Abstract
Genomic islands (GIs) and integrative conjugative elements (ICEs) are major players in bacterial evolution since they encode genes involved in adaptive functions of medical or environmental importance. Here we performed the genomic analysis of ICEVchBan8, an unusual ICE found in the genome of a clinical non-toxigenic Vibrio cholerae O37 isolate. ICEVchBan8 shares most of its genetic structure with SXT/R391 ICEs. However, this ICE codes for a different integration/excision module is located at a different insertion site, and part of its genetic cargo shows homology to other pathogenicity islands of V. cholerae.
Keywords: Integrative conjugative elements, Genomic islands, Lateral gene transfer, Vibrio cholerae
1. Introduction
Recent comparative genomics studies revealed that prokaryotic genomes are dynamic entities capable of acquiring and discarding large amounts of genetic material via lateral gene transfer (LGT) and recombination [1]. The role of LGT in the evolution of Vibrio cholerae, an autochthonous inhabitant of riverine and marine environments, as well as a human pathogen, is extensively documented [1–4]. In this species, major virulence genes and several important adaptive functions are known to be clustered in regions of the chromosome, laterally acquired from conspecific or distantly related organisms [5,6]. The extent of LGT was confirmed by the presence of Integrative conjugative elements (ICEs) and a large set of Genomic Islands (GIs) in V. cholerae sequenced genomes, contributing to genomic plasticity and generating a significantly heterogeneous group of strains in this species [1,5].
GIs are not self-transmitting gene clusters, since they are devoid of transfer genes, but they can excise and form circular intermediates [7–9]. They are of significant interest since they often encode genes of clinical importance, such as virulence factors, otherwise known as pathogenicity islands (PAI) [8]. Among all the V. cholerae associated PAIs, Vibrio pathogenicity island 2 (VPI-2) is present in toxigenic O1 strains, and occasionally in non-O1, non-O139 V. cholerae [10,11]. VPI-2 is a 57.3 kb island integrated at a tRNA-Ser, encoding 52 ORFs including proteins for release, transport, and catabolism of sialic acid [11].
Integrative conjugative elements (ICEs) are a class of mobile elements integrated into the prokaryotic host chromosome. They comprise mosaic genetic element with plasmid-, phage-, and transposon-like features organized in functional modules and are capable of self-transfer from a donor to a recipient cell via conjugation [12]. These elements were first described in V. cholerae with the discovery of the SXT element [13] and have since been found in the majority of V. cholerae and related Vibrio spp. [14,15]. All ICEs that have been described in V. cholerae to date belong to the SXT/R391 family [16], which share a conserved genetic scaffold of 52 genes that incorporates unique sequences coding for resistance to antibiotics and heavy metals, new toxin/antitoxin systems, restriction/modification systems, and alternative metabolic pathways [15,16].
In this study, we report the genomic analysis of ICEVchBan8, an unusual ICE present in the genome of the non-toxigenic clinical isolate V. cholerae O37 MZO-3, collected in Bangladesh in 2001 [1,15]. Unlike most V. cholerae non-O1, non-O139 isolates, strains of serogroup O37 have been shown to have epidemic potential [17,18]. V. cholerae MZO-3 lacks CTXΦ [1] but shows the presence and/or major rearrangements of two of the main pathogenicity islands found in V. cholerae 7th pandemic isolates: a novel version of Vibrio seventh pandemic island 2 (VSP-2) [19] and the replacement of VPI-2 with ICEVchBan8, which is the subject of the present work.
2. Materials and methods
2.1. ICE assembly, annotation and comparative genomics
Nucleotide sequence of ICEVchBan8 was obtained from the genome sequence of V. cholerae O37 strain MZO-3 (AAUU00000000) [1]. Gaps between two contigs were closed by manual editing using Consed and subjected to custom primer walk and PCR amplification. Complete nucleotide sequence of ICEVchBan8 was deposited in GenBank under Accession no. JQ345361.
