Abstract
This research examined the psychophysiology of emotional arousal anticipatory to potentially aversive and highly pleasant outcomes. Human brain reactions (event-related potentials) and body reactions (heart rate, skin conductance, the probe startle reflex) were assessed along motivational gradients determined by apparent distance from sites of potential punishment or reward. A predator-prey survival context was simulated using cues that signaled possible money rewards or possible losses; the cues appeared to loom progressively closer to the viewer, until a final step when a rapid key response could ensure reward or avoid a punishing loss. The observed anticipatory response patterns of heightened vigilance and physiological mobilization are consistent with the view that the physiology of emotion is founded on action dispositions that evolved in mammals to facilitate survival by dealing with threats or capturing life-sustaining rewards.
Research with animals shows that brain and reflex reactions change systematically with the proximity of aversive cues. When prey animals perceive a predator in the distance, limbic circuitry in their brains initiates a range of defensive reactions, including motor inhibition, focused attention to the threat, and decelerating heart rate (fear bradycardia; Campbell, Wood, & McBride, 1997); if the predator gets closer, these reactions are augmented. Studying conditioned fear, Fanselow (1994) found that initial motor “freezing” depended on projections from the amygdala to the brain’s ventral central gray, and Kapp, Whalen, Supple, and Pascoe (1992) found that bradycardia evoked by a fear cue covaried closely with cell firing in the amygdala. Further understanding of this defense circuit was provided by M. Davis (1998), who demonstrated that fearful animals were hyperreactive to startling stimuli presented during conditioned fear cues—a response also mediated by projections from the amygdala.
As the distance from the predator is further reduced, prey animals increasingly mobilize for action. This “alarm” reaction (Masterson & Crawford, 1982) involves yet higher vigilance, sympathetic activation of glands and smooth muscles, movement of blood to the gross muscles, and cardiac acceleration. With close confrontation (the circa-strike zone), prey show active defense (fight or flight), mediated by the dorsal central gray (Fanselow, 1994).
The reactions of a stalking predator are similar to the anticipatory responses of threatened prey—in the initial inhibition of movement and augmented vigilance, but also in physiological mobilization with increasing proximity; as the predator approaches the strike zone, instead of fight or flight, there is a final rush to capture. Furthermore, research on anticipated reward has implicated many of the same brain structures activated in defense (e.g., the amygdala; Zald, 2003), as well as other structures (e.g., nucleus accumbens and medial prefrontal cortex; see Schultz, 2000) that may be unique to reward. Thus, the confrontation between predator and prey can be seen as an antagonistic dance, involving common actions and competing motives that may be part of humans’ mixed emotional inheritance.
PICTURE PROCESSING
Lang, Bradley, and Cuthbert (1997) proposed that humans viewing aversive, unpleasant picture stimuli are engaged by these cues in the same way that nonhuman mammals are engaged by a threatening stimulus seen at a distance. From this perspective, the psychophysiologies of human emotions are action dispositions (Lang et al., 1997; see also Frijda, 1987), that is, states of heightened vigilance and physiological mobilization that are primitively associated with survival goals and occur in mammals when overt action is delayed or inhibited. Thus, parallel somatic and autonomic reflexes are deployed by similar defense circuits in animal and human brains. When pictures are more aversive or arousing, defensive responding increases, as it does when animals are closer to threatening stimuli.
Studies of picture perception support this view: A sudden noise prompts a larger startle when people are viewing unpleasant pictures, as it does when animals are under threat. Unpleasant images (of mutilation or attack) prompt parasympathetic-mediated bradycardia associated with fear vigilance and also engage the sympathetic system, enhancing skin conductance. All of these responses are larger for pictures rated higher in emotional arousal (e.g., Bradley, Hamby, Löw, & Lang, 2007).
Highly fearful participants viewing pictures of the feared objects (e.g., snakes or spiders) show sympathetically mediated heart rate acceleration (e.g., Hamm, Cuthbert, Globisch, & Vaitl, 1997; Klorman, Weissbert, & Wiessenfeld, 1977), as if the threat were close, requiring mobilization of the body for action prior to fight or flight. Additionally, they show greater skin conductance increases and probe reflex potentiation compared with low-fear control participants, again consistent with the idea that fearful participants apprehend greater threat in these images (Sabatinelli, Bradley, & Lang, 2001).
Arousing pleasant pictures (e.g., erotica) also strongly modulate psychophysiological reactivity (Bradley, Codispoti, Cuthbert, & Lang, 2001), engaging appetitive motive circuits and reflex changes that denote increased vigilance and autonomic arousal. This reaction again follows the animal model: A hidden predator, lying in wait for approaching prey, also shows heightened attention, followed by increasing mobilization for action as distance diminishes.
THE PRESENT RESEARCH
The main goal of the present research was to more directly assess this distance hypothesis (i.e., that reflex patterns denoting vigilance and action mobilization change with increasing threat imminence) in human participants, measuring anticipatory somatic and autonomic reflexes—and changes in evoked brain responses—as a threatening stimulus appeared to loom progressively closer, with opportunity for escape delayed until the culminating step.
A further aim was to compare gradient response patterns for threat of punishment with those for anticipated reward. On the basis of classic experiments with rodents, Miller (1951) concluded that when an organism is distant from a goal, approach motivation to achieve a reward is greater than motivation to avoid punishment, but that as the goal becomes more proximal, avoidance motivation rises steeply, surpassing approach. Analogous phenomena were found in studies of evaluative judgments (Cacioppo, Gardner, & Berntson, 1997), and research on risk behavior (see Kahneman & Tversky, 1979, 2000) suggests that loss aversion may be stronger than the motivation to gain rewards.
