Abstract
CTCL is responsive at all stages to immunotherapy. We determined whether a novel agonist for TLR 7/8 (3M007) combined with either IFN-γ or IL-15 enhanced patients' immune responses in vitro. Our data demonstrate synergism between IFN-γ and 007 in the activation of patients' NK cytolytic activity against CTCL tumor cell lines and increased production of cytokines by dendritic cells compared to 007 alone. Microarray studies of gene expression of patients' PBMC primed with IFN-γ followed by stimulation with 007 identified significant upregulation of expression of IL-12- p35 (α-chain), IL-12-p40 (β-chain) and nine IFN-α genes. Importantly, the underlying mechanism of increased levels of IFN-α and IL-12 from combined treatment appears to involve IFN regulatory factor 8 (IRF-8). These results further support our hypothesis that combinations of biological modifiers activating different arms of the immune system may provide significant therapeutic benefits for patients with advanced CTCL.
Keywords: CTCL, immunotherapy, TLR7/8 agonist, IFN-γ, IRF-8
INTRODUCTION
Cutaneous T-cell lymphomas (CTCL) are typically clonal proliferative disorders of skin-trafficking T-cells. The most common form is mycosis fungoides characterized by cutaneous patches, plaques and tumors and occasionally erythroderma. Sezary syndrome is a less common leukemic variant of CTCL associated with erythroderma, lymphadenopathy and circulating malignant T-cells. 1,2Although the vast majority of cases of mycosis fungoides and Sezary syndrome are associated with a CD4 phenotype, rarely, the skin-invasive malignant population expresses a CD8 phenotype.3 Lack of CD26 and, less frequently, the lack of CD7 on circulating malignant T-cells are quite characteristic.4,5
Advanced forms of CTCL, such as Sezary syndrome, are typified by the overt finding of circulating malignant T-cells and are associated with an array of disorders of cell mediated immunity. Circulating malignant T-cells have been observed to express features of Th2 cells with production of varying amounts of IL-4, IL-5 and IL-10. Other findings, that typify Th2 features of the malignant population, include the absence of expression of the IL-12 -β2 receptor, which is the signaling receptor for IL-12, in addition to the high expression of Gata 3, a transcription factor critical for Th2 expression. Moreover, the malignant T-cells are poor producers of IFN-γ.6–10
Profound defects in other components of the cellular immune system, which are critical for the anti-tumor response, have also been observed including the depressed production of the dendritic cell derived cytokines IFN-α, IL-12 and IL-15. In addition, CD8 T cell and NK cell functions become disordered with advancing leukemic involvement in the absence of exogenous medications including steroids and chemotherapeutics.11,12 Undoubtedly, also contributing to the immune depression is the loss of the normal T-cell repertoire that has been observed, even in early stages of disease, but which becomes more profound with advancing stages of CTCL.13,14 It is quite clear that the extent of the immune dysregulation appears to be correlated with the burden of circulating malignant T-cells.
Substantial emerging evidence supports the hypothesis that the host immune response may be critical for control of disease progression.15,16 Recent efforts using a multimodality immunotherapy approach for Sezary syndrome have been associated with a high clinical response rate and, in some cases, quite durable remissions.17 Thus, efforts have been directed at the development of immune augmentative therapies of the type that might support anti-tumor immunity.18,19 In that regard, IFN-α and IFN-γ as well as recombinant IL-12 have all exhibited evidence of clinical efficacy.20 As each of these cytokines are components of the innate immune response, IFN-α from plasmacytoid dendritic cells (pDCs), IL-12 from myeloid DCs (mDCs) and IFN-γ from natural killer (NK) cells, one rational approach to therapy of CTCL might be the use of agents which can activate multiple cell populations of the innate immune system, more closely mimicking the natural sequence of events.21 Recently, Wysocka et al. published that in vitro activation of antigen presenting cells through the use of TLR agonists can lead to the potent augmentation of the cellular immune response of highly immune depressed patients with Sezary syndrome.12,22 In this study we sought to determine whether priming of antigen presenting cells with IFN-γ or IL-15 might lead to the synergistic enhancement of cell mediated immune responses when combined with the novel TLR 7/8 agonist 007, which is being considered for a phase II clinical trial for CTCL. Our study, which includes microarray analysis of global gene expression, indicates that multifactor stimulation indeed significantly enhances immune responses by activating several arms of the immune system of Sezary syndrome patients. These findings suggest that a novel therapeutic approach to CTCL might include treatment with a TLR agonist in combination with IFN-γ or other immune potentiating cytokine with similar effects.
MATERIAL AND METHODS
Patients
Sezary syndrome (SS) patients were diagnosed on the basis of clinical, histopathologic and immunohistologic criteria.23 Flow cytometric analysis of peripheral blood samples with assessment of numbers of CD4+/CD26−/CD7− cells was routinely used to quantify the numbers of circulating malignant T-cells.4 Presence of circulating malignant T-cells was verified by examination of one-micron sections of formalin-fixed peripheral blood buffy coats for lymphocytes with atypical ceribriform appearing nuclei. Patients with erythroderma and circulating malignant T-cells were defined to have Sezary syndrome (SS). Donation of peripheral blood samples by patients was undertaken according to protocols approved by the University of Pennsylvania Institutional Review Board.
