Abstract
More comprehensive, and efficient, mapping strategies are needed to avoid post-operative language impairments in patients undergoing epilepsy surgery. Conservative resection of dominant anterior frontal or temporal cortex frequently results in post-operative naming deficits despite standard pre-operative electrocortical stimulation mapping of visual object (picture) naming. Naming to auditory description may better simulate word retrieval in human conversation but is not typically tested, in part due to the time demands of electrocortical stimulation mapping. Electrocorticographic high gamma (60-150 Hertz) activity, recorded simultaneously through the same electrodes used for stimulation mapping, has recently been used to map brain function more efficiently, and has at times predicted deficits not anticipated based on stimulation mapping alone. The present study investigated electrocorticographic mapping of visual object naming and auditory descriptive naming within conservative dominant temporal or frontal lobe resection boundaries in 16 patients with 933 subdural electrodes implanted for epilepsy surgery planning. A logistic regression model showed that electrodes within traditional conservative dominant frontal or temporal lobe resection boundaries were significantly more likely to record high gamma activity during auditory descriptive naming than during visual object naming. Eleven patients ultimately underwent resection and 7 demonstrated post-operative language deficits not anticipated based on electrocortical stimulation mapping alone. Four of these patients underwent a resection that included sites where high gamma activity was observed during auditory naming. These findings indicate that electrocorticographic mapping of auditory descriptive naming may reduce the risk of permanent post-operative language deficits following dominant temporal or frontal resection.
Keywords: language mapping, epilepsy surgery, high gamma, electrocorticography, electrocortical stimulation, surgical outcome
1. Introduction
Patients with debilitating pharmacoresistant partial seizures arising from the dominant hemisphere may be candidates for epilepsy surgery. This procedure often follows intracranial monitoring with implanted subdural electrodes to identify appropriate surgical boundaries by mapping the epileptogenic zone and functional language cortex prior to resection. The current gold-standard for language mapping in this setting is electrocortical stimulation mapping (ESM), during which a small electrical current is sequentially passed through adjacent electrodes and inhibition of function is interpreted to indicate functional language cortex. ESM has been shown to provide accurate mapping of visual object naming (Anderson et al., 1999; Hamberger et al., 2001; Krauss et al., 1996; Sinai et al., 2005b). However, studies have also shown that avoiding visual naming regions alone does not prevent all post-operative language deficits (Cervenka et al., 2011b; Davies et al., 2005; Hamberger et al., 2005; Kojima et al., 2012; Sinai et al., 2005b), and in one study over 50% of patients had post-operative language deficits despite ESM of language prior to surgery (Davies et al., 2005).
Researchers have postulated that auditory descriptive naming, during which patients respond to auditory stimuli such as “an instrument you beat with sticks”, rather than naming an object, is a more “ecologically valid” measure of naming than visual object naming since individuals encounter naming by auditory description more frequently during normal conversation than naming of objects (Hamberger and Seidel, 2003; Hamberger et al., 2005). Auditory descriptive naming requires not only utilization of a different sensory modality, but also sentence and syntax processing, which are not utilized during visual object naming. Therefore, one plausible explanation for why patients experience post-operative language deficits despite mapping of visual object naming is that regions responsible for sentence and syntax processing may not be comprehensively mapped or preserved.
Studies demonstrate that ESM can also interfere with auditory descriptive naming (Hamberger et al., 2001; Hamberger et al., 2005; Kojima et al., 2012; Malow et al., 1996). However, electrical impulses are delivered in long trains over several seconds and can disrupt function during many different components of a task (stimulus presentation, sentence processing, word retrieval, or word expression). It is therefore difficult at times to interpret ESM results to determine which functions are performed by different cortical regions since the outcome is an all-or-nothing response. In addition, studies have demonstrated that both auditory and visual naming deficits have resulted from resection of sites deemed nonessential for visual naming but essential for auditory descriptive naming using ESM(Hamberger et al., 2005). This finding illustrates a potential limitation of ESM of language.
Administration of an exhaustive battery of language tasks using ESM is often not feasible. ESM is time-consuming and can be limited by poor patient motivation or performance, or by pain caused by stimulation of the trigeminal efferent fibers, preventing comprehensive evaluation. ESM can also provoke after-discharges and seizures, especially during stimulation near a seizure focus, which is typically the region of greatest interest with regard to localizing cortical function for surgery planning. Less invasive and less time intensive language mapping techniques may improve surgical planning and post-operative functional outcomes.
Analysis of recordings of event-related electrocorticographic (ECoG) changes that occur during language tasks provides an alternative method for detecting cortical activation and mapping functional language cortex with high spatial and temporal resolution (Cervenka et al., 2011b; Chang et al., 2010; Crone et al., 1998; Crone et al., 2001; Edwards et al., 2010; Flinker et al., 2010; Jung et al., 2008; Kojima et al., 2012; Mainy et al., 2008; Ritaccio et al., 2010; Sinai et al., 2005a; Tanji et al., 2005; Towle et al., 2008; Wu et al., 2010). Because this is a passive recording technique, ECoG mapping does not increase the risk of seizures or after-discharges and cannot inflict pain. Investigations of changes in ECoG power within the time-frequency domain have revealed that a broadband increase in power in the high gamma (> 60 Hz) frequency range is a reliable marker of cortical activation (Cervenka et al., 2012; Crone et al., 1998). Studies have recently shown improving sensitivity and specificity of ECoG mapping of high gamma activity compared to ESM of motor function (Brunner et al., 2009; Leuthardt et al., 2007) as well as both receptive and expressive components of language (Cervenka et al., 2011b; Kojima et al., 2012; Sinai et al., 2005a; Sinai et al., 2009; Towle et al., 2008), and of auditory perception (Boatman-Reich et al., 2010; Cervenka et al., 2011a; Cervenka et al., 2012). In certain circumstances, ECoG mapping has also been shown to predict post-operative deficits not predicted by ESM (Cervenka et al., 2011b; Kojima et al., 2012).
We studied 16 patients with a total of 933 electrodes implanted over dominant cortex and compared localization of auditory descriptive naming and visual object naming using ECoG mapping of high gamma activity. We hypothesized that sites specific to auditory descriptive naming but not visual object naming as identified by ECoG mapping of high gamma activity would fall within the boundaries of a typical dominant temporal lobectomy (up to 4.5 centimeters from the anterior temporal tip) or anterior frontal resection (BA 8-11, 46, or 47) sparing Broca’s area, primary, and premotor cortices.
2. Material and methods
2.1 Subjects
Seventeen consecutive patients with subdural electrodes implanted for clinical purposes over the left hemisphere were recruited for this study between January 2009 and January 2012. Language lateralization was determined by pre-operative intracarotid amobarbital procedure, ESM results, or based on handedness if no other language evaluation was performed pre-operatively (right-handed patients were assumed to be left hemisphere dominant for language(Knecht et al., 2000)). Patients who were right hemisphere dominant for language were excluded. Intracarotid amobarbital procedure (IAP) revealed that 10 subjects were left hemisphere dominant for language (8 right-handed, 1 left-handed, and one ambidextrous), one left-handed subject had bilateral language representation, and six right-handed patients did not undergo IAP. Patients with full-scale IQ less than 70 or with significant auditory or visual impairment were excluded and those unable to complete the testing battery were excluded from further analyses.
Subjects were admitted to the Johns Hopkins Hospital Epilepsy Monitoring Unit once deemed stable post-operatively (1 to 3 days following electrode implantation) and remained under continuous video electrocorticographic monitoring for one to two weeks, followed by removal of intracranial electrodes and resection of the seizure onset zone when applicable.
The study was approved by the Johns Hopkins Medicine Institutional Review Board. Written informed consent was obtained from each patient (and their parent or guardian when appropriate) during enrollment in accordance with the standards of the Declaration of Helsinki.
