Abstract
Guided by the diagnostic nosology, anxiety patients are expected to show defensive hyperarousal during affective challenge, irrespective of the principal phenotype. In the current study, patients representing the whole spectrum of anxiety disorders (i.e., specific phobia, social phobia, panic disorder with or without agoraphobia, obsessive-compulsive disorder, generalized anxiety disorder (GAD), posttraumatic stress disorder(PTSD)), and healthy community control participants, completed an imagery-based fear elicitation paradigm paralleling conventional intervention techniques. Participants imagined threatening and neutral narratives as physiological responses were recorded. Clear evidence emerged for exaggerated reactivity to clinically relevant imagery—most pronounced in startle reflex responding. However, defensive propensity varied across principal anxiety disorders. Disorders characterized by focal fear and impairment (e.g., specific phobia) showed robust fear potentiation. Conversely, for disorders of long-enduring, pervasive apprehension and avoidance with broad anxiety and depression comorbidity (e.g., PTSD secondary to cumulative trauma, GAD), startle responses were paradoxically diminished to all aversive contents. Patients whose expressed symptom profiles were intermediate between focal fearfulness and broad anxious-misery in both severity and chronicity exhibited a still heightened but more generalized physiological propensity to respond defensively. Importantly, this defensive physiological gradient—the inverse of self-reported distress—was evident not only between but also within disorders. These results highlight that fear circuitry could be dysregulated in chronic, pervasive anxiety, and preliminary functional neuroimaging findings suggest that deficient amygdala recruitment could underlie attenuated reflex responding. In summary, adaptive defensive engagement during imagery may be compromised by long-term dysphoria and stress—a phenomenon with implications for prognosis and treatment planning. Depression and Anxiety 29:264–281, 2012.
Keywords: imagery, anxiety disorders, specific phobia, social phobia, panic, agoraphobia, GAD, comorbidity, depression, PTSD, trauma, chronicity, emotional reactivity, blunting, diagnostic subtypes, psychophysiology, startle, fMRI, neuroimaging
EMOTIONS AS ACTION DISPOSITIONS
From the perspective of natural science, human emotions include three measurable response classes: verbal reports of experience, overt actions, and associated physiological mobilization.[1] Several theorists have proposed[2–4] that primitive survival reflexes are the foundation for emotion’s physiological mobilization and action. That is, humans and other animals approach elements that sustain life (appetitive motivation) and fight or flee amidst threats to continued existence (defensive motivation). Humans, however, seldom react as directly as do less complex species. With the development of higher order cortices, emerged enhanced capacity for inhibition and delay, and evaluation of alternatives and outcomes. Nevertheless, primitive reactance is adumbrated in muscles and glands, supported by neural circuits deep within the brain and widely shared among species. For this reason, emotions (fear and anger; joy and desire) are considered action dispositions[5] and as such are often most evident when humans are overtly passive, but mobilized somatically and autonomically for actions that may never actually manifest.
ANXIETY, DEFENSIVE PHYSIOLOGY, AND THE DSM-IV
Throughout the diagnostic nosology,[6] anxiety disorders are fundamentally conceptualized as disruptions of emotional processing, more specifically of exaggerated propensities to respond defensively to stimuli typically perceived as mildly threatening or even innocuous. Based on the supposition that emotional experience of fear and/or anxiety includes not only subjective distress and behavior (i.e., escape/avoidance) but accompanying physiological activation, the canon lists for each diagnosis at least one physiological symptom intended to reflect arousal secondary to disorder-related distress. Notably, prototypical physiological activation patterns have not been identified for respective disorders, suggesting that physiological hyperarousal is an undifferentiated constituent of clinical anxiety—similarly heightened across symptom presentations.
INTERNALIZING SYMPTOMATOLOGY: A DISCERNIBLE UNDERLYING STRUCTURE?
Epidemiological phenotypic[7–11] and genotypic[12–14] factor analytic studies of anxiety and mood disorder comorbidity have revealed a common internalizing dimension, typically expressed as one of two classes of disorders, circumscribed fear versus pervasive anxiety and sadness. Phobic disorders (specific and social phobia) are conceptualized as expressions of underlying fear pathology whereas generalized anxiety disorder (GAD), dysthymia, and depression, showing strong common affinity, reflect latent anxious-misery/distress.
Although epidemiological data have provided compelling evidence of focal fearfulness and broad anxiety as organizing concepts, there is a paucity of complementary objective evidence. Recently, the National Institute of Mental Health has initiated the Research Domain Criteria Project (RDoC[15–18]) to promote investigation of basic mechanisms underlying mental illness, unconstrained by conventional diagnostic boundaries, with the aim of explicating endophenotypes[19] or response measures (e.g., reaction time, autonomic and startle reflexes, brain circuit connectivity)—presumably informative indices of the elementary organic dysfunction (e.g., gene expression).
Consistent with the aims of the RDoC, in this review, we integrate recent results from a psychophysiological investigation of the full range of anxiety spectrum disorders, evaluating reflex outputs from the brain’s fear/defense circuitry during aversive imagery. Following a brief review of somatovisceral and functional neuroimaging patterns elicited by imagery, we consider observed defensive profiles both within as well as between anxiety disorders toward the goal of determining the relative influence of disorder-specific as well as nonspecific features in defensive mobilization.
MENTAL IMAGERY AS A WINDOW INTO EMOTIONAL EXPERIENCE
Mental imagery, especially of an affectively laden narrative, in which the participant imagines the engaging role of active protagonist, has been a productive means of instantiating observable affective dispositions.[20–22] From hundreds of unselected participants, we have collected normative ratings of pleasure and arousal for a wide range of narrative scripts (Affective Norms for English Text,[23]) and found the same pattern that emerged for pictures (International Affective Picture System,[24]). These data attest that despite the effort involved in generating a mental image, emotional narratives prime motivational dispositions analogously to external percepts. In fact, physiological arousal during threat imagery parallels anticipatory reactions to in vivo threat,[21] similarly mobilizing the autonomic nervous system (e.g., heart rate, skin conductance), communicating distress through facial musculature (e.g., corrugator “frown” muscle), prompting somatic reflexive action (e.g., startle potentiation),[22] enhancing attention allocation (e.g., electrocortical activity[25]), and yielding verbal evaluations of intense aversion. Not surprisingly, imagery has been incorporated into empirically supported treatments of fear and anxiety to selectively prompt clinically relevant distress with the goal of promoting habituation and, ultimately, extinction. Aversive imagery, indeed, is a component in numerous conventional behavioral methodologies implemented to treat the entire anxiety spectrum (e.g., specific phobia,[26] panic disorder,[27] posttraumatic stress disorder (PTSD),[28, 29] GAD[30]). Script-driven imagery is an especially flexible tool in experimental and therapeutic contexts as it permits presentation of not only standard, but also personal threat challenges, the latter being essential given the idiosyncratic nature of human experience and consequent fear and apprehension.
MOTIVATIONAL CIRCUITRY AND EMOTIONAL IMAGERY
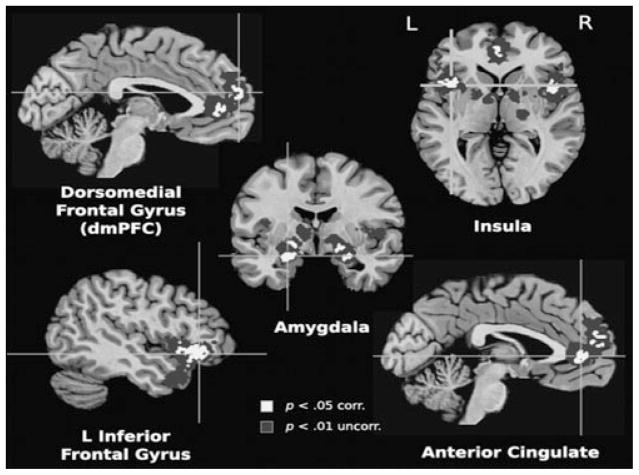
The use of imagery to elicit clinically pertinent distress is premised on the expectation that imagining frightening narratives activates brain circuitry implicated in animal models of human fear.[31] In a series of functional neuroimaging studies[32–34] from our laboratory, we have observed a corticolimbic network that reliably activates during aversive relative to neutral imagery even in nonclinical samples. These distributed neural sources are consistent with Bioinformational Theory,[35] which conceptualizes emotional imagery as episodes encoded in memory via associative networks including (1) stimulus or sensory representations (cues perceived in the context), (2) meaning representations (semantic information “about” the context), and (3) response representations (behavior and physiology that occur in the context). The response representation or procedural knowledge is of particular significance for the action mobilization characteristic of emotional engagement. It is these efferent memories that are most directly connected to the brain’s motivational system (appetitive or defensive), presumably intrinsic to the pronounced physiological arousal evidenced in the psychophysiological laboratory. Guided by this conception, response dispositions primed during aversive imagery should be observable in neural substrates. Accordingly, blood-oxygen level dependent (BOLD) signal increases have reliably emerged in supplementary and presupplementary motor areas, precentral gyrus, and cerebellum[31–33]—regions involved in planning and executing motor action. Corresponding enhancements have been demonstrated in ventrolateral inferior frontal gyrus and dorsal medial prefrontal and dorsal parietal cortex, perhaps reflecting semantic elaboration and sensory integration subserving the generation of a vivid mental image of the self. Importantly, in contrast to imagery of simple visual scenes[36] or motor execution,[37] enhanced defensive motivation was clearly evidenced by middle temporal lobe, insula, and amygdala recruitment—activation that strongly covaried with subjective arousal (Fig. 1). Functional connectivity analyses further confirmed that these premotor and motivational regions contribute to a network of coordinated cortico-cortical and corticolimbic loops sensitive to the emotional intensity of imagined narratives.[38]
Figure 1.
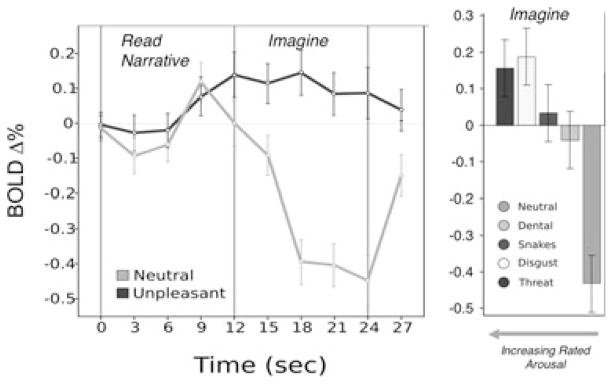
On the left, mean event-related bold signal change (percent) in the amygdala during read (12 s) and imagery (12 s) of neutral and unpleasant narratives. On the right, average signal change in the amygdala during the imagery epoch for neutral and individual unpleasant content categories, arranged from right to left in order of increasing mean subjective arousal. Error bars represent 95% confidence intervals.[146] Adapted from Costa and colleagues.[32]
The aforementioned patterns were observed in studies conducted at the UF Center for the Study of Emotion and Attention, and similar activations have been observed across laboratories. For a recent meta-analysis,[39] we selected whole-brain functional neuroimaging studies (i.e., magnetic resonance imaging, positron emission tomography, spectroscopy) of narrative emotional imagery paradigms, in particular those that used auditory or visual cues to prompt imagined participation in emotional events. These included procedures typically classified as script-driven imagery,[40] symptom provocation,[41] and/or autobiographical recall.[42] Across 74 studies and more than 1,300 participants, increased activation for emotionally arousing compared to neutral imagery highlighted a distributed network underlying imaginal engagement including inferior frontal gyrus, insula, dorsal medial prefrontal cortex, anterior cingulate, and, again, a region fundamentally implicated in emotional responding—the amygdala (Fig. 2).
Figure 2.
