Abstract
Studies of cognition often use an “oddball” paradigm to study effects of stimulus novelty and significance on information processing. However, an oddball tends to be perceptually more novel than the standard, repeated stimulus as well as more relevant to the ongoing task, making it difficult to disentangle effects due to perceptual novelty and stimulus significance. In the current study, effects of perceptual novelty and significance on ERPs were assessed in a passive viewing context by presenting repeated and novel pictures (natural scenes) that either signaled significant information regarding the current context or not. A fronto-central N2 component was primarily affected by perceptual novelty, whereas a centro-parietal P3 component was modulated by both stimulus significance and novelty. The data support an interpretation that the N2 reflects perceptual fluency and is attenuated when a current stimulus matches an active memory representation and that the amplitude of the P3 reflects stimulus meaning and significance.
INTRODUCTION
Studies of orienting and attention often use a “repetition-change” or “oddball” paradigm to study effects of perceptual novelty on cognitive processing. In this paradigm, an infrequent oddball target is presented amidst a series of repetitive, frequently presented “standard” stimuli. The brain’s response to the novel stimulus includes an anterior negativity occurring around 200 msec (N2) and a later positivity over centro-parietal sensors (P3), prompting the hypothesis that the N2–P3 complex may index orienting to novel stimuli (e.g., Näätänen & Gaillard, 1983; Duncan-Johnson & Donchin, 1977; Squires, Petuchowski, Wickens, & Donchin, 1977; Snyder & Hillyard, 1976).
Stimulus novelty has been operationalized not only in terms of infrequent or rare presentation but also more broadly in terms of exposure to unfamiliar stimuli (Chong et al., 2008; Nittono, Shibuya, & Hori, 2007; Daffner, Mesulam, et al., 2000; Daffner, Scinto, et al., 2000; Daffner et al., 1998; Courchesne, Courchesne, & Hillyard, 1978; Courchesne, Hillyard, & Galambos, 1975). For instance, Daffner, Scinto, et al. (2000) covaried the frequency of stimulus occurrence (rare/standard) as well as stimulus familiarity (unusual pattern/simple geometric figures) and found the largest N2 for infrequent unfamiliar targets, whereas P3 amplitude was primarily modulated by frequency. Similarly, Breton, Ritter, Simson, and Vaughan (1988) found a larger N2 for novel infrequent targets that varied across the experiment compared with identical infrequent targets, whereas P3 was again primarily modulated by frequency in the oddball paradigm.
In the oddball paradigm, the change to a rare target following a series of standard stimuli involves both perceptual novelty compared with the repetitive stream of preceding standard stimuli as well as change in significance because the rare stimulus is often relevant to the ongoing task. Thus, rare stimuli in oddball tasks tend to be both perceptually more novel as well as more significant than the standard background stimulus, making it difficult to disentangle effects of perceptual novelty and stimulus significance on information processing.
The present study was designed to assess effects of perceptual novelty and significance on information processing when people view pictures of natural scenes. Perceptual novelty was manipulated by repeatedly presenting the same picture or by presenting a series of novel pictures. Participants were simply instructed to view each picture while it was on the screen. The first repetition of a picture (i.e., “change-to-repeated”) was meaningful in that it always signaled the beginning of a repetitive series. Relatedly, the first presentation of a novel picture (i.e., “change-to-novel”) was meaningful in that it always necessarily signaled the end of a repetitive series and the beginning of a novel series. Thus, these two types of pictures signaled meaningful information regarding upcoming events. Following presentation of these informative pictures, the “simply novel” and the “simply repeated” pictures in each series allowed an assessment of the effect of perceptual novelty on information processing.
We measured ERPs as indices of cognitive processing that might index perceptual novelty, significance, or both. Among the numerous ERP subcomponents belonging to the broad N2 family (N2a, N2b, N2c, and N2pc; Folstein & Van Petten, 2008; Luck, 2005), a centro-frontal negative component labeled N2b (Pritchard, Shappell, & Brandt, 1991) is reliably modulated by stimulus novelty, when defined by frequency in the experimental series (Folstein & Van Petten, 2008; Daffner et al., 1998; Czigler, Csibra, & Ambró, 1996; Breton et al., 1988; Courchesne et al., 1975; Squires, Squires, & Hillyard, 1975), and has been interpreted as resulting from a perceptual mismatch between the repetitive standard and the infrequent target (Breton et al., 1988; Näätänen & Gaillard, 1983; Courchesne et al., 1975).