2.2 Language battery
Two naming instruments were used during a 30 to 60 minute electrocorticographic recording session: a visual object naming test (VNT) derived from the 60-item Boston Naming Test (Goodglass et al., 1983) and a 50-item Auditory Naming Test (ANT)(Hamberger and Seidel, 2003). The VNT is a widely used visual confrontation naming test that requires subjects to name black-and-white line drawings presented on a white background. The ANT is an auditory descriptive naming test consisting of 50 spoken phrases that describe specific objects, such as “an insect that makes honey” (Hamberger and Seidel, 2003).
During the VNT, stimuli were presented in pseudo-randomized order on a computer monitor placed 1 meter in front of the subject (subtending a maximum visual angle of 4.4 degrees), and were instructed to name items aloud as quickly as possible. Stimuli were displayed for 1 second. During the ANT, a pre-recorded audio file was played at a normal conversational volume and duration (1.02 to 2.08 seconds), consisting of auditory descriptive stimuli. Subjects were instructed to name items aloud as quickly as possible. They were asked to minimize movements or gestures during testing.
Subjects were tested during stable interictal periods when their neurologic capabilities were at their functional baseline. During both tasks, if subjects could not immediately name the test item, they were asked to say “pass”. Trials were discarded if the subject responded incorrectly, “passed”, did not respond, or if there was any distraction in the room, unsolicited subject speech, or significant subject movement during the trial or during the baseline period prior to the trial.
2.3 ECoG recordings
Subdural electrode arrays consisted of 1.5-mm-thick soft Silastic sheets (Ad-Tech, Racine, Wisconsin) imbedded with platinum-iridium disks (4-mm in diameter, 2.3-mm exposed surface, 10-mm apart) of various configurations determined by the epilepsy and neurosurgical team based on previous neuroimaging, continuous video EEG results, and any anatomic constraints. Electrode locations were determined by co-registration of each subject’s post-implantation CT to a pre-surgical volumetric MRI (1- to 1.8-mm coronal slice thickness; see also (Boatman-Reich et al., 2010; Boatman and Miglioretti, 2005; Crone et al., 2001; Sinai et al., 2005b) for a complete description of co-registration procedures). Electrode locations were documented with intraoperative digital photographs pre- and post- operatively to assess for any electrode displacement from implantation to explanation.
Electrocorticographic recordings were amplified and digitally recorded using an intracranial referential montage at a sampling rate of 1000 Hz and a bandwidth of 0.3-300 Hz. Reference electrodes were selected over cortical regions of less clinical interest. The digital ECoG recordings were inspected by a trained epileptologist (MCC) to exclude electrodes or individual trials from analysis if signals were contaminated by significant artifact or epileptiform activity. Signals were re-montaged to an average reference (Boatman-Reich et al., 2010) then low-pass filtered to 250 Hz and down-sampled by a factor of 2.
Marker channels were used to record stimulus onset and offset. During the VNT, a marker for stimulus onset time was digitally recorded. Stimulus onset during the ANT and verbal responses during both tasks were digitally recorded.
2.4 Electrocortical stimulation mapping
When deemed appropriate for clinical purposes, ESM was performed on pairs of electrodes using routine clinical procedures (Lesser et al., 1987). At a given electrode pair, alternating polarity square-wave pulses (0.3 ms duration, 50 Hz) were delivered in 2 to 5 second trains in 0.5 mA increments from 1 mA up to a maximum of 12 mA, or the highest amperage that did not produce after-discharges at a given electrode pair. Behavioral mapping was performed once this threshold was established (typically between 7 and 12 mA). Language tasks included a visual naming test, sentence comprehension, paragraph reading, and spontaneous speech. Sites were defined as ESM(+) if stimulation at a given site temporarily interfered with language performance (produced no response, an incorrect response such as a paraphasia, or a delayed response).
2.5 Post-operative language outcomes
Subject medical records were reviewed post-operatively for report of any language deficits described by their treating physician(s) in follow-up clinic notes. Follow-up neuropsychological evaluations were reviewed when available, typically within a year following surgery. Whenever possible, subjects repeated the ANT and VNT post-operatively prior to discharge and at subsequent outpatient follow up visits. They were also asked to participate in a telephone interview that included a letter (FAS, (Tombaugh et al., 1999)) and category (Animals, Supermarket (Schretlen and Vannorsdall, 2010)) verbal fluency tests, the Hopkins Verbal Learning Test-Revised (HVLT-R, (Brandt and Benedict, 2001)), and the ANT((Hamberger and Seidel, 2003)).
2.6 Data Analyses
2.6.1 Language battery performance
For each trial, the subject’s response time (RT), i.e. the latency of verbal response onset after stimulus presentation, was determined by visual inspection of the microphone channel auditory waveform of the subject’s response from the time of visual stimulus onset during the VNT and from completion of the final word in the stimulus description in the ANT. Stimulus onset was chosen for the VNT because all of the information necessary for naming the stimulus was presented immediately at onset. The end of stimulus presentation was chosen for the ANT because there was no available method for determining at what time during stimulus presentation subjects became aware of the correct response (whether this required presentation of the entire stimulus or only a portion) and subjects listened to the entire sentence before responding.
For all subjects, the number and percentage of correct responses on the VNT and ANT were measured, and RTs were recorded for each trial and then averaged over all correct trials. Means and standard deviations for RT’s were calculated. Relative performance on the VNT and ANT was determined by comparing the number of correct trials and mean RT’s within subjects using paired t-tests (p<0.05). The goal in selecting these particular tasks and the number of stimulus items was to accumulate the same number of correct responses with the same average response latency for each task within subjects for the purpose of accurate ECoG analyses.
2.6.2 ECoG analyses
ECoG signals were decomposed into time-frequency atoms using a matching pursuit algorithm(Boatman-Reich et al., 2010; Franaszczuk et al., 1998; Mallat and Zhang, 1993; Ray et al., 2003; Sinai et al., 2009; Sinai et al., 2005b). Time-frequency decomposition was performed on segments of ECoG that included a pre-stimulus interval of 1000 ms just prior to stimulus onset, and a post-stimulus interval of 3096 or 6144 ms, depending on the maximum duration between stimulus onset and time to verbal response. Event-related power changes relative to the baseline were compared between an 800-ms portion of the prestimulus baseline (-100 to -900 ms) and the first 2000 or 4000 ms following stimulus onset. Power line noise (60 Hz and 120 Hz) was excluded from the spectral power analysis. Baseline time points were averaged to determine baseline power at each frequency and were then compared to post-stimulus power at each post-stimulus time-frequency point for all selected trials using a paired t-test (p = 0.05) after natural logarithmic transformation, assuming unequal variances (Zygierewicz et al., 2005). To correct for multiple within-subject comparisons, a false discovery rate control (q = 0.05) was used. Magnitudes of statistically significant power changes from baseline to post-stimulus onset were plotted with respect to time and frequency. All signal processing was performed using a distributed computing software system, ANIN, implemented on a cluster of 16 single processor nodes (Franaszczuk and Jouny, 2004). Electrodes that revealed statistically significant increase in high gamma activity in the 60-150 Hz frequency range were defined as ECoG(+) and sites that revealed no statistically significant high gamma activity were designated as ECoG(−). The test-retest reliability of this method has been demonstrated (Cervenka et al., 2012).
2.6.3 Electrode localization
Pre-surgical volumetric MRIs that were co-registered with post-implantation CT scans to determine electrode localization were normalized onto a Montreal Neurological Institute (MNI) brain atlas using the BioImage Suite (Yale University School of Medicine, New Haven, Connecticut, (Papademetris et al., 2012)). Each ECoG(+) electrode was assigned a coordinate on the MNI brain which was converted into Talairach X, Y, and Z coordinates. Talairach coordinates were analyzed using the Talairach client software (Lancaster et al., 1997; Lancaster et al., 2000) to assign appropriate Brodmann Areas (BAs). BAs that the client was unable to assign were analyzed using the Yale Brodmann Areas Atlas Tool from the BioImage Suite. All assigned BAs were checked by visual inspection. If electrodes appeared outside of any BA using either method, electrode coordinates were adjusted manually to match most closely the cortical position of the electrode on the subject’s 3D MRI CT reconstructed images and operative photographs.