The laboratory task we studied took the form of a computer-game simulation in which picture cues—signaling that money could be gained or lost—first appeared at a distance, then loomed progressively closer, finally arriving in a “strike zone” where reward capture or escape from loss depended on reaction speed. We expected this simulation to evoke three related anticipatory processes: (a) heightened attention to motivationally significant cues, with increasing demand for vigilance as the imminence of reward or punishment increases; (b) mobilization of the body for action, preparatory to avoiding loss or achieving a reward; and (c) emotional arousal, which is induced in contexts of both threat and appetite. The experimental design was predicated on the defense cascade model (Lang et al., 1997), which construes emotional perception in the context of an evolved predator-prey interaction. In this view, anticipatory processing (and increasing emotional arousal) is reflected in staged patterns of autonomic reflexes and startle probe modulation. We also explored evoked brain potentials from first appearance of a distant, potential danger or reward; to initial looming, when encounter is uncertain (maybe the danger or reward will go away); to a later closing of the gap, when an encounter is certain and imminent; and then to the final confrontation, which requires coping action.
Autonomic Reflexes
Both heightened vigilance and response mobilization prompt activation of the sympathetic branch of the autonomic nervous system, mediating glandular responses that include changes in the palmar sweat glands (Wallin, 1981). Considering that arousal increases with imminence (Lang et al., 1997), we expected that both reward and punishment (relative to motivationally neutral stimuli) would be associated with a tonic, progressively augmented skin conductance response over the anticipatory period.
Heart rate is differently modulated by the sympathetic and parasympathetic branches of the autonomic nervous system (Cacioppo et al., 1994). On the one hand, motivationally relevant stimuli prompt anticipatory response mobilization and an associated heart rate acceleration, either through vagal release or through direct sympathetic activation (R.C. Davis, Buchwald, & Frankman, 1955). On the other hand, orienting is associated with cardiac deceleration, and there are considerable data showing that tasks demanding vigilance for response cues result in parasympathetic activation and a decrease in heart rate (Graham, 1992; Lacey & Lacey, 1978; Somsen, Jennings, & Van der Molen, 2004). We anticipated, as proposed in the defense cascade model, that subsequent to the initial appearance of a motivational cue, heart rate would show a tonic, progressive deceleration associated with increasing vigilance as an encounter becomes more imminent; however, we expected that immediately prior to the anticipated confrontation, heart rate would abruptly accelerate during mobilization for a rapid response.
The Startle Probe
During the task, brief noise probes were presented periodically. Much previous research has shown that startle reflexes evoked by such probes are modulated by both affect and attention (Bradley, Codispoti, & Lang, 2006). Thus, during free viewing of picture stimuli, startle probe reflexes are potentiated when participants view unpleasant stimuli and inhibited (relative to responses to neutral cues) when participants view pleasant stimuli (e.g., Lang, Bradley, & Cuthbert, 1990). It has also been shown that startle responses are reduced when attention is directed to a task-relevant stimulus (e.g., Anthony, 1985; Graham, 1992). We anticipated that both affect and attention would modulate the startle probe response: Previous data suggest that reflexes initially elicited during arousing, appetitive cues will be relatively inhibited and, conversely, that reflexes initially elicited during arousing, aversive cues will be potentiated—a pattern reflecting hedonic valence. However, it has not been demonstrated whether or not this pattern is sustained in the looming context, when vigilance increases markedly.
Brain Event-Related Potentials
A continuous stream of discrete, briefly presented images paced the participants’ reactions to the task. Thus, it was possible to systematically assess picture-by-picture changes in event-related potentials (ERPs) as they developed in anticipation of reward or threat of loss. Previous studies of emotional picture processing have defined a late positive-going potential (300–600 ms after cue onset), dominant over the central-parietal area, that is greater in amplitude as the image content is judged to be more emotionally arousing (e.g., Cuthbert, Schupp, Bradley, Birbaumer, & Lang, 2000; Keil et al., 2002; Schupp et al., 2000). These data suggest that motivationally relevant images (cues signaling potential reward or loss) will prompt a larger late positive potential than neutral pictures, and that, as reward or loss becomes more imminent and emotional arousal is augmented, this potential will show a progressive increase with sequential presentation of motivationally relevant pictures.
METHOD
The study was approved by the institutional review board of the University of Florida. Thirty-two paid volunteers (15 males, 17 females; mean age = 20 years; 29 right-handed) participated. The final artifact-free sample sizes were 31 for the ERP data, 30 for the startle probe and skin-conductance data, and 29 for the heart rate data.
After recording sensors were placed on participants, they received instructions, practiced the task, and were allowed to ask questions about the procedure. Participants began play with an initial stake of $10 that could be augmented or reduced, depending on performance.