Preparation and Culture of Mononuclear Cells
Peripheral blood mononuclear cells (PBMC) from patients were collected from blood as previously described.24 Cells were cultured in RPMI 1640 (Life Technologies, Inc., Gaitheresburg, MD), supplemented with 10% fetal bovine serum (FBS, Hyclone, Utah, endotoxin level <0.06 EU/ml), penicillin/ streptomycin, and L-glutamine (all reagents purchased from Gibco-BRL, Grand Island, NY). To induce immune responses in vitro, PBMC from patients were cultured in 24-well plates at a density of 2×106/ml/well for 24 hours (h) with 007 at concentrations of 10μg/ml. Cell free supernatants were then collected to determine cytokine levels and cells were harvested for flow cytometric analysis. To assess IFN-γ or IL-15 priming effects on IL-12 p70 or IFN-α production induced by 007, PBMC were stimulated with medium, IFN-γ (10ng/ml) or IL-15 (1ng/ml) for 4h followed by stimulation with 007 (10ug/ml) for an additional 20h. At that point supernatants were collected and stored for further analysis.
Reagents
The synthetic imidazoquinoline 007 was a gift of 3M Pharmaceuticals, (St. Paul, MN).25 Recombinant IFN-γ and IL-15 were purchased from R&D Systems, Inc. (Minneapolis, MN). CR51 was purchased from Perkin Elmer, Wellesley, MA.
Detection of Cytokines
Culture supernatants were harvested and tested for the presence of IFN-α and IL-12p70 by ELISA according to the manufacturer's recommendations, using antibody pairs from Endogen (IFN-α, Woburn, MA, sensitivity 10 pg/ml) or R&D Systems Inc (L-12p70 Minneapolis, MN, sensitivity: 10 pg/ml).
Flow Cytometric Analysis
To analyze expression of CD69 on NK cells or T-cells, PBMC were stained with anti-CD3-PerCp, anti-CD56/CD16-APC, anti-CD69-FITC or anti-CD8-PerCp and anti-CD69-FITC. Murine immunoglobulins of appropriate isotypes were used as a control. All antibodies were purchased from BD Biosciences, San Jose, CA.
Cells were analyzed with the FACS Calibur (Becton Dickinson) flow cytometer using CELLQuest software (Becton Dickinson, San Jose, CA) at the Flow Cytometry and Cell Sorting Core, Abramson Cancer Center, University of Pennsylvania, Philadelphia, PA. 50,000 events were collected to analyze NK cells and CD8 T-cells.
Natural Killer Cytotoxicity Assay
PBMC stimulated with 007 +/− IFN-γ or IL-15, were the source of activated NK cells. Priming with IFN-γ or IL-15 was performed as described above. After 24h stimulation, cells were harvested, washed with PBS (Gibco-BRL, Grand Island, NY) and plated at different concentrations. Human lymphoblastoma K562 cells or the SEZ4 malignant T-cell line derived from the peripheral blood of a leukemic CTCL patient were used as targets. A standard 4h Cr51-release assay was performed as previously described.24
Statistical Analysis
Data are expressed as means ± SD of tested individuals. Statistical significance was determined using the Wilcoxon signed-rank test.
Microarray Studies: Preparation of PBMC RNA
Gene expression was analyzed in PBMC from four SS patients. They included three CTCL CD4 patients: one with low circulating tumor cell burden (<20% of lymphocytes) and two with medium circulating tumor cell burden (20–50% of lymphocytes), the fourth patient was a CTCL CD8 SS patient with medium circulating tumor cell burden. The four PBMCs were treated as described above with IFN-γ and/or 007 and the cells were then harvested for RNA extraction. Samples were collected from PBMC maintained in culture in growth medium alone at 4h and at 7h, cells treated with IFN-γ alone collected at 4h and at 7h, cells treated alone with 007 for 7h and from cells first treated with IFN-γ for 4h and then with 007 for an additional 3h. For each patient, six different samples were available. RNA was processed as previously described and analyzed using Illumina HumanHT-12 v4 microarray chips according to Illumina.26 All arrays were processed in the Wistar Institute Genomics Facility.
Data Preprocessing
The gene-wise, median correlation of each array compared to all other arrays was computed to ensure that no outliers existed within the data. All arrays were first quantile normalized and the data filtered to remove non-informative probes. A probe was considered to be non-informative if its intensity was lower that the background in the majority of the samples, or if the maximum ratio between any two samples was below 1.2, resulting in 19,294 probes to be used for further analysis.
Gene Expression Statistics
An ANOVA test was conducted on the data to identify differentially expressed probes associated with all treatments. A total of 5,326 probes passed the p-value cutoff of 0.001 with a zero false discovery rate. Tukey's honestly significant difference test was then used to determine the within-group differences with a 99% confidence interval. All preprocessing and microarray data analysis were conducted in MATLAB 7.10.0.
Synergistic Response to Treatments
Arrays from cells kept in medium for 7h were used as the baseline control. There was essentially no significant difference in the 4h and 7h results, but only 7h samples were used for further comparisons. We first selected those genes that exhibited significant differential expression with the combined IFN-γ/007 treatment compared to the medium control to identify those genes that potentially displayed synergistic response due to the combined treatment. The gene expression levels of the selected genes from medium versus IFN-γ/007 treatments were compared to the expression levels of samples treated 7h with only IFN-γ and only 007. A gene was designated as being synergistically affected if the combined IFN-γ/007 expression level was at least 50% greater than the sum of the expression levels of the individual treatments.
Enrichment of Cell Type Specific Genes
Specificity of expression of a specific gene to lymphoid cell types (dendritic cells, monocytes, myeloid cells, T-cells, B cells, NK cells and Neutrophils) was obtained from the IRIS database.27 IRIS classifies markers as cell-type-specific if they are expressed three-fold or more in one specific immune cell type or lineage compared to expression in other immune cells. The data is based on large numbers of gene expression studies on isolated, specific cell types. Significance of enrichment of genes of a specific cell type in a list of changed genes was tested using Fisher Exact Test with significance level set at P<0.05.