Sites were assigned to anterior frontal and temporal lobe regions of interest that could potentially fall within the margins of a standard resection. ECoG(+) electrodes falling within 3.5, 4.0, and 4.5 cm from the left anterior temporal tip (designated Temporal 3.5, 4.0, and 4.5, respectively) were identified as anterior temporal sites. To do so, the anterior temporal tip of the MNI brain was identified and ECoG(+) sites within 3.5, 4, or 4.5 cm from the Y coordinate within temporal lobe Brodmann areas (BA 20, 21,, 22, 27, 28, 34, 35, 36, 37, 38, 41, and 42) were assigned to these regions of interest (Temporal 3.5, Temporal 4, and Temporal 4.5, respectively). Three distinct distances were selected as previous studies have defined different boundaries for conservative dominant anterior temporal lobe resection (Engel, 1993; Hamberger et al., 2001; Hermann et al., 1999; Wiebe et al., 2001). In addition, electrodes anterior to primary motor and premotor/supplementary motor cortex in the frontal lobe (anterior to BA 4 and 6, respectively) and excluding pars opercularis (BA 44) and pars triangularis (BA 45) were also identified and defined as “Anterior Frontal” (BA 8,9,10,11,46, and 47).
2.6.4 Statistical modeling
For each of the regions of interest within the frontal or temporal lobe, the probability of an ECoG(+) site or sites to be present during a given task was determined using a logistic regression model (Wolfinger and O’Connell, 1993) including random effects for subjects and for electrodes nested in each subject as follows:
where Yijk if the electrode j for subject i was ECoG(+) during task k and Yijk = 0 otherwise, Xk =1 for VNT and 0 for ANT, ui and uij are the random effect terms for subject i, electrode j, and εjk are the error terms. The “lme4” package in the R software was used for the analysis (Bates et al., 2011).
The random effect terms were included in the model to account for the between subject variability of the response, as well as the variability of the outcome (ECoG(+) during ANT, VNT, or both) in the electrodes within each subject. The nested model used here assumed that the electrodes were different for each subject; in other words electrode 1 for subject 1 was different from electrode 1 for subject 2. Parameters in the model were estimated using the electrodes in temporal lobe regions of interest (Temporal 3.5, 4, and 4.5), as well as the anterior frontal lobe.
2.6.5 Post-operative language outcomes
Subjects were defined as having a post-operative language deficit if a decline in language performance was documented in post-operative follow-up clinic notes, or if there was a significant decline in language performance on language measures during neuropsychological testing or post-operative telephone interview. Subject performance on language measures examined pre- and post-operatively were compared to established norms (Brandt and Benedict, 2001; Hamberger and Seidel, 2003; Mitrushina et al., 2005; Roberts et al., 2002; Schretlen and Vannorsdall, 2010; Tombaugh et al., 1999). A significant decline in performance was defined as a decline in T-score by one standard deviation or more(Hannay and Lezak, 2004). If post-operative deficits were found, operative photographs, post-operative MRIs, EMU summaries, and operative reports were reviewed to identify electrodes that were within resection boundaries. Any ECoG(+) or ESM(+) sites that were resected were identified.
Positive and negative predictive values, sensitivity and specificity for predicting post-operative language deficits were calculated for ECoG language mapping and ESM. For ESM, positive predictive values and specificity were expected to be 100% based on the clinical practice of avoiding ESM(+) sites, leading to a false positive rate of 0 in most cases, i.e. there would not typically be a situation where ESM(+) sites were resected and no deficit was seen. Subjects were excluded from these analysis if they received amygdalohippocampectomy and demonstrated post-operative language impairment because hippocampal resection has been shown to produce naming deficits independent of the degree of neocortical resection (Hamberger et al., 2010). Subjects in whom no post-operative data were available (lost to follow up) were also excluded.
3. Results
Sixteen epilepsy patients (ages 14-50, mean age 29.9 +/− 11.2 years) were studied (Table 1). One additional subject was consented and completed the ANT but was not able to complete the VNT. This subject was excluded from further analysis.
Table 1.
Subject Characteristics
| Subject | Gender | Age | Age at sz- onset |
Handed- ness |
IAP | FIQ | VIQ | MRI |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 47 | 8 | R | NT | NT | NT | L STG CD |
| 2 | M | 14 | 6 | R | NT | NT | NT | Normal |
| 3 | F | 28 | 10 | R | NT | NT | NT | L F gliosis (prior resection) |
| 4 | F | 50 | 46 | R | L | 91 | 86 | L F,T,insular infiltrating lesion |
| 5 | M | 31 | 27 | R | L | 75 | 75 | B F T2/FLAIR hyperintensities |
| 6 | F | 27 | 7 | R | NT | NT | NT | Normal |
| 7 | F | 36 | 20 | R | L | 90 | 92 | Normal |
| 8 | M | 14 | 7 | R | NT | NT | NT | Left periventricular CD |
| 9 | F | 50 | 31 | B | L | 81 | 85 | Nonspecific T2/FLAIR subcortical changes |
| 10 | F | 29 | 15 | R | L | 85 | 81 | Normal |
| 11 | F | 28 | 0 | R | L | 84 | 83 | L MTS |
| 12 | F | 25 | 7 | R | L | 106 | 93 | L F gliosis (prior resection) |
| 13 | M | 32 | 2 | L | B | 79 | 81 | normal |
| 14 | M | 20 | 14 | L | L | 90 | 89 | normal |
| 15 | M | 24 | 20 | R | L | 124 | 119 | normal |
| 16 | F | 23 | 13 | R | L | 118 | 112 | normal |
Sz = seizure; IAP = intracarotid amobarbital procedure results for language; R = right; L = left; B = bilateral; NT = not tested; FIQ = full scale intelligence quotient; VIQ = verbal intelligence quotient; STG = superior temporal gyrus; CD = cortical dysplasia; F = frontal; T = temporal; MTS = mesial temporal sclerosis
3.1 Language battery performance
Average number of correct responses was 37/50 (75%, range 40-98%) for the ANT and 38/60 (64%, range 20-88%) on the VNT. No subjects provided all correct responses, indicating that there was no ceiling effect for either task. There was a statistically significant difference in percent of correct responses between the ANT and the VNT within subjects (p < 0.005). However, the overall number of correct responses did not vary between tasks within subjects (p = 0.6).
3.2 ECoG analyses
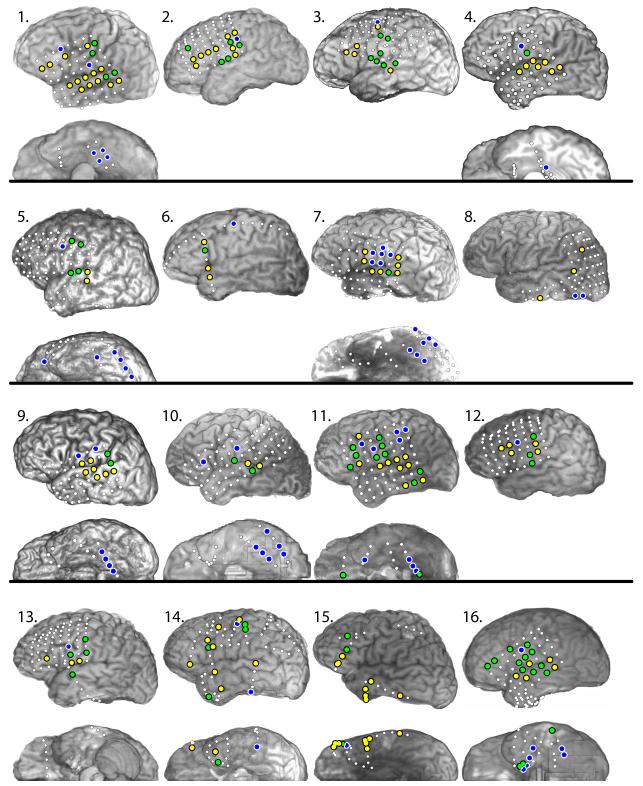
Fourteen patients received implantation of dominant hemisphere grid and/or strip electrodes, 2 patients received bilateral strip electrodes (Figure 1). A total of 933 electrodes were tested and the median number of electrodes examined per patient was 63.5 (mean 58, range 9 to 83). Of these, 230 electrodes were ECoG(+), 162 during the ANT (97 during ANT only), 133 during the VNT (68 during VNT only), and 65 during both tasks.