ICE genetic analysis was accomplished in four steps: (i) ICEVchBan8 nucleotide sequence was aligned with SXT using NUCmer [20]; (ii) ORFs were identified and annotated using the RAST annotation pipeline (http://rast.nmpdr.org); (iii) ICE sequence and genetic organization was compared with published ICEs using the Artemis Comparative Tool (ACT) (www.sanger.ac.uk/Software/ACT); and (iv) similarity in nucleotide and protein sequences for ICEVchBan8 was determined as % nucleotide or amino acid identity with other ICEs in GenBank employing BLASTN and BLAST-PSI [21].
2.2. PCR assays
Chromosomal DNA was extracted as previously described [22]. Amplification was performed in an automated thermocycler (Bio-Rad MJ-Mini), and PCR conditions were performed in 50 µl reaction mix contained 1.3 U of DreamTaq DNA polymerase (Fermentas), according to manufacturer’s instructions. See Supplementary Table 1 for primer and amplicon details. To confirm accurate assembly of the two contigs and to close gaps between them, we designed different primers: pMZO_1F/pMZO3_1R for contig 1 assembly; pMZO3_1F2 /pMZO3_54R for contigs 1 and 54 junction. EnterotoxinB-F/s063R primer pair was designed to localize the 3′-end of Hotspot 4 insertion and AcriF/AcriR to confirm the presence of the acriflavine resistance gene.
Primers Ban8attP-F/Ban8attP-R were designed to investigate the capacity of ICEVchBan8 to form a circular intermediate (attP), and primers Ban8attB-F/Ban8attB-R were designed to detect the empty site on the bacterial chromosome (attB). The four primers were specifically designed to allow the combination of Ban8attP-F/Ban8attB-R and Ban8attP-R/Ban8attB-F in order to amplify the attL and attR junctions of the integrated ICE, respectively. Positive controls for excision experiments were strains V. cholerae O139 MO10 carrying SXT [13] and V. cholerae O1 7452 carrying ICEVchInd5 [23], using primers P4/P5 for attP amplification, as previously described [24].
Amplicons to be sequenced were directly purified from PCR by Nucleospin extract kit (Macherey–Nagel), according to manufacturer’s instructions. DNA sequences were obtained using an Applied Biosystems DNA sequencer 3730.
3. Results
3.1. Assembly and genomic organization of ICEVchBan8
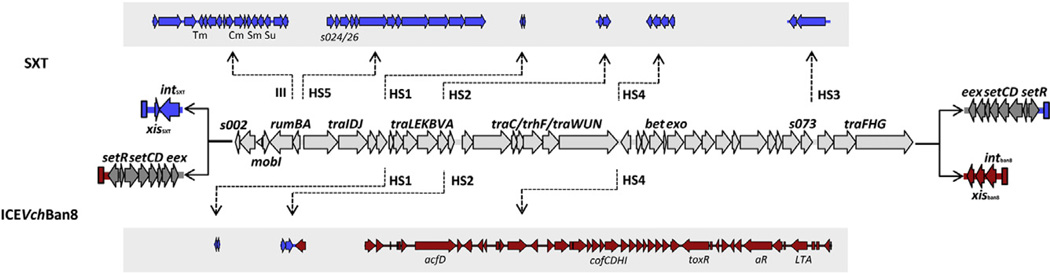
Comparative analysis of the genome of V. cholerae O37 MZO-3 revealed sequences belonging to the genetic backbone of ICEVchBan8 on two contigs: nt. 27439–105212 of contig 1 (Accession no. NZ_AAUU01000001.1), and nt. 1–25409 of contig 54 (Accession no. NZ_AAUU01000001.54). The overall genetic organization of this element had already been identified as similar to SXT/R391 ICEs, and named ICEVchBan8 [15]. Assembly of the ICE was accomplished using NUCmer with SXTM010 as reference and it revealed one gap at the junction between the two contigs that was resolved by PCR and confirmed by sequencing (Fig. 1). Our analysis revealed a 103.381 bp sequence (Accession no. JQ345361). The complete sequence of ICEVchBan8 was submitted to RAST pipeline for annotation and resulted in identification of 106 ORFs. Annotation was refined manually, comparing it with SXT and other ICEs of the SXT/R391 family found in V. cholerae [15]. ICEVchBan8 contains 47 out of 52 conserved core genes responsible for transfer and regulation of integration/excision in the SXT/R391 ICEs backbone (Fig. 1) [15]. Genes xis and int were replaced by a new integration/excision module (as described in the next section), whereas genes s024, s025 and s026 are missing. Genes s024 to s026 were previously annotated as hypothetical proteins, and reported as not involved in ICE conjugative transfer [25], suggesting a nonfunctional role in SXT biology.