Pathway Analysis
Pathway analysis was conducted using the Ingenuity Pathway Analysis (IPA; Ingenuity Systems, www.ingenuity.com) using the data from the comparison of the IFN-γ/007 treatment compared to samples in medium. The final list used as an input into IPA was limited to those probes that exhibited an absolute fold change greater than 3. Similarly, probe lists were obtained from the individual IFN-γ and 007 treated arrays versus the medium control. To determine statistically significant canonical pathways, the Benjamini and Hochberg multiple test corrected p-value was restricted to 0.01.
Role of the Funding Source
This work was supported by grants from the National Cancer Institute and the Leukemia and Lymphoma Society. The funding sources were not involved in the design or completion of this study. They were not involved in the preparation or submission of this manuscript.
RESULTS
Activation of NK Cells in Response to 007:Enhancement of Cytolytic Activity by IFN-γ or IL-15
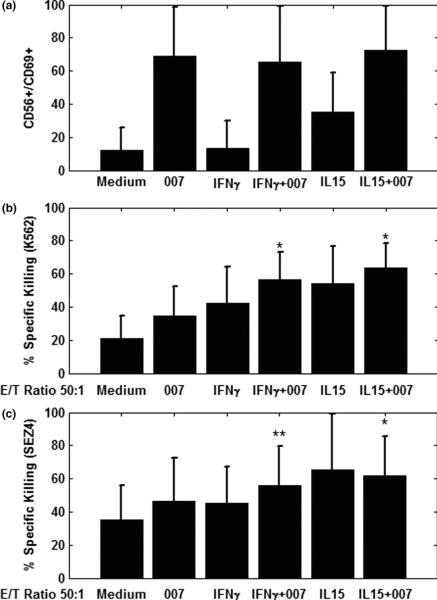
We have previously shown that synthetic agonists for TLR7 or TLR8 alone were capable of activating the NK and CD8 T-cells of Sezary syndrome patients leading to upregulation of CD69, an early activation molecule on the cell surface.22 Consistent with these findings, stimulation with 007 also upregulated the expression of CD69 on NK cells (Figure1 A) and CD8 T-cells (data not shown). Priming of patients' PBMC with IFN-γ or IL-15 prior to stimulation with 007 did not seem to further significantly enhance expression of CD69 on NK or CD8 T cells (data not shown). However, priming with IFN-γ or IL-15 significantly increased (p<0.05) the cytolytic activity of NK cells compared to 007 alone. (Figure 1 B, -C). As shown in Figure 1B, the lysis of the universal NK cell target, K562 was significantly increased after cytokine priming (p<0.05). Importantly, we also observed a marked increase in killing of SEZ4, a malignant T-cell line derived from a Sezary syndrome patient. (p<0.05; Figure 1C).28 The enhanced killing of the SEZ4 cell line following priming with cytokines and triggering with 007, provided a strong indication that the NK cells of Sezary syndrome patients are capable of eliminating CTCL tumor cells.
Figure 1.
A. 007, a TLR7/8 agonist, potently activates the NK cells of Sezary syndrome patients and upregulates CD69 expression.
PBMC from Sezary syndrome patients were cultured with media, IFN-γ, or IL-15 for 4h followed by 20h stimulation with 007, a TLR7/8 agonist. Cells were then harvested, stained with appropriate antibodies and analyzed for the expression of CD69 on CD56/CD16 NK cells. Data represent mean ± standard deviation of tested individuals and are presented as a percentage of NK cells positive for CD69 (n=12).
B and C. IFN-γ and IL-15 significantly upregulate the 007-induced cell-mediated cytolytic killing activity of the PBMC of Sezary syndrome patients against tumor cell lines.
PBMC from patients were stimulated as described in Figure 1A, followed by a 4h Cr51 assay using as targets either K562 leukemic cells (B) or the SEZ4, a Sezary-derived T cell line (C). Data represent mean ± standard deviation of tested individuals (n=9)
Enhancement of IL-12 and IFN-α Production in Response to the TLR7/8 Agonist 007 and IFN-γ or IL-15
Since the activation of NK cells and T cells by the TLR 7/8 agonist is indirect and depends upon the activation of dendritic cells and the cytokines produced by these cells, we analyzed the production of IFN-α and IL-12p70. We previously showed that despite the decreased number of circulating DCs in CTCL patients compared to healthy volunteers, the production of these DC-dependent cytokines could be markedly enhanced by subjecting the DCs to priming with IFN-γ or IL-15, prior to stimulation with a TLR agonist.11 Therefore, we examined the ability of the TLR agonist 007 to induce either IFN-α or IL-12 from the PBMCs of Sezary syndrome patients and whether priming with IFN-γ or IL-15 led to further increases in production. As shown in Figure 2A, 007 alone induced 108.3 pg/ml of IL-12 p70. Importantly, there was a synergistic enhancement of IL-12p70 production if PBMCs were first primed with IFN-γ, resulting in production of 4475.9 pg/ml of IL-12 p70 corresponding to a statistically significant (p< 0.001) 41.3 fold increase compared to 007 alone. Priming the PBMCS with IL-15 produced 301.6 pg/ml of IL-12 p70 which was a statistically significant increase of 2.7 fold compared to medium (p<0.05) but IL-15 was less potent than IFN-γ. Priming with IFN-γ also significantly enhanced IFN-α production in response to 007. A 1.9 fold increase in production of IFN-α was detected upon priming of PBMC with IFN-γ (1364.5 pg/ml; p<0.05), compared to levels induced by 007 alone (694.8 pg/ml) (Figure 2B). In contrast, priming with IL-15 did not significantly enhance production of IFN-α in response to 007.
Figure 2.