Later positive components (around 300 msec) that are reliably modulated by infrequent stimuli typically include two subcomponents, a slightly earlier frontal P3 (e.g., P3a; Polich, 2007; Comerchero & Polich, 1998, 1999; Knight, 1984; Courchesne et al., 1975; Squires et al., 1975) and a later, centro-parietal P3 (e.g., P3b or P300; Kok, 2001; Donchin & Coles, 1988; Courchesne et al., 1978). A well-established finding is a larger centro-parietal P3b for rare compared with standard stimuli, which is often interpreted as reflecting a process that is sensitive to changes in the current context (e.g., context updating; Donchin & Coles, 1988). An unexpected as well as unusual stimulus can also elicit a more frontal P3-like potential (P3a; Courchesne et al., 1975; Squires et al., 1975), which depends not only on probability (rare) but also on its status as a nontarget in an oddball paradigm. Importantly, its appearance also appears to depend upon the difficulty of the target discrimination (Sawaki & Katayama, 2006, 2007; Polich & Comerchero, 2003; Katayama & Polich, 1998), which suggests that it would not be easily apparent in a passive viewing context such as that used here.
In the current design, pictures in the simply repeated condition are somewhat similar to standard stimuli in the oddball design in that they involve contiguous repetition of nontarget stimuli, whereas pictures in the change-to-novel condition more closely resemble the oddball stimuli in that they immediately follow a series of repeated stimuli (i.e., standards) and are meaningful in the experimental context. Thus, consistent with previous studies, we expected to find both components of the N2–P3 complex when ERPs in these two conditions were compared. The more interesting questions concern conditions in which perceptual novelty and stimulus significance are controlled: Will the same N2–P3 complex be found when comparing novel and repeated pictures that are equated for significance? Or will N2 and P3 vary with specific factors? If perceptual novelty differentially affects processing, we expected that N2 would differ for novel and repeated pictures, regardless of significance. On the other hand, if stimulus significance is more likely to be reflected in the P3, we expected larger amplitude for stimuli signaling a change, regardless of whether these were novel or repeated.
METHODS
Participants
Participants were 25 students (13 women) from a General Psychology course at the University of Florida who participated for course credit.
Materials and Design
The stimuli were 168 pictures selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005), depicting a wide variety of contents (e.g., indoor and outdoor scenes, pictures of food, cars, animals, and people) and representing an equal number of emotionally arousing (pleasant and unpleasant) and neutral pictures.
Repeated pictures were presented from five to eight times in a row; novel pictures were presented in series of six pictures. Throughout the study, there were 24 sets of repeated pictures and 24 sets of novel pictures (300 trials), with a set of novel pictures following sets of repeated pictures.
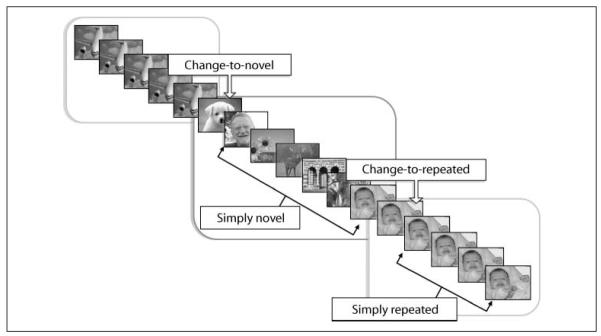
Novel and repeated pictures were either informative in terms of signaling a change in the upcoming block or not. More specifically (see Figure 1), the first presentation of a novel picture always signaled the end of a repetitive series and the beginning of a series of novel pictures (change-to-novel); similarly, the second presentation (i.e., first repetition) of an identical picture always signaled that a series of repetitive pictures was beginning (change-to-repeated). Following each “informative” cue, novel (simply novel) and repeated (simply repeated) varied primarily in terms of perceptual novelty.
Figure 1.
Schematic representation showing the sequence of experimental events. The first presentation of a novel picture always signaled the end of a repetitive series and the beginning of a series of novel pictures (“change-to-novel”); succeeding novel pictures were “simply novel.” The second presentation (i.e., first repetition) of an identical picture always signaled that a series of repetitive pictures was beginning (“change-to-repeated”); succeeding repeated pictures were “simply repeated.”
Using the same 168 pictures, three presentation orders were constructed that varied the specific pictures presented in the repeated and novel sets across participants. The order of picture presentation of novel pictures was balanced such that there were three pictures of each affective category in each block of six. In all conditions, there were an equal number of emotionally arousing and neutral pictures.