Fig. 1.
Structural comparison between SXT and ICEVchBan8. A genetic map of core genes shared between the two ICEs (light gray) is shown. Specific regions for SXT and ICEVchBan8 are depicted in blue and red, respectively. Dark gray indicates inverted genes setR/eex. Hotspot insertions are indicated by dotted arrows, as shown in the above (SXT) and below (ICEVchBan8) the shared backbone.
Four operons of tra genes, encoding the conjugative apparatus (traIDJ, traLEKBA, traCFWUN, and traFHG) share at least 97% similarity at the nucleotide level with the tra clusters in the conserved SXT/R391 ICE backbone. As noted for all SXT-related ICEs, specific inserted genes were identified in five hotspots and four variable regions within the core backbone (Fig. 1) [15]. In ICEVchBan8, Hotspot 1 and Hotspot 2 have the same molecular arrangement as in SXT, with an additional gene in Hotspot 2 coding for a hypothetical protein, also found in ICEVspPor2 and ICEValPor1 from V. splendidus and V. alginolyticus [26]. Hotspot 3, and variable regions I, II and III do not have any insertions. At the 3′ end of the element, the variable region IV is disrupted by the rearrangement of setR/eex backbone genes. Overall, ICEVchBan8 holds some major rearrangements in its genetic structure, such as the substitution of the integration/excision module, location of the regulation module, and presence of a large cluster of genes in Hotspot 4 (see Section 3.4 for further description) never described before.
3.2. ICEVchBan8 integration/excision and regulation modules
To date, all ICEs described in V. cholerae belong to the SXT/R391 family, the distinguishing traits of which is the presence of a conserved integrase (intSXT) and the insertion at the prfC locus [16]. Analysis of ICEVchBan8 insertion site into MZO-3 genome showed its insertion at a different locus, a tRNA-Ser, leaving the prfC gene intact. In epidemic V. cholerae strains the tRNA-Ser locus is the insertion site of VPI-2 [11]. V. cholerae O37 strain MZO-3 lacks VPI-2 and is replaced by the ICE under study. The two 23 bp inverted sequences (attL and attR) flanking the element were almost identical to the flanking sequences of VPI-2. Furthermore, alignment of the nucleotide sequence of the flanking genes showed that insertion of new DNA occurs at the same base pair for both VPI-2 and ICEVchBan8, suggesting the two elements insert via a similar recombination event and by similar integrase activity.
The P4-like integrase gene, intBAN8, located at the 3′-end of ICEVchBan8, is different from intSXT. IntBAN8 showed low similarity with the integrase genes of V. parahaemolyticus RIMD 2210633 and V. cholerae VPI-2 integrase. At the amino acid level, intBAN8 was 75% and 74% similar to the phage integrases of Shewanella sp. MR-7 and V. cholerae VPI-2, respectively. Notably, there was no significant similarity at the amino acid level with any of the ICE integrases. In a recent study, Boyd and colleagues [7] reported results of a phylogenetic analysis of integrases distributed among different bacterial groups. Their finding showed that, with only a few exceptions, GI integrases cluster together and separate from phage, plasmid, integron, and ICE integrases. In their study intBAN8 clustered within the GI group instead of the SXT-like int group, yet on a separate branch from the V. cholerae VPI-2 integrases [7].