Figure 1.
Left lateral and basal view showing electrode placement and ECoG(+) sites in each subject. White circles = ECoG(-) sites; Yellow circles = ECoG(+) sites during the auditory naming task only; Blue circles = ECoG(+) sites during the visual naming task only; Green circles = ECoG(+) sites during both ANT and VNT.
3.3 Electrode localization
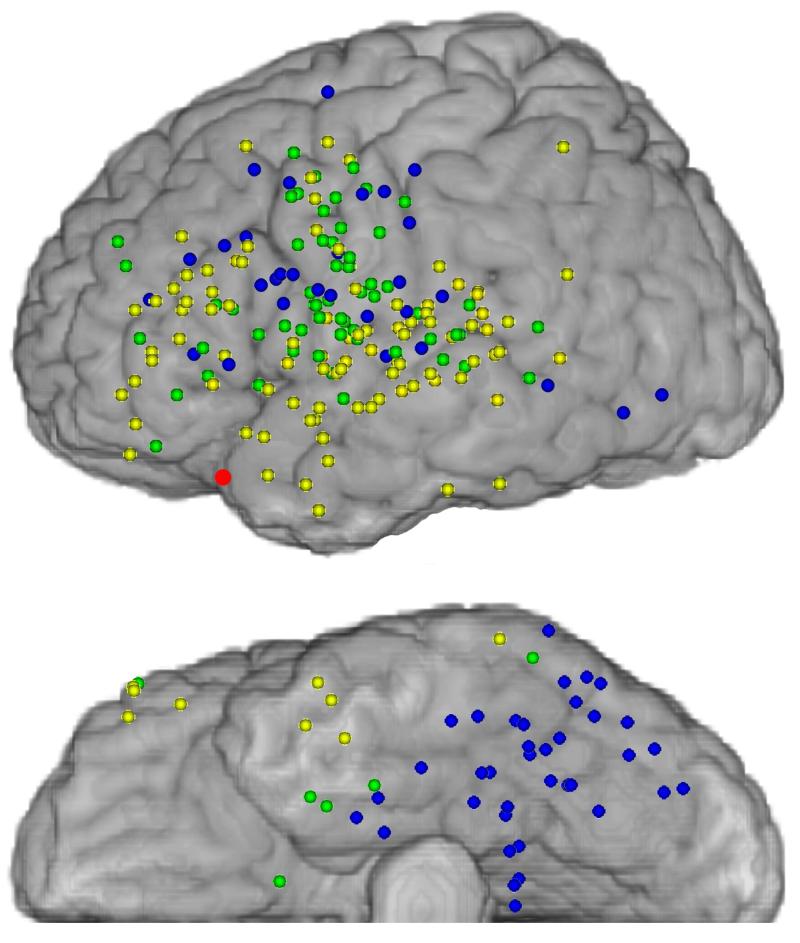
After normalization onto an MNI brain atlas for within-group comparisons (Figure 2), over half of ECoG(+) electrodes during ANT localized to superior or middle temporal gyrus (BA 21, 22, 38, 41, and 42; 63 sites) or to anterior frontal cortex (26 sites). Fewer ECoG(+) electrodes during VNT localized to middle or superior temporal gyrus (18 sites) or anterior frontal cortex (14 sites) and the largest proportion of ECoG(+) electrodes during VNT were seen over basal temporal or occipital cortex (BA 19 and 37; 36 sites). Brodmann areas that contained the largest number of sites that were ECoG(+) during both ANT and VNT were in ventral premotor cortex (BA 6; 17 sites) and superior temporal gyrus (BA 22; 8 sites), followed by motor areas (BA 4; 6 sites). This was consistent with the functional importance of these areas for both tasks. Nevertheless, these areas also contained sites, albeit fewer, that were ECoG(+) for ANT or VNT only.
Figure 2.
Left lateral and basal views of electrode localization normalized onto a Montreal Neurological Institute brain atlas for all subjects. Colored circles represent electrode localization and color conventions are the same as in Figure 1. The red circle indicates the location of the anterior temporal tip.
Sites with significant high gamma activity during ANT, VNT, and during both tasks were identified within designated regions of interest (Temporal 3.5, Temporal 4.0, Temporal 4.5 and Anterior Frontal). These regions were selected because of the likelihood that a dominant resection could be performed in those regions during routine epilepsy surgery. Sites that were ECoG(+) were found during ANT-only, VNT-only, and during both tasks (ANT+VNT) within all regions of interest. In all ROIs, the number of ECoG(+) sites during ANT exceeded the number during VNT and in all Temporal ROIs, the number of ANT-only sites exceeded both VNT-only and ANT+VNT sites (Table 2), even within the ROI that represented the most conservative resection boundaries (Temporal 3.5).
Table 2.
ECoG(+) sites within each region of interest (ROI), probability that ECoG(+) sites were (+) during ANT only or VNT only, and the odds ratios of ECoG(+) sites being (+) during VNT compared with ANT in each ROI.
| Region of Interest |
Total | ANT | VNT | ANT + VNT |
Probability ANT only |
Probability VNT only |
Odds VNT vs ANT |
P-value |
|---|---|---|---|---|---|---|---|---|
| Temporal 3.5 |
27 | 25 | 7 | 5 | 92% | 25% | 2% | <0.0001 |
| Temporal 4.0 |
32 | 29 | 9 | 6 | 90% | 28% | 4% | <0.0001 |
| Temporal 4.5 |
42 | 37 | 13 | 8 | 88% | 31% | 6% | <0.0001 |
| Anterior Frontal |
40 | 32 | 19 | 11 | 80% | 47% | 22% | 0.003 |
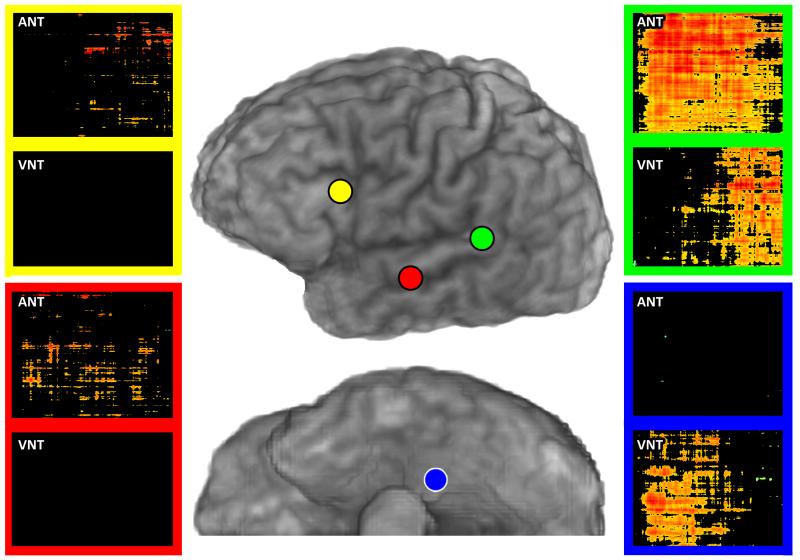
Figure 3 shows time-frequency plots of significant high gamma power increases during ANT only in the Temporal 4.5 and Anterior Frontal ROIs, during VNT only in basal temporal cortex, and during both ANT and VNT in posterior superior temporal gyrus, in a representative subject (Subject 1). Of note, timing differences of high gamma power increases vary between regions and between tasks. For example, during auditory naming, the high gamma power increase in posterior superior temporal gyrus begins early and continues throughout the auditory stimulus. During visual naming, the power increase occurs later, during spoken responses (auditory feedback).