Increased activations during emotional imagery resulting from multikernal density analysis of peak coordinates for emotional versus neutral processing in 74 whole-brain studies (N = 1,325). Lighter regions are family-wise error corrected for single voxels and significant extent at p < .05 and darker regions are significant at p = .05 after correction for spatial extent at p < .01. Adapted from Costa and colleagues.[39]
DEFENSIVE ACTIVATION AND THE STARTLE REFLEX
Given the significance of this defensive network during emotional processing, not only during imagery but a range of affective challenges,[43–45] and its well-established contribution to clinical fear and anxiety,[46–48] the ability to index circuit output in the psychophysiological laboratory is of utmost value for research and, potentially, clinical assessment. The potentiated probe startle reflex, a response readily quantified by the magnitude of its first component—the eyeblink—has provided such a measure. As defined over several decades of infrahuman neuroscience research,[49–53] startle potentiation is mediated by the brain’s fear/defense circuit, centered on the amygdala. Circuit activation begins when the lateral and basolateral nuclei of the amygdala receive threat-relevant information from sensory/memory systems. These nuclei project to the central nucleus of the amygdala and bed nucleus of the stria terminalis (BNST), which in turn project to a variety of hypothalamic sites, the central gray, facial motor nucleus, and brainstem target areas, initiating a range of defensive reflexes that evolved to counter imminent threats to survival.[54] Importantly, the amygdala/BNST also project to the central pontine site of the startle circuit, increasing the magnitude of the startle reaction during threat/fear states. Startle reactions are elicited by any abrupt sensory stimulus and serve as a primitive escape response in many species.
In the case of human fear, recording the startle response to a brief acoustic probe (e.g., 95 decibel (dB) white noise) has provided a productive, cost-effective, and noninvasive measure of defensive neural activation. Heightened reflex responding is reliably observed during aversive contexts such as picture viewing,[55] anticipation,[56] exteroceptive threat,[57, 58] conditioning, [59] and narrative imagery,[22] closely corresponding to rated arousal and withstanding even massed repetition[60] and competing cognitive tasks.[61] Furthermore, across these elicitation paradigms, fear potentiation has been further amplified by individual differences in fearfulness and anxiety.[62–67] In the ensuing discussion, we consider whether anxiety invariably confers exaggerated startle responsivity during imagery, or alternatively, if the defensive profile varies as a function of foremost clinical problem (i.e., principal disorder) and comorbid symptom constellations.
AVERSIVE IMAGERY, STARTLE, AND THE ANXIETY SPECTRUM: CURRENT SAMPLE
In research recently undertaken[68–72] at the UF Fear and Anxiety Disorders Clinic and the Center for the Study of Emotion and Attention, treatment-seeking adults (N = 393; mean age 33 years; 64% female) and a demographically matched, healthy community comparison sample (N = 68) completed the Anxiety Disorder Interview Schedule for DSM-IV (ADIS-IV; 73), a semistructured interview for assessing current anxiety, mood, substance use, and somatoform disorders and for screening psychosis and major physical disease. In the case of multiple axis I disorders, diagnostic primacy was determined by clinician-rated severity reflecting both distress and interference. Controls denied current or lifetime diagnoses of psychiatric illness.
At a subsequent session, participants completed a narrative emotional imagery procedure. This entailed listening to a 6-s description of a standard aversive and arousing experience (e.g., being attacked by an animal) or quotidian, neutral event (e.g., reading a magazine). Participants were instructed to imagine being actively engaged in the narrative, as a participant rather than observer, until a tone cue signaled the end of imagery after 12 s. Included in the series of standard scripts were two idiographic, “personal” threat narratives representing each patient’s primary clinical fear; or for controls, their “worst fear” experience (Table 1).
TABLE 1.
Sample personal threat scene exemplars by principal disorder
| Principal disorder | Personal threat exemplar |
|---|---|
| Control | I clench my jaw and fist as the nurse prepares the needle. I quickly close my eyes to avoid seeing the injection. |
| Specific phobia | As I move closer to the cage, I see a large hairy spider. My heart is pounding and my body is shaking. |
| Social phobia | I don’t know anyone at this party. I feel sweaty and clammy as I realize that everyone is staring at me. |
| Panic disorder without agoraphobia | I wake up suddenly—frozen in fear. My heart pounds, I’m dizzy, nauseous, short of breath, choking. |
| Panic disorder with agoraphobia | I am sweaty and I feel like I am about to faint, standing in the middle of a crowded mall. I need to get out. |
| OCD | My heart pounds as liquid from the garbage drips on my hands. The germs are spreading. I need to get clean |
| GAD | As I watch the ambulance drive away my heart pounds and I begin to panic. What if my children are hurt? |
| PTSD | My leg is trapped between the seats. This is it, the van is full of smoke and I am going to die in this fire. |
Startle probes consisting of 50-ms 95 dB white noise with instantaneous rise-time were presented during the imagery epoch and on a subset of intertrial intervals. Startle blinks from orbicularis oculi electromyography (EMG) represented the magnitude difference between onset and peak muscle potential,[74] standardized within subject in relation to the mean and standard deviation of intertrial probe responses.[75] Following imagery assessment, participants rated each scene for experienced pleasure and emotional arousal.[76]
Patterns of startle reflex responding elicited during imagery and concomitant patterns of momentary as well as preexisting distress were assessed. Illustrated in Figure 3 (top panel) are the group means by principal disorder (no diagnosis N = 68; specific phobia N = 67; social phobia N = 71; panic disorder without agoraphobia (PD) N = 39; panic disorder with agoraphobia (PDA) N = 62; obsessive-compulsive disorder (OCD) N = 43; GAD N = 65; PTSD N = 42) on the Beck Depression Inventory (BDI-II),[77] indicating that cognitive and somatic symptoms of depression were within the normal range among control participants and no more than minimally elevated among anxiety patients endorsing the most focal dysfunction (specific phobia). Symptom levels then progressively increased with the generalization of anxiety and apprehension, Group F(7,444) = 35.86, p < .001. Controlling for multiple comparisons, specific phobia patients reported less symptomatic depression than all patients except those with social phobia and panic disorder without agoraphobia, whereas principal PTSD endorsed distress that exceeded all other groups. As shown in Figure 3 (top panel), intermediate symptom severity characterized PDA, differing reliably only from the extremes of the continuum (i.e., control, specific phobia, PTSD, ps < .001).
Figure 3.
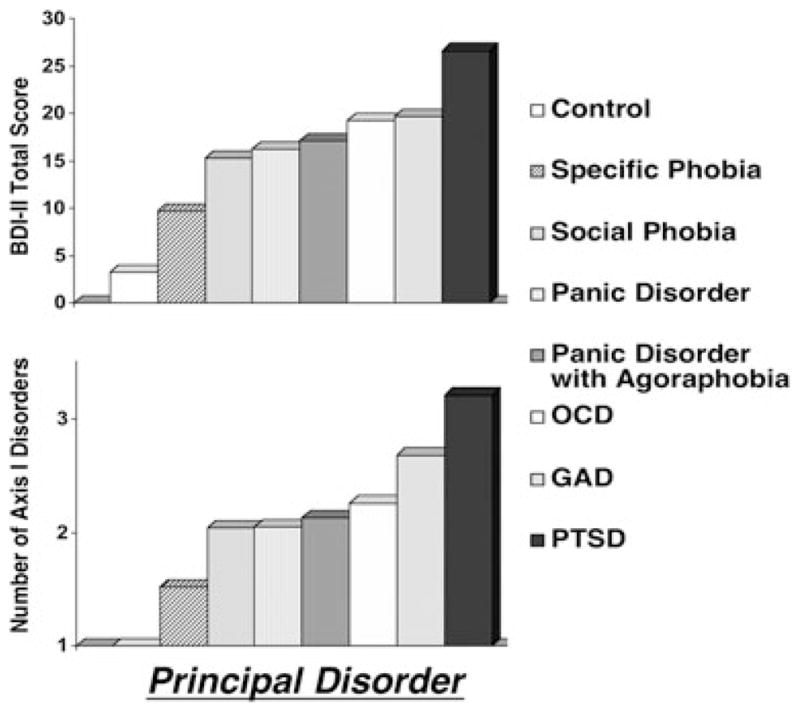
Top panel. Mean total score reported on the BDI-II[77] by principal disorder (OCD, obsessive compulsive disorder; GAD, generalized anxiety disorder; PTSD, posttraumatic stress disorder) as determined with the ADIS-IV[73]. Bottom panel. Total number of axis I disorders conferred based on clinician judgment subsequent to ADIS-IV interview, arranged by principal disorder (control participants excluded).
The BDI total score was depicted here for ease of comparison with the extant literature. Importantly, this pattern emerged across nearly all symptom domains including unspecified anxiety (State-Trait Anxiety Inventory, STAI[78]), generalized fearfulness (Fear Survey Schedule, FSS[79]), anger (State-Trait Anger Inventory[80]), and functional interference (Illness Intrusiveness Rating Scale [81]) as well as interview-based frequency of additional anxiety, mood, and other axis I disorders (Fig. 3 bottom panel).
The confluence of dimensional and categorical dysphoria observed here, as well as innumerable preceding studies[82, 83] is centrally important to the ensuing discussion. Corresponding symptom exacerbation (or amelioration) is the rule, rather than the exception, for anxiety and depressive disorders and accompanying symptomatology. As such, we have termed this nonspecific self-reported symptom array negative affectivity [68] to highlight the synergy of multiple pathologies as opposed to isolated disorders in modulating defensive reflex physiology. In the subsequent section of this report, we examine the effects of this general variable as it modulates imagery-driven fear-potentiated startle within each of the diagnoses of the anxiety disorder spectrum.
POSTTRAUMATIC STRESS, TRAUMA RECURRENCE, AND STARTLE RESPONSES DURING AVERSIVE IMAGERY
We will first explore fear-potentiated startle at the extreme of the self-reported distress continuum—principal PTSD, addressing in particular a potential dose–response relationship between trauma recurrence and intensity of physiological reactions to trauma memories. Epidemiological work has confirmed that exposure to multiple compared to single traumatic events more strongly predisposes the development of PTSD.[84] Cumulative exposure is associated with more severe[85] and chronic posttraumatic stress,[86] more generalized symptomatology,[87] and poorer sociooccupational functioning.[88] In effect, multiple compared to single traumatic exposure more perniciously sensitizes individuals to subsequent stress, prolonging pathological emotional processing across numerous subjective symptom domains. Are corresponding sequelae observed during physiological activation to imagery challenge?
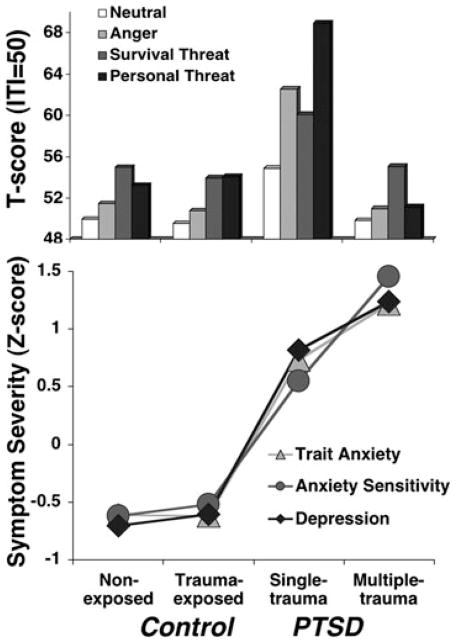
Compared to control participants, when PTSD is considered as a homogeneous diagnostic entity,[70, 89] clear “exaggerated startle” reactivity was observed, not to baseline startle probes, rather to those administered in the midst of idiographic (i.e., trauma relevant) as well as standard aversive (i.e., anger, survival threat) imagery. No evidence emerged among control participants to implicate trauma exposure per se in the etiology of this heightened reactivity (Fig. 4 top panel). Conversely, PTSD patients differed dramatically according to whether lifetime trauma exposure was limited to a single, discrete event (e.g., rape, industrial accident) or distributed across multiple occurrences (e.g., childhood sexual and/or physical abuse, domestic violence). The physiological hyperreactivity observed for the PTSD group as a whole was driven by patients with discrete traumatic exposure, whereas those with cumulative traumatization showed blunted startle reactivity—failing even to recruit responses during trauma-related imagery that exceeded those during neutral imagery. Importantly, this biomarker difference was not reflected in subjective fear—all patients reported equivalently intense aversive arousal during imagery of their trauma narratives.