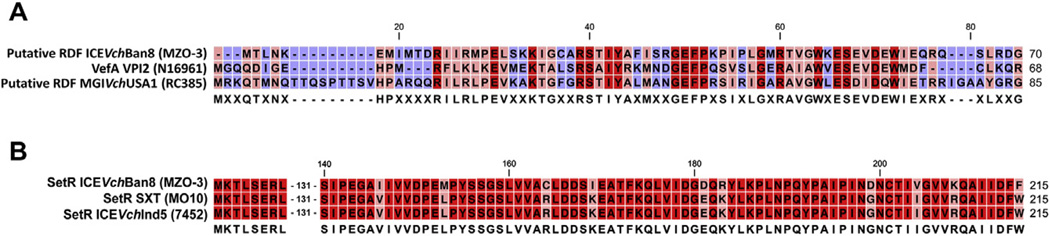
Downstream from the integrase gene, there is a small ORF of 189nt (AFD29101.1) showing no similarity at the nucleotide level with other ORFs in the databases. Considering its position and length, we interpreted the role of this gene as a putative recombination directionality factor (RDF). Low conservation at the nucleotide level is considered a general feature of RDF, as described for the xis gene encoded by SXT/R391 ICEs, which shares only general biochemical characteristics with other RDF [27,28]. At the amino acid level (see Fig. 2a), Blast-PSI revealed an alpA superfamily domain and significant similarity with several proteins annotated as alpA phage transcriptional regulators. Notably, the putative RDF showed 47% (28/60) similarity with vefA of V. cholerae N16961 VPI-2, recently recognized as the main RDF involved in the excision of VPI-2 [29]. This ORF was also 55% (30/55) similar to the putative RDF of MGIVchUsa1, a newly described genetic element in V. cholerae mobilized by SXT/R391 ICEs [30]. This suggests the gene functions as RDF during ICEVchBan8 excision, therefore annotated as xisBAN8, and belongs to a large family of proteins functionally related to the RDF of Escherichia coli prophage alpA, potentially acquired and exchanged by lateral gene transfer by phages, GI and ICEs.
Fig. 2.
Muscle alignment of the predicted amino acid sequences of: (a) xisBAN8, VefA of VPI-2 from V. cholerae N16961 and putative RDF of MGIVchUSA1 from V. cholerae RC385 and (b) SetR of ICEVchBan8, SetR of SXT from V. cholerae MO10 and SetR of ICEVchInd5 from V. cholerae 7452. The first 145aa of SetR are identical in all the three sequences. Amino acids conserved in all sequences are shown in red; amino acids conserved in two of three sequences are shown in pink. Gaps and amino acids divergent in all three sequences are shown in blue. Consensus sequence is depicted below the alignment.
The regulation module (setRCD/eex) of ICEVchBan8 showed a major rearrangement since it is unusually located at the 5′-end of the element, with setR being the first ORF of the element (Fig. 1). This peculiar genetic arrangement differs from the conserved backbone of SXT-related ICEs, in which the setCDR operon is located at the 3′-end of the element. Interestingly, gene setR shows a very high mutation rate at the C-terminal (Fig. 2b) which is surprising considering the high level of conservation of setR in all SXT/R391 ICEs [15]. Being setR the key regulator of SXT/R391 ICEs mobility, these mutations together with the presence of a different int/xis module might have an important role in the ICE transfer gene regulation.
3.3. ICEVchBan8 is able to form a circular intermediate
ICEs of the SXT/R391 family integrate and excise from the chromosome via a circular extrachromosomal intermediate [24]. Although sharing a common backbone with this family of ICEs, ICEVchBan8 shows important rearrangements in the integration/excision module that might affect this functional feature of the element. PCR was used to determine whether a similar circular intermediate is formed after excision of ICEVchBan8 from the host chromosome. Primers oriented towards the right and left ICEVchBan8-chromosome junctions were designed (Ban8attP-F/Ban8attP-R) to detect an extrachromosomal intermediate detected by the attP site. Furthermore, primers Ban8attB-F/Ban8attB-R were used to amplify the empty insertion site on the chromosome (attB). Using these primers, a PCR product of 283 bp was amplified for attP, whereas an amplicon of 613 bp was retrieved for attB (Supplementary Fig. 1, lanes 1 and 2, respectively), thus indicating the ability of ICEVchBan8 to excise from the chromosome. As a further control, we performed a PCR assay combining Ban8attP-F with Ban8attB-R, and Ban8attP-R with Ban8attB-F, to identify attL (524 bp) and attR (373 bp) junctions of the integrated ICEVchBan8 in the host chromosome, respectively (Supplementary Fig. 1, lane 3). These data indicate that a circular extrachromosomal form of ICEVchBan8 is present in cells harboring the integrated form of the element.