Each trial consisted of a fixation cross presented at the center of the screen for 500 msec before picture onset. Each picture was displayed for 2 sec followed by a 2-sec intertrial interval. Pictures were presented on a 19-in. CRT monitor situated approximately 100 cm from the participant.
An acoustic startle probe was presented at 1200 msec after picture onset on 16% of the trials, both during repeated and novel picture viewing. These data are not reported here.
Physiological Recording and Data Reduction
Electroencephalographs were collected from the scalp using a 128-channel system (Electrical Geodesics, Inc., Eugene, OR) running NetStation software on a Macintosh computer. Scalp impedance for each sensor was kept below 50 kΩ. The EEG was recorded continuously with a sampling rate of 250 Hz, the vertex sensor as reference electrode, and on-line band-pass filtered from 0.01 to 100 Hz. EEG data were analyzed off-line using a MATLAB-based program (Junghöfer & Peyk, 2004; Junghöfer, Elbert, Tucker, & Rockstroh, 2000). Continuous EEG data were low-pass filtered at 40 Hz using digital filtering, and artifact detection was performed by means of a dedicated algorithm that uses statistical parameters to determine trials with artifacts (Junghöfer et al., 2000). Processed data were then transformed to an average reference and baseline corrected (200 msec before picture onset) before subject averaging and analysis.
Procedure
After arrival at the laboratory, participants signed an informed consent form. Participants were then seated in a recliner in a small, sound-attenuated, dimly lit room, and the EEG sensor net was attached. The participant was instructed that a series of pictures would be presented and that each picture should be viewed the entire time it was on the screen and that brief noises heard over the headphones could be ignored. After the picture series was finished, a postexperimental questionnaire was completed, and the subject was debriefed.
Data Analysis
For each subject, trials were averaged separately based on the factorial combination of perceptual novelty and stimulus significance resulting in four conditions: (1) change-to-novel, the first picture of a novel series, which was both novel and signaled a change in the experimental structure; (2) simply novel, the remaining pictures in a novel set, which were novel, but did not signal a change in the experimental structure; (3) change-to-repeated, the second presentation (i.e., first repetition) of a repeated picture, which was repeated and signaled a change in the experimental structure; and (4) simply repeated, the remaining repetitions of a repeated picture.
Statistical analyses were performed by averaging a group of sensors over the area where each ERP component showed its maximal amplitude (Picton et al., 2000). Thus, N2 amplitude was assessed over centro-frontal sensors (see Figure 2, top) in a 250- to 350-msec time window following picture onset, and P3 amplitude was assessed over centro-parietal sensors (see Figure 2, bottom) in a 350- to 550-msec time window following picture onset.
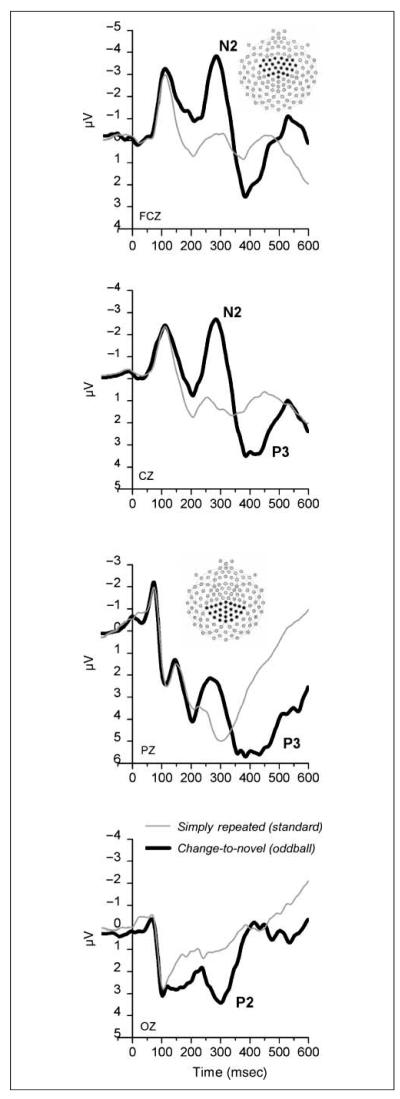
Figure 2.
Grand average ERPs at four midline electrode sites for the conditions most similar to standard (i.e., “simply repeated”) and rare (i.e., “change-to-novel”) stimuli in the oddball design. As expected, change-to-novel stimuli (i.e., oddball) showed a larger N2–P3 complex compared with simply repeated stimuli (i.e., standards). Insets show the group of sensors used for statistical analysis.