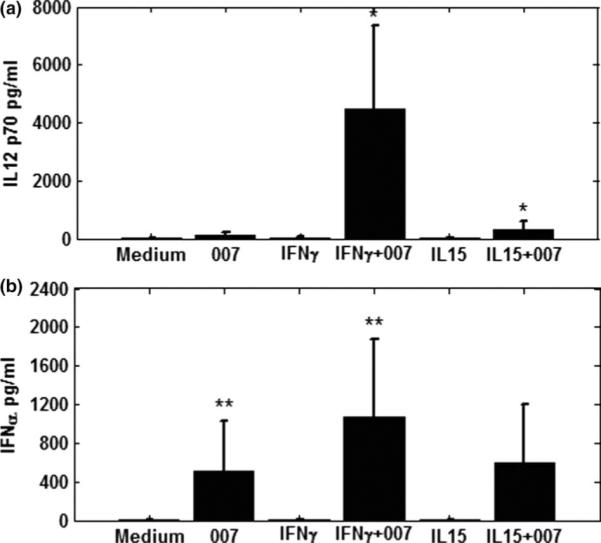
A and B. Priming of PBMCs with IFN-γ and IL-15 prior to stimulation with the TLR7/8 agonist 007 significantly increases IL-12 p70 production (A) but only priming with IFN-γ significantly increases levels of IFN-α (B).
PBMC from Sezary syndrome patients were cultured with media, IFN-γ, or IL-15 for 4h followed by 20h stimulation with 007, a TLR7/8 agonist. Cytokine levels were measured in cell-free supernatants by ELISA: A. IL-12p70; B. IFN-α. Data represent mean ± standard deviation of tested individuals (n=10). ** p<0.005; * p<0.05
Analysis of Gene Expression Using Microarrays
A. IFN-γ priming followed by stimulation with 007 results in a significant increase in IL-12 and IFN-α gene expression
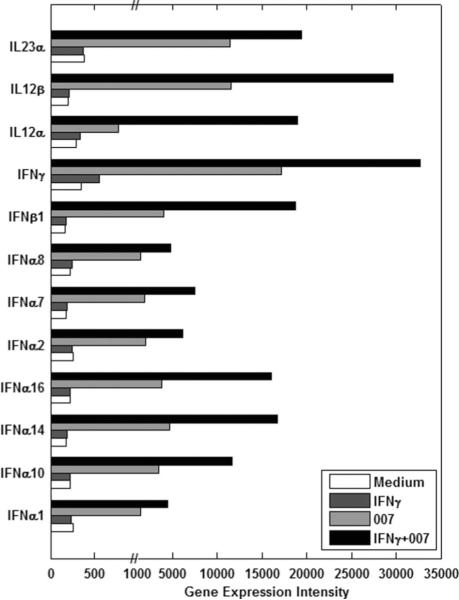
To further characterize the underlying mechanism of TLR7/8 agonist-induced responses and IFN-γ enhancing effect on these responses, we analyzed global gene expression changes in cultured PBMC from four Sezary syndrome patients (see methods). The changes in gene expression in response to treatment were highly significant and relatively uniform across the four patient samples regardless of circulating tumor cell burden or type. Although many genes changed due to the single and combined treatments (4,910 genes), we first concentrated on those genes whose expression was most significantly and synergistically affected by the combined treatment. The most significantly affected genes with the most highly induced mRNA levels as a result of the single and combined treatments are shown in Figure 3. Nine IFN genes were highly induced by the dual treatment including 7 different IFN-α genes, as well as IFN-γ and IFN-β. The IFN-α and the IFN-β genes were not affected by treatment with IFN-γ alone, but responded to 007 and were significantly increased in transcription by the dual treatment. Only IFN-γ showed a small increase in message levels in response to IFN-γ. In agreement with the cytokine assays, expression of message for IL12-α (p35) and IL-12-β (p40) chains were upregulated by treatment with the most significant effect on p40 message levels.
Figure 3.
Gene expression intensities of interferons and interleukins that were synergistically increased by the combined treatment of IFN-γ and 007 compared to single treatments.
B. IFN-γ priming followed by stimulation with 007 results in significant upregulation of IFN regulatory factor (IRF)-8
We examined expression patterns of transcription factors that could be associated with the increased IFN-α and IL-12 gene expression. We observed 63 transcription factors that were induced by single or combined treatments. Within the signal transducer and activator of transcription (STAT) family, STAT-1 and 2 were induced relatively equivalently by IFN-γ, 007, and combined treatment while STAT 4 and 5A were primarily induced by 007. All treatments reduced expression of the IRF2BP2 transcriptional repressor (data not shown).
Interferon regulatory factor-8 (IRF-8) was the only member of the IRF family that was induced by both IFN-γ and 007 and synergistically upregulated by the combined treatment, suggesting that it is the major transcription factor that contributes to increased transcription of IL-12 and IFN-α genes and is essential to dendritic cell development.29,30 As shown in Figure 4, priming of patients' PBMC with IFN-γ or stimulation with 007 increased IRF-8 message more than 4 and 5 fold respectively (compared to medium alone) but the combination of IFN-γ + 007 resulted in more than a 12-fold increase in IRF-8 mRNA expression. Stimulation with 007 induced expression of IRFs 2, 4, and 7 messages while levels of IRF-1 message were similar regardless of treatment (Figure 4, data not shown).
Figure 4.