3.4. Hotspot 4 organization
Hotspot 4, located between genes traN and s063, is characterized by a unique 49.5 kb region (Fig. 1) that includes 45 ORFs (see Supplementary Table 2 for a complete description of the hotspot content). Overall this hotspot merited more detailed analysis. Bioinformatics analysis of its genetic content revealed several ORFs related to known virulence determinants and pathogenic cellular properties.
Cellular transport
HS4 encodes for a replication protein and three membrane permeases containing ABC-transporter domain common in OmpA/B protein found in several Vibrio species (AFD29034.1 to AFD29037.1). Furthermore, three putative accessory colonization factors, with similarity to Acf found in other Vibrios, are interspersed in the hotspot (AFD29040.1, AFD29043.1, and AFD29065.1).
Type IV pilus assembly cluster
This cluster contains 11 ORFs (AFD29051.1 to AFD29061.1), with similarity to proteins involved in the formation of pili, i.e., Longus, the CFA III of ETEC, the toxin-coregulated pilin (TCP) of V. cholerae, and the bundle-forming pilin (BFP) found in enteropathogenic E. coli (EPEC). These structures are known to be involved in essential features of pathogenicity such as twitching, motility/adherence to host cells, biofilm formation, cell signaling, invasion, and evasion of the immune system.
Metallopeptidase
A putative ORF of 2922 bp (AFD29067.1) was annotated as a tagA gene, encoding a ToxR-activated A protein with low similarity to gene stcE found in plasmids of E. coli O157:H7 and Shigella boydii. StcE belongs to the family of metallopeptidases involved in the pathogenesis of these organisms.
Acriflavine resistance family protein
This ORF (AFD29072.1) is 71–78% similar to the AcrB/AcrD/AcrF family proteins involved in acriflavine resistance. In V. cholerae MZO3, the transcriptional regulator usually associated with this gene is truncated (AFD29071.1).
CtxA
This 1806 bp ORF (AFD29075.1) contains a functional domain found in enterotoxins like the heat labile (LT) toxin of E. coli and the CTX subunit A of V. cholerae. The second part of the protein showed weak similarity with the PspC and CbpA surface proteins involved in immune response activation and host-pathogen relationship of Streptococcus pneumoniae in pneumococcal bacteremia.
4. Discussion
Based on the results reported here, ICEVchBan8 displays a unique genetic organization, yet conserving 47 of the 52 core genes of the SXT/R391 ICEs.
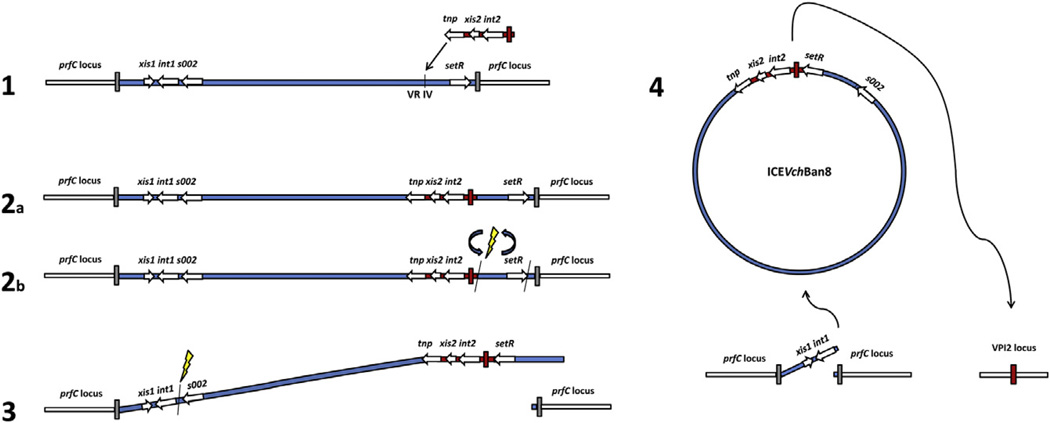
The presence of a new integration/excision module (xis/int) and the complete inversion and rearrangement of the regulation cluster (eex/setR), raise questions about evolution and classification. We propose a hypothetical model to elucidate the mechanism that might have led to the formation of this hybrid element. The model is organized into four subsequent steps (Fig. 3), with an initial recombination event involving acquisition of new genetic material into the variable region IV of a prototype SXT/R391 ICE. This initial recombination event is followed by substitution of the integration/excision module, an anomalous rearrangement and excision of the ICE, and reintegration at a different site.