Both N2 and the P3 mean amplitudes were analyzed in separate repeated measure ANOVAs, which covaried perceptual novelty (2, Novel and Repeated) and stimulus significance (2, Change and No change). Greenhouse–Geisser corrections were applied where relevant. The partial eta squared statistic (η2), indicating the proportion between the variance explained by one experimental factor and the total variance, has been calculated and reported.
RESULTS
Figure 2 illustrates the grand average ERPs at four midline electrode sites in the simply repeated and change-to-novel conditions, which are most similar to “standard” and “rare” stimuli in the typical oddball design. Consistent with the expectation that there would be evidence of differences in both N2 and P3 in this comparison, there was a clear difference in the amplitude of the N2 component, which showed a maximum peak around 280 msec over centro-frontal electrode sites1 and was larger in the change-to-novel compared with the simply repeated condition, F(1,24) = 50.4, p < .0001, η2 = .68. In addition, P3 amplitude, which peaked around 400 msec over centro-parietal electrode sites, was also larger in the change-to-novel compared with the simply repeated condition, F(1,24) = 70.5, p < .0001, η2 = .75.
Centro-frontal N2
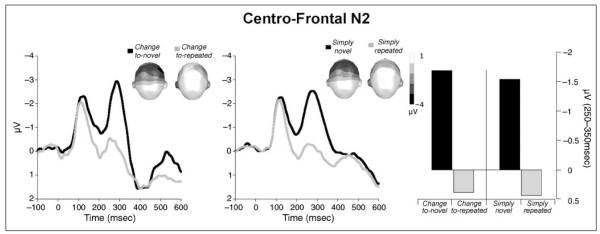
When both perceptual novelty and stimulus significance were included as factors in an ANOVA of N2 amplitude,2 only perceptual novelty modulated the amplitude of the N2 component, F(1,24) = 73.94, p < .0001, η2 = .75. As illustrated in Figure 3, N2 amplitude was smaller for repeated compared with novel pictures regardless of stimulus significance. Thus, N2 amplitude was smaller for repeated compared with novel pictures when these pictures signaled a change in the experimental structure (i.e., change-to-novel vs. change-to-repeated), F(1,24) = 41.61, p < .0001, η2 = .63, as well as when they did not signal a change (i.e., simply novel vs. simply repeated), F(1,24) = 49.72, p < .0001, η2 = .67.
Figure 3.
Grand average ERP waveforms over centro-frontal sensors for novel and repeated pictures that signaled a significant change in the experimental context (left) or did not (middle). Topographic plots (top view) of the scalp voltage in the N2 window (250–350 msec) are included in each figure. Right panel: Mean N2 amplitude over centro-frontal sensors was attenuated for repeated compared with novel pictures, regardless of significance.
On the other hand, stimulus significance had no effect on the N2 amplitude, and there was no interaction between perceptual novelty and stimulus significance.
Centro-parietal P3
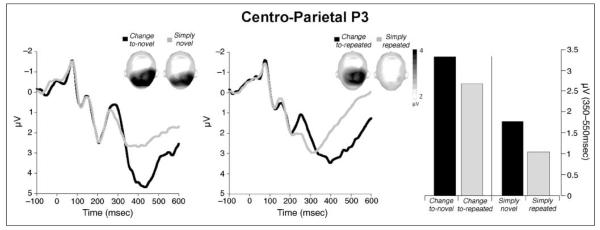
When stimulus novelty and stimulus significance were included as factors in an ANOVA of P3 amplitude,3 stimuli signaling a change in the experimental structure elicited a larger centro-parietal P3 than those that did not signal a change, F(1,24) = 54.55, p < .0001, η2 = .69. As illustrated in Figure 4, P3 amplitude was larger in the change-to-novel compared with the simply novel condition, F(1,24) = 41.68, p < .0001, η2 = .63, and in the change-to-repeated compared with the simply repeated condition, F(1,24) = 23.86, p < .0001, η2 = .49.
Figure 4.
Grand average ERP waveforms over centro-parietal sensors for novel pictures (left) and repeated pictures (middle) that signaled a significant change in the experimental context or did not. Topographic plots (top view) of the scalp voltage in the P3 window (350–550 msec) are included in each figure. Right panel: Mean P3 amplitude was larger for pictures that signaled a change compared with those that did not.
Moreover, stimulus novelty also affected P3 amplitude, F(1,24) = 17.95, p < .0001, η2 = .43, with larger P3 amplitudes for novel compared with repeated pictures, whether these signaled a change, F(1,24) = 6.2, p < .05, or did not, F(1,24) = 8.6, p < .01.