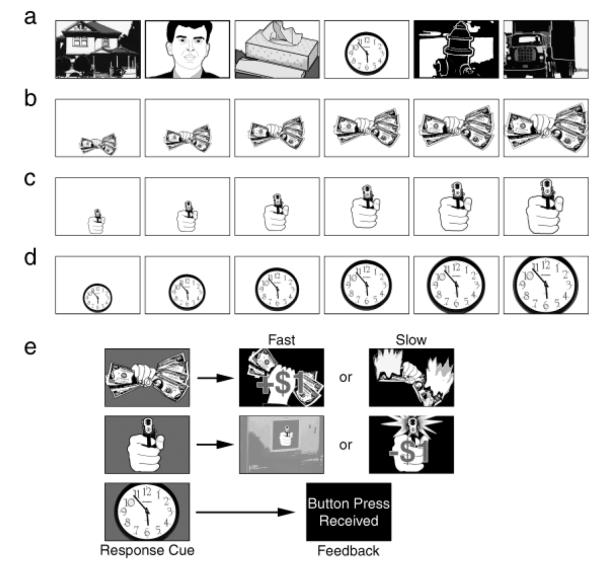
The picture stimuli were selected from the International Affective Picture System (Lang, Bradley, & Cuthbert, 2005). They were continuously projected (1.5 s each, 0.5-s interpicture interval) on a screen 200 cm in front of the participant. Four blocks of trials were administered (see Fig. 1). Each block included 160 neutral (nonlooming) images. Looming images (i.e., images that were used in looming sequences) occurred at random positions in the stream. Within a block, each looming image (picture of money, picture of a gun, neutral picture) occurred four times as an isolated picture, four times as a sequence of three images of increasing size (i.e., foils); four times as a sequence of six pictures of increasing size, and four times as a sequence of seven pictures of increasing size. For the six- and seven-picture sequences, participants were required to respond (by computer mouse) when the final picture was presented (signaled by a change in background color). The same motive pictures (money, gun) appeared throughout the experiment. The looming neutral pictures varied, but were readily distinguishable from the neutral stream at first appearance (by virtue of their background, small size, and screen location; see Fig. 1). Participants gained a reward ($1) for responding to a money cue within the response window and avoided a loss ($1) by responding to a gun cue within the response window. Responses to neutral cues were acknowledged, but had no speed requirement and resulted in no gains or losses.
Fig. 1.
Diagram of the experimental design. A continuous stream of pictures was presented throughout the experiment. Most of these pictures were emotionally neutral in content (a). However, a fist holding money or a gun aimed at the viewer occasionally appeared in the stream. On response trials, the money (b), the gun (c), or one of the neutral images (d) loomed, becoming increasingly large over six or seven presentations. Participants were instructed to press a key at the end of this picture sequence, when the picture background changed color (e). The reaction time response prompted immediate feedback, as shown. A slow response on gun trials resulted in a loss of $1, and a fast response on money trials added $1 to the participant’s stake; all responses to neutral cues were acknowledged without regard to latency. Money and gun pictures could also appear briefly in the neutral stream—singly or in a short looming sequence of three images. For these brief presentations, there was no subsequent response cue.
The response window was 400 ms for the initial trial block. So that the task would stay challenging, the response window for the second through fourth blocks was the participant’s mean response time on the preceding block. Responses preceding the color-change cue resulted in no gain (money) or loss (gun).
Startle probes (98-dB white noise, duration of 50 ms) were presented over headphones. Within the looming sequences of each block, money, gun, and neutral cues were each probed (1,100 ms after picture onset) 12 times: 4 times at Picture 1, 4 times at Picture 3, and 4 times at either Picture 5 or Picture 6 (chosen independently of the length of the sequence).
Physiological Recording
The electroencephalogram (EEG) was sampled at 100 Hz with a 129-channel system (Electrical Geodesics, Eugene, OR); a vertex reference was used, and the signal was filtered on-line (0.01–40 Hz). Bad channels were later interpolated using spherical splines, filtered at 0.1 through 30 Hz, corrected for blink artifacts (Ille, Berg, & Scherg, 2002), and rereferenced to the average reference. ERPs were averaged in 1-s segments (including a 100-ms prepicture baseline) for each cue type and position in the looming sequence.
Skin-conductance sensors were placed adjacent to one another on the hypothenar eminence of the palm of the nondominant hand. A Coulborn conductance coupler provided a constant voltage (0.5 V) across the two electrodes. Change relative to a 1-s presequence baseline was computed in half-second bins.
Heart rate from forearm sensors was recorded in milliseconds. Change scores were computed in half-second bins from a 1-s baseline.
Magnitude (base to peak) of the eyeblink was determined from electromyograph (EMG) recording of the orbicularis oculi muscle. Raw signals were filtered (13–150 Hz), rectified, integrated (100-ms time constant), and sampled at 1 kHz.
Data Reduction and Analysis
Participants were not instructed when response signals might occur during a looming sequence, but soon learned that danger and opportunity increased the closer the cue appeared to be. For purposes of analysis, the anticipatory sequences (money, gun, or neutral) were divided into an early maybe stage (Pictures 1–3), when distance was greater and continuation of the sequence was uncertain, and a later imminent stage (Pictures 4–6), when the response requirement was temporally proximal and finally inevitable. The final picture of a sequence, when a response occurred, was not included in the analysis; therefore, Picture 6 was always penultimate (i.e., the picture just previous to the one evoking a motor response in a seven-picture sequence).
In analyzing autonomic reactions (heart rate and skin conductance) during the looming sequence, we performed separate trend tests for the early maybe stage of the sequence (Pictures 1–3), when a confrontation was uncertain, and the later imminent stage, when a final response would be required (Pictures 4–6). Each test included the factors of cue type (money, gun, neutral) and response sequence (12 half-second bins) over that stage. Additionally, skin conductance and phasic heart rate responses were assessed within each picture in the imminent stage. These analyses included the factors of time (four half-second bins for each picture), picture (picture position in the sequence: 4, 5, 6), and cue type.