Fig. 3.
Hypothetical model of ICEVchBan8 formation. (1) A fragment of an unknown mobile genetic element (shown in red) transposes into the variable region IV (VR IV) of the SXT/R391 precursor of ICEVchBan8 (shown in blue), with the same mechanism responsible for acquisition of the new genetic material in the hotspots. (2a or 2b) During transposition or in an unknown subsequent event, the 3′ region of ICEVchBan8 precursor undergoes inversion rearrangement. This inversion causes loss of the old attR site, triggering excision of the rearranged element from the prfC locus. (3) During abnormal excision, the ICE loses the 5′ region containing the original integration module (intSXT). This event may also result from competition between two integration modules in the linear or circular form. (4) Reintegration of the newborn ICEVchBan8 in VPI-2 locus is catalyzed by the new integrase (intBan8).
Even though the model is speculative, it is supported by the well-documented ability of the SXT/R391 ICEs to incorporate new genetic material into conserved hotspots and recombine during integration/excision [31]. It is also known that SXT/R391 ICEs encode an additional recombination machinery involved in hybrid formation, which is conserved in the ICEVchBan8 backbone (bet and exo) [31]. In contrast to ICEVchBan8, recombination events in other SXT/R391 ICEs maintain synteny of the backbone without loss of core genes [15]. However, the ICE is integrated at a different locus of the V. cholerae genome than the prfC locus and has an integrase gene not related to intSXT. Therefore, ICEVchBan8 could not be classified as a member of the SXT/R391 family that includes all of the ICEs that have been described in V. cholerae to date. However, we chose to retain the proposed nomenclature [15,16] since, most likely, ICEVchBan8 is an atypical member of the SXT/R391 family that has undergone major recombination events. Moreover, differences between the SXT-related elements are not that important to classify ICEVchBan8 as a new type of ICE or GI.
ICEVchBan8 holds a GI integration module and is inserted in a t-RNA-Ser. This peculiar organization suggests that recombination events may have generated this ‘hybrid’ element compromising the boundaries between the different classes of mobile elements. Previous studies investigating correlation between GIs and ICEs have shown the pathogenicity islands of Legionella pneumophila, Agrobacterium tumefaciens, and Bartonella tribocorum, to be either ICEs or defective ICEs [32]. The hypothesis that some GIs are derived from ICEs that have lost their transfer ability is also supported by the discovery of ICEEc1, an ICE found in a strain of E. coli. ICEEc1 includes the high pathogenicity island HPI, previously described in Yersinia, but with an additional transfer module able to self-mobilize by conjugation with the entire element [33].
It is concluded that mobile elements previously believed to be divergent in evolution may have instead followed the same path in selected genomes and, thus, recombination can occur between elements otherwise assigned to different families.
Supplementary Material
Acknowledgments
Support for the research was provided by National Institutes of Health Grant No. 1 R01 A139129-01 (R.R.C.) and by Ministero dell’ Istruzione, dell’ Universita e della Ricerca – Italy (M.M.C.).
E.T. received a PhD graduate assistentship from the Graduate School of University of Maryland, USA, and was supported by the NIH Grant 2RO1A1039129-11A2. M.S. is the recipient of a PhD fellowship from the Doctorate School in Cellular and Developmental Biology, Sapienza Università di Roma. D.C. was supported by a fellowship from Institute Pasteur – Fondazione Cenci Bolognetti, Italy.
Appendix A
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.febslet.2012.03.064.