Figure 3.
Time-frequency plots of ECoG high gamma power changes from matching pursuit analyses. Examples are shown for two recording sites (colored circles) that were ECoG(+)for ANT only (red circle, within the Temporal 4.5 region of interest; yellow circle, within the Anterior Frontal region of interest), for a site that was ECoG(+) for both ANT and VNT (green circle in posterior superior temporal gyrus), and for a site that was ECoG(+) for VNT only (blue circle in basal temporal cortex). Color coded rectangles surround two time-frequency plots corresponding to each recording site: plots for ANT are on top, and plots for VNT are on the bottom. In each plot, the X-axis is time ranging from 0 to 2 seconds, and the Y-axis is frequency ranging from 60 to 150 Hz. High gamma power increases are depicted with a color map ranging from yellow (smallest increase) to dark red (largest increase); black indicates no significant difference for a given time-frequency point compared to the 1 second pre-stimulus baseline.
3.4 Statistical modeling results
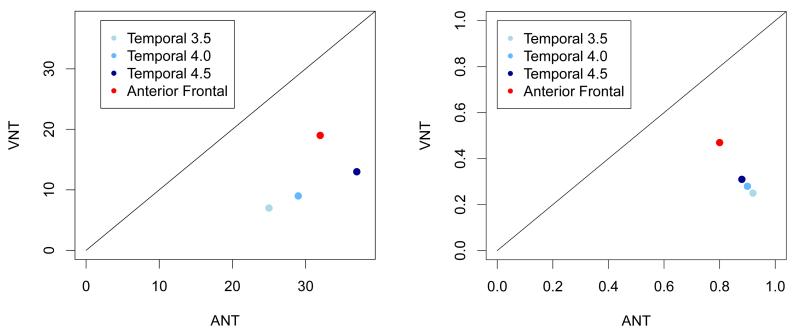
Regions of interest were examined further to determine the odds that ECoG(+) sites were present during ANT and during VNT within these regions in order to assess whether there was a significant difference in the overall number of ECoG(+) sites during each task. A logistic regression model with random effect terms was chosen to account for between subject variability of the response as well as variability of the outcome (ECoG(+) during ANT only, VNT only, or both) in the electrodes within each subject (Table 2, Figure 4). Within all anterior temporal and anterior frontal ROIs, the odds that ECoG(+) were present during the ANT exceeded the odds that ECoG(+) sites were present during the VNT and these differences were statistically significant, even within the boundaries of the most conservative resection region (Temporal 3.5).
Figure 4.
Summary comparing auditory descriptive naming (ANT) versus visual object naming (VNT) within each region of interest. Left- scatterplot depicting the number of ECoG(+) sites for ANT versus VNT within each region of interest (Temporal 3.5, Temporal 4.0, Temporal 4.5, and Anterior Frontal). Right- scatterplot depicting the probability that ECoG(+) sites for ANT versus VNT were within each region of interest.
In ROI Temporal 3.5, the between-subject and within-subject variability in response were negligible, i.e. the odds of activation of the electrodes in this area were similar between subjects and within the electrodes for each subject. The estimate for the odds of ECoG(+) sites in this region during the ANT was 12.5. This implies that the estimated probability of finding that ECoG(+) sites were present within this ROI during only the ANT, based on the logistic regression model, was 92%. The probability of finding sites within this ROI that were ECoG(+) only during the VNT, was 25%. The estimated slope of the logistic regression was −3.57. This implies that the odds ratio of ECoG(+) sites during the VNT versus during the ANT was 0.02. In other words, the odds of finding high gamma activity during the VNT were only 2% of the odds of finding this activity during the ANT. A test for significance of the difference of the estimated odds ratio as compared to 1 (the odds of finding ECoG(+) sites were the same during the two tasks) resulted in a p-value < 0.0001, indicating a significant difference.
Similar results were observed for Temporal 4.0 and 4.5. The probability of identifying ECoG(+) sites located within 4.0 cm and 4.5 cm of the anterior temporal tip during only the ANT was estimated as 90% and 88%, respectively (28% and 31%, respectively, during only the VNT). For these two regions, the estimated parameters of the random effects showed that the between-subjects variability of the response was higher than the variability within subjects. However, in both cases, the variability was negligible (with a p-value close to 1). The odds ratios of finding ECoG(+) sites during the ANT versus during the VNT were significant for both regions. The odds of finding ECoG(+) sites during the VNT were only 4% of the odds of finding significant ECoG activity during the ANT in the Temporal 4.0 region and 6% in the Temporal 4.5 region (p < 0.0001).
The analysis of the electrodes located in the anterior frontal lobe showed a probability of significant high gamma ECoG activity of 80% during only the ANT and 47% during only the VNT. The variability within subjects was close to 0, however, the standard deviation of variability between subjects was 0.12 (the p-value was 0.95, implying no significant difference from zero). The odds of finding ECoG(+) sites during the VNT were 22% of the odds of identifying ECoG(+) sites during the ANT in Anterior Frontal with a p-value of 0.003 showing a significant difference from an odds ratio of 1.
A subgroup analysis was attempted to determine whether seizure localization (anterior frontal versus anterior temporal versus other) impacted these results, however, the study was not adequately powered to make this assessment.
3.5 Post-operative language outcomes
Of 16 subjects, 11 proceeded to resection (see Table 3). Post-operative records were reviewed in all subjects for documentation of a post-operative language deficit. Of the 11 patients who underwent resection, 8 reported a subjective language impairment post-operatively and/or a language decline was demonstrated during post-operative evaluation. Four patients who demonstrated post-operative deficits had received an anterior temporal lobectomy with amygdalohippocampectomy (Subjects 7,9,11, and 15) and were excluded from further analysis because prior studies have shown that hippocampal resection alone can produce these deficits(Hamberger et al., 2010). Current techniques cannot distinguish whether deficits are produced by cortical or hippocampal resection, or both. One was lost to follow up and therefore no post-operative data were available and this subject was excluded from further analysis as well. Two subjects demonstrated no post-operative deficits (Subjects 8 and 16).
Table 3.
Language mapping and post-operative outcomes including whether or not ESM was performed prior to resection, resection type (if any), pathology results, whether or not ANT or VNT ECoG(+) sites were resected and language outcomes. Decline in language performance is shown in bold.