Blink-reflex t scores (based on each participant’s distribution) were analyzed for differences in magnitude among cue types and for change in magnitude over the picture sequence, in a 3 (cue type: money, gun, neutral cue) × 3 (picture position in the sequence: Picture 1, Picture 3, Pictures 5 and 6 combined) analysis.
After considering analyses used in previous research (see Cuthbert et al., 2000; Schupp et al., 2000) and inspecting the obtained voltage maps, we used the average ERP of 13 central-parietal electrodes in the EEG analysis. ERP late positive potentials (mean amplitude 300–600 ms after picture onset) were evaluated for differences among the cue types and for changes within the maybe and imminent stages, in 3 (cue type) × 3 (picture position in the sequence: Picture 1, Picture 2, Picture 3 or Picture 4, Picture 5, Picture 6) analyses. All p values are Greenhouse-Geisser corrected.
RESULTS
Performance: Strike and Escape
Cue type had a significant effect on response time, F(2, 62) = 6.22, p < .01, η2 = .167. Response time was faster for money cues (M = 372 ms, SD = 78 ms) than for neutral cues (M = 392 ms, SD = 73 ms) and was also faster for gun cues (M = 368 ms, SD = 77 ms) than for neutral cues, ps < .01; response times did not differ between money and gun cues. Response were faster when the cue occurred at Picture 7 (M = 358 ms, SD = 76 ms) than when it occurred at Picture 6 (M = 397 ms, SD = 72 ms), F(1, 31) = 91.5, p < .0001, η2 = .747. On average, participants made $23.85 (SD = 4.77, range: $7–$30). Regardless of performance, participants received a minimum of $20.
Skin Conductance
During the maybe stage, tonic skin-conductance level (see Fig. 2) diminished progressively for neutral pictures, but was sustained for motive cues. Thus, during this stage, skin conductance showed a main effect of cue type, F(2, 58) = 4.12, p < .04, η2 = .124, as well as a Cue Type × Response Sequence interaction, F(22, 638) = 3.50, p < .03, η2 = .108. There was also a main effect of cue type at Picture 4, F(2, 58) = 5.36, p < .01; neutral cues differed from both money cues, p < .01, and gun cues, p < .02. This difference between motive and neutral cues increased further over the imminent stage, resulting in a Cue Type × Response Sequence interaction, F(22, 638) = 5.68, p < .003. Both money and gun cues—but not neutral cues—showed a dramatic increase in skin conductance at penultimate Picture 6. This increase was reflected in the interaction between cue type and time, F(6, 174) = 6.49, p < .003. The test for linear trend was highly significant, F(1, 29) = 11.34, p < .003.
Fig. 2.
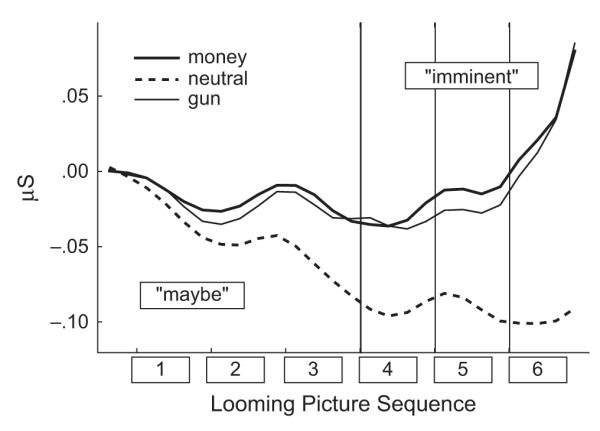
Averaged changes in skin conductance for money, gun, and neutral cues as a function of the cue’s position in the picture sequence preceding the response cue (maybe stage: Pictures 1–3; imminent stage: Pictures 4–6).
Heart Rate
During the maybe stage, heart rate increased for both the gun and the money cues, peaking at Picture 2, and then decelerating during Picture 3; this pattern was not present for neutral cues (see Fig. 3). Thus, there was a main effect of cue type, F(2, 56) = 6.27, p < .005, η2 = .183, as well as a Cue Type × Response Sequence interaction, F(22, 616) = 4.64, p < .002, η2 = .142. The quadratic trend was significant for money cues and gun cues, Fs(1, 28) > 25.55, ps < .001, but not for neutral cues, F(1, 18) < 1, n.s. Over the imminent stage, all cue types prompted a general deceleration, with a nadir at Picture 6, resulting in a significant effect of response sequence, F(11, 308) = 30.82, p < .001, η2 = .524. Greater deceleration was found for gun and neutral cues than for money cues, as reflected in a main effect of cue type, F(2, 56) = 4.06, p < .03, η2 = .127, and the results of post hoc contrasts; that is, both gun and neutral cues differed from money cues (ps < .02), but gun and neutral cues showed a similar, more marked deceleration (n.s.).
Fig. 3.