There was no interaction between perceptual novelty and stimulus significance.
DISCUSSION
In this study, we assessed the effects of stimulus novelty and stimulus significance on the amplitude of the N2–P3 complex. In the classic oddball design, these two factors typically covary because a rare stimulus tends to be both perceptually more novel as well as more significant to the ongoing task than the standard, repetitive stimulus. In fact, when pictures in the simply repeated condition (i.e., standard) were compared with those in the change-to-novel condition (oddball), a robust difference in both N2 and P3 were obtained, consistent with previous studies. On the other hand, when significance was controlled, the N2 component of the ERP was strongly affected by perceptual novelty. P3 amplitude, however, was significantly modulated by both stimulus significance and perceptual novelty.
N2
The data suggest that the N2 is specifically modulated by perceptual novelty, consistent with other recent studies that have found no effects of task relevance on N2 amplitude when novelty is controlled (Chong et al., 2008; Folstein, Van Petten, & Rose, 2008). Whereas the heightened N2 amplitude for novel stimuli is often interpreted as reflecting a mismatch between the current stimulus and a mental template (Folstein & Van Petten, 2008; Daffner, Mesulam, et al., 2000; Daffner, Scinto, et al., 2000; Breton et al., 1988; Näätänen & Gaillard, 1983; Courchesne et al., 1975), the current data suggest that the emphasis might be better placed on the dramatic reduction in the N2 for repeated stimuli. That is, following a single repetition (i.e., change-to-repeated), N2 attenuation was equivalent to when a picture had been repeated up to six times (i.e., simply repeated). A similar dramatic absence of N2 following a single repetition of a stimulus was reported by Wang, Cui, Wang, Tian, and Zhang (2004) who presented serial pairs of cues in a same–different task.
Taken together, the data suggest that the attenuating effect of stimulus repetition on the amplitude of the centro-frontal N2 component might reflect heightened perceptual fluency (Jacoby & Dallas, 1981), in which an active memory representation contributes, in a top–down fashion, to the perceptual processing of the current stimulus. According to this hypothesis, attenuation of centro-frontal N2 amplitude primarily reflects a template match in which perceptual information available in memory facilitates current processing. If perceptual fluency modulates N2 amplitude, effects of stimulus familiarity on this component are also expected, consistent with data reported by Daffner, Scinto, et al. (2000) in which the largest N2 amplitudes occurred for targets that were both infrequent and unusual. Moreover, Daffner, Scinto, et al. (2000) found larger N2 amplitudes for very unusual standard stimuli compared with rare oddballs that were simple geometric figures, suggesting that perceptual encoding of unusual or extremely unfamiliar stimuli may not benefit greatly from mere stimulus repetition. On the other hand, perceptual fluency (based on experience) should be high for very simple stimuli, such as common geometric figures, even if these are only rarely presented in a specific experimental context. Taken together, the data are consistent with the hypothesis that N2 amplitude reflects perceptual fluency and is attenuated when encoding is facilitated in a top–down manner by active memory representations and is amplified when perception is driven by bottom–up processing.
P3
Over centro-parietal sensors, a larger P3 was found for stimuli that signaled a significant context change compared with those that did not signal a change. Thus, for both novel and repeated pictures, those that signaled a change in the structure of the current series elicited an enhanced P3 over centro-parietal sensors. These data are consistent with previous studies reporting increases in P3 amplitude as the information content of the cue increases (e.g., Gratton et al., 1990; Duncan-Johnson & Donchin, 1977; Donchin, Kubovy, Kutas, Johnson, & Herning, 1973; Sutton, Tueting, Zubin, & John, 1967; Sutton, Braren, Zubin, & John, 1965). For instance, Johnson (1986) found an enhanced P3 when a repeated stimulus signaled the identity of the next stimulus. Relatedly, Gratton et al. (1990) found that P3 amplitude to an identical warning stimulus varied as a function of the amount of information it contained regarding an upcoming trial, leading these researchers to suggest that “the amplitude of the P300 … reflects the utilization of information.” Whereas these studies varied the predictive nature of an informative cue, others have emphasized instead that the P3 is modulated by a violation of expectancies based on past events (Duncan-Johnson & Donchin, 1977; Squires, Wickens, Squires, & Donchin, 1976). In the current design, this interpretation would propose that, because novel pictures are expected in novel series, disconfirmation of this expectancy (i.e., when a picture is repeated) elicits an enhanced P3 and vice versa for the repeated blocks.
