.2.329. [DOI] [PubMed] [Google Scholar]
- Main J, Moss-Morris R, Booth R, Kaptein AA, Kolbe J. The use of reliever medication in asthma: The role of negative mood and symptom reports. The Journal of Asthma. 2003;40:357–365. doi: 10.1081/jas-120018635. [DOI] [PubMed] [Google Scholar]
- Put C, Van den Bergh O, Van Ongeval E, De Peuter S, Demedts M, Verleden G. Negative affectivity and the influence of suggestion on asthma symptoms. Journal of Psychosomatic Research. 2004;57:249–255. doi: 10.1016/S0022-3999(03)00541-5. [DOI] [PubMed] [Google Scholar]
- Quanjer PH, Tammeling GJ, Cotes, J.E, Pedersen OF, Peslin R, Yernault JC. Lung volumes and forced ventilatory flows. Report Working Party Standardization of Lung Function Tests, European Community for Steel and Coal. Official Statement of the European Respiratory Society. The European Respiratory Journal. 1993;6(suppl 16):5–40. [PubMed] [Google Scholar]
- Redolfi S, Raux M, Donzel-Raynaud C, Morelot-Panzini C, Zelter M, Derenne JP, et al. Effects of upper airway anaesthesia on respiratory-related evoked potentials in humans. European Respiratory Journal. 2005;26:1097–1103. doi: 10.1183/09031936.05.00139804. [DOI] [PubMed] [Google Scholar]
- Revelette WR, Davenport PW. Effects of timing of inspiratory occlusion on cerebral evoked potentials in humans. Journal of Applied Physiology. 1990;68:282–288. doi: 10.1152/jappl.1990.68.1.282. [DOI] [PubMed] [Google Scholar]
- Rietveld S. Symptom perception in chronic asthma: Learning for better or worse? In: Brown ES, editor. Asthma: Social and psychological factors and psychosomatic syndromes. Advances in psychosomatic medicine. Base: Karger; 2003. pp. 115–130. [DOI] [PubMed] [Google Scholar]
- Rietveld S, Prins PJ. The relationship between negative emotions and acute subjective and objective symptoms of childhood asthma. Psychological Medicine. 1998;28:407–415. doi: 10.1017/s0033291797006387. [DOI] [PubMed] [Google Scholar]
- Ritz T, George C, Dahme B. Respiratory resistance during emotional stimulation: Evidence for a nonspecific effect of experienced arousal? Biological Psychology. 2000;52:143–160. doi: 10.1016/s0301-0511(99)00026-5. [DOI] [PubMed] [Google Scholar]
- Schön D, Rosenkranz M, Regelsberger J, Dahme B, Büchel C, von Leupoldt A. Reduced perception of dyspnea and pain after right insular cortex lesions. American Journal of Respiratory and Critical Care Medicine. 2008;178:1173–1179. doi: 10.1164/rccm.200805-731OC. [DOI] [PubMed] [Google Scholar]
- Smith JC, Bradley MM, Lang PJ. State anxiety and affective physiology: Effects of sustained exposure to affective pictures. Biological Psychology. 2005;69:247–260. doi: 10.1016/j.biopsycho.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. State-Trait Anxiety Inventory, manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologist Press; 1970. [Google Scholar]
- VanDiest I, Janssens T, Bogaerts K, Fannes S, Davenport PW, VanDen Bergh O. Affective modulation of inspiratory motor drive. Psychophysiology. 2009;46:12–16. doi: 10.1111/j.1469-8986.2008.00715.x. [DOI] [PubMed] [Google Scholar]
- Vögele C, von Leupoldt A. Mental disorders in chronic obstructive pulmonary disease (COPD) Respiratory Medicine. 2008;102:764–773. doi: 10.1016/j.rmed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Mertz C, Kegat S, Burmester S, Dahme B. The impact of emotions on the sensory and affective dimension of perceived dyspnea. Psychophysiology. 2006;43:382–386. doi: 10.1111/j.1469-8986.2006.00415.x. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Riedel F, Dahme B. The impact of emotions on the perception of dyspnea in pediatric asthma. Psychophysiology. 2006;43:641–644. doi: 10.1111/j.1469-8986.2006.00453.x. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Büchel C. The unpleasantness of perceived dyspnea is processed in the anterior insula and amygdala. American Journal of Respiratory and Critical Care Medicine. 2008a;177:1026–1032. doi: 10.1164/rccm.200712-1821OC. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Sommer T, Kegat S, Baumann HJ, Klose H, Dahme B, Büchel C. Breathlessness and pain: The same in the brain? Psychophysiology. 2008b;45(S1):S92. [Google Scholar]
- von Leupoldt A, Dahme B. Psychological aspects in the perception of dyspnea in obstructive pulmonary diseases. Respiratory Medicine. 2007;101:411–422. doi: 10.1016/j.rmed.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The relationship between respiratory-related evoked potentials and the perception of inspiratory resistive loads. Psychophysiology. 2000a;37:831–841. [PubMed] [Google Scholar]
- Webster KE, Colrain IM. The respiratory-related evoked potential: Effects of attention and occlusion duration. Psychophysiology. 2000b;37:310–318. [PubMed] [Google Scholar]
- Whitelaw WA, Derenne JP. Airway occlusion pressure. Journal of Applied Physiology. 1993;74:1475–1483. doi: 10.1152/jappl.1993.74.4.1475. [DOI] [PubMed] [Google Scholar]