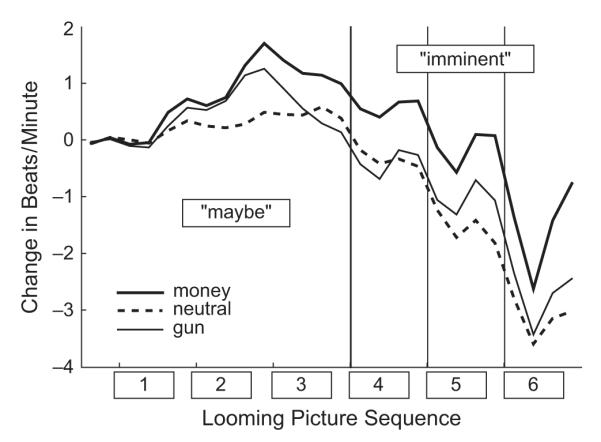
Averaged changes in heart rate for money, gun, and neutral cues as a function of the cue’s position in the picture sequence preceding the response cue (maybe stage: Pictures 1–3; imminent stage: Pictures 4–6).
Furthermore, all cue types prompted similar phasic responses to each of the successively more imminent pictures. That is, after the initial deceleration at the onset of a cue, there was a brief acceleration that was sustained until the next cue’s onset: This phasic quadratic trend was significant, F(1, 28) = 83.98, p < .0001. The acceleratory limb of the phasic heart rate response (see Fig. 3) increased over the imminent stage, significantly more for motive cues than for neutral cues, resulting in a significant Time × Cue Type × Picture interaction, F(4, 112) = 2.79, p < .05. At the penultimate picture (6), the acceleratory response was significantly greater for the money cue than for both the gun (p < .02) and the neutral (p < .005) cues.
Somatic Reflex: The Startle Probe Reflex
As illustrated in Figure 4, cue type had a main effect on the startle reflex, F(2, 58) = 3.88, p < .03, η2 = .118. Relative to the startle reflex for neutral cues, the startle reflex for money cues was inhibited, and the startle reflex for gun cues was potentiated. Reflex magnitude showed a main effect of picture, however, decreasing dramatically with increasing imminence, F(2, 58) = 26.63, p < .001, η2 = .479. Thus, for the first picture, the pattern of results showed the same ordering as in free-viewing studies (i.e., relative potentiation for unpleasant pictures and inhibition for pleasant pictures; the difference between money and gun cues was significant, p < .02, but the difference between neutral cues and each type of motive cue was not). At Picture 3, there was a general diminution in startle (most apparent for gun cues). Nevertheless, reflexes for the money cues were significantly inhibited relative to reflexes for both the gun stimuli, p < .02, and the neutral stimuli, p < .04. At the imminent stage, startle magnitude was dramatically inhibited, showing no significant differences among cue types.
Fig. 4.
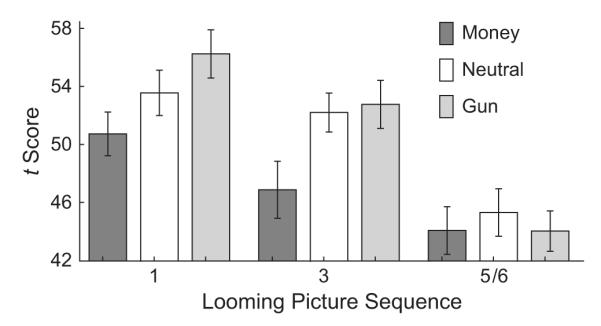
Average magnitude of the startle probe reflex for money, gun, and neutral images over the looming sequence. Probes were presented 1,100 ms after picture onset at three points in the sequence: during Pictures 1 and 3 (maybe stage) and during either Picture 5 or Picture 6 (imminent stage). Mean t scores and their standard errors are shown.
ERPs
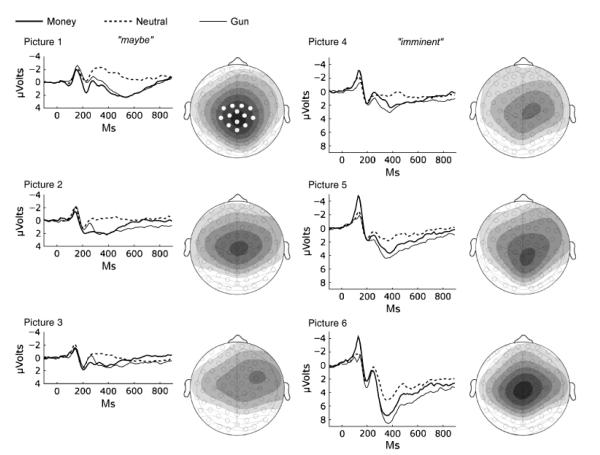
The ERPs for money, gun, and neutral cues were similarly distributed over the scalp. Figure 5 shows voltage maps (motive cues minus neutral cues), 300 to 600 ms after picture onset, for each picture position in the looming sequences. As expected, the late positive potential was focused in the central-parietal area. It was initially strong, then weakened, but developed again over the imminent stage.
Fig. 5.
Event-related potentials (average waveform across 13 central-parietal sensors) for money, gun, and neutral cues at each position in the six-picture looming sequence preceding a response cue. The graph for each picture position is accompanied by a voltage map that illustrates the scalp distribution of differences in the late positive potential (300–600 ms after picture onset) between motive and neutral pictures (i.e., gun pictures minus neutral pictures, money pictures minus neutral pictures). A 0.5-μV change is represented by a contour line; darker shading indicates greater positive voltage. Data from the 13 sensors highlighted in white in the map for Picture 1 were averaged for statistical analysis. The early components of the event-related potential waveform (prior to 200 ms), which primarily reflect sensory features of the stimulus, were not statistically reliable for analyzing differences between cue types.