References
- 1.Chun J, et al. Comparative genomics reveals mechanism for short-term and long-term clonal transitions in pandemic Vibrio cholerae. Proc. Natl. Acad. Sci. USA. 2009;106:15442–15447. doi: 10.1073/pnas.0907787106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Colwell R, Spira W. In: Cholera. Barua D, Greenough W, editors. New York: Plenum Medical Book Co; 1992. pp. 107–127. [Google Scholar]
- 3.Faruque SM, Albert MJ, Mekalanos JJ. Epidemiology, genetics, and ecology of toxigenic Vibrio cholerae. Microbiol. Mol. Biol. Rev. 1998;62:1301–1314. doi: 10.1128/mmbr.62.4.1301-1314.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaper JB, Glenn Morris JR, Levine MM. Cholera. Clin. Microbiol. Rev. 1995;8:48–86. doi: 10.1128/cmr.8.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho YJ, Yi H, Lee JH, Kim DW, Chun J. Genomic evolution of Vibrio cholerae. Curr. Opin. Microbiol. 2010;13:646–651. doi: 10.1016/j.mib.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Davis BM, Waldor MK. CTXphi contains a hybrid genome derived from tandemly integrated elements. Proc. Natl. Acad. Sci. USA. 2000;97:8572–8577. doi: 10.1073/pnas.140109997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boyd EF, Almagro-Moreno S, Parent MA. Genomic islands are dynamic, ancient integrative elements in bacterial evolution. Trends Microbiol. 2009;17:47–53. doi: 10.1016/j.tim.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Dobrindt U, Hochhut B, Hentschel U, Hacker J. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2004;2:414–424. doi: 10.1038/nrmicro884. [DOI] [PubMed] [Google Scholar]
- 9.Hentschel U, Hacker J. Pathogenicity islands: the tip of the iceberg. Microbes Infect. 2001;3:545–548. doi: 10.1016/s1286-4579(01)01410-1. [DOI] [PubMed] [Google Scholar]
- 10.Jermyn WS, Boyd EF. Characterization of a novel Vibrio pathogenicity island (VPI-2) encoding neuraminidase (nanH) among toxigenic Vibrio cholerae isolates. Microbiology. 2002;148:3681–3693. doi: 10.1099/00221287-148-11-3681. [DOI] [PubMed] [Google Scholar]
- 11.Murphy RA, Boyd EF. Three pathogenicity islands of Vibrio cholerae can excise from the chromosome and form circular intermediates. J. Bacteriol. 2008;190:636–647. doi: 10.1128/JB.00562-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wozniak RAF, Waldor MK. Integrative and conjugative elements: mosaic mobile genetic elements enabling dynamic lateral gene flow. Nat. Rev. Microbiol. 2010;8:552–563. doi: 10.1038/nrmicro2382. [DOI] [PubMed] [Google Scholar]
- 13.Waldor MK, Tschape H, Mekalanos JJ. A new type of conjugative transposon encodes resistance to sulfamethoxazole, trimethoprim, and streptomycin in Vibrio cholerae O139. J. Bacteriol. 1996;178:4157–4165. doi: 10.1128/jb.178.14.4157-4165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taviani E, Ceccarelli D, Lazaro N, Bani S, Cappuccinelli P, Colwell RR, Colombo MM. Environmental Vibrio spp., isolated in Mozambique, contain a polymorphic group of integrative conjugative elements and class 1 integrons. FEMS Microbiol. Ecol. 2008;64:45–54. doi: 10.1111/j.1574-6941.2008.00455.x. [DOI] [PubMed] [Google Scholar]
- 15.Wozniak RAF, et al. Comparative ICE genomics: insights into the evolution of the SXT/R391 family of ICEs. PLoS Genet. 2009;5:e1000786. doi: 10.1371/journal.pgen.1000786. http://dx.doi.org/1000710.1001371/journal.pgen.1000786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrus V, Marrero J, Waldor MK. The current ICE age: biology and evolution of SXT-related integrating conjugative elements. Plasmid. 2006;55:173–183. doi: 10.1016/j.plasmid.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Aldová E, Láznicková K, Stěpánková E, Lietava J. Isolation of nonagglutinable vibrios from an enteritis outbreak in Czechoslovakia. J. Infect. Dis. 1968;118:25–31. doi: 10.1093/infdis/118.1.25. [DOI] [PubMed] [Google Scholar]