| Subject | ESM performed | Resection | Pathology | ANT ECoG(+) sites resected |
VNT ECoG(+) sites resected |
Language Outcome |
|---|---|---|---|---|---|---|
| 1 | YES | Nonea | NA | NA | NA | NA |
| 2 | YES | L F MSTa | NA | NA | NA | NA |
| 3 | YES | L F | Post-grid implantation changes only |
YES | NO | Lost to follow up |
| 4 | YESb | L F,T, insular tumor resection | oligodendroglioma | YES | NO |
“More word-finding
difficulties” |
| 5 | YES | L AT and L FP resection |
L F: neocortex with hypercellularity |
NO | YES |
↓VNT on post-op
day 1 |
| 6 | NO | None- nonlocalizing |
NA | NA | NA | NA |
| 7 | YES | LAT + AH | L temporal lobe: neocortex with no specific histologic changes |
NO | NO | ↓HVLT-D |
| 8 | YES | L PO resection | dysembryoplastic neuroepithelial tumor (DNT) |
NO | NO | No post-op deficits reported |
| 9 | YES | LAT + AH | L temporal lobe: mesial temporal sclerosis |
NO | NO |
“new word-finding
difficulties” |
| 10 | YES | Nonea | N/A | NA | NA | NA |
| 11 | YES | LAT + AH | L temporal lobe: MTS |
NO | NO | ↓HVLT-D, Category-S |
| 12 | YES | L F | Balloon cells | YES | NO | “Alexia and agraphia” |
| 13 | YES | L F | osteomyelitis | YES | NO | ↓HVLT-I, -D, -Di |
| 14 | YES | Nonea | NA | NA | NA | NA |
| 15 | NO | R AT + AH | Normal | NA | NA | ↓HVLT-I, -D, FAS |
| 16 | YES | L LT | Balloon cells | YES | YES | No deficits on ANT, VNT at 1 month post- op. |
L = left, R = right, F = frontal resection, FP = frontopolar resection, AT = anterior temporal resection, LT = lateral temporal resection, AH = amygdalohippocampectomy, PO = parieto-occipital resection, MST = multiple subpial transection, HVLT = Hopkins Verbal Learning Test, −I = Immediate, −D = Delay, Di = Discrimination, FAS = Verbal Fluency Test, Category-S = Category supermarket, VNT = Visual Naming Test
Seizures localized to language cortex based on ESM and therefore no resection was performed
ESM sites resected out of clinical necessity
Therefore, six patients that underwent resection were included when determining number of true and false positive and negative results for ECoG mapping and for ESM (4 with deficits and no amygdalohippocampectomy, and 2 without deficits). Of the remaining four subjects with post-operative deficits, one (Subject 4) received a resection that included both ECoG(+) and ESM(+) sites based on clinical necessity. The patient was counseled prior to resection regarding anticipated post-operative language deficits, which did subsequently occur. Three patients with decline in post-operative language performance had received a resection that included ECoG(+) sites but spared all sites deemed critical based on ESM. ESM results, but not ECoG results, were taken into account for clinical decision-making purposes, given that ECoG mapping remains experimental. One of these (Subject 12) was diagnosed during formal post-operative evaluation to have a “severe transcortical motor aphasia, with alexia and agraphia” that has persisted for 16 months, requiring continued outpatient speech therapy and cognitive rehabilitation. One (Subject 16) received a resection that included ECoG(+) sites and no ESM(+) sites, but did not exhibit any post-operative deficits. In summary, of these 6 subjects, the number of true positives for ESM was 1 with 0 false positives, 2 true negatives and 3 false negatives. For ECoG mapping there were 4 true positives, 1 false positive, 1 true negative and 1 false negative.
Based on these findings, the positive predictive value and the specificity of ESM in predicting post-operative language impairment were 100%, but this was expected based on clinical practice of avoiding ESM(+) sites during resection in most cases. The sensitivity of ESM was only 25%, and its negative predictive value was 40%. For ECoG language mapping, the positive predictive value was 80% with a negative predictive value of 100%. The sensitivity and specificity of ECoG for predicting post-operative language impairments were 100% and 50%, respectively.
4. Discussion
Patients undergoing focal cortical resection for medically resistant seizures arising from the dominant hemisphere frequently have post-operative language deficits, especially a decline in naming performance, that are not predicted by functional language mapping. These findings highlight the need for a better understanding of the functional anatomic organization of language cortex as well as more sensitive functional language mapping techniques to predict and prevent post-operative language deficits.
This study used experimental electrocorticographic mapping based on event-related increases in high gamma activity, to examine the functional anatomic organization of cortical regions that are often targeted during epilepsy surgery. We compared cortical activation patterns during visual object naming, the task most commonly performed during functional language mapping, and during auditory descriptive naming. Previous studies have shown performance on one or more of these tasks to be impaired following dominant cortical resection (Cervenka et al., 2011b; Davies et al., 2005; Hamberger et al., 2005; Kojima et al., 2012; Sinai et al., 2005a). We showed that cortical activation occurred within dominant anterior temporal (within 3.5-4.5 cm posterior to the dominant anterior temporal tip) and anterior frontal (anterior to premotor cortex and excluding Broca’s area) neocortex more often during auditory descriptive naming than during visual object naming. When regions with significant cortical activation were resected, language deficits were seen in 4 out of 5 patients. All of these patients underwent standard functional mapping of visual object naming with electrocortical stimulation prior to resection, and language deficits were predicted using this technique in only one patient. These findings may have significant clinical implications when planning epilepsy surgery and selecting appropriate language mapping strategies.
This study showed that the odds of identifying cortical activation in dominant anterior temporal neocortex were significantly greater during auditory descriptive naming than during visual object naming. The odds that ECoG(+) sites were present only during auditory naming increased within more conservative resection boundaries. In other words, the odds of finding ECoG(+) sites during auditory naming versus visual naming were greater within 3.5 centimeters from the anterior temporal tip compared to 4.0 cm and 4.5 cm. These findings suggest that conservative dominant temporal resections may not spare language cortex and even more concerning, that auditory naming areas are at an even greater risk of resection relative to visual naming areas during more conservative resections.
The finding that auditory and visual naming sites are often distinct in anterior temporal cortex are in agreement with a prior study using ESM of dominant temporal cortex to map auditory descriptive naming and visual object naming (Hamberger et al., 2005). Hamberger et al. (Hamberger et al., 2005) identified dominant temporal regions in 11 patients in whom stimulation interfered with auditory descriptive naming. In 7 patients, stimulation did not interfere with visual object naming. Six of these seven patients demonstrated post-operative naming deficits following resection of auditory naming sites within temporal cortex. However, post-operative naming impairment included visual object naming as well. In addition, three patients in whom no auditory or visual naming sites were resected demonstrated post-operative naming deficits, illustrating a potential limitation in the sensitivity of ESM of naming during either task.
In Hamberger et al. (2005), the majority of patients that demonstrated post-operative naming deficits underwent dominant amygdalohippocampectomy as well as anterior temporal neocortical resection. A more recent study by the same group showed that the dominant hippocampus plays an important role in naming (Hamberger et al., 2010), which may also account for these earlier findings. In the present study, all patients who underwent a resection that included the mesial temporal structures exhibited declines in language performance as well. One of these patients underwent a non-dominant resection, so decline in language performance was not expected. However, he showed a nearly global decline in performance, possibly suggesting poor attention, or medication effect.
Although several studies have focused on ESM and ECoG language mapping of dominant temporal neocortex, fewer studies have investigated localization of naming in the dominant anterior frontal lobe (DeLeon et al., 2007; Ilmberger et al., 2001; Lesser et al., 1984; Ojemann et al., 2002). It is widely accepted that pars opercularis and pars triangularis, portions of Broca’s area (BA 44 and 45, respectively) are critical for language production (Dronkers et al., 2007). In routine clinical practice, dominant frontal resections may be performed without intracranial monitoring by sparing these regions as well as primary and premotor cortices (BA 4 and 6, respectively) in an effort to avoid post-operative language and motor deficits. However, lesion studies have demonstrated transcortical aphasia in patients with damage to prefrontal cortex (DeLeon et al., 2007). ESM studies have also described speech arrest, interference with sentence comprehension, verb generation, and with visual naming during dominant frontal stimulation, outside of Broca’s area, primary motor, or premotor cortices (Ilmberger et al., 2001; Lesser et al., 1984; Ojemann et al., 2002). In the present study, both auditory and visual naming sites were identified in the anterior frontal cortex, outside of BA 4, 6, 44, and 45, and more auditory descriptive naming sites compared to visual object naming sites were found. These sites were often not identified using traditional ESM. Two patients that underwent anterior frontal resection developed post-operative language deficits, indicating that ESM may not be optimal for predicting post-operative language deficits in patients undergoing frontal lobectomy. These findings also suggest that more extensive pre-operative mapping of dominant frontal cortex, including auditory descriptive naming, may be warranted.
Prior studies have also suggested that in certain circumstances, ECoG mapping may predict naming deficits not identified by ESM (Cervenka et al., 2011b; Kojima et al., 2012; Sinai et al., 2005b). Kojima et al. (2012) recently described a patient that underwent dorsolateral-premotor and inferior-Rolandic resection including language cortex identified by ECoG mapping but not identified during ESM. This patient developed dysphasia requiring speech therapy. In the present study, the sensitivity of ESM was only 25% with a negative predictive value of 40%. However, the positive predictive value of ECoG mapping was 80% with a negative predictive value of 100%. A test with a false negative rate of zero is most desirable for surgery planning to avoid post-operative deficits, however, false positives can also prevent resection of epileptogenic tissue. Combining these two techniques may improve post-operative language and seizure outcomes in this patient population.