ERPs to the first picture resembled those observed for emotional and neutral pictures during free viewing (Cuthbert et al., 2000; Schupp et al., 2000): Both money and gun cues showed a significantly greater late positive potential than neutral pictures (see Figs. 5 and 6), resulting in a significant effect of cue type, F(2, 60) = 65.31, p < .0001, η2 = .685, and the test for a quadratic trend (emotional vs. neutral cues) was highly significant, F(1, 30) = 87.43, p < .0001. This difference diminished over the picture sequence in the maybe stage, resulting in a main effect of picture, F(2, 60) = 25.58, p < .0001, as well as a Cue Type × Picture interaction, F(4, 120) = 4.78, p < .02. During the subsequent imminent stage, however, the late positive potential increased, yielding a significant main effect of picture, F(2, 60) = 85.93, p < .0001, and the ERP response was most strongly positive for the penultimate pictures, just prior to responding. ERPs for all three cue types differed from each other, as indicated by a main effect of cue type, F(2, 60) = 40.51, p < .0001; all subsequent comparisons between cue types were significant (ps < .003). This late positive response to the penultimate pictures was particularly large for the gun cues; Analysis yielded main effects of cue type during separate tests for Picture 5, F(2, 60) = 29.44, p < .0001, and Picture 6, F(2, 60) = 23.02, p < .0001; all pair-wise comparisons between cues were again significant, p < .007 (i.e., there was greater late positivity for money cues than for neutral cues, but late positivity for gun cues exceeded late positivity for both neutral and money cues).
Fig. 6.
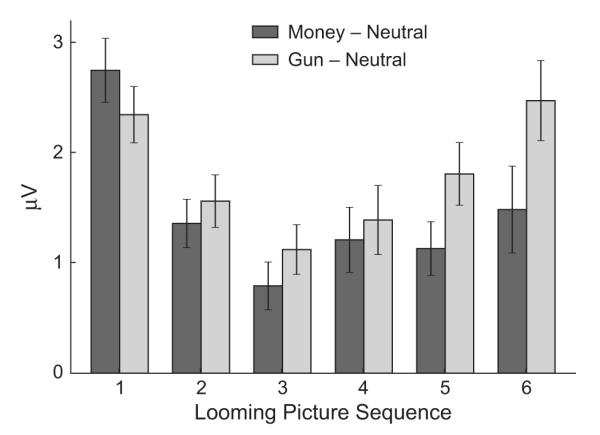
Late positive potentials (300–600 ms after picture onset). The graph shows the amplitude difference between gun cues and neutral cues and between money cues and neutral cues as a function of the picture’s position in the looming sequence. Means and standard errors are shown.
DISCUSSION
Results were consistent with the animal model: Looming picture sequences prompted systematic modulation of somatic, autonomic, and brain responses that varied reliably with apparent distance from the goal. Thus, when motive cues first appeared and were remote from the action signal, emotional reactions were typical of those during free viewing of arousing pleasant and unpleasant pictures. The startle reflex indexed cue differences in hedonic valence (Bradley et al., 2001); as with animals, comparisons with the response to neutral stimuli indicated that the reflex was potentiated when viewers were confronted with an aversive cue (M. Davis, 1998; M. Davis & Lang, 2003) and inhibited when they viewed an appetitive cue (Koch, Schmid, & Schnitzler, 1996). ERPs when cues first appeared were also consistent with ERPs observed in free-viewing studies, showing enhanced positivity for emotionally arousing pictures, regardless of affective valence (Cuthbert et al., 2000; Schupp et al., 2000). This late positive response has been associated with heightened activation in the amygdala and visual sensory areas of the brain (Sabatinelli, Lang, Keil, & Bradley, 2007), a pattern consistent with increased processing of motivationally pertinent cues.
Maybe Stage
Increasing sympathetic activation was apparent over the maybe period. Skin conductance was greater for motivational than for neutral cues, and this difference was progressively augmented as the picture sequence continued. Heart rate initially accelerated (vagal release) in response to the motivationally relevant cues—both money and gun cues—but was not sustained. Rather, a marked decelerative trend was apparent by Picture 3 (see Fig. 3), and this trend was somewhat greater for threat than for reward; this deceleration indicates a reassertion of modulation by the parasympathetic system and is suggestive of fear bradycardia, as observed in animals confronting a predator (Campbell et al., 1997).
Curiously, the initially large ERP difference between the motivationally relevant and neutral cues showed a modest diminution during the maybe stage, perhaps reflecting a somewhat lessened affective reaction after first impact (considering the uncertainty of an eventual confrontation). The startle probe reflex showed an overall reduction in magnitude at the end of this period. Previous research (see Anthony, 1985) has found a similar inhibition of the probe reflex that accompanies anticipatory heart rate deceleration when participants anticipate a reaction time cue. This deceleration (Lacey & Lacey, 1978) and reduced startle reflex (Anthony, 1985) suggest increasing vigilance for the action signal, such that fewer attentional resources are available for the task-irrelevant startle stimulus.
Imminent Stage
The imminent stage was characterized by coactivation of the sympathetic and parasympathetic systems, as manifested in a marked tonic heart rate deceleration (parasympathetic system) for all cue stimuli and a coincident progressive, tonic increase in skin conductance (sympathetic system) for motivational cues. Effects of both autonomic branches were also apparent in phasic heart rate responses to the final three imminent cues; that is, an initial parasympathetic deceleration associated with orienting to the new, more proximal stimulus (Graham & Clifton, 1966) was followed by an abrupt sympathetic acceleration, mobilizing the organism for the increasingly imminent, required response.