- 18.Kamal A. Outbreak of gastroenteritis by non-agglutinable (NAG) vibrios in the Republic of Sudan. J. Egypt Public Health Assoc. 1971;46:125–173. [Google Scholar]
- 19.Taviani E, Grim CJ, Choi J, Chun J, Haley B, Hasan NA, Huq A, Colwell RR. Discovery of novel Vibrio cholerae VSP-II genomic islands using comparative genomic analysis. FEMS Microbiol. Lett. 2010;308:130–137. doi: 10.1111/j.1574-6968.2010.02008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delcher AL, Salzberg SL, Phillippy AM. Current Protocols in Bioinformatics. John Wiley & Sons, Inc; 2002. [Google Scholar]
- 21.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. In: Current Protocols in Molecular Biology. Sons JWA, editor. New York: Green Publishing Associates and Wiley; 1990. [Google Scholar]
- 23.Ceccarelli D, et al. ICEVchInd5 is prevalent in epidemic Vibrio cholerae O1 El Tor strains isolated in India. Int. J. Med. Microbiol. 2011;301:318–324. doi: 10.1016/j.ijmm.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Hochhut B, Waldor MK. Site-specific integration of the conjugal Vibrio cholerae SXT element into prfC. Mol. Microbiol. 1999;32:99–110. doi: 10.1046/j.1365-2958.1999.01330.x. [DOI] [PubMed] [Google Scholar]
- 25.Beaber JW, Hochhut B, Waldor MK. Genomic and functional analyses of SXT, an integrating antibiotic resistance gene transfer element derived from Vibrio cholerae. J. Bacteriol. 2002;184:4259–4269. doi: 10.1128/JB.184.15.4259-4269.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Blanco A, Lemos ML, Osorio CR. Integrating Conjugative Elements (ICEs) as vectors of antibiotic, mercury and quaternary ammonium compounds resistance in marine aquaculture environments. Antimicrob. Agents Chemother. 2012;56:2619–2626. doi: 10.1128/AAC.05997-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burrus V, Waldor MK. Control of SXT integration and excision. J. Bacteriol. 2003;185:5045–5054. doi: 10.1128/JB.185.17.5045-5054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Halloran JA, McGrath BM, Pembroke JT. The orf4 gene of the enterobacterial ICE, R391, encodes a novel UV-inducible recombination directionality factor, Jef, involved in excision and transfer of the ICE. FEMS Microbiol. Lett. 2007;272:99–105. doi: 10.1111/j.1574-6968.2007.00747.x. [DOI] [PubMed] [Google Scholar]
- 29.Almagro-Moreno S, Napolitano M, Boyd E. Excision dynamics of Vibrio pathogenicity island-2 from Vibrio cholerae : role of a recombination directionality factor VefA. BMC Microbiol. 2010;10:306. doi: 10.1186/1471-2180-10-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daccord A, Ceccarelli D, Burrus V. Integrating conjugative elements of the SXT/R391 family trigger the excision and drive the mobilization of a new class of Vibrio genomic islands. Mol. Microbiol. 2010;78:576–588. doi: 10.1111/j.1365-2958.2010.07364.x. [DOI] [PubMed] [Google Scholar]
- 31.Garriss G, Waldor MK, Burrus V. Mobile antibiotic resistance encoding elements promote their own diversity. PLoS Genet. 2009;5:e1000775. doi: 10.1371/journal.pgen.1000775. http://dx.doi.org/1000710.1001371/journal.pgen.1000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burrus V, Waldor MK. Shaping bacterial genomes with integrative and conjugative elements. Res. Microbiol. 2004;155:376–386. doi: 10.1016/j.resmic.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 33.Schubert S, Dufke S, Sorsa J, Heesemann J. A novel integrative and conjugative element (ICE) of Escherichia coli: the putative progenitor of the Yersinia high-pathogenicity island. Mol. Microbiol. 2004;51:837–848. doi: 10.1046/j.1365-2958.2003.03870.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.