Abstract
Objectives
The purpose of this study was to investigate early markers of risk for neurobehavioral compromise in congenital heart disease (CHD) survivors.
Methods
Fetuses < 24 wks gestational age (GA) were enrolled in this prospective pilot study for serial Doppler assessment of the middle cerebral and umbilical artery. The cerebral-to-placental resistance ratio (CPR) and MCA pulsatility index (PI) z-scores for GA were calculated. After birth, subjects underwent high-density (128-lead) electroencephalogram (EEG) and beta frequency (12–24Hz) band EEG power, a measure of local neural synchrony, was analyzed. Neurodevelopment was assessed at 18-months with the Bayley Scales of Infant Development III (BSID).
Results
13 subjects were enrolled: 4 with hypoplastic left heart syndrome (HLHS), 4 with transposition of the great arteries (TGA), and 5 with tetralogy of Fallot (TOF). Compared with subjects with normal CPR, those with CPR<1(N=7) had lower mean BSID cognitive scores (91.4±4.8 vs. 99.2±3.8, p=.008). Fetal MCA PI z-score also correlated with BSID cognitive score (r=.589, p=0.044) as did neonatal EEG left frontal polar (r=.58, p=.037) and left frontal (r=.77,p=.002) beta power. Furthermore, fetal Doppler measures were associated with EEG power: fetuses with CPR<1 had lower left frontal polar (t=2.36, p=.038) and left frontal (t=2.85, p=.016) beta power as newborns compared with fetuses with normal CPR, and fetal MCA PI z-score correlated with neonatal EEG left frontal polar (r=.596, p=.04) and left frontal (r=.598, p=.04) beta power.
Conclusions
In CHD fetuses with HLHS, TGA, and TOF, abnormal cerebrovascular resistance predicted decreased neonatal EEG left frontal beta power and lower 18-mo cognitive development scores.
Keywords: congenital heart disease, neurodevelopment, fetal cerebral Doppler, fetal cerebrovascular resistance, hypoplastic left heart syndrome, tetralogy of Fallot, transposition of the great arteries, neonatal high-density electroencephalogram
INTRODUCTION
Children with severe forms of congenital heart disease (CHD) are at high risk for a spectrum of neurocognitive difficulties that include learning disabilities, behavioral problems and mental retardation.1, 2 The etiology of these neurodevelopmental problems is poorly understood but is presumed to be multifactorial, arising from a combination of genetic factors along with insults that occur in early infancy and childhood secondary to both altered physiology and medical interventions.
Fetuses with severe forms of CHD exhibit abnormal cerebral Doppler measurements. Donofrio et al. reported that fetuses with complex CHD evidenced brain-sparing, where the cerebral to placental resistance ratio (CPR) was less than one.3 Kaltman et al. reported diminished middle cerebral artery pulsatility index (MCA PI) in the setting of left-sided obstructive lesions and increased PI in the setting of rightsided obstructive lesions.4 Both groups hypothesized that cardiac structural alterations led to changes in fetal cerebral blood delivery and speculated that these changes impact cerebral development in a manner similar to what has been seen in fetuses with intra-uterine growth retardation. 5 The purpose of this prospective pilot study was to test the hypothesis that abnormal fetal cerebral Doppler measures in CHD fetuses would be associated with impaired neurodevelopment.
METHODS
This was a prospective, observational pilot investigation of fetuses and infants with complex CHD. Following parental consent, consecutively encountered fetuses < 24 weeks gestational age (GA) who presented for fetal echocardiogram with the following three cardiac diagnoses were enrolled: hypoplastic left heart syndrome (HLHS), transposition of the great arteries (TGA), and tetrology of Fallot (TOF).
Fetal Doppler Measurements
Fetuses were assessed serially every 4 to 6 weeks through pregnancy by fetal echocardiography and blood flow velocities in the umbilical (UA) and the middle cerebral artery (MCA) were recorded via pulse-wave Doppler. Using the peak systolic (PSV), the end diastolic (EDV), and the time averaged mean (TAMX) velocities, the Resistance Index (RI) and the Pulsatility Index (PI) were calculated for each artery [RI=(PSV-EDV)/EDV; PI = (PSV-EDV)/TAMX]. Using published normal values, 6 the MCA PI z-score for GA was calculated which allowed correction for the expected change in cerebral blood flow that occurs across gestation. For example, a MCA PI z-score of -1 indicates that the value for that subject is 1 SD less than the expected mean value at that GA. Low PI values, indicative of diminished pulsatility, can be alternatively interpreted as increased blood flow in diastole. In addition, the cerebral-to-placental resistance ratio (CPR), a measure of the relative differences in resistance between the cerebral and the placental vascular beds, was calculated [CPR=MCA RI/UA RI]. A CPR<1 indicates that resistance is lower in the cerebral vascular bed than in the placenta and is always considered to be abnormal, regardless of gestational age. We considered both a negative MCA PI z-score and/or a CPR<1 to represent increased cerebral blood flow in the fetus, a presumed autoregulatory response to diminished oxygen delivery in the setting of disordered blood flow secondary to the structural heart defect.