The penultimate cue evoked the largest phasic responses for nearly all measures: Skin conductance increased dramatically, as did the phasic heart rate response and the ERP late positive wave. This was the point of greatest threat or greatest hope for reward, characterized by both maximum action mobilization and vigilance for the expected response signal. As in studies of simple reaction time (e.g., Anthony, 1985), however, enhanced vigilance markedly inhibited the startle reflex, eliminating modulation by hedonic valence.
Punishment Versus Reward
Overall, anticipatory physiological patterns for reward and threat of loss were remarkably similar, reflecting the common sensory and motor requirements of the responses. However, differences were apparent during the late imminent stage, a finding that is consistent with motivational theory: Miller’s (1951) animal model proposes that strength of avoidance increases markedly near a site of punishment, relative to the increment in appetitive approach when the animal is equivalently proximal to a reward; Kahneman and Tversky (1979, 2000) similarly predicted that motivation to avoid a loss is greater than motivation to achieve a gain. The ERP findings lend support to these views, showing greater late positivity for imminent punishment cues than for imminent reward cues.
A parallel difference was found for heart rate—greater deceleration for anticipated punishment than for anticipated reward. This result is consistent with the enhanced parasympathetic activation observed in animals under threat (fear bradycardia; Campbell et al., 1997). Heart rate is, of course, the sum of sympathetic acceleratory and parasympathetic deceleratory influences (Sokolov & Cacioppo, 1997). Thus, given the skin-conductance findings suggesting equivalent sympathetic activation for punishment and reward, one might conclude that the defense and appetitive motive systems prompt different balances between the two branches of the autonomic nervous system, with defense showing stronger vagal effects.
This interpretation is not straightforward, however, as deceleration was similar for the threatening gun cue and the looming neutral cue. To shed light on this issue, future experiments could manipulate relative monetary values for gains and losses, modulating the comparative strength of appetitive and defensive motivation. Another potentially fruitful avenue for future work would be to reinforce cues that are initially neutral and assess anticipatory learning for reward and punishment. An immediate goal would be to use neural imaging (Maren, 2007; Mobbs et al., 2007) to assess motivational brain circuits that mediate changing patterns in heart rate and the other reflex measures.
These data have broad implications for emotion theory. They show clearly that human emotions are not static states that can be captured by a single measure. Nor are increments in emotional intensity reflected in monotonic enhancement of physiological reactivity. The view highlighted here is that emotions are fundamentally action dispositions (Frijda, 1987; Lang et al., 1997), that is, dynamic reflex postures that evolved to ensure survival and the propagation of an individual’s genetic inheritance and that are instantiated when anticipated action is inhibited or delayed.
The physiological changes seen with increased emotional arousal are determined by the probability of overt action (fight or flight, strike or capture). Increasing imminence of confrontation unleashes a cascade of physiological responses that do not so much correspond to specific affects (pleasant vs. unpleasant), but rather facilitate coping behaviors, increasing vigilance and mobilizing the body for action. Implications for emotion theorists are clear: The physiology of emotion is dynamic and adaptive to context. Furthermore, the considerable similarity in the physiologies of emotional arousal or imminence in punishment and reward encourages doubts that specific affects are discriminable by a Jamesian (see also Damasio, 1994) apprehension of the ongoing physiology. The present data encourage the view that emotional states arise in the context of delayed or inhibited action, prompting heightened vigilance and physiological mobilization, mediated by brain circuits that evolved to confront survival threats and facilitate acquisition of life-sustaining rewards.
Acknowledgments
This work was supported by National Institute of Mental Health Grant P50 MH-72850 (P.J. Lang, principal investigator).