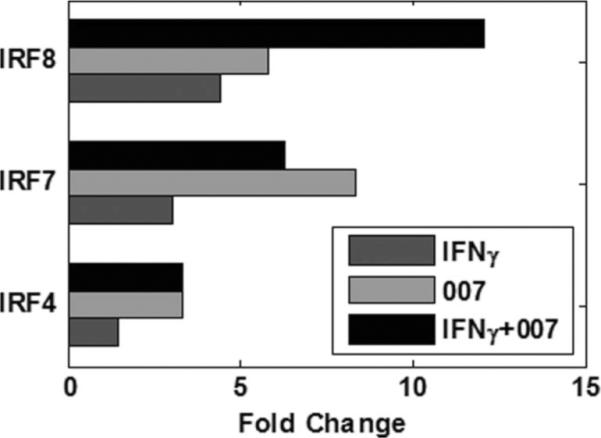
Fold changes in gene expression of IRF-4, IRF-7, and IRF-8 in samples treated with IFN-γ, 007, and IFN-γ+007 compared to untreated samples. Combined treatment displays synergistic effects of IFN-γ+007 on IRF-8 gene expression. IRF-7 fold changes are shown as an average of two microarray probes.
We were also able to identify transcription factor binding sites. Gene set enrichment analysis (GSEA) of promoter regions of significantly changed gene expression from the microarray data revealed the significant overexpression of genes binding to IRF1 (35%), IRF2 (31%), IRF7 (38%) IRF8 (45%) and NFκB (35%), in combined treatment, consistent with the up-regulation of these transcription factors in IFN-γ + 007 treated samples compared to untreated samples.31
C. Induced transcription is primarily associated with the myeloid lineage
To identify the cell types that were specifically affected by treatment with IFN-γ, 007, or IFN-γ + 007, we examined all the genes differentially expressed in response to the single or combined treatments using the IRIS database of immune specific genes (See Materials and Methods).27 We found that genes specific to myeloid lineages were significantly overrepresented in our gene list. In particular, dendritic cell specific genes were overrepresented in the list of significant genes for all three treatments, while genes identified as monocyte specific were overrepresented only if 007 was present. Lymphoid genes specific for T cells, B cells, or NK cells were not significantly different as a result of any of the treatments (Table 1).
Table 1.
Significantly increased numbers of specific cell type genes changed by each treatment. ns = non-significant increase.
| total number of cell type specific genes in IRIS | induced by IFN-γ | induced by 007 | induced by IFN-γ + 007 | |
|---|---|---|---|---|
| DC | 52 | 11 | 19 | 24 |
| Myeloid | 295 | 50 | 116 | 126 |
| Monocyte | 64 | NS | 32 | 26 |
DISCUSSION
Our results demonstrate the ability of the novel TLR agonist 007 to potently activate immune cellular responses in vitro in the peripheral blood cells of immunodepressed Sezary syndrome patients. NK cells, as well as CD8 T cells, were rapidly activated by 007. Moreover, the cytolytic activity of NK cells was also markedly upregulated by 007. As these cells are critical to the host immune response against the tumor cells these effects are likely to mediate a beneficial clinical effect.32
More importantly, we demonstrate a marked synergistic enhancement of IL-12 and IFN-α cytokine production when patients' PBMCs were primed for 4 hours with IFN-γ prior to stimulation with 007. Previously, our group, as well as others, has demonstrated similar effects of IFN-γ synergism with multiple different TLR agonists.22,33 The cytokines that are induced have the capacity to enhance functions of cytolytic T cells and NK cells. Moreover, IFN-α mediates the anti-proliferative and pro-apoptotic effects on the malignant T cells.34–36
IFN-γ's enhancement of IL-12 production is a result of an increase in both IL-12-p35 and IL-12-p40 gene transcription and, as previously shown by our group and others, from a decrease in the production of IL-10--a critical cytokine which counterbalances IL-12 synthesis.22,37–40 Our data also demonstrate the enhancing effect of IFN-γ on IFN-α gene transcription following culture with single and combined treatments. Of note, the gene expression of other members of the IL-12 family has been induced as well; both IL-23-a/p19 (which interacts with the IL-12 p40 chain) and IL-27 were significantly induced by both 007 and 007+IFN-γ (data not shown). Only IL-27 was found to be induced by IFN-γ alone. These 3 related heterodimeric cytokines have overlapping and unique roles in regulating the innate immune response41,42. Additional genes of interest include IDO2, a presumptive immunomodulatory gene related to IDO1 that is expressed in monocytes/macrophages and in antigen-presenting cells. Although it has not been clear as to whether IDO2 transcription, like IDO1, is induced by IFN-γ, we find IDO2 expression to be induced by IFN-γ in these studies.
Our data suggests that IFN-γ-dependent increases in IFN-α and IL-12 production may result, at least in part, from enhanced expression of IRF-8. The IRF family has been implicated in the regulation of IFN-α and IL-12 gene transcription.43 Interestingly, IL-12 and IFN-α have in turn been suggested to up-regulate mRNA/protein expression of IRF-8 creating a positive feedback loop.44 Among IRFs, IRF-1, in synergy with IRF-8, was found to regulate transcription of the IL-12p35 gene whereas IRF-7 and IRF-8 have been considered the major regulators of IFN-α gene transcription.29,30,45 Constitutively expressed in plasmacytoid dendritic cells, IRF-7 and IRF-8 can be rapidly upregulated in response to viral infection, TLR7/8 agonists such as single-stranded RNA or imidazoquinolines (007) or CpGs, which are TLR-9 agonists. Additionally, IRF-8 expression is inducible by IFN-γ.46 Importantly IRF-8 −/− mice lack plasmacytoid dendritic cells as well as CD8α+ myeloid dendritic cells that correspond to the recently described population of CD11c/CD141 dendritic cells in humans.30,47 The remaining CD8α− myeloid DC in these mice failed to mature upon stimulation with TLR agonists further supporting the importance of IRF-8 in their activation. These data strongly support the notion that IRF-8 is essential to the development of both plasmacytoid and myeloid dendritic cells. In this context, the increase in the IRF-8 expression produced by the combination of 007 and IFN-γ not only provides an explanation for the increased levels of IFN-α and IL-12, but also emphasizes the immunotherapeutic benefit of combining 007, a TLR7/8 agonist, with IFN-γ for treatment of CTCL patients with limited numbers of dendritic cells.