Electrocorticographic analyses carry several limitations. Only patients with implanted subdural electrodes for surgical planning can be studied. Therefore, findings cannot be generalizable to healthy individuals given that an underlying seizure focus or lesion may cause unanticipated functional reorganization. In the present study, the sample size did not allow for a subgroup analysis to determine the effects of seizure localization on cortical activation patterns and further studies are warranted to assess this. Furthermore, ECoG mapping identifies cortical regions that participate in a given task but may not be essential for that task as illustrated by one patient in whom ECoG(+) sites were resected and no post-operative deficits were demonstrated. This may account for the relatively low specificity of ECoG compared to ESM in predicting post-operative language deficits in this study. The magnitudes of activation appear to vary between ECoG(+) sites (as seen in Figure 3), and the significance of this finding when predicting post-operative language outcomes (i.e. whether a greater change in power predicts a greater likelihood of post-operative impairment if that region is resected) has not yet been studied in detail. Larger studies examining the predictive values of ECoG mapping in predicting post-operative language outcomes will likely be needed to support its future clinical application. Finally, the time course of ECoG activation during various language tasks (Chang et al., 2010; Edwards et al., 2010)and the dynamics of connectivity between activated regions (Korzeniewska et al., 2011) can provide valuable insight into the underlying functions of individual regions and warrants further investigation as well.
5. Conclusion
Electrocorticographic language mapping revealed regions in dominant anterior frontal and temporal neocortex that were recruited during auditory descriptive naming but not during visual object naming and not identified during traditional electrocortical stimulation mapping. Resection of these regions produced language deficits that were at times debilitating and persistent. These findings suggest that supplementing ESM with ECoG mapping of language, and expanding traditional language mapping batteries to include an auditory descriptive naming test, may improve post-operative language performance.
Highlights.
Standard dominant temporal or frontal resections can cause language deficits
Deficits can also occur after resections tailored by cortical stimulation mapping
Electrocorticographic (ECoG) high gamma activity maps cortex critical for naming
ECoG maps auditory more often than visual naming within standard resection boundaries
Naming deficits can occur if ECoG maps are not taken into account
Acknowledgements
We thank the research participants that made this study possible, Jakir Hossain for creating a MATLAB program to localize electrodes, Noelle Stewart, Rebecca Fisher, Viktar Kanasevich, and Karen Walters for administering ESM, Drs. Frederick Lenz, George Jallo, and William S. Anderson for performing all neurosurgical procedures, and Dr. Marla Hamberger for providing auditory descriptive naming stimuli.
Funding
This work was supported by the National Institutes of Neurological Disorders and Stroke [NS40596 to N.E.C.]; National Institutes of Health- National Institute of Deafness and Other Communication Disorders [DC010028 and DC005645 to D.F.B.]; The Epilepsy Foundation [Research and Training Fellowships for Clinicians to M.C.C.].
Abbreviations
- ESM
electrocortical stimulation mapping
- ECoG
electrocorticography or electrocorticographic
- IAP
intracarotid amobarbital procedure
- VNT
visual object naming test
- ANT
auditory descriptive naming test
- RT
response time
- MNI
Montreal Neurological Institute
- BA
Brodmann’s area
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson JM, Gilmore R, Roper S, Crosson B, Bauer RM, Nadeau S, et al. Conduction aphasia and the arcuate fasciculus: A reexamination of the Wernicke-Geschwind model. Brain Lang. 1999;70:1–12. doi: 10.1006/brln.1999.2135. [DOI] [PubMed] [Google Scholar]
- Bates D, Chambers J, Dalgaard P, Falcon S, Gentleman R, Hornik K, et al. R: a language and environment for statistical computing. [online]. Available at: http://www.R-project.org/. ISBN 3-900051-07-0.
- Boatman-Reich D, Franaszczuk PJ, Korzeniewska A, Caffo B, Ritzl EK, Colwell S, et al. Quantifying auditory event-related responses in multichannel human intracranial recordings. Front Comput Neurosci. 2010;4:4. doi: 10.3389/fncom.2010.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boatman DF, Miglioretti DL. Cortical sites critical for speech discrimination in normal and impaired listeners. J Neurosci. 2005;25:5475–80. doi: 10.1523/JNEUROSCI.0936-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Benedict RHB. Hopkins Verbal Learning Test-Revised. Professional manual. Psychological Assessment Resources, Inc.; Lutz, FL: 2001. [Google Scholar]
- Brunner P, Ritaccio AL, Lynch TM, Emrich JF, Wilson JA, Williams JC, et al. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–86. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka MC, Nagle S, Boatman-Reich D. Cortical high-gamma responses in auditory processing. American journal of audiology. 2011a;20:171–80. doi: 10.1044/1059-0889(2011/10-0036). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka MC, Boatman-Reich DF, Ward J, Franaszczuk PJ, Crone NE. Language mapping in multilingual patients: electrocorticography and cortical stimulation during naming. Front Hum Neurosci. 2011b;5:13. doi: 10.3389/fnhum.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka MC, Franaszczuk PJ, Crone NE, Hong B, Caffo BS, Bhatt P, et al. Reliability of early cortical auditory gamma-band responses. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EF, Rieger JW, Johnson K, Berger MS, Barbaro NM, Knight RT. Categorical speech representation in human superior temporal gyrus. Nat Neurosci. 2010;13:1428–32. doi: 10.1038/nn.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–15. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, Hao L, Hart J, Jr., Boatman D, Lesser RP, Irizarry R, et al. Electrocorticographic gamma activity during word production in spoken and sign language. Neurology. 2001;57:2045–53. doi: 10.1212/wnl.57.11.2045. [DOI] [PubMed] [Google Scholar]
- Davies KG, Risse GL, Gates JR. Naming ability after tailored left temporal resection with extraoperative language mapping: increased risk of decline with later epilepsy onset age. Epilepsy Behav. 2005;7:273–8. doi: 10.1016/j.yebeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- DeLeon J, Gottesman RF, Kleinman JT, Newhart M, Davis C, Heidler-Gary J, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain. 2007;130:1408–22. doi: 10.1093/brain/awm011. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Plaisant O, Iba-Zizen MT, Cabanis EA. Paul Broca’s historic cases: high resolution MR imaging of the brains of Leborgne and Lelong. Brain. 2007;130:1432–41. doi: 10.1093/brain/awm042. [DOI] [PubMed] [Google Scholar]
- Edwards E, Nagarajan SS, Dalal SS, Canolty RT, Kirsch HE, Barbaro NM, et al. Spatiotemporal imaging of cortical activation during verb generation and picture naming. Neuroimage. 2010;50:291–301. doi: 10.1016/j.neuroimage.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J., Jr. Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992) Neurology. 1993;43:1612–17. doi: 10.1212/wnl.43.8.1612. [DOI] [PubMed] [Google Scholar]
- Flinker A, Chang EF, Barbaro NM, Berger MS, Knight RT. Sub-centimeter language organization in the human temporal lobe. Brain Lang. 2010 doi: 10.1016/j.bandl.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franaszczuk PJ, Jouny CC. Software system for data management and distributed processing of multichannel biomedical signals. Conf Proc IEEE Eng Med Biol Soc. 2004;2:983–5. doi: 10.1109/IEMBS.2004.1403326. [DOI] [PubMed] [Google Scholar]