REFERENCES
- Anthony BJ. In the blink of an eye: Implications of reflex modification for information processing. In: Ackles PK, Jennings JR, Coles MGH, editors. Advances in psychophysiology. Vol. 1. JAI Press; Greenwich, CT: 1985. pp. 167–218. [Google Scholar]
- Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: Defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Hamby S, Löw A, Lang PJ. Brain potentials in perception: Picture complexity and emotional arousal. Psychophysiology. 2007;44:364–373. doi: 10.1111/j.1469-8986.2007.00520.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG, Binkley PF, Quigley KS, Uchino BN, Fieldstone A. Autonomic cardiac control: II. Noninvasive indices and basal response as revealed by autonomic blockades. Psychophysiology. 1994;31:586–598. doi: 10.1111/j.1469-8986.1994.tb02351.x. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Gardner WL, Berntson GG. Beyond bipolar conceptualizations and measures: The case of attitudes and evaluative space. Personality and Social Psychology Review. 1997;1:3–25. doi: 10.1207/s15327957pspr0101_2. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Wood G, McBride T. Origins of orienting and defensive responses: An evolutionary perspective. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Hillsdale, NJ: 1997. pp. 41–67. [Google Scholar]
- Cuthbert BN, Schupp HT, Bradley MM, Birbaumer N, Lang PJ. Brain potentials in affective picture processing: Covariation with autonomic arousal and affective report. Biological Psychology. 2000;52:95–111. doi: 10.1016/s0301-0511(99)00044-7. [DOI] [PubMed] [Google Scholar]
- Damasio A. Descartes’ error. Putnam’s Sons; New York: 1994. [Google Scholar]
- Davis M. Anatomic and physiologic substrates of emotion in an animal model. Journal of Clinical Neurophysiology. 1998;15:378–387. doi: 10.1097/00004691-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Davis M, Lang PJ. Emotional experience and emotion science. In: Gallagher M, Nelson RJ, editors. Comprehensive handbook of psychology: Vol. 3. Biological psychology. Wiley; New York: 2003. pp. 405–439. [Google Scholar]
- Davis RC, Buchwald AM, Frankman RW. Autonomic and muscular responses and their relation to simple stimuli. Psychological Monographs. 1955;69:1–71. [Google Scholar]
- Fanselow MS. Neural organization of the defensive behavior system responsible for fear. Psychonomic Bulletin & Review. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- Frijda NH. Emotion, cognitive structure, and action tendency. Cognition and Emotion. 1987;1:115–143. [Google Scholar]
- Graham FK. Attention: The heartbeat, the blink, and the brain. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults: Perspectives from human and animal research. Erlbaum; Hillsdale, NJ: 1992. pp. 3–29. [Google Scholar]
- Graham FK, Clifton RK. Heart rate change as a component of the orienting response. Psychological Bulletin. 1966;65:305–320. doi: 10.1037/h0023258. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Cuthbert BN, Globisch J, Vaitl D. Fear and startle reflex: Blink modulation and autonomic response patterns in animal and mutilation fearful subjects. Psychophysiology. 1997;34:97–107. doi: 10.1111/j.1469-8986.1997.tb02420.x. [DOI] [PubMed] [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. Journal of Clinical Neurophysiology. 2002;19:113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Kahneman D, Tversky A. Choices, values, and frames. Cambridge University Press; New York: 2000. [Google Scholar]
- Kapp BS, Whalen PJ, Supple WF, Pascoe JP. Amygdaloid contributions to conditioned arousal and sensory information processing. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 229–254. [Google Scholar]
- Keil A, Bradley MM, Hauk O, Rockstroh B, Elbert T, Lang PJ. Large-scale neural correlates of affective picture processing. Psychophysiology. 2002;39:641–649. doi: 10.1017.S0048577202394162. [DOI] [PubMed] [Google Scholar]
- Klorman R, Weissbert RP, Wiessenfeld AR. Individual differences in fear and autonomic reactions to affective stimulation. Psychophysiology. 1977;14:45–51. doi: 10.1111/j.1469-8986.1977.tb01154.x. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler H-U. Pleasure-attenuation of the startle response is disrupted by 6-hydroxydopamine lesion of the nucleus accumbens. NeuroReport. 1996;7:1442–1446. doi: 10.1097/00001756-199605310-00024. [DOI] [PubMed] [Google Scholar]
- Lacey BC, Lacey JI. Two-way communication between the heart and the brain: Significance of time within the cardiac cycle. American Psychologist. 1978;33:99–113. doi: 10.1037//0003-066x.33.2.99. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, attention, and the startle reflex. Psychological Review. 1990;97:377–395. [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Motivated attention: Affect, activation and action. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Hillsdale, NJ: 1997. pp. 97–135. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective ratings of pictures and instruction manual (Tech. Rep. No. A-6) University of Florida; Gainesville: 2005. [Google Scholar]
- Maren S. The threatened brain. Science. 2007;317:1043–1044. doi: 10.1126/science.1147797. [DOI] [PubMed] [Google Scholar]
- Masterson FA, Crawford M. The defense motivation system: A theory of avoidance behavior. The Behavioral and Brain Sciences. 1982;5:661–696. [Google Scholar]
- Miller NE. Comments on theoretical models illustrated by the development of a theory of conflict behavior. Journal of Personality. 1951;20:82–100. doi: 10.1111/j.1467-6494.1951.tb01514.x. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Petrovic P, Marchant JL, Hassabis D, Weiskopf N, Seymour B, et al. When fear is near: Threat imminence elicits prefrontal-periaqueductal gray shifts in humans. Science. 2007;317:1079–1083. doi: 10.1126/science.1144298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38:719–722. [PubMed] [Google Scholar]
- Sabatinelli D, Lang PJ, Keil A, Bradley MM. Emotional perception: Correlation of functional MRI and event related potentials. Cerebral Cortex. 2007;17:1085–1091. doi: 10.1093/cercor/bhl017. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: The late positive potential is modulated by motivational relevance. Psychophysiology. 2000;37:257–261. [PubMed] [Google Scholar]
- Sokolov EN, Cacioppo JT. Orienting and defense reflexes: Vector coding the cardiac response. In: Lang PJ, Simons RF, Balaban MT, editors. Attention and orienting: Sensory and motivational processes. Erlbaum; Hillsdale, NJ: 1997. pp. 1–22. [Google Scholar]
- Somsen RJ, Jennings JR, Van der Molen MW. The cardiac cycle time effect revisited: Temporal dynamics of the central-vagal modulation of heart rate in human reaction time tasks. Psychophysiology. 2004;41:941–953. doi: 10.1111/j.1469-8986.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- Wallin BG. Sympathetic nerve activity underlying electrodermal and cardiovascular reactions in man. Psychophysiology. 1981;18:470–476. doi: 10.1111/j.1469-8986.1981.tb02483.x. [DOI] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]