We also examined whether the effects on gene expression we detected with the single and combined treatments had different affects on the PBMCs derived from CTCL patients with malignant CD4 cells versus those with malignant cells of the CD8 phenotype. To address this possibility, we analyzed the CD8 CTCL samples separately and re-evaluated the differentially expressed genes based on similar criteria used in the initial analysis. Our results revealed that the treatments were equally effective on CD8 CTCL as well as CD4 CTCLs. Among the genes that significantly changed after single treatment with IFN-γ or 007 when considering both CD4 and CD8 CTCLs, 72% and 81% of the genes exhibited similar change when we excluded the CD8 samples from the analysis (data not shown). This correlation further increased to 83% when we examined the genes affected by the combined IFN-γ + 007 treatment suggesting the treatments would be equally effective for both CTCL types.
Our findings have important implications for the treatment of patients with advanced CTCL. It appears to be possible to potently boost immune responses of this patient population by combining a TLR agonist with IFN-γ. Both TLR agonists and IFN-γ are available in the clinic and each has become standard of care for various forms of CTCL (see National Comprehensive Cancer Network guidelines). Importantly, emerging evidence supports the use of several different TLR agonists in the treatment of CTCL. Suchin and colleagues were the first to report the efficacy of imiquimod, a topical TLR7 agonist for the treatment of early skin disease associated with CTCL.48 This has been followed by additional reports.49–52 Furthermore, a recent phase I trial using a systemically administered recombinant CpG, a TLR9 agonist, demonstrated a 35% response rate among highly refractory, heavily pretreated CTCL patients with advanced disease. Interestingly, complete responses with resolution of all disease were noted among several patients in this trial.53 Based upon our in vitro observations in this study, it is now potentially possible to consider the additive or synergistic boosting of immune responses among Sezary syndrome patients using combined therapy with a TLR agonist and IFN-γ. The possibility of observing a corresponding enhancement of clinical responses will be tested in a clinical trial.
Supplementary Material
Acknowledgements
This work was supported in part by grants from the National Cancer Institute R01 CA122569, R01 CA132098 and, a Translational Research grant from the Leukemia and Lymphoma Society. A.V.K. is supported by NCI T32 CA09171. The project used the Wistar Genomics facility supported by P30 CA010815.
We are grateful to Dr. Giorgio Trinchieri for his suggestions.
REREFENCES
- [1].Haynes BF, Bunn P, Mann D, et al. Cell surface differentiation antigens of the malignant T cell in Sezary syndrome and mycosis fungoides. J Clin Invest. 1981;67(2):523–530. doi: 10.1172/JCI110062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Diamandidou E, Cohen PR, Kurzrock R. Mycosis fungoides and Sezary syndrome. Blood. 1996;88(7):2385–2409. [PubMed] [Google Scholar]
- [3].Gormley RH HS, Anand D, Junkins-Hopkins J, Rook AH, Kim EJ. Primary cutaneous aggressive epidermotropic CD8+ T cell lymphoma. J Am Acad Dermatol. 2010;62(2):300–307. doi: 10.1016/j.jaad.2009.02.035. [DOI] [PubMed] [Google Scholar]
- [4].Introcaso CE, Hess SD, Kamoun M, Ubriani R, Gelfand JM, Rook AH. Association of change in clinical status and change in the percentage of the CD4+CD26− lymphocyte population in patients with Sezary syndrome. J Am Acad Dermatol. 2005;53(3):428–434. doi: 10.1016/j.jaad.2005.06.001. [DOI] [PubMed] [Google Scholar]
- [5].Vonderheid EC. On the diagnosis of erythrodermic cutaneous T-cell lymphoma. J Cutan Pathol. 2006;33(Suppl 1):27–42. doi: 10.1111/j.0303-6987.2006.00541.x. [DOI] [PubMed] [Google Scholar]
- [6].Rook AH, Vowels BR, Jaworsky C, Singh A, Lessin SR. The immunopathogenesis of cutaneous T-cell lymphoma. Abnormal cytokine production by Sezary T cells. Arch Dermatol. 1993;129(4):486–489. [PubMed] [Google Scholar]
- [7].Showe LC, Fox FE, Williams D, Au K, Niu Z, Rook AH. Depressed IL-12-mediated signal transduction in T cells from patients with Sezary syndrome is associated with the absence of IL-12 receptor beta 2 mRNA and highly reduced levels of STAT4. J Immunol. 1999;163(7):4073–4079. [PubMed] [Google Scholar]
- [8].Vowels BR, Cassin M, Vonderheid EC, Rook AH. Aberrant cytokine production by Sezary syndrome patients: cytokine secretion pattern resembles murine Th2 cells. J Invest Dermatol. 1992;99(1):90–94. doi: 10.1111/1523-1747.ep12611877. [DOI] [PubMed] [Google Scholar]
- [9].Vowels BR, Lessin SR, Cassin M, et al. Th2 cytokine mRNA expression in skin in cutaneous T-cell lymphoma. J Invest Dermatol. 1994;103(5):669–673. doi: 10.1111/1523-1747.ep12398454. [DOI] [PubMed] [Google Scholar]