A recent interpretation of P3 (both P3a and P3b) is that it reflects “template updating” (e.g., Polich, 2007), in which attention allocation is heightened (hence, P3 amplitude) when a degraded template must be updated. Much of the data contributing to this interpretation indicate a systematic increase in P3 amplitude as a function of the time between task-relevant targets (i.e., target-to-target interval). This template-updating account of the P3 resembles, except for its emphasis on mismatch, the “template-matching” hypothesis we previously suggested as possibly mediating N2 amplitude. Two points are relevant. First, as noted by Polich (2007) and others (e.g., Roth, 1983; Pritchard, 1981), P3 amplitude has been proposed to be enhanced not only when a cue mismatches an existing template but also when it matches a template, which raises difficult issues for any explanation that relies on a template mechanism for the P3 (see also Bradley, 2009). Second, in an oddball paradigm, the template is presumed to arise from the active maintenance of the task-relevant target in memory. In the current study, the absence of an explicit task argues against active maintenance of a specific stimulus, as does the large number of novel stimuli that were presented.
Rather, in the current design, short-term memory presumably includes representations of recently presented stimuli. In this case, a template-updating (i.e., mismatch) hypothesis would predict a large P3 when a novel picture follows a repeated picture (i.e., change-to-novel), which is quite consistent with the current data but would not expect, as found here, a similar P3 enhancement in the change-to-repeated condition, in which the current stimulus physically matches the preceding cue. Rather than reflecting properties related to a perceptual template, the data instead suggest that P3 is sensitive to a cue’s information content and, depending upon the current context, can be elicited for stimuli that mismatch (e.g., change-to-novel) or match (e.g., change-to-repeated) an active memory representation.
Thus, rather than specifically indexing template matching (or mismatching), the P3 primarily varied with what might be called “significance” and is consistent with earlier studies emphasizing the relationship between P3 and information content (e.g., Kok, 2001; Gratton et al., 1990; Duncan-Johnson & Donchin, 1977; Donchin et al., 1973; Sutton et al., 1965, 1967). The hypothesis that P3 amplitude is associated with “significance” (Donchin, 1981), “meaning” ( Johnson, 1986), or “information value” (Sutton et al., 1965) is encompassed by the “context-updating” hypothesis, in which P3 is considered a manifestation of a “process by which the subject interacts with the environment” (Donchin, Ritter, & McCallum, 1978, p. 38) or more colloquially depends on “the information that exists in the subject’s head about the ongoing environment” (Donchin, 1981).
Thus, depending upon the context, significant information can be gleaned from a predictive cue, an expectancy violation, and indeed even novelty. That is, overall, novel pictures in the current study elicited an enhanced P3 compared with repeated pictures, replicating previous data (e.g., Codispoti, Ferrari, & Bradley, 2006, 2007). Moreover, pictures that signaled relevant information in the current study also heightened P3 amplitude. For picture cues, both affect and task relevance also independently amplify late centro-parietal positivities during viewing (Ferrari, Codispoti, Cardinale, & Bradley, 2008). Based on data such as these, Bradley (2009) suggested that a late positive potential measured over centro-parietal sensors primarily reflects the significance of a cue in the current context. Whereas “stimulus significance” is a difficult term to operationally define (Johnson, 1986), it can be viewed as reflecting activation in fundamental motivational systems that have evolved to direct attention and action (Bradley, 2009). In this view, a significant stimulus is one that is motivationally relevant in the current context, and P3 amplitude may reflect processing of these informationally rich events.
Summary
Taken together, the present findings provide evidence that centro-frontal N2 amplitude varies primarily with perceptual novelty and that the amplitude of the centro-parietal P3 is heightened for both significant and novel stimuli. N2 amplitude was drastically attenuated following a single contiguous repetition of a picture, interpreted here as reflecting enhanced perceptual fluency when an active memory representation contributes information in a top–down manner. P3 was enhanced for stimuli that were informative regarding upcoming events, regardless of repetition, as well as for novel compared with repeated stimuli, suggesting that it is specifically enhanced by stimulus significance.
Acknowledgments
This research was supported in part by a grant from the National Institute of Mental Health (P50 MH 72850) to the Center for the Study of Emotion and Attention (CSEA) at the University of Florida. Vera Ferrari is now at the University of Bologna, Italy.
Notes
The posterior P2 was similarly modulated by novelty as the centro-frontal N2, with smaller amplitudes when viewing repeated, compared with novel pictures.
A three-way ANOVA involving the factors of picture content (emotional or neutral), stimulus novelty, and stimulus significance on N2 amplitude indicated a significant effect of picture content, F(1,24) = 19.52, p < .0001, η2 = .449, showing less negativity overall for emotional compared with neutral pictures. There were no interactions involving novelty or significance.