- Franaszczuk PJ, Bergey GK, Durka PJ, Eisenberg HM. Time-frequency analysis using the matching pursuit algorithm applied to seizures originating from the mesial temporal lobe. Electroencephalogr Clin Neurophysiol. 1998;106:513–21. doi: 10.1016/s0013-4694(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT. Auditory and visual naming tests: normative and patient data for accuracy, response time, and tip-of-the-tongue. J Int Neuropsychol Soc. 2003;9:479–89. doi: 10.1017/s135561770393013x. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Goodman RR, Perrine K, Tamny T. Anatomic dissociation of auditory and visual naming in the lateral temporal cortex. Neurology. 2001;56:56–61. doi: 10.1212/wnl.56.1.56. [DOI] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, Goodman RR, McKhann GM., 2nd Does cortical mapping protect naming if surgery includes hippocampal resection? Ann Neurol. 2010;67:345–52. doi: 10.1002/ana.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberger MJ, Seidel WT, McKhann GM, 2nd, Perrine K, Goodman RR. Brain stimulation reveals critical auditory naming cortex. Brain. 2005;128:2742–9. doi: 10.1093/brain/awh621. [DOI] [PubMed] [Google Scholar]
- Hannay HJ, Lezak MD. The Neuropsychological Examination: Interpretation. In: Lezak MD, Howieson DB, Loring DW, editors. The Neuropsychological Assessment. 4th ed. Oxford University Press; New York, New York: 2004. pp. 133–56. [Google Scholar]
- Hermann BP, Perrine K, Chelune GJ, Barr W, Loring DW, Strauss E, et al. Visual confrontation naming following left anterior temporal lobectomy: a comparison of surgical approaches. Neuropsychology. 1999;13:3–9. doi: 10.1037//0894-4105.13.1.3. [DOI] [PubMed] [Google Scholar]
- Ilmberger J, Eisner W, Schmid U, Reulen HJ. Performance in picture naming and word comprehension: evidence for common neuronal substrates from intraoperative language mapping. Brain Lang. 2001;76:111–8. doi: 10.1006/brln.2000.2415. [DOI] [PubMed] [Google Scholar]
- Jung J, Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, et al. The neural bases of attentive reading. Hum Brain Mapp. 2008;29:1193–206. doi: 10.1002/hbm.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, et al. Handedness and hemispheric language dominance in healthy humans. Brain. 2000;123(Pt 12):2512–8. doi: 10.1093/brain/123.12.2512. [DOI] [PubMed] [Google Scholar]
- Kojima K, Brown EC, Rothermel R, Carlson A, Matsuzaki N, Shah A, et al. Multimodality language mapping in patients with left-hemispheric language dominance on Wada test. Clin Neurophysiol. 2012 doi: 10.1016/j.clinph.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewska A, Franaszczuk PJ, Crainiceanu CM, Kus R, Crone NE. Dynamics of large-scale cortical interactions at high gamma frequencies during word production: event related causality (ERC) analysis of human electrocorticography (ECoG) Neuroimage. 2011;56:2218–37. doi: 10.1016/j.neuroimage.2011.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss GL, Fisher R, Plate C, Hart J, Uematsu S, Gordon B, et al. Cognitive effects of resecting basal temporal language areas. Epilepsia. 1996;37:476–83. doi: 10.1111/j.1528-1157.1996.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Rainey LH, Summerlin JL, Freitas CS, Fox PT, Evans AC, et al. Automated labeling of the human brain: a preliminary report on the development and evaluation of a forward-transform method. Hum Brain Mapp. 1997;5:238–42. doi: 10.1002/(SICI)1097-0193(1997)5:4<238::AID-HBM6>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, et al. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10:120–31. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser RP, Lueders H, Dinner DS, Hahn J, Cohen L. The location of speech and writing functions in the frontal language area: results of extraoperative cortical stimulation. Brain. 1984;107:275–91. doi: 10.1093/brain/107.1.275. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Lüders H, Klem G, Dinner DS, Morris HH, Hahn JF, et al. Extraoperative cortical functional localization in patients with epilepsy. J Clin Neurophysiol. 1987;4(1):27–53. doi: 10.1097/00004691-198701000-00003. [DOI] [PubMed] [Google Scholar]
- Leuthardt EC, Miller K, Anderson NR, Schalk G, Dowling J, Miller J, et al. Electrocorticographic frequency alteration mapping: a clinical technique for mapping the motor cortex. Neurosurgery. 2007;60:260–70. doi: 10.1227/01.NEU.0000255413.70807.6E. discussion 70-1. [DOI] [PubMed] [Google Scholar]
- Mainy N, Jung J, Baciu M, Kahane P, Schoendorff B, Minotti L, et al. Cortical dynamics of word recognition. Hum Brain Mapp. 2008;29:1215–30. doi: 10.1002/hbm.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallat S, Zhang Z. Matching pursuit with time-frequency dictionaries. IEEE Trans Signal Process. 1993;41:3397–415. [Google Scholar]
- Malow BA, Blaxton TA, Sato S, Bookheimer SY, Kufta CV, Figlozzi CM, et al. Cortical stimulation elicits regional distinctions in auditory and visual naming. Epilepsia. 1996;37:245–52. doi: 10.1111/j.1528-1157.1996.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Mitrushina M, Boone KB, Razani J, D’Elia LF. Handbook of Normative Data for Neuropsychological Assessment. Second Edition ed. Oxford University Press; New York: 2005. [Google Scholar]
- Ojemann JG, Ojemann GA, Lettich E. Cortical stimulation mapping of language cortex by using a verb generation task: effects of learning and comparison to mapping based on object naming. 2002;97:33–38. doi: 10.3171/jns.2002.97.1.0033. 2002. [DOI] [PubMed] [Google Scholar]
- Yale School of Medicine. Section of Bioimaging Sciences, Department of Diagnostic Radiology; New Haven, CT: 2012. Bioimage Suite: An integrated medical image analysis suite [computer program] [Google Scholar]
- Ray S, Jouny CC, Crone NE, Boatman D, Thakor NV, Franaszczuk PJ. Human ECoG analysis during speech perception using matching pursuit: a comparison between stochastic and dyadic dictionaries. IEEE Trans Biomed Eng. 2003;50:1371–3. doi: 10.1109/TBME.2003.819852. [DOI] [PubMed] [Google Scholar]
- Ritaccio A, Brunner P, Cervenka MC, Crone N, Guger C, Leuthardt E, et al. Proceedings of the first international workshop on advances in electrocorticography. Epilepsy Behav. 2010;19:204–15. doi: 10.1016/j.yebeh.2010.08.028. [DOI] [PubMed] [Google Scholar]
- Roberts PM, Garcia LJ, Desrochers A, Hernandez D. English performance of proficient bilingual adults on the Boston Naming Test. Aphasiology. 2002;16:634–45. [Google Scholar]
- Schretlen DJ, Vannorsdall TD, Calibrated Ideational Fluency Assessment (CIFA) Professional manual. Psychological Assessment Resources, Inc.; Lutz, FL: 2010. [Google Scholar]
- Sinai A, Franaszczuk P, Crone NE. Electrocorticographic spectral responses during auditory vs. Epilepsia. 2005a;46:71–2. [Google Scholar]
- Sinai A, Crone NE, Wied HM, Franaszczuk PJ, Miglioretti D, Boatman-Reich D. Intracranial mapping of auditory perception: event-related responses and electrocortical stimulation. Clin Neurophysiol. 2009;120:140–9. doi: 10.1016/j.clinph.2008.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, et al. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005b;128:1556–70. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency gamma-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25:3287–93. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–77. [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, et al. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–27. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiebe S, Blume WT, Girvin JP, Eliasziw M. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- Wolfinger R, O’Connell M. Generalized linear mixed models: A pseudo-likelihood approach. Journal of Statistical Computation and Stimulation. 1993;48:233–43. [Google Scholar]
- Wu M, Wisneski K, Schalk G, Sharma M, Roland J, Breshears J, et al. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010;66:E407–9. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- Zygierewicz J, Durka PJ, Klekowicz H, Franaszczuk PJ, Crone NE. Computationally efficient approaches to calculating significant ERD/ERS changes in the time-frequency plane. J Neurosci Methods. 2005;145:267–76. doi: 10.1016/j.jneumeth.2005.01.013. [DOI] [PubMed] [Google Scholar]