- [10].Nebozhyn M, Loboda A, Kari L, et al. Quantitative PCR on 5 genes reliably identifies CTCL patients with 5% to 99% circulating tumor cells with 90% accuracy. Blood. 2006;107(8):3189–3196. doi: 10.1182/blood-2005-07-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wysocka M, Zaki MH, French LE, et al. Sezary syndrome patients demonstrate a defect in dendritic cell populations: effects of CD40 ligand and treatment with GM-CSF on dendritic cell numbers and the production of cytokines. Blood. 2002;100(9):3287–3294. doi: 10.1182/blood-2002-01-0231. [DOI] [PubMed] [Google Scholar]
- [12].Wysocka M, Benoit BM, Newton S, Azzoni L, Montaner LJ, Rook AH. Enhancement of the host immune responses in cutaneous T-cell lymphoma by CpG oligodeoxynucleotides and IL-15. Blood. 2004;104(13):4142–4149. doi: 10.1182/blood-2004-03-1190. [DOI] [PubMed] [Google Scholar]
- [13].Yamanaka K, Yawalkar N, Jones DA, et al. Decreased T-cell receptor excision circles in cutaneous T-cell lymphoma. Clin Cancer Res. 2005;11(16):5748–5755. doi: 10.1158/1078-0432.CCR-04-2514. [DOI] [PubMed] [Google Scholar]
- [14].Yawalkar N, Ferenczi K, Jones DA, et al. Profound loss of T-cell receptor repertoire complexity in cutaneous T-cell lymphoma. Blood. 2003;102(12):4059–4066. doi: 10.1182/blood-2003-04-1044. [DOI] [PubMed] [Google Scholar]
- [15].Wood GS. Lymphocyte activation in cutaneous T-cell lymphoma. J Invest Dermatol. 1995;105(1 Suppl):105S–109S. doi: 10.1111/1523-1747.ep12316249. [DOI] [PubMed] [Google Scholar]
- [16].Rook AH, Wood GS, Yoo EK, et al. Interleukin-12 therapy of cutaneous T-cell lymphoma induces lesion regression and cytotoxic T-cell responses. Blood. 1999;94(3):902–908. [PubMed] [Google Scholar]
- [17].Richardson SK, Lin JH, Vittorio CC, et al. High clinical response rate with multimodality immunomodulatory therapy for Sezary syndrome. Clin Lymphoma Myeloma. 2006;7(3):226–232. doi: 10.3816/CLM.2006.n.063. [DOI] [PubMed] [Google Scholar]
- [18].Rook AH, Junkins-Hopkins JM, McGinnis KS, et al. Cytokines and other biologic agents as immunotherapeutics for cutaneous T-cell lymphoma. Adv Dermatol. 2002;18:29–43. [PubMed] [Google Scholar]
- [19].Gardner JM, Evans KG, Musiek A, Rook AH, Kim EJ. Update on treatment of cutaneous T-cell lymphoma. Curr Opin Oncol. 2009;21(2):131–137. doi: 10.1097/CCO.0b013e3283253190. [DOI] [PubMed] [Google Scholar]
- [20].Rook AH, Kuzel TM, Olsen EA. Cytokine therapy of cutaneous T-cell lymphoma: interferons, interleukin-12, and interleukin-2. Hematol Oncol Clin North Am. 2003;17(6):1435–1448. doi: 10.1016/s0889-8588(03)00109-6. [DOI] [PubMed] [Google Scholar]
- [21].Ito T, Amakawa R, Kaisho T, et al. Interferon-alpha and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J Exp Med. 2002;195(11):1507–1512. doi: 10.1084/jem.20020207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wysocka M, Newton S, Benoit BM, et al. Synthetic imidazoquinolines potently and broadly activate the cellular immune response of patients with cutaneous T-cell lymphoma: synergy with interferon-gamma enhances production of interleukin-12. Clin Lymphoma Myeloma. 2007;7(8):524–534. doi: 10.3816/clm.2007.n.037. Prepublished on 2007/11/21 as DOI. [DOI] [PubMed] [Google Scholar]
- [23].Murphy GF. Cutaneous T-cell lymphoma. Adv Pathol. 1988;1:131–156. [Google Scholar]
- [24].Rook AH, Kubin M, Cassin M, et al. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J Immunol. 1995;154(3):1491–1498. [PubMed] [Google Scholar]
- [25].Gorden KB, Gorski KS, Gibson SJ, et al. Synthetic TLR agonists reveal functional differences between human TLR7 and TLR8. J Immunol. 2005;174(3):1259–1268. doi: 10.4049/jimmunol.174.3.1259. [DOI] [PubMed] [Google Scholar]
- [26].Showe MK, Vachani A, Kossenkov AV, et al. Gene expression profiles in peripheral blood mononuclear cells can distinguish patients with non-small cell lung cancer from patients with nonmalignant lung disease. Cancer Res. 2009;69(24):9202–9210. doi: 10.1158/0008-5472.CAN-09-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Abbas AR, Baldwin D, Ma Y, et al. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes Immun. 2005;6(4):319–331. doi: 10.1038/sj.gene.6364173. [DOI] [PubMed] [Google Scholar]
- [28].Abrams JT, Lessin S, Ghosh SK, et al. A clonal CD4-positive T-cell line established from the blood of a patient with Sezary syndrome. J Invest Dermatol. 1991;96(1):31–37. doi: 10.1111/1523-1747.ep12514693. Prepublished on 1991/01/01 as DOI. [DOI] [PubMed] [Google Scholar]
- [29].Kawai T, Sato S, Ishii KJ, et al. Interferon-alpha induction through Toll-like receptors involves a direct interaction of IRF7 with MyD88 and TRAF6. Nat Immunol. 2004;5(10):1061–1068. doi: 10.1038/ni1118. [DOI] [PubMed] [Google Scholar]
- [30].Tsujimura H, Tamura T, Ozato K. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 2003;170(3):1131–1135. doi: 10.4049/jimmunol.170.3.1131. Prepublished on 2003/01/23 as DOI. [DOI] [PubMed] [Google Scholar]