A three-way ANOVA involving the factors of picture content (emotional or neutral), stimulus novelty, and stimulus significance on P3 amplitude indicated a significant effect of picture content, F(1,24) = 43.2, p < .0001, η2 = .64, with more positivity for emotional compared with neutral pictures. A Novelty × Picture Content interaction, F(1,24) = 6.72, p < .05, η2 = .219, indicated larger affective modulation of the P3 for novel compared with repeated pictures, F(1,24) = 20.43, p < .0001, η2 = .46.
REFERENCES
- Bradley MM. Natural selective attention: Orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breton F, Ritter W, Simson R, Vaughan HG., Jr. The N2 component elicited by stimulus matches and multiple targets. Biological Psychology. 1988;27:23–44. doi: 10.1016/0301-0511(88)90003-8. [DOI] [PubMed] [Google Scholar]
- Chong H, Riis JL, McGinnis SM, Williams DM, Holcomb PJ, Daffner KR. To ignore or explore: Top–down modulation of novelty processing. Journal of Cognitive Neuroscience. 2008;20:120–134. doi: 10.1162/jocn.2008.20003. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetitive picture processing: Autonomic and cortical correlates. Brain Research. 2006;1068:213–220. doi: 10.1016/j.brainres.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Codispoti M, Ferrari V, Bradley MM. Repetition and event-related potentials: Distinguishing early and late processes in affective picture perception. Journal of Cognitive Neuroscience. 2007;19:577–586. doi: 10.1162/jocn.2007.19.4.577. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a, perceptual distinctiveness, and stimulus modality. Cognitive Brain Research. 1998;7:41–48. doi: 10.1016/s0926-6410(98)00009-3. [DOI] [PubMed] [Google Scholar]
- Comerchero MD, Polich J. P3a and P3b from typical auditory and visual stimuli. Clinical Neurophysiology. 1999;110:24–30. doi: 10.1016/s0168-5597(98)00033-1. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Courchesne RY, Hillyard SA. The effect of stimulus deviation on P3 waves to easily recognized stimuli. Neuropsychologia. 1978;16:189–199. doi: 10.1016/0028-3932(78)90106-9. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Czigler I, Csibra G, Ambró A. Aging, stimulus identification and the effect of probability: An event-related potential study. Biological Psychology. 1996;43:27–40. doi: 10.1016/0301-0511(95)05173-2. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Calvo V, Faust R, Holcomb PJ. An electrophysiological index of stimulus unfamiliarity. Psychophysiology. 2000;37:737–747. [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Cohen LG, Kennedy BP, West WC, et al. Regulation of attention to novel stimuli by frontal lobes: An event-related potential study. NeuroReport. 1998;9:787–791. doi: 10.1097/00001756-199803300-00004. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LF, Calvo V, Faust R, Mesulam MM, West WC, et al. The influence of stimulus deviance on electrophysiologic and behavioral responses to novel events. Journal of Cognitive Neuroscience. 2000;12:393–406. doi: 10.1162/089892900562219. [DOI] [PubMed] [Google Scholar]
- Donchin E. Surprise…Surprise? Psychophysiology. 1981;18:493–513. doi: 10.1111/j.1469-8986.1981.tb01815.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MGH. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:406–425. [Google Scholar]
- Donchin E, Kubovy M, Kutas M, Johnson R, Herning RI. Graded changes in evoked response (P300) amplitude as a function of cognitive activity. Perception and Psychophysics. 1973;14:319–324. [Google Scholar]
- Donchin E, Ritter W, McCallum C. Cognitive psychophysiology: The endogenous components of the ERP. In: Callaway E, Tueting P, Koslow SH, editors. Brain event-related potentials in man. Academic Press; New York: 1978. pp. 349–411. [Google Scholar]
- Duncan-Johnson CC, Donchin E. On quantifying surprise: The variation of event-related potentials with subjective probability. Psychophysiology. 1977;14:456–467. doi: 10.1111/j.1469-8986.1977.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Ferrari V, Codispoti M, Cardinale R, Bradley MM. Directed and motivated attention during processing of natural scenes. Journal of Cognitive Neuroscience. 2008;20:1753–1761. doi: 10.1162/jocn.2008.20121. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: A review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C, Rose SA. Novelty and conflict in the categorization of complex stimuli. Psychophysiology. 2008;45:467–479. doi: 10.1111/j.1469-8986.2007.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Bosco CM, Kramer AF, Coles MG, Wickens CD, Donchin E. Event-related brain potentials as indices of information extraction and response priming. Electroencephalography and Clinical Neurophysiology. 1990;75:419–432. doi: 10.1016/0013-4694(90)90087-z. [DOI] [PubMed] [Google Scholar]