Figure 4.
Top panel. Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals) during standard neutral, anger, and survival threat as well as personal threat imagery for nonexposed and trauma-exposed control groups and patients with principal PTSD secondary to single or cumulative trauma exposure. Bottom panel. Group means (standardized across participants) on the trait form of the STAI,[78] Anxiety Sensitivity Index,[107] and BDI-II.[77] Adapted from McTeague and colleagues.[70]
Regarding phenotypic features that might elucidate this hypoactive modulation, these patients sustained more numerous, higher magnitude, and interpersonal traumatic events (including, but not limited to the index trauma) beginning earlier in life. Consequent posttraumatic stress persisted on average over three times longer than those with single incident exposure (i.e., 17 and 5 years, respectively). Referring again to Figure 3, similar to the symptom generalization gradient observed across our entire anxiety disorder sample, recurrent compared to single traumatization was also associated with more severe PTSD that, importantly, was concomitant with more excessive comorbid negative affectivity as quantified by questionnaires (e.g., cognitive and somatic symptoms of depression, trait anxiety, and anger) as well as additional diagnoses (Fig. 4 bottom panel). In fact, 74% of the recurrently traumatized group met criteria for an additional anxiety disorder and 85% surpassed threshold for comorbid depression, far exceeding the prevalence in the single trauma group (anxiety disorder: 32%; depressive disorder: 52%). Importantly, previous PTSD samples that demonstrated exaggerated defensive reactivity during script-driven imagery were generally characterized by comorbidity incidence at[90, 91] or below[92] the level of the reactive, single-trauma group—far below the multiple-trauma group.
Returning to the hierarchical structure identified in large-scale epidemiological investigations,[7–14] PTSD has been more closely linked to the anxious-misery dimension[93] Pertinent to our startle findings, Watson[94] has qualified this conclusion, highlighting that PTSD has been less robust an indicator than the other disorders with which it typically coheres (i.e., GAD, depression). Intrinsically, the conventional PTSD diagnosis may encompass patients with a clear fear diathesis (and heightened defensive reflexes) as well as those with a more complex anxious-misery diathesis (and defensive compromise).[95] The current findings also implicate disorder duration such that exaggerated reactivity may develop in the immediate aftermath of discrete trauma, whereas cumulative traumatization and the associated context may prompt sustained stress and hyperarousal that ultimately impair defensive physiological reflexes and broaden subjective symptomatic distress.
SOCIAL PHOBIA, SYMPTOM GENERALIZATION, AND STARTLE RESPONSES DURING AVERSIVE IMAGERY
Is a similar within-diagnosis spectrum evident, even for a predominantly fearful disorder? To address this question, we consulted the provenance of the social phobia nomenclature. The syndrome was initially conceptualized as a disorder of circumscribed fear, similar to specific phobia but typically manifest in a singular performance situation, in which scrutiny is possible.[96] However, findings subsequent to the publication of DSM-III suggested that the presence of multiple social fears, including interaction situations, were more common than a singular performance fear[97] leading to the addition in DSM-III-R[98] and maintenance in DSM-IV[6] of a “generalized” specifier for social phobia to denote fears of “most” social situations. These conceptual revisions prompted the question of whether subtypes of social phobia represent qualitatively distinct syndromes or a continuum of severity and, in effect, across an array of symptom measures, generalized patients have consistently exceeded those with nongeneralized or circumscribed social phobia.[97, 99–101]
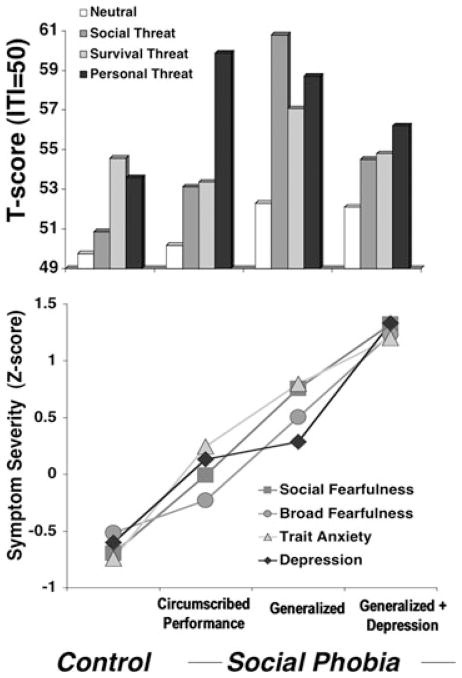
During narrative imagery social phobia patients, considered irrespective of diagnostic subtypes, surpassed controls in startle reflex responding during imagery of standard and idiographic social threat whereas the groups displayed commensurate reactivity to contents depicting commonly shared, adaptive fears (survival threat).[69] Deviating from the impression denoted by the overall group means, circumscribed performance phobia patients responded similarly to controls with the exception of a uniquely strong reaction to personal threat scenarios. Unlike control participants, this exceeded reflex responding to all other scenes, including standard social threat (Fig. 5 top panel). In short, defensive hyperreactivity in circumscribed phobia patients was limited to their personal performance fears. Social phobia patients with fear of scrutiny generalized across routine social interactions in addition to structured performances showed strong startle potentiation for all social threat contents, both idiographic and standard, as well as a substantial response to survival scenarios. In a final parsing of diagnostic variants, generalized social phobia patients were classified according to comorbid depression status, revealing that the broad defensive hyperreactivity of the generalized group as a whole was attributable to patients without depression, as those with depression evidenced relative reflex attenuation.
Figure 5.
Top panel. Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals) during standard neutral, social threat, and survival threat as well as personal threat imagery for controls and patients with principal social phobia limited to performance situations, social phobia generalized to routine interaction as well as performance situations, and generalized social phobia patients with comorbid depression. Bottom panel. Group means (standardized across participants) on the social fear subscale of the FSS,[69] total score of FSS,[79] trait form of the State-Trait Anxiety Inventory,[78] and BDI-II.[77] Adapted from McTeague and colleagues.[69]
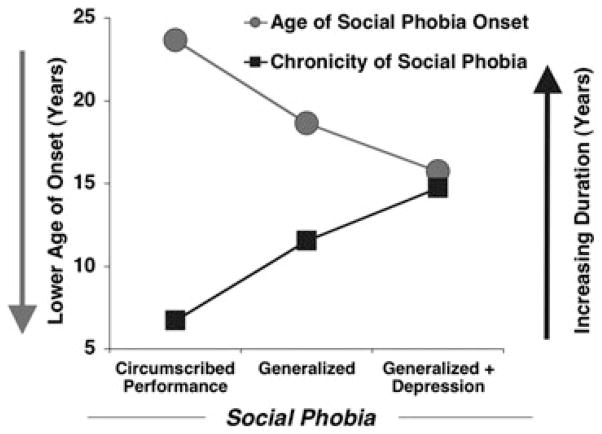
As shown in Figure 5 (bottom panel), this pattern of fear-potentiated startle within social phobia covaried with a stark linear increase in dimensional (i.e., questionnaire measures of fear, trait anxiety, and depression) and categorical (i.e., comorbid disorders) negative affectivity, as well as clinician-rated social phobia severity. In parallel to the PTSD findings, as symptom severity increased among social phobia patients, clinician-rated prognosis reciprocally worsened, age of onset decreased, and chronicity lengthened (Fig. 6).
Figure 6.
For principal social phobia limited to performance situations, social phobia generalized to routine interaction and performance situations, and generalized social phobia patients with comorbid depression, retrospective recall of age of onset of social phobia (gray circles), and duration of disorder (black squares) accounting for age at evaluation, the latter which did not differ across groups. Adapted from McTeague and colleagues.[69]
Interestingly, whereas in the principal PTSD sample we observed consistent startle hyper- and hyporeactivity, for the discrete and recurrently traumatized patients, respectively, within this primary fear disorder we observed finer distinctions concerning defensive propensities. The socially fearful patients with highly focused fears, minimal comorbid symptomatology, and otherwise high functional status exhibited targeted defensive hyperreactivity. With generalization of interpersonal apprehension (without concomitant depression), exaggerated defensive responding was similarly extreme, but now observed to a broader range of aversive contents. Finally, the most generalized social phobia marked by profound comorbid, broad negative affectivity, and the most enduring dysfunction (i.e., disorder chronicity) displayed relative defensive impairment. Essentially, even when interpersonal fear is at the forefront of the clinical presentation, those individuals whose comorbid symptom profile suggest pervasive apprehension and dysphoria exhibit attenuated defensive action possibly more akin to disorders phenotypically predominated by anxious-misery.
PANIC DISORDER, AGORAPHOBIA, AND STARTLE RESPONSES DURING AVERSIVE IMAGERY
Now that we have explored a disorder representing each end of the negative affectivity continuum in Figure 3, we turn to an intermediate syndrome—fear of uncued panic attacks and associated situational apprehension and avoidance. Panic disorder with versus without agoraphobia is typically associated with more profound comorbidity,[102–104] greater sociooccupational impairment,[105] and poorer treatment outcome[106] We additionally sought to distinguish agoraphobia severity, a practice with advent in DSM-III-R,[98] although reduced to a dichotomous distinction in the DSM-IV.[6] Guided by our findings for PTSD and social phobia, we expected a reactivity spectrum covarying according to comorbidity, with severe agoraphobia showing response diminution.
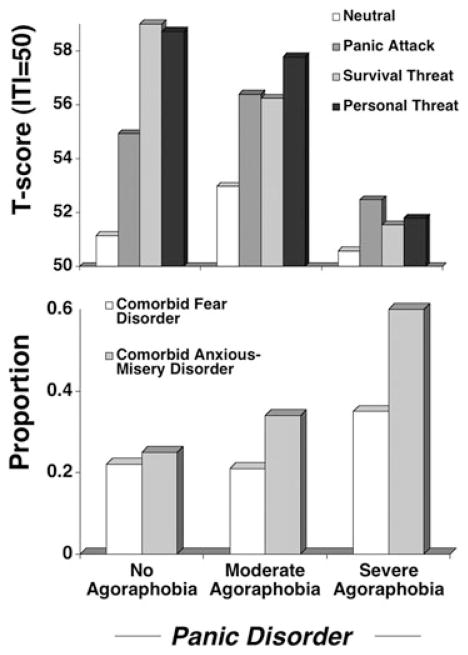
Startle potentiation during aversive imagery was generally more pronounced in PD than PDA—a pattern even more dramatic when patients without agoraphobia were compared to those who endorsed severe agoraphobic apprehension and avoidance during interview (the moderate group was intermediate) (Fig. 7 top panel). Again, seemingly paradoxically, the panic disorder group with severe agoraphobia failed to engage reflex responses greater than those to neutral during aversive imagery of any content—as observed in PTSD secondary to recurrent traumatization.
Figure 7.
Top panel. Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals) during standard neutral, panic attack, and survival threat as well as personal threat imagery for patients with principal panic disorder without agoraphobia, with moderate agoraphobia, and with severe agoraphobia. Bottom panel. Proportion of each group with additional (nonprincipal) fear disorders (specific and social phobias) and anxious-misery disorders (GAD, PTSD, recurrent major depression). Adapted from McTeague and colleagues.[71]
As a parallel and substantiation of the within-diagnosis findings for social phobia and PTSD, a subjective distress dimension emerged, the inverse of fear potentiation—as defensive reflexes diminished, dimensional and categorical comorbid negative affectivity increased, and prognosis worsened. Notably, nonspecific distress and interference more closely corresponded to startle modulation than did trait measures of interoceptive hypersensitivity (i.e., anxiety sensitivity,[107] anxious arousal[108]) putatively specific to panic disorder. Complementing this profile of broadly generalized apprehension and avoidance, panic disorder with severe agoraphobia recalled a history of distress more than twice as long as the panic disorder without agoraphobia group.
Overall, axis I comorbidity mirrored dimensional measures. However, analyses refined by disorder focus indicated that severe agoraphobia patients did not exceed the other groups in comorbid circumscribed fearfulness (i.e., specific or performance phobia diagnoses), transient, single episode major depression, or more enduring mild dysphoria (dythymia). Rather, this group endorsed elevations in concurrent, intractable, and enduring broad anxious distress (i.e., GAD, PTSD) and recurrent major depression (Fig. 7 bottom panel). Referring back to Figure 3 illustrates that principal PD and PDA are situated, adjacently in the middle of the self-reported distress continuum. Interestingly, it is in this intermediate range that comorbidity with the fear versus sustained anxious-misery disorders, and not just gross anxiety and/or mood disorder prevalence, is reflected in the patients’ reflex physiology.
In summary, these findings prompt the speculation that principal panic proneness may, at one extreme, prompt enhanced defensive startle reactivity in the absence of significant agoraphobia. However, with the development of extensive conditioned avoidance secondary to anxious anticipation of panic,[109] the consequent stress of chronic hyperarousal and accompanying dysphoria may function over time to attenuate or even impair active defensive responding—a pathology trajectory that, although with different target fears, may approximate disorder progression in PTSD and social phobia.
COMMON FEATURES ACROSS DISPARATE DISORDERS: OBJECTIVE AND SUBJECTIVE INDICES
As an interim summary, we have observed that although these three samples were phenotypically dissimilar in foremost clinical complaint (i.e., trauma-cue sensitivity, interpersonal apprehension, interoceptive hyperawareness) and associated principal disorder, symptom profiles reflecting more circumscribed fear demonstrated startle potentiation specific to fear-relevant imagery. Alternately, fear generalization without accompanying pervasive dysphoria was associated with broadened or “generalized’ defensive reflexes. Finally, at profound levels of long-enduring nonspecific negative affectivity, startle responses were uniformly diminished amidst standard and idiographic aversive imagery.
SPECIFIC PHOBIA, FEAR FOCUS, AND STARTLE RESPONSES DURING AVERSIVE IMAGERY
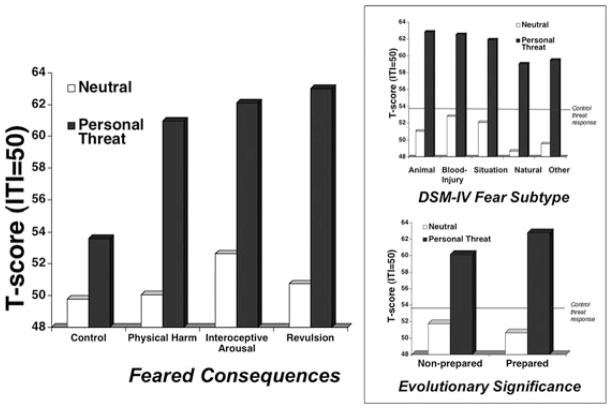
We now move back to the left side of Figure 3 where comorbid negative affectivity is negligible, in particular for specific phobia, the prototypical exaggerated fear disorder. Within principal specific phobia we, again, explored whether variables germane to the clinical presentation and treatment planning would yield differential sensitivities in defensive reflex responding.[72] Whereas control participants responded with similar robustness to both standard survival (attacking animals and humans) and personal threat imagery, principal phobia patients reacted most prominently during personal threat imagery—much greater than control participants responded to their personal fear imagery. This heightened sensitivity to idiographic phobic threat, greater even than to standard attack scenarios, for which defensive engagement is adaptive, might be a function of a subset of patients intensely fearful of physical harm upon confrontation with their phobic object and/or context. Thus, we distinguished principal phobia patients according to their feared primary consequence.[110] Interestingly, equivalent exaggerated reactivity was observed regardless of whether patients feared that encountering the phobic object/context would result in threat to physical integrity, panic-like physiological arousal, or revulsion. For example, a dog phobic who feared ultimately “being seriously bitten” or a height phobic apprehensive of “falling to [their] death,” showed commensurate defensive profiles to a patient afraid of cockroaches due to the possibility of being “grossed out.” These data suggested that for principal specific phobia, fear circuitry might be invariably sensitized across fear foci. In separate subsequent analyses, we compared the DSM-IV subtypes (animal, blood-injury, situational, natural environment, other) and evolutionarily prepared (i.e., dangerous to pretechnological humans) versus nonprepared fears[111] Again, a punctate augmentation to phobia-relevant imagery was evidenced across all diagnostic variants (Fig. 8).
Figure 8.
Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals) during standard neutral and personal threat imagery for principal specific phobia patients distinguished by the ultimate consequence feared upon encounter with phobic object/context (i.e., threat to physical integrity, physiological arousal, or revulsion/disgust). Inset. Top panel. Mean startle reflex responses for principal specific phobia patients distinguished by DSM-IV subtype. Bottom panel. Mean startle reflex responses for principal specific phobia patients distinguished by evolutionarily prepared versus unprepared fears. Black line refers to mean response to personal threat imagery for control participants.
In light of the robustness of clinically pertinent fear potentiation across these principal specific phobia presentations, we were prompted to examine whether the presence of specific phobia confers defensive hyperreactivity, irrespective of diagnostic primacy. Thus, we included 88 more participants for whom specific phobia was an additional/nonprincipal diagnosis.[72] Principal disorders ranged the entire anxiety spectrum (except specific phobia) as well as major depressive and adjustment disorders. Figure 9 illustrates the group means for individuals with principal phobia, showing robust fear potentiation. Conversely, individuals with additional phobia displayed relatively obtunded responding to their foremost clinical fears. In light of the aforementioned results in other anxiety disorders, this pattern is not surprising as 86% of the additional phobia group was diagnosed with a principal anxious-misery disorder. Furthermore, categorical and dimensional negative affectivity and functional interference progressively increased from controls at the minimum, to principal phobia and finally additional phobia at the extreme. Marginal differences were observed across patient subgroups for transient depression in comparison to stark differences in refractory depression, with much greater prevalence of the latter in additional phobia patients.
Figure 9.
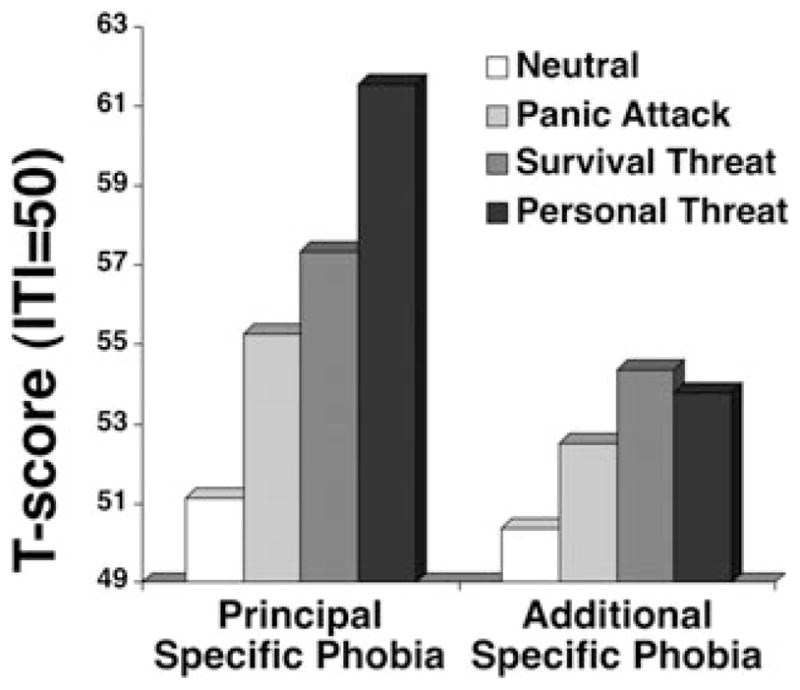
Mean startle reflex responses (standardized to the distribution of responses during intertrial intervals) during standard neutral, panic attack, and survival threat as well as personal threat imagery for patients with principal specific phobia and patients for whom specific phobia was not the most impairing disorder present (nonprincipal/additional phobia). Adapted from McTeague and colleagues.[72]
Interestingly, as found with panic disorder, features more pertinent to meeting threshold for specific phobia diagnosis did not distinguish subgroups. When queried about their particular phobic object/context during interview, patients expressed equivalently severe fear and avoidance and consequent distress and functional interference, suggesting that relative hyper- and hypostartle reactivity did not reflect differences in phobia intensity. Furthermore, principal and nonprincipal phobia patients recalled experiencing phobia-related dysfunction of similar duration, on average 16 years. In other words, unlike the effects we have thus far observed for more generalized distress, the duration of phobic fearfulness does not appear to strongly co-vary with the symptom severity and physiological gradients.
Furthermore, the specific phobia results highlight that simply considering the presence or absence of focal fearfulness versus broad anxiety within the clinical presentation does not fully explain the associated defensive reactivity. In a recent latent class analysis of two epidemiological samples, Vaidyanathan and colleagues[112] found that specific phobia diagnoses typically occur in one of two diagnostic profiles, one marked by multiple phobic disorders (and few other disorders)—the other by a wide array of axis I disorders concurrent with specific phobia. The former group is more akin to the highly reactive principal phobia patients in the current study, whereas the latter group resembles the non-reactive, nonprincipal phobia patients. Vaidyanathan and colleagues speculated that rather than reflecting gross severity of psychopathology, these classes distinguished individuals fundamentally disposed to different disorder combinations—and our findings suggest different defensive propensities. Taken together, the primacy of focal fears and the extent of comorbid negative affectivity must be considered simultaneously to appreciate the associated phenotypic and endophenotypic constellation.
THE REFLEX PHYSIOLOGY AND THE ANXIETY SPECTRUM
To this point, we have considered all anxiety disorders but OCD and GAD. In hierarchical epidemiological investigations, no consensus has emerged regarding whether OCD may be better construed as a predominantly fear disorder or anxious-misery disorder,[11, 14, 113, 114] whereas previously stipulated,[94] GAD has been reliably related to the latter. Returning again to Figure 3, in our sample these disorders were adjacent and situated on the more broadly distressed end of the symptom continuum. Depicted in Figure 10 are the fear potentiation scores (i.e., personal threat minus neutral) for the overall sample and as implicated in the means, defensive startle responsivity reliably differed across the disorder spectrum, F(8,379) = 3.30, p < .01. Targeted comparisons with the single-trauma PTSD at the reactive antipode revealed reliable attenuations for both OCD and GAD, ps < .05. Corresponding to their conveyed levels of vast negative affectivity, both disorders demonstrated relative defensive hyporeactivity.
Figure 10.
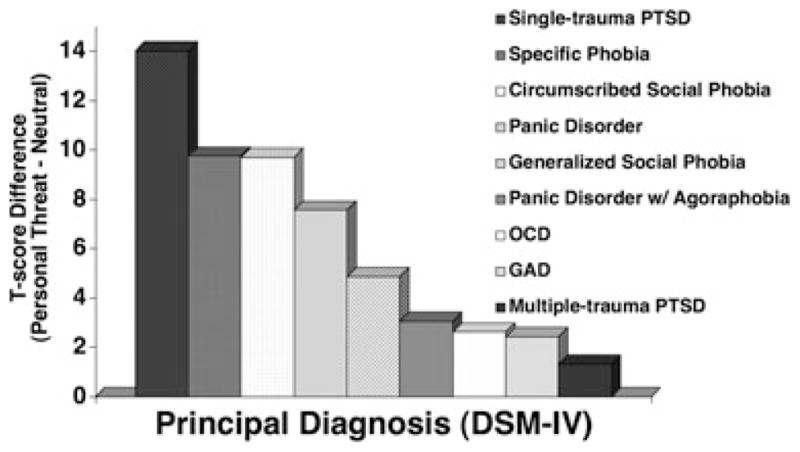
Mean fear potentiation of startle reflexes (startle response magnitude during personal threat minus neutral imagery) for patients by principal disorder (OCD, obsessive-compulsive disorder; GAD, generalized anxiety disorder; PTSD, posttraumatic stress disorder) as determined with the ADIS-IV,[73] arranged in order of decreasing response magnitude.
A FEAR/DEFENSE CIRCUIT DIMENSION: POSSIBLE MECHANISMS
In the current sample, diminished startle reactivity was evident in patients who endured the longest standing disorder and the highest comorbid negative affectivity, whereas more focally fearful, less symptomatic patients demonstrated robust fear potentiation, specific to clinically relevant personal narratives. Patients intermediate on both the subjective and physiological continua exhibited a more generalized propensity to respond defensively and to endorse secondary diffuse symptomatology. At first glance, obtunded startle during aversive imagery in clinical anxiety seems paradoxical. However, in a smaller, though sizable anxiety spectrum sample (N = 130), Cuthbert and colleagues[75] observed the same phenomenon with fear disorders (specific and social phobia) at one end and PDA and PTSD at the non-reactive antipode of the startle reflex spectrum—with an inverse subjective distress gradient.
The reason for this consistent attenuation amidst persistent broad negative affectivity is unclear. Although dissociation is a viable candidate process, multiple investigators have found no impact of high dissociative tendencies on defensive physiology during imagery.[115–117] Furthermore, considering that in the current sample, those displaying the most impaired reflex responding endorsed as much or more extreme aversion and arousal than the other groups, the data do not strongly support a dissociation/disengagement interpretation.[69–72] In fact, the sole subjective index for which no group differences, F(7,437) = 1.38, ns, were observed across the entire sample was trait ability to generate vivid images of sensorimotor experience (Questionnaire Upon Mental Imagery[118]). A related candidate mechanism driving the response diminution is impaired cognitive-attentional capacity. However, in addition to the aforementioned contradictory subjective data, concurrent recording of facial expressivity, in particular corrugator EMG, which is sensitive to subovert frowning challenges such an explanation. This is most clearly demonstrated in the case of PTSD[70]—despite profound differences in startle responsivity, patients with discrete versus recurrent traumatization showed equivalently exaggerated corrugator increases sustained throughout personal threat imagery. Diminished fear potentiation of startle may reflect dysfunction specific to the automatic recruitment of the motivational neurocircuitry underlying this reflex. At the same time, facial expressivity, more closely under instrumental control remains effectively mobilized.
The reciprocal relationship between negative affectivity and fear potentiation of the startle reflex was also not attributable to baseline or intertrial startle reactivity as, consistent with prior investigations,[75] no group differences were observed. In terms of the influence of psychotropic medication usage, selective serotonin reuptake inhibitors (SSRIs; 30.7%) and benzodiazepines (28.8%) were the most frequently endorsed and at rates that differed across the anxiety spectrum (SSRIs, χ2 (6) = 38.56, P < .001; benzodiazepines χ2 (6) = 31.25, P < .001). Importantly, this pattern did not covary meaningfully with the startle reflex or negative affectivity continua (SSRIs: Specific phobia 13.6% social phobia 18.3% PD 40.5% PDA 40.3% OCD 60% GAD 24.3% PTSD 35.7%; benzodiazepines: Specific phobia 17.5%, social phobia 21.1%, PD 43.8%, PDA 54.2%, OCD 16.2%, GAD 24.3%, PTSD 28.2%). The effects of these and less frequently endorsed compounds (e.g., serotonin-norepinephrine reuptake inhibitors 5%, norepinephrine-dopamine reuptake inhibitors 2.3%, tricyclics 1.8%) were assessed by comparing resting and imagery reactivity among the medicated and nonmedicated patients. Considering either general psychotropic usage or more specific classes of drugs, no reliable effects emerged, consistent with prior psychophysiological studies of anxiety and depression.[75, 118–121] Furthermore, reported usage of prescription and over-the-counter medications for physical health, as well as recreational substances similarly exerted no discernible impact on startle reflex responding.
A FEAR/DEFENSE CIRCUIT DIMENSION: THE CONVERGENCE OF NEGATIVE AFFECTIVITY, CHRONICITY, AND STRESS
Given that the startle circuit is part of the somatic system, reduced fear potentiation may reflect the psychomotor retardation and behavioral inhibition associated with high negative affectivity. Similar results during threat of shock,[122] affective picture viewing,[123, 124] and anticipation[119] suggest that this blunting phenomenon may persist across affective challenges. The current data further highlight that even across different anxiety disorders, common features predict response attenuation: (1) the breadth and severity of negative affectivity (i.e., generalized anxiety and depressive symptoms, (2) the duration (i.e., chronicity) of this pervasive distress. In short, the stress of severe, broad, and enduring dysphoria is related to response diminution. Importantly, the symptoms defined by diagnosis are not the only burden. Mental illness is typically accompanied by lifespan sociocon-textual adversity, the magnitude and frequency of which often covary with anxiety/depression severity.[125–128] As such, the current data tentatively implicate the debilitating effects of cumulative, chronic stress on the integrity of the underlying fear/defense system.
Animal data corroborate that variations in stressor intensity, duration, and recurrence can result in dampened defensive responses.[129–134] For example, Davis and Astrachan[135] observed a nonmonotonic relationship between conditioned fear-potentiated startle and shock intensity: Rats exposed to light cues paired with intermediate levels of shock displayed pronounced conditioned potentiation, low shock intensities elicited modest augmentation, and those exposed to the highest shock intensity showed no discernible increase in startle magnitude. Chalmers and colleagues [136] found a similar absence of conditioned fear potentiation among rats exposed to highly intense, prolonged, and inescapable shock. More specific to stressor chronicity, animals exposed to brief (i.e., 10 days) and/or less severe “resident/intruder” stress demonstrated hypervigilance and hyperarousal, whereas those exposed to longer duration stress (20–30 days) developed more generalized anxiety and depressive-like symptoms—including passivity, limited movement, and reduced communication and consumption behaviors—that persisted even in the absence of the aggressor.[129, 131] Essentially, in the current data diminished fear potentiation may reflect neuromorphological and/or functional connectivity alterations in fear/defense neurocircuitry secondary to the accumulation of disorder-related as well as broader contextual stressors.
DYSFUNCTION OF THE FEAR/DEFENSE NEURAL CIRCUIT?
The question arises as to whether the attenuated fear potentiation coincident with enduring broad negative affectivity is actually the downstream expression of disrupted neurocircuit activation. In a preliminary study, we recruited 31 individuals who consistently endorsed extreme or normative symptom levels across a battery of measures assessing dimensional anhedonia, anxious arousal, nonspecific anxiety, and depression (Mood and Anxiety Symptom Questionnaire,[108] STAI,[77] BDI-II[78]). Based on our findings of deficient fear-potentiated startle responses among patients with pervasive negative affect, we expected that undifferentiated elevated dysphoria would be related to impaired amygdala activation during aversive narrative imagery. Although a nonclinical sample, the symptom level differences were marked (e.g., BDI-II total scores: high symptom group M = 24.9; low symptom group M = 4.6) with the elevations of the high negative affect group on par with the dysphoria conveyed by our patients showing fear-potentiated startle deficits.[68–72] With a restricted anterior prescription, we oversampled the BOLD response at a temporal resolution of 500 ms, thus enabling finer estimation of the temporal dynamics of amygdala activation and to determine whether the magnitude as well as the trajectory of affective engagement covaried with negative affectivity.[137, 138] The low symptom group showed reliably enhanced amygdala activation (Fig. 11) relative to baseline that emerged by 3 s into the presentation of aversive narratives, only to heighten throughout the duration of imagery (24 s from text onset to imagery offset). In contrast, the high negative affectivity group failed to show reliable amygdala activation at any time point.
Figure 11.
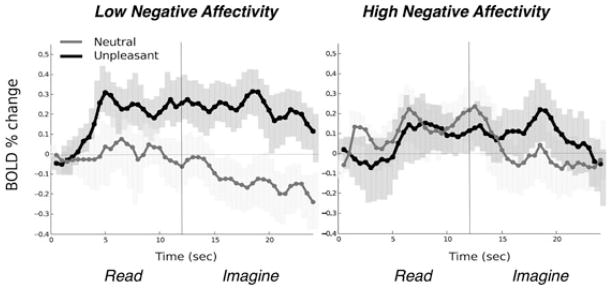
Mean event-related bold signal change (percent) in the amygdala during read (12 s) and imagery (12 s) of neutral and unpleasant narratives for participants low and high in negative affectivity. Error bars represent 95% confidence intervals.[146] Adapted from Costa and colleagues.[33]
Although the generalizabilty of these results is limited by the use of a nonclinical sample, amygdala hypoactivation has been observed during script-driven aversive imagery among subsets of broadly distressed anxiety patients.[139–142] Furthermore, some of the pioneering neuroimaging studies of pathological anxiety and aversive imagery have limited their patient samples to participants who showed palpable autonomic responses (i.e., “responders”) during a preceding imagery assessment.[143, 144] This has been an especially productive approach for elucidating the neurocircuit activation among individuals showing concordant, exaggerated defensive reactivity across multiple systems, whereas much less is understood about system discordance and hypoactivation. Hopefully, efforts will increase to examine possible deficiencies in neurocircuit activation and/or connectivity underlying the blunted physiological reflex activation coincident with severe negative affectivity.[68, 145]
CONCLUSION
In a large-scale study of anxiety spectrum patients, focally fearful individuals with minimal comorbidity demonstrated robust startle potentiation during imagery, specific to clinically relevant personal narratives. In contrast, marked diminution for all aversive contents was observed in patients who endured far more sustained dysfunction and conveyed diagnostic presentations marked by pervasive apprehension and comorbid negative affectivity (i.e., simultaneous elevations across categorical and dimensional measures of anxiety and mood symptomatology). Patients with symptom profiles interposed between focal fearfulness and broad anxious-misery exhibited a still heightened but more generalized physiological propensity to respond defensively and indicated intermediate disorder duration. Consistent with the aims of the RDoC initiative, fear-potentiated startle during narrative imagery appears to be a dimensional biomarker of fear-defense circuit activation—meaningfully cross-cutting conventional diagnostic distinctions to highlight common dysfunction.
Longitudinal examination is necessary to determine whether these defensive and subjective response profiles are stable, time-invariant dispositions throughout the trajectories of anxious dysfunction, potentially reflecting genetic underpinnings.[147] Imagery-driven fear-potentiated startle might even be a useful endophenotype in investigations of genetic liability, preceding disorder onset.[148, 149] Alternatively or additionally, we may be observing individuals at temporally different junctures in pathogenesis. A predominantly fearful, hyperreactive presentation may evolve with progressive generalization and resistance to extinction, to chronic stress/hyperarousal and a constellation of refractory negative affectivity, which adversely impact motivational neurocircuitry integrity and thus, ultimately impair defensive action (startle).
Our patient groups did not differ on age at evaluation; so reports of more persistent distress in the hyporeactive patients indicate that these individuals suffer much longer than focally fearful patients prior to seeking treatment and as such, outreach to promote earlier intervention is clearly warranted. Importantly, regardless of the etiology of response diminution, a challenge arises: does this biomarker reflect mechanisms that impede therapeutic gains? For many patients, imaginal exposure and other treatments aimed at extinguishing exaggerated fear response are clearly effective in promoting symptom amelioration. Among patients whose defensive startle reflex is impaired, and presumably the brain’s fear/defense circuitry is compromised, can the reflex physiology of fear be readily accessed for extinction? As a preamble to exposure, it might be beneficial to train patients to reengage their reflex physiology, promoting concordance between subjective and physiological experience, and thus enabling patients to maximize treatment and ensure meaningful recovery.
Acknowledgments
This work was supported in part by a National Institute of Mental Health grants P50 MH 72850 to the Center for the Study of Emotion and Attention (CSEA), University of Florida, Gainesville, FL (P.J. Lang Director & Principal Investigator), R21 MH082702 to Peter J. Lang, and NRSA Research Fellowship (F31 MH069048) to the first author. Special thanks to Vincent D. Costa, Bruce N. Cuthbert, Andreas Keil, and Margaret M. Bradley for assistance with study design and interpretation of findings. Special thanks to the following individuals for their assistance in data collection: Marie-Claude Laplante, Cyd C. Strauss, Denise M. Sloan, Eleni Dimoulas, Jose M. Soler-Baillo, Greg Perlman, Bethany C. Wangelin, Joshua R. Shumen, Esther Jean-Baptiste, and Hailey W. Bulls.
Footnotes
Conflict of Interest Statement: Neither author has any conflicts of interest to report.
References
- 1.Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: brain mechanisms and psychophysiology. Biol Psychiatry. 1998;44(12):1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- 2.Konorski J. Integrative Activity of the Brain: An Interdisciplinary Approach. Chicago, IL: University of Chicago Press; 1967. [Google Scholar]
- 3.Dickinson A, Dearing MF. Appetitive-aversive interactions and inhibitory processes. In: Dickinson A, Boakes RA, editors. Mechanisms of Learning and Motivation. Hillsdale, NJ: Erlbaum; 1979. pp. 203–231. [Google Scholar]
- 4.Lang PJ, Bradley MM. Emotion and the motivational brain. Biol Psychol. 2010;84:437–450. doi: 10.1016/j.biopsycho.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frijda NH. Impulsive action and motivation. Biol Psychol. 2010;84:570–579. doi: 10.1016/j.biopsycho.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4. Washington, DC: American Psychiatric Press; 2000. (DSM-IV-TR) [Google Scholar]
- 7.Krueger RF. The structure of common mental disorders. Arch Gen Psychiatry. 1999;56:921–926.64. doi: 10.1001/archpsyc.56.10.921. [DOI] [PubMed] [Google Scholar]
- 8.Vollebergh WA, Iedema J, Bijl RV, de Graaf R, Smit F, Ormel J. The structure and stability of common mental disorders: the NEMESIS study. Arch Gen Psychiatry. 2001;58:597–603. doi: 10.1001/archpsyc.58.6.597. [DOI] [PubMed] [Google Scholar]
- 9.Slade T, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychol Med. 2006;36:1593–1600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- 10.Krueger RF, Markon KE. Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. Annu Rev Clin Psychol. 2006;2:111–133. doi: 10.1146/annurev.clinpsy.2.022305.095213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox BJ, Clara IP, Hills AL, Sareen J. Obsessive-compulsive disorder and the underlying structure of anxiety disorders in a nationally representative sample: confirmatory factor analytic findings from the German Health Survey. J Anxiety Disord. 2010;24:30–33. doi: 10.1016/j.janxdis.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 13.Hettema JM, Prescott CA, Myers JM, Neale MC, Kendler KS. The structure of genetic and environmental risk factors for anxiety disorders in men and women. Arch Gen Psychiatry. 2005;62:182–189. doi: 10.1001/archpsyc.62.2.182. [DOI] [PubMed] [Google Scholar]
- 14.Tambs K, Czajkowsky N, Røysamb E, Neale MC, Reichborn-Kjennerud T, Aggen SH, Harris JR, Ørstavik RE, Kendler KS. Structure of genetic and environmental risk factors for dimensional representations of DSM-IV anxiety disorders. Br J Psychiatry. 2009;195:301–307. doi: 10.1192/bjp.bp.108.059485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute of Mental Health. The National Institute of Mental Health Strategic Plan. Bethesda, MD: National Institute of Mental Health; 2008. NIH publication 08-6368. Available at: http://www.nimh.nih.gov/about/strategic-planning-reports/index.shtml. [Google Scholar]
- 16.Cuthbert BN. Dimensional models of psychopathology: research agenda and clinical utility. J Abnorm Psychol. 2005;114:565–569. doi: 10.1037/0021-843X.114.4.565. [DOI] [PubMed] [Google Scholar]
- 17.Insel TR, Cuthbert BN. Endophenotypes: bridging genomic complexity and disorder heterogeneity. Biol Psychiatry. 2009;66:988–989. doi: 10.1016/j.biopsych.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Sanislow CA, Pine DS, Quinn KJ, Kozak MJ, Garvey MA, Heinssen RK, Wang PS, Cuthbert BN. Developing constructs for psychopathology research: research domain criteria. J Abnorm Psychol. 2010;119:631–639. doi: 10.1037/a0020909. [DOI] [PubMed] [Google Scholar]
- 19.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 20.Cook EW, 3rd, Melamed BG, Cuthbert BN, McNeil DW, Lang PJ. Emotional imagery and the differential diagnosis of anxiety. J Consult Clin Psychol. 1988;56:734–740. doi: 10.1037//0022-006x.56.5.734. [DOI] [PubMed] [Google Scholar]
- 21.Lang PJ, Kozak MJ, Miller GA, Levin DN, McLean A., Jr Emotional imagery: conceptual structure and pattern of somatovisceral response. Psychophysiology. 1980;17(2):179–192. doi: 10.1111/j.1469-8986.1980.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 22.Vrana SR, Lang PJ. Fear imagery and the startle-probe reflex. J Abnorm Psychol. 1990;99(2):189–197. doi: 10.1037//0021-843x.99.2.189. [DOI] [PubMed] [Google Scholar]
- 23.Bradley MM, Lang PJ. Technical Report D-1. Gainesville, FL: University of Florida; 2007. Affective Norms for English Text (ANET): Affective ratings of text and instruction manual. [Google Scholar]
- 24.Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. Gainesville, FL: University of Florida; 2008. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. [Google Scholar]
- 25.McTeague LM, Löw A, Wangelin, Sivinki RG, Bradley MM, Lang PJ. Emotional imagery: probe P3, beta band activity & motivational engagement. Psychophysiology. 2008;45(Supplement 1):S107. [Google Scholar]
- 26.Craske MG, Antony MM, Barlow DH. Mastering Your Fears and Phobias: Therapist Guide. 2. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 27.Craske MG, Barlow DH. Mastery of Your Anxiety & Panic: Therapist Guide. 4. New York: Oxford University Press; 2006. [Google Scholar]
- 28.Foa EB, Hembree E, Rothbaum BO. Prolonged Exposure Therapy for PTSD: Emotional Processing of Traumatic Experiences: Therapist Guide. New York, NY: Oxford University Press; 2007. [Google Scholar]
- 29.Cloitre M, Cohen LR, Koenen KC. Treating Survivors of Childhood Abuse: Psychotherapy for the Interrupted Life. New York, NY: Guilford Press; 2006. [Google Scholar]
- 30.Zinbarg RE, Craske MG, Barlow DH. Mastery of Your Anxiety and Worry: Therapist Guide. 2. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 31.Lang PJ, Davis M. Emotion, motivation, and the brain: reflex foundations in animal and human research. Prog Brain Res. 2006;156:3–29. doi: 10.1016/S0079-6123(06)56001-7. [DOI] [PubMed] [Google Scholar]
- 32.Costa VD, Lang PJ, Sabatinelli D, Versace F, Bradley MM. Emotional imagery: assessing pleasure and arousal in the brain’s reward circuitry. Hum Brain Mapp. 2010;31:1446–1457. doi: 10.1002/hbm.20948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Costa VD, McTeague LM, Bradley MM, Lang PJ. Timing of hedonic discrimination in mesolimbic reward centers. Psychophysiology. 2010;47(Supplement 1):S29. [Google Scholar]
- 34.Sabatinelli D, Lang PJ, Bradley MM, Flaisch T. The neural basis of narrative imagery: emotion and action. Prog Brain Res. 2006;156:93–103. doi: 10.1016/S0079-6123(06)56005-4. [DOI] [PubMed] [Google Scholar]
- 35.Lang PJ. Presidential address, 1978. A bio-informational theory of emotional imagery. Psychophysiology. 1979;16:495–512. doi: 10.1111/j.1469-8986.1979.tb01511.x. [DOI] [PubMed] [Google Scholar]
- 36.Ganis G, Thompson WL, Kosslyn SM. Brain areas underlying visual mental imagery and visual perception: an fMRI study. Brain Res Cogn Brain Res. 2004;20:226–241. doi: 10.1016/j.cogbrainres.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 37.Ehrsson HH, Geyer S, Naito E. Imagery of voluntary movement of fingers, toes, and tongue activates corresponding body-part-specific motor representations. J Neurophysiol. 2003;90:3304–3316. doi: 10.1152/jn.01113.2002. [DOI] [PubMed] [Google Scholar]
- 38.Costa VD, Bradley MM, Sabatinelli, Lang PJ. Reward circuit activation and connectivity during emotional imagery and perception. Psychophysiology. 2010;47(Supplement 1):S2. [Google Scholar]
- 39.Costa VD, Bradley MM, Lang PJ. A meta-analysis of narrative emotional imagery. Psychophysiology. 2010;47(Supplement 1):S29. [Google Scholar]
- 40.Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional cerebral blood flow in the amygdala and medial prefrontal cortex during traumatic imagery in male and female Vietnam veterans with PTSD. Arch Gen Psychiatry. 2004;61(2):168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- 41.Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry. 1994;51(1):62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- 42.George MS, Ketter TA, Parekh PI, Horwitz B, Herscovitch P, Post RM. Brain activity during transient sadness and happiness in healthy women. Am J Psychiatry. 1995;152(3):341–351. doi: 10.1176/ajp.152.3.341. [DOI] [PubMed] [Google Scholar]
- 43.Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–348. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- 44.Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;42:998–1031. doi: 10.1016/j.neuroimage.2008.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sabatinelli D, Fortune EE, Li Q, Siddiqui A, Krafft C, Oliver WT, Beck S, Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. Neuroimage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Etkin A. Functional neuroanatomy of anxiety: a neural circuit perspective. Curr Top Behav Neurosci. 2010;2:251–277. doi: 10.1007/7854_2009_5. [DOI] [PubMed] [Google Scholar]
- 47.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- 48.Shin LM, Liberzon I. The neurocircuitry of fear, stress, and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Davis M. The role of the amygdala in conditions and unconditioned fear and anxiety. In: Butters AP, editor. The Amygdala: A Functional Analysis. 2. New York, NY: Oxford University Press; 2000. pp. 213–288. [Google Scholar]
- 50.Fendt M, Fanselow MS. The neuroanatomical and neuro-chemical basis of conditioned fear. Neurosci Biobehav Rev. 1999;23:743–760. doi: 10.1016/s0149-7634(99)00016-0. [DOI] [PubMed] [Google Scholar]
- 51.Ledoux JE, Schiller D. The human amygdala: insights from other animals. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York, NY: Guilford Press; 2009. pp. 43–60. [Google Scholar]
- 52.Davis M, Antoniadis EA, Amaral DG, Winslow JT. Acoustic startle reflex in rhesus monkeys: a review. Rev Neurosci. 2008;19:171–185. doi: 10.1515/revneuro.2008.19.2-3.171. [DOI] [PubMed] [Google Scholar]
- 53.Davis M, Walker DL, Miles L, Grillon C. Phasic vs sustained fear in rats and humans: role of the extended amygdala in fear vs anxiety. Neuropsychopharmacology. 2010;35:105–135. doi: 10.1038/npp.2009.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lang PJ, Davis M, Ohman A. Fear and anxiety: animal models and human cognitive psychophysiology. J Affect Disord. 2000;61:137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- 55.Bradley MM, Codispoti M, Cuthbert BN, Lang PJ. Emotion and motivation I: defensive and appetitive reactions in picture processing. Emotion. 2001;1:276–298. [PubMed] [Google Scholar]
- 56.Sabatinelli D, Bradley MM, Lang PJ. Affective startle modulation in anticipation and perception. Psychophysiology. 2001;38:719–722. [PubMed] [Google Scholar]
- 57.Bradley MM, Moulder B, Lang PJ. When good things go bad: the reflex physiology of defense. Psychol Sci. 2005;16:468–473. doi: 10.1111/j.0956-7976.2005.01558.x. [DOI] [PubMed] [Google Scholar]
- 58.Lang PJ, Wangelin BC, Bradley MM, Versace F, Davenport PW, Costa VD. Threat of suffocation and defensive reflex activation. Psychophysiology. 2011;48:393–396. doi: 10.1111/j.1469-8986.2010.01076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Weike AI, Schupp HT, Hamm AO. Fear acquisition requires awareness in trace but not delay conditioning. Psychophysiology. 2007;44(1):170–180. doi: 10.1111/j.1469-8986.2006.00469.x. [DOI] [PubMed] [Google Scholar]
- 60.Ferrari V, Bradley MM, Codispoti M, Lang PJ. Repetitive exposure: brain and reflex measures of emotion and attention. Psychophysiology. 2011;48(4):515–522. doi: 10.1111/j.1469-8986.2010.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wangelin BC, Löw A, McTeague LM, Bradley MM, Lang PJ. Aversive picture processing: effects of a concurrent task on sustained defensive system engagement. Psychophysiology. 2011;48(1):112–116. doi: 10.1111/j.1469-8986.2010.01041.x. [DOI] [PubMed] [Google Scholar]
- 62.Wendt J, Lotze M, Weike AI, Hosten N, Hamm AO. Brain activation and defensive response mobilization during sustained exposure to phobia-related and other affective pictures in spider phobia. Psychophysiology. 2008;45:205–215. doi: 10.1111/j.1469-8986.2007.00620.x. [DOI] [PubMed] [Google Scholar]
- 63.Melzig CA, Michalowski JM, Holtz K, Hamm AO. Anticipation of interoceptive threat in highly anxiety sensitive persons. Behav Res Ther. 2008;46:1126–1134. doi: 10.1016/j.brat.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Bradley MM, Silakowski T, Lang PJ. Fear of pain and defensive activation. Pain. 2008;137:156–163. doi: 10.1016/j.pain.2007.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lissek S, Levenson J, Biggs AL, Johnson LL, Ameli R, Pine DS, Grillon C. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. Am J Psychiatry. 2008;165:124–132. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Limberg A, Barnow S, Freyberger HJ, Hamm AO. Emotional vulnerability in borderline personality disorder is cue specific and modulated by traumatization. Biol Psychiatry. 2011;69:574–582. doi: 10.1016/j.biopsych.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 67.Vaidyanathan U, Patrick CJ, Cuthbert BN. Linking dimensional models of internalizing psychopathology to neurobiological systems: affect-modulated startle as an indicator of fear and distress disorders and affiliated traits. Psychol Bull. 2009;135:909–942. doi: 10.1037/a0017222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lang PJ, McTeague LM. The anxiety disorder spectrum: fear imagery, physiological reactivity, and differential diagnosis. Anxiety Stress Coping. 2009;22:5–25. doi: 10.1080/10615800802478247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Strauss CC, Bradley MM. Fearful imagery in social phobia: generalization, comorbidity, and physiological reactivity. Biol Psychiatry. 2009;65:374–382. doi: 10.1016/j.biopsych.2008.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 2010;67:346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McTeague LM, Lang PJ, Laplante MC, Bradley MM. Aversive imagery in panic disorder: agoraphobia severity, comorbidity, and defensive physiology. Biol Psychiatr. 2011;70:415–24. doi: 10.1016/j.biopsych.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McTeague LM, Lang PJ, Wangelin BC, Laplante MC, Bradley MM. Aversive imagery in specific phobia: fear focus, comorbidity, and defensive physiology. Under review. [Google Scholar]
- 73.Brown TA, Barlow DH, DiNardo PA, Barlow DH. The Anxiety Disorder Interview Schedule for DSM-IV: Adult Version. New York, NY: Oxford University Press; 1994. [Google Scholar]
- 74.Globisch J, Hamm A, Schneider R, Vaitl D. A computer program for scoring reflex eyeblink and electrodermal responses written in Pascal. Psychophysiology. 1993;39:S30. [Google Scholar]
- 75.Cuthbert BN, Lang PJ, Strauss C, Drobes D, Patrick CJ, Bradley MM. The psychophysiology of anxiety disorder: fear memory imagery. Psychophysiology. 2003;40:407–422. doi: 10.1111/1469-8986.00043. [DOI] [PubMed] [Google Scholar]
- 76.Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. J Behav Ther Exp Psychiatr. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 77.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- 78.Spielberger CD, Gorsuch RL, Lushene PR, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory (STAI) Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 79.Wolpe J, Lang PJ. A fear survey schedule for use in behavior therapy. Behav Res Ther. 1964;2:27–30. doi: 10.1016/0005-7967(64)90051-8. [DOI] [PubMed] [Google Scholar]
- 80.Spielberger CD. Manual for the State-Trait Anger Expression Inventory. Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- 81.Devins GM. Using the illness intrusiveness ratings scale to understand health-related quality of life in chronic disease. J Psychosom Res. 2010;68:591–602. doi: 10.1016/j.jpsychores.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 82.Griffith JW, Zinbarg RE, Craske MG, Mineka S, Rose RD, Waters AM, Sutton JM. Neuroticism as a common dimension in the internalizing disorders. Psychol Med. 2010;40:1125–1136. doi: 10.1017/S0033291709991449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Krueger RF, Finger MS. Using item response theory to understand comorbidity among anxiety and unipolar mood disorders. Psychol Assess. 2001;13:140–151. [PubMed] [Google Scholar]
- 84.Breslau N, Chilcoat HD, Kessler RC, Davis GC. Previous exposure to trauma and PTSD effects of subsequent trauma: results from the Detroit Area Survey of trauma. Am J Psychiatr. 1999;156:902–907. doi: 10.1176/ajp.156.6.902. [DOI] [PubMed] [Google Scholar]
- 85.Dohrenwend BP, Turner JB, Turse NA, Adams BG, Koenen KC, Marshall R. The psychological risks of Vietnam for U.S. veterans: a revisit with new data and methods. Science. 2006;313:979–982. doi: 10.1126/science.1128944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Clancy CP, Graybeal A, Tompson WP, Badgett KS, Feldman ME, Calhoun PS, Erkanli A, Hertzberg MA, Beckham JC. Lifetime trauma exposure in veterans with military-related posttraumatic stress disorder: association with current symptomatology. J Clin Psychiatr. 2006;67:1346–1353. doi: 10.4088/jcp.v67n0904. [DOI] [PubMed] [Google Scholar]
- 87.Mollica RF, McInnes K, Poole C, Tor S. Dose-effect relationships of trauma to symptoms of depression and post-traumatic stress disorder among Cambodian survivors of mass violence. Br J Psychiatr. 1998;173:482–488. doi: 10.1192/bjp.173.6.482. [DOI] [PubMed] [Google Scholar]
- 88.Cloitre M, Cohen LR, Edelman RE, Han H. Posttraumatic stress disorder and extent of trauma exposure as correlates of medical problems and perceived health among women with childhood abuse. Women Health. 2001;3:1–17. doi: 10.1300/J013v34n03_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lang PJ, McTeague LM. Discrete and recurrent traumatization in PTSD: fear vs. anxious misery. J Clin Psychol Med Settings. 2011;18:209–9. doi: 10.1007/s10880-011-9252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pitman RK, Orr SP, Forgue DF, de Jong JB, Claiborn JM. Psychophysiologic assessment of posttraumatic stress disorder imagery in Vietnam combat veterans. Arch Gen Psychiatry. 1987;44:970–975. doi: 10.1001/archpsyc.1987.01800230050009. [DOI] [PubMed] [Google Scholar]
- 91.Keane TM, Kolb LC, Kaloupek DG, Orr SP, Blanchard EB, Thomas RG, Hsieh FY, Lavori PW. Utility of psychophysiological measurement in the diagnosis of posttraumatic stress disorder: results from a Department of Veterans Affairs Cooperative Study. J Consult Clin Psychol. 1998;66:914–923. doi: 10.1037//0022-006x.66.6.914. [DOI] [PubMed] [Google Scholar]
- 92.Shalev AY, Orr SP, Pitman RK. Psychophysiologic assessment of traumatic imagery in Israeli civilian patients with posttraumatic stress disorder. Am J Psychiatry. 1993;150:620–624. doi: 10.1176/ajp.150.4.620. [DOI] [PubMed] [Google Scholar]
- 93.Cox BJ, Clara IP, Enns MW. Posttraumatic stress disorder and the structure of common mental disorders. Depress Anxiety. 2002;15(I):168–171. doi: 10.1002/da.10052. [DOI] [PubMed] [Google Scholar]
- 94.Watson D. Rethinking the mood and anxiety disorders: a quantitative hierarchical model for DSM-V. J Abnorm Psychol. 2005;114:522–536. doi: 10.1037/0021-843X.114.4.522. [DOI] [PubMed] [Google Scholar]
- 95.Forbes D, Parslow R, Creamer M, O’Donnell M, Bryant R, Mc-Farlane A, Silove D, Shalev A. A longitudinal analysis of post-traumatic stress disorder symptoms and their relationship with fear and anxious-misery disorders: implications for DSM-V. J Affect Disord. 2010;127:147–152. doi: 10.1016/j.jad.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 96.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Press; 1980. (DSM-III) [Google Scholar]
- 97.Turner SM, Beidel DC, Townsley RM. Social phobia: a comparison of specific and generalized subtypes and avoidant personality disorder. J Abnorm Psychol. 1992;101:326–331. doi: 10.1037//0021-843x.101.2.326. [DOI] [PubMed] [Google Scholar]
- 98.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3. Washington, DC: American Psychiatric Press; 1987. revised. (DSM-III-R) [Google Scholar]
- 99.Heimberg RG, Hope DA, Dodge CS, Becker RE. DSM-III-R subtypes of social phobia. Comparison of generalized social phobics and public speaking phobics. J Nerv Ment Dis. 1990;178:172–179. doi: 10.1097/00005053-199003000-00004. [DOI] [PubMed] [Google Scholar]
- 100.Holt CS, Heimberg RG, Hope D, Liebowitz MR. Situational domains of social phobia. J Anxiety Disord. 1992;6:63–77. [Google Scholar]
- 101.Boone ML, McNeil DW, Masia CL, Turk CL, Carter LE, Ries BJ, Lewin MR. Multimodal comparisons of social phobia subtypes and avoidant personality disorder. J Anxiety Disord. 1999;13:271–292. doi: 10.1016/s0887-6185(99)00004-3. [DOI] [PubMed] [Google Scholar]
- 102.Andrews G, Slade T. Agoraphobia without a history of panic disorder may be part of the panic disorder syndrome. J Nerv Ment Dis. 2002;190:624–630. doi: 10.1097/00005053-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 103.Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. J Abnorm Psychol. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- 104.Kessler RC, Chiu WT, Jin R, Ruscio AM, Shear K, Walters EE. The epidemiology of panic attacks, panic disorder, and agoraphobia in the National Comorbidity Survey replication. Arch Gen Psychiatry. 2006;63:415–424. doi: 10.1001/archpsyc.63.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wittchen HU, Nocon A, Beesdo K, Pine DS, Hofler M, Lieb R, Gloster AT. Agoraphobia and panic. Prospective-longitudinal relations suggest a rethinking of diagnostic concepts. Psychother Psychosom. 2008;77:147–157. doi: 10.1159/000116608. [DOI] [PubMed] [Google Scholar]
- 106.Slaap BR, den Boer JA. The prediction of nonresponse to pharmacotherapy in panic disorder: a review. Depress Anxiety. 2001;14:112–122. doi: 10.1002/da.1053. [DOI] [PubMed] [Google Scholar]
- 107.Reiss S, Peterson RA, Gursky DM, McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. doi: 10.1016/0005-7967(86)90143-9. [DOI] [PubMed] [Google Scholar]
- 108.Watson D, Weber K, Assenheimer JS, Clark LA, Strauss ME, McCormick RA. Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol. 1995;104:3–14. doi: 10.1037//0021-843x.104.1.3. [DOI] [PubMed] [Google Scholar]
- 109.Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- 110.LeBeau RT, Glenn D, Liao B, Wittchen HU, Beesdo-Baum K, Ollendick T, Craske MG. Specific phobia: a review of DSM-IV specific phobia and preliminary recommendations for DSM-V. Depress Anxiety. 2010;27:148–167. doi: 10.1002/da.20655. [DOI] [PubMed] [Google Scholar]
- 111.Mineka S, Ohman A. Phobias and preparedness: the selective, automatic, and encapsulated nature of fear. Biol Psychiatry. 2002;52(10):927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- 112.Vaidyanathan U, Patrick CJ, Iacono WG. Patterns of comorbidity among mental disorders: a person-centered approach. Compr Psychiatry. 2011;52:527–535. doi: 10.1016/j.comppsych.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Slade T, Watson D. The structure of common DSM-IV and ICD-10 mental disorders in the Australian general population. Psychol Med. 2006;36:1593–1600. doi: 10.1017/S0033291706008452. [DOI] [PubMed] [Google Scholar]
- 114.Wittchen HU, Beesdo-Baum K, Gloster AT, Höfler M, Klotsche J, Lieb R, Beauducel A, Bühner M, Kessler RC. The structure of mental disorders re-examined: is it developmentally stable and robust against additions? Int J Methods Psychiatr Res. 2009;18(4):189–203. doi: 10.1002/mpr.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kaufman ML, Kimble MO, Kaloupek DG, McTeague LM, Bachrach P, Forti AM, Keane TM. Peritraumatic dissociation and physiological response to trauma-relevant stimuli in Vietnam combat veterans with posttraumatic stress disorder. J Nerv Ment Dis. 2002;190:167–174. doi: 10.1097/00005053-200203000-00005. [DOI] [PubMed] [Google Scholar]
- 116.Nixon RD, Resick PA, Griffin MG. Panic following trauma: the etiology of acute posttraumatic arousal. J Anxiety Disord. 2004;18:193–210. doi: 10.1016/S0887-6185(02)00290-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Halligan SL, Michael T, Wilhelm FH, Clark DM, Ehlers A. Reduced heart rate responding to trauma reliving in trauma survivors with PTSD: correlates and consequences. J Trauma Stress. 2006;19(5):721–734. doi: 10.1002/jts.20167. [DOI] [PubMed] [Google Scholar]
- 118.Sheehan PW. A shortened form of Betts’ questionnaire upon mental imagery. J Clin Psychol. 1967;223:380–389. doi: 10.1002/1097-4679(196707)23:3<386::aid-jclp2270230328>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 119.Dichter GS, Tomarken AJ, Shelton RC, Sutton SK. Early-and late-onset startle modulation in unipolar depression. Psychophysiology. 2004;41:433–440. doi: 10.1111/j.1469-8986.00162.x. [DOI] [PubMed] [Google Scholar]
- 120.Forbes EE, Miller A, Cohn JF, Fox NA, Kovacs M. Affect-modulated startle in adults with childhood-onset depression: relations to bipolar course and number of lifetime depressive episodes. Psychiatr Res. 2005;134:11–25. doi: 10.1016/j.psychres.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 121.Rottenberg J, Gross JJ, Gotlib IH. Emotion context insensitivity in major depressive disorder. J Abnorm Psychol. 2005;114:627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- 122.Melzig CA, Weike AI, Zimmermann J, Hamm AO. Startle reflex modulation and autonomic responding during anxious apprehension in panic disorder patients. Psychophysiology. 2007;44:846–854. doi: 10.1111/j.1469-8986.2007.00560.x. [DOI] [PubMed] [Google Scholar]
- 123.Kaviani H, Gray JA, Checkley SA, Raven PW, Wilson GD, Kumari V. Affective modulation of the startle response in depression: influence of the severity of depression, anhedonia, and anxiety. J Affect Disord. 2004;83:21–31. doi: 10.1016/j.jad.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 124.Taylor-Clift A, Morris BH, Rottenberg J, Kovacs M. Emotion-modulated startle in anxiety disorders is blunted by co-morbid depressive episodes. Psychol Med. 2011;41:129–139. doi: 10.1017/S003329171000036X. [DOI] [PubMed] [Google Scholar]
- 125.Galea S, Brewin CR, Gruber M, Jones RT, King DW, King LA, McNally RJ, Ursano RJ, Petukhova M, Kessler RC. Exposure to hurricane-related stressors and mental illness after Hurricane Katrina. Arch Gen Psychiatry. 2007;64:1427–1434. doi: 10.1001/archpsyc.64.12.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.McLaughlin KA, Conron KJ, Koenen KC, Gilman SE. Childhood adversity, adult stressful life events, and risk of past-year psychiatric disorder: a test of the stress sensitization hypothesis in a population-based sample of adults. Psychol Med. 2010;40:1647–1658. doi: 10.1017/S0033291709992121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Moitra E, Dyck I, Beard C, Bjornsson AS, Sibrava NJ, Weisberg RB, Keller MB. Impact of stressful life events on the course of panic disorder in adults. J Affect Disord. 2011;134:373–376. doi: 10.1016/j.jad.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Spinhoven P, Elzinga BM, Hovens JG, Roelofs K, Zitman FG, van Oppen P, Penninx BW. The specificity of childhood adversities and negative life events across the life span to anxiety and depressive disorders. J Affect Disord. 2010;126:103–112. doi: 10.1016/j.jad.2010.02.132. [DOI] [PubMed] [Google Scholar]
- 129.Avgustinovich DF, Kovalenko IL, Kudryavtseva NN. A model of anxious depression: persistence of behavioral pathology. Neurosci Behav Physiol. 2005;35:917–924. doi: 10.1007/s11055-005-0146-6. [DOI] [PubMed] [Google Scholar]
- 130.Rygula R, Abumaria N, Flügge G, Fuchs E, Rüther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 131.Radley JJ, Morrison JH. Repeated stress and structural plasticity in the brain. Ageing Res Rev. 2005;4:271–287. doi: 10.1016/j.arr.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 132.Dwivedi Y, Mondal AC, Payappagoudar GV, Rizavi HS. Differential regulation of serotonin (5HT)2A receptor mRNA and protein levels after single and repeated stress in rat brain: role in learned helplessness behavior. Neuropharmacology. 2005;48:204–214. doi: 10.1016/j.neuropharm.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 133.Waddell J, Morris RW, Bouton ME. Effects of bed nucleus of the stria terminalis lesions on conditioned anxiety: aversive conditioning with long-duration conditional stimuli and reinstatement of extinguished fear. Behav Neurosci. 2006;120:324–336. doi: 10.1037/0735-7044.120.2.324. [DOI] [PubMed] [Google Scholar]
- 134.Uchida S, Hara K, Kobayashi A, Funato H, Hobara T, Otsuki K, Yamagata H, McEwen BS, Watanabe Y. Early life stress enhances behavioral vulnerability to stress through the activation of REST4-mediated gene transcription in the medial prefrontal cortex of rodents. J Neurosci. 2010;30:15007–15018. doi: 10.1523/JNEUROSCI.1436-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Davis M, Astrachan DI. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used in training. J Exp Psychol Anim Behav Process. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- 136.Chalmers DV, Hohf JC, Levine S. The effects of prior shock stimulation on the behavioral and physiological responses to intense acoustic stimuli. Physiology and Behavior. 1974;12:711–718. doi: 10.1016/0031-9384(74)90004-3. [DOI] [PubMed] [Google Scholar]
- 137.Siegle GJ, Carter CS, Thase ME. Use of FMRI to predict recovery from unipolar depression with cognitive behavior therapy. Am J Psychiatry. 2006;163:735–738. doi: 10.1176/ajp.2006.163.4.735. [DOI] [PubMed] [Google Scholar]
- 138.Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ. Neural mechanisms of cognitive reappraisal of negative self-beliefs in social anxiety disorder. Biol Psychiatry. 2009;66:1091–1099. doi: 10.1016/j.biopsych.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without post-traumatic stress disorder: a positron emission tomography study. Biol Psychiatry. 1999;45(7):806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57(8):832–840. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 141.Kilts CD, Kelsey JE, Knight B, Ely TD, Bowman FD, Gross RE, Selvig A, Gordon A, Newport DJ, Nemeroff CB. The neural correlates of social anxiety disorder and response to pharmacotherapy. Neuropsychopharmacology. 2006;31:2243–2253. doi: 10.1038/sj.npp.1301053. [DOI] [PubMed] [Google Scholar]
- 142.Lanius RA, Williamson PC, Boksman K, Densmore M, Gupta M, Neufeld RW, Gati JS, Menon RS. Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic resonance imaging investigation. Biol Psychiatry. 2002;52:305–311. doi: 10.1016/s0006-3223(02)01367-7. [DOI] [PubMed] [Google Scholar]
- 143.Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR, Fischman AJ, Jenike MA, Pitman RK. A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry. 1996;53:380–387. doi: 10.1001/archpsyc.1996.01830050014003. [DOI] [PubMed] [Google Scholar]
- 144.Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: a PET investigation. Am J Psychiatr. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- 145.Lanius RA, Vermetten E, Loewenstein RJ, Brand B, Schmahl C, Bremner JD, Spiegel D. Emotion modulation in PTSD: clinical and neurobiological evidence for a dissociative subtype. Am J Psychiatr. 2010;167:640–647. doi: 10.1176/appi.ajp.2009.09081168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Loftus GR, Masson MEJ. Using confidence intervals in within-subject designs. Psychol Bull Rev. 1994:476–490. doi: 10.3758/BF03210951. [DOI] [PubMed] [Google Scholar]
- 147.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biol Psychiatry. 1998;44:990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- 149.Grillon C, Warner V, Hille J, Merikangas KR, Bruder GE, Tenke CE, Nomura Y, Leite P, Weissman MM. Families at high and low risk for depression: a three-generation startle study. Biol Psychiatry. 2005;57:953–960. doi: 10.1016/j.biopsych.2005.01.045. [DOI] [PubMed] [Google Scholar]