- [31].Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Bouaziz JD, Ortonne N, Giustiniani J, et al. Circulating natural killer lymphocytes are potential cytotoxic effectors against autologous malignant cells in sezary syndrome patients. J Invest Dermatol. 2005;125(6):1273–1278. doi: 10.1111/j.0022-202X.2005.23914.x. [DOI] [PubMed] [Google Scholar]
- [33].Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6(8):769–776. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- [35].Kadowaki N, Antonenko S, Lau JY, Liu YJ. Natural interferon alpha/beta-producing cells link innate and adaptive immunity. J Exp Med. 2000;192(2):219–226. doi: 10.1084/jem.192.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Takaoka A, Hayakawa S, Yanai H, et al. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 2003;424(6948):516–523. doi: 10.1038/nature01850. [DOI] [PubMed] [Google Scholar]
- [37].Hermann P, Rubio M, Nakajima T, Delespesse G, Sarfati M. IFN-alpha priming of human monocytes differentially regulates gram-positive and gram-negative bacteria-induced IL-10 release and selectively enhances IL-12p70, CD80, and MHC class I expression. J Immunol. 1998;161(4):2011–2018. [PubMed] [Google Scholar]
- [38].D'Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86(2):646–650. [PubMed] [Google Scholar]
- [40].Libraty DH, Airan LE, Uyemura K, et al. Interferon-gamma differentially regulates interleukin-12 and interleukin-10 production in leprosy. J Clin Invest. 1997;99(2):336–341. doi: 10.1172/JCI119162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Hunter CA, Villarino A, Artis D, Scott P. The role of IL-27 in the development of T-cell responses during parasitic infections. Immunol Rev. 2004;202:106–114. doi: 10.1111/j.0105-2896.2004.00213.x. [DOI] [PubMed] [Google Scholar]
- [42].Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. Prepublished on 2007/02/13 as DOI 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- [43].Honda K, Taniguchi T. IRFs: master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol. 2006;6(9):644–658. doi: 10.1038/nri1900. [DOI] [PubMed] [Google Scholar]
- [44].Lehtonen A, Lund R, Lahesmaa R, Julkunen I, Sareneva T, Matikainen S. IFN-alpha and IL-12 activate IFN regulatory factor 1 (IRF-1), IRF-4, and IRF-8 gene expression in human NK and T cells. Cytokine. 2003;24(3):81–90. doi: 10.1016/j.cyto.2003.07.001. [DOI] [PubMed] [Google Scholar]
- [45].Liu J, Guan X, Tamura T, Ozato K, Ma X. Synergistic activation of interleukin-12 p35 gene transcription by interferon regulatory factor-1 and interferon consensus sequence-binding protein. J Biol Chem. 2004;279(53):55609–55617. doi: 10.1074/jbc.M406565200. [DOI] [PubMed] [Google Scholar]
- [46].Politis AD, Ozato K, Coligan JE, Vogel SN. Regulation of IFN-gamma-induced nuclear expression of IFN consensus sequence binding protein in murine peritoneal macrophages. J Immunol. 1994;152(5):2270–2278. Prepublished on 1994/03/01 as DOI. [PubMed] [Google Scholar]
- [47].Bachem A, Guttler S, Hartung E, et al. Superior antigen cross-presentation and XCR1 expression define human CD11c+CD141+ cells as homologues of mouse CD8+ dendritic cells. J Exp Med. 2010;207(6):1273–1281. doi: 10.1084/jem.20100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Suchin KR, Junkins-Hopkins JM, Rook AH. Treatment of stage IA cutaneous T-Cell lymphoma with topical application of the immune response modifier imiquimod. Arch Dermatol. 2002;138(9):1137–1139. doi: 10.1001/archderm.138.9.1137. [DOI] [PubMed] [Google Scholar]
- [49].Urosevic M, Dummer R, Conrad C, et al. Disease-independent skin recruitment and activation of plasmacytoid predendritic cells following imiquimod treatment. J Natl Cancer Inst. 2005;97(15):1143–1153. doi: 10.1093/jnci/dji207. [DOI] [PubMed] [Google Scholar]
- [50].Majewski S, Marczak M, Mlynarczyk B, Benninghoff B, Jablonska S. Imiquimod is a strong inhibitor of tumor cell-induced angiogenesis. Int J Dermatol. 2005;44(1):14–19. doi: 10.1111/j.1365-4632.2004.02318.x. [DOI] [PubMed] [Google Scholar]
- [51].Huber MA, Staib G, Pehamberger H, Scharffetter-Kochanek K. Management of refractory early-stage cutaneous T-cell lymphoma. Am J Clin Dermatol. 2006;7(3):155–169. doi: 10.2165/00128071-200607030-00002. [DOI] [PubMed] [Google Scholar]
- [52].Martinez-Gonzalez MC, Verea-Hernando MM, Yebra-Pimentel MT, Del Pozo J, Mazaira M, Fonseca E. Imiquimod in mycosis fungoides. Eur J Dermatol. 2008;18(2):148–152. doi: 10.1684/ejd.2008.0352. [DOI] [PubMed] [Google Scholar]
- [53].Kim YH, Girardi M, Duvic M, et al. Phase I trial of a Toll-like receptor 9 agonist, PF-3512676 (CPG 7909), in patients with treatment-refractory, cutaneous T-cell lymphoma. J Am Acad Dermatol. 2010;63(6):975–983. doi: 10.1016/j.jaad.2009.12.052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.