Neonatal EEG
Electroencephalogram (EEG) is a sensitive measure of cerebral function and injury. EEG is also a good measure of brain maturity in preterm infants,7, 8 and in infants exposed to prenatal stressors such as maternal alcohol consumption.9 The origins of the electricocortical activity recorded by EEG are groups of neurons that depolarize together, creating electric dipoles that lead to electric potentials measured at the scalp. Local synchronous depolarization of neurons is measured as EEG power. Synchronous depolarization of neurons from distant sites is quantified as EEG coherence, a measure of functional connectivity. These EEG measures of neurologic function have been shown to be associated with cognitive performance in older children and adults.10, 11
Neuronal networks measured by EEG oscillate at different frequencies which are thought to represent different neuronal sources and functions.12 Thus, total EEG activity (power) is typically partitioned into different frequency bands ranging from 1–50 Hz. Coherence can also be measured in these frequency ranges. Different methods exist to record electroencephalographic output. The high-density, 128-lead method we use offers enhanced assessment of neurologic function, compared with traditional EEG (e.g. 19 lead, International 10–20 System). Moreover, these high impedance electrodes (EGI, Inc.) which do not require scalp preparation, can be applied in less than 5 minutes.
In this study, infants underwent high-density (128 lead) electroencephalogram (EEG) recordings prior to discharge from the neonatal intensive care unit. Two measures of electrocortical function were calculated in the frontal brain regions at different frequency bands: 1) power, a measure of local neural synchrony and 2) coherence, a measure of functional connectivity between regions using the techniques previously described by Grieve et al.13 Initial analyses were carried out using data recorded in the beta frequency (12–24Hz) band as described by Scher et al.14 Additional data exploration was carried out in 5 other spectral bands: delta (1–4 Hz), theta (4–8 Hz), alpha (8–12 Hz), low gamma (24–40 Hz), and gamma (40–50 Hz). Frontal brain regions were chosen due to the known importance of this region on executive function and attention in human development, known domains of deficit among survivors with congenital heart disease.
18-month Neurodevelopmental Assessment
At 18 months of age, subjects returned for a developmental assessment consisting of the Bayley Scales of Infant Development, 3rd edition (BSID).15 A trained psychologist blinded to cardiac diagnosis, as well as fetal blood flow and neonatal EEG results, administered the test and calculated Cognitive, Language and Motor composite scores. The BSID-III has been validated in at risk populations and can be used between the ages of 1 and 42 months of age. The normalized population mean and SD of each composite score is 100±15.
Statistical Analysis
Baseline subject characteristics are reported as means (SD) or medians (IQR) as appropriate. Associations were investigated between fetal cerebrovascular Doppler measures (MCA PI z-score, and CPR), EEG measures (power, coherence), and 18-month developmental score (Cognitive, Language, Motor). Fetuses were categorized as abnormal if the subject demonstrated a CPR<1. Differences in mean BSID scores between fetuses with CPR≥1 and fetuses with CPR<1 were assessed using a Student’s t-test. Correlations between MCA PI z-score and BSID score were tested using Pearson’s correlation coefficient. Correlations between EEG power and coherence and BSID scores were also assessed using Pearson’s correlation coefficient. In order to minimize multiple comparisons testing with EEG analysis, we initially focused on EEG power and coherence in the beta frequency (12–24Hz) band given previously published correlations between these measures and neurodevelopmental outcomes.8 We also focused on the frontal brain regions due to the established role of the frontal cortex in higher level cognitive functioning.16 For the purposes of the EEG analyses, a per-test (8 tests were considered a priori) alpha value of 0.006 was used to ensure a family-wise Type I error rate of 0.05. We investigated associations between fetal cerebral blood flow and EEG power and coherence using similar techniques: Student’s t-tests were used to compare mean values of EEG power and coherence between fetuses with CPR≥1 and fetuses with CPR<1 and Pearson correlation coefficients were calculated between EEG power and coherence values and MCA PI z-scores. Multivariate linear regression was used to investigate the combined association of fetal cerebral Doppler and neonatal EEG on 18-month development score.
RESULTS
From 3/08–3/09, 16 subjects (2 female, 14 male) were enrolled: 5 with hypoplastic left heart syndrome (HLHS), 5 with transposition of the great arteries (d-TGA), and 6 with tetralogy of Fallot (TOF). The mean GA at enrollment was 22±2 weeks (Table 1). Of this group, 3 subjects were excluded due to either death (1 HLHS, 1 TOF with extreme prematurity) or severe disability (1 TGA with stroke). Therefore, complete data was available in 13 subjects.
Table 1.
Cardiac Diagnosis and Fetal Cerebral Doppler Measures Across Gestation
| Cardiac Dx | N | MCA PI z-score (mean±SD) |
CPR<1 at any time |
|---|---|---|---|
| HLHS | 5 | −2.4±0.72 | 3/5 |
| TGA | 6 | −0.75±0.46 | 2/6 |
| TOF | 5 | −2.01±0.87 | 4/5 |
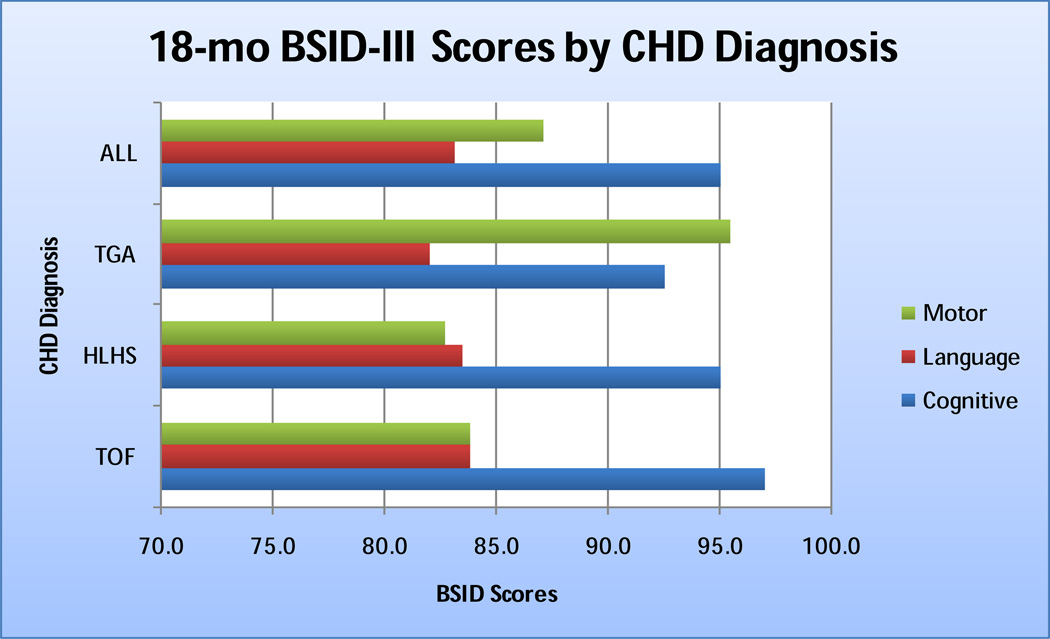
The mean age at the time of the BSID assessment was 18.7±0.8 months. Cognitive, language and motor scores means for the cohort were below average, with language and motor scores among the HLHS and TOF subgroups > 1 SD below the population mean. [Figure 1]
Figure 1.
18-month BSID-III cognition, motor, and language scores by CHD diagnosis
Fetal Cerebrovascular Doppler Measures
The mean number of assessments across pregnancy was 3.32 and the mean GA at the time of the first fetal assessment was 22.8±2.6 weeks. Mean MCA PI z-score of the group was −1.7±1.1 (range −3.37 to −0.3) and 9/16 (56%) subjects had a cerebral to placental resistance ratio (CPR)<1 (3/5 (60%) HLHS, 2/6 (30%) TGA, and 4/5 (80%) TOF). The abnormal CPR was more likely to occur early in pregnancy: 7/9 CPR<1 measures were between 18–24 wks and therefore this data point was used in subsequent analyses. Mean MCA PI z-scores differed between the HLHS and TGA groups (p=0.001) and between the TOF and TGA groups (p=0.013). There was no difference between the HLHS and the TOF groups in mean MCA PI z-score. [Table 1]
Fetal Cerebrovascular Doppler Measures and Neurodevelopment
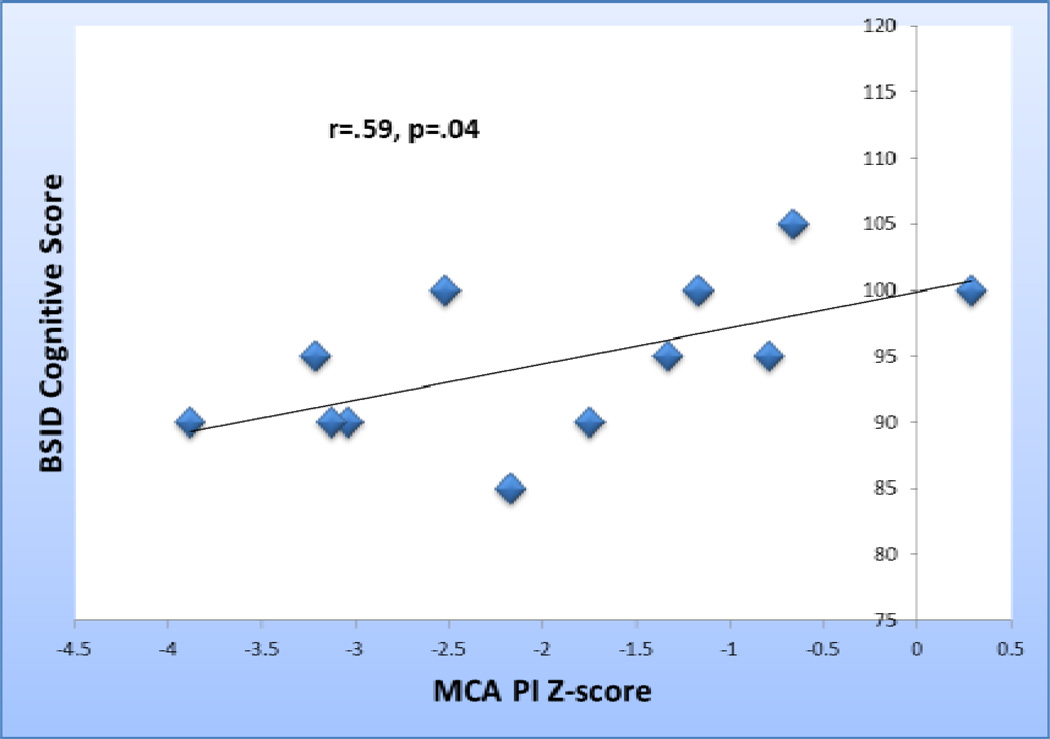
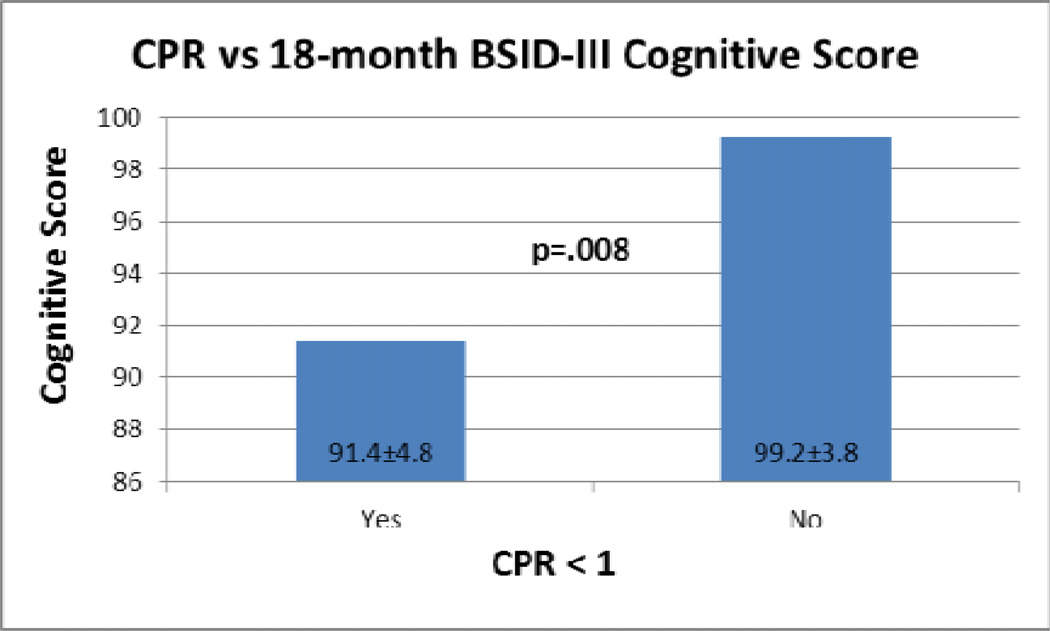
Among the 13 subjects with fetal cerebral Doppler data and 18-month neurodevelopmental assessments, fetal MCA PI z-score at the time of the first fetal echocardiogram correlated with BSID cognitive score (r=.589, p=0.044). [Figure 2] Compared with fetuses with normal CPR, those with CPR<1(N=7) had lower mean BSID cognitive scores (91.4±4.8 vs. 99.2±3.8, p=.008)[Figure 3]. These associations were seen only with the earliest echocardiogram data (mean GA 23.2±2.8). No significant associations were detected between fetal blood flow and Language or Motor scores. While mean Language scores were 10 points lower among the group with a CPR<1 at the time of the first assessment (78.9±19.6 vs 88.2±5.8), this difference was not statistically significant.
Figure 2.
MCA PI z-score at the time of the first fetal echocardiogram versus BSID cognition scores
Figure 3.
CPR vs. 18-month cognitive score
Neonatal EEG and Neurodevelopment