- Jacoby L, Dallas M. On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology: General. 1981;3:300–324. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Johnson R., Jr. A triarchic model of P300 amplitude. Psychophysiology. 1986;23:367–384. doi: 10.1111/j.1469-8986.1986.tb00649.x. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker D, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Junghöfer M, Peyk P. Analyzing electrical activity and magnetic fields in the brain. MATLAB News & Notes. 2004;2/04:14–15. [Google Scholar]
- Katayama J, Polich J. Stimulus context determines P3a and P3b. Psychophysiology. 1998;35:23–33. [PubMed] [Google Scholar]
- Knight RT. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalography and Clinical Neurophysiology. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective rating of measures and instruction manual (Tech. Rep. No. A-6) University of Florida; Gainesville, FL: 2005. [Google Scholar]
- Luck SJ. An introduction to the event-related potential technique. MIT Press; Cambridge, MA: 2005. [Google Scholar]
- Näätänen R, Gaillard AWK. The orienting reflex and the N2 deflection of the event related potential (ERP) In: Gaillard AWK, Ritter W, editors. Tutorials in event related potential research: Endogenous components. 10th ed North Holland; Amsterdam: 1983. [Google Scholar]
- Nittono H, Shibuya Y, Hori T. Anterior N2 predicts subsequent viewing time and interest rating for novel drawings. Psychophysiology. 2007;44:687–696. doi: 10.1111/j.1469-8986.2007.00539.x. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Jr., et al. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Comerchero MD. P3a from visual stimuli: Typicality, task, and topography. Brain and Topography. 2003;15:141–152. doi: 10.1023/a:1022637732495. [DOI] [PubMed] [Google Scholar]
- Pritchard WS. Psychophysiology of P300. Psychological Bulletin. 1981;89:506–540. [PubMed] [Google Scholar]
- Pritchard WS, Shappell SA, Brandt ME. Psychophysiology of N200/N400: A review and classification scheme. In: Ackles PK, Coles MG, editors. Advances in psychophysiology. Jessical Kingsley Publishers; London: 1991. pp. 43–106. [Google Scholar]
- Roth WT. A comparison of P300 and skin conductance response. In: Gaillard AWK, Ritter W, editors. Tutorials in event related potential research: Endogenous components. 10th ed North Holland; Amsterdam: 1983. [Google Scholar]
- Sawaki R, Katayama J. Stimulus context determines whether nontarget stimuli are processed as task-relevant or distractor information. Clinical Neurophysiology. 2006;117:2532–2539. doi: 10.1016/j.clinph.2006.06.755. [DOI] [PubMed] [Google Scholar]
- Sawaki R, Katayama J. Difficulty of discrimination modulates attentional capture for deviant information. Psychophysiology. 2007;44:374–382. doi: 10.1111/j.1469-8986.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- Snyder E, Hillyard SA. Long-latency evoked potentials to irrelevant, deviant stimuli. Behavioral Biology. 1976;16:319–331. doi: 10.1016/s0091-6773(76)91447-4. [DOI] [PubMed] [Google Scholar]
- Squires KS, Petuchowski C, Wickens C, Donchin E. The effects of stimulus sequence on event-related potentials: A comparison of visual and auditory sequences. Perception & Psychophysics. 1977;22:31–40. [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Squires NK, Wickens C, Squires NK, Donchin E. The effect of stimulus sequence on the waveform of the cortical event-related potential. Science. 1976;193:1142–1146. doi: 10.1126/science.959831. [DOI] [PubMed] [Google Scholar]
- Sutton S, Braren M, Zubin J, John ER. Evoked-potential correlates of stimulus uncertainty. Science. 1965;150:1187–1188. doi: 10.1126/science.150.3700.1187. [DOI] [PubMed] [Google Scholar]
- Sutton S, Tueting P, Zubin J, John ER. Information delivery and the sensory evoked potential. Science. 1967;155:1436–1439. doi: 10.1126/science.155.3768.1436. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cui L, Wang H, Tian S, Zhang X. The sequential processing of visual feature conjunction mismatches in the human brain. Psychophysiology. 2004;41:21–29. doi: 10.1111/j.1469-8986.2003.00134.x. [DOI] [PubMed] [Google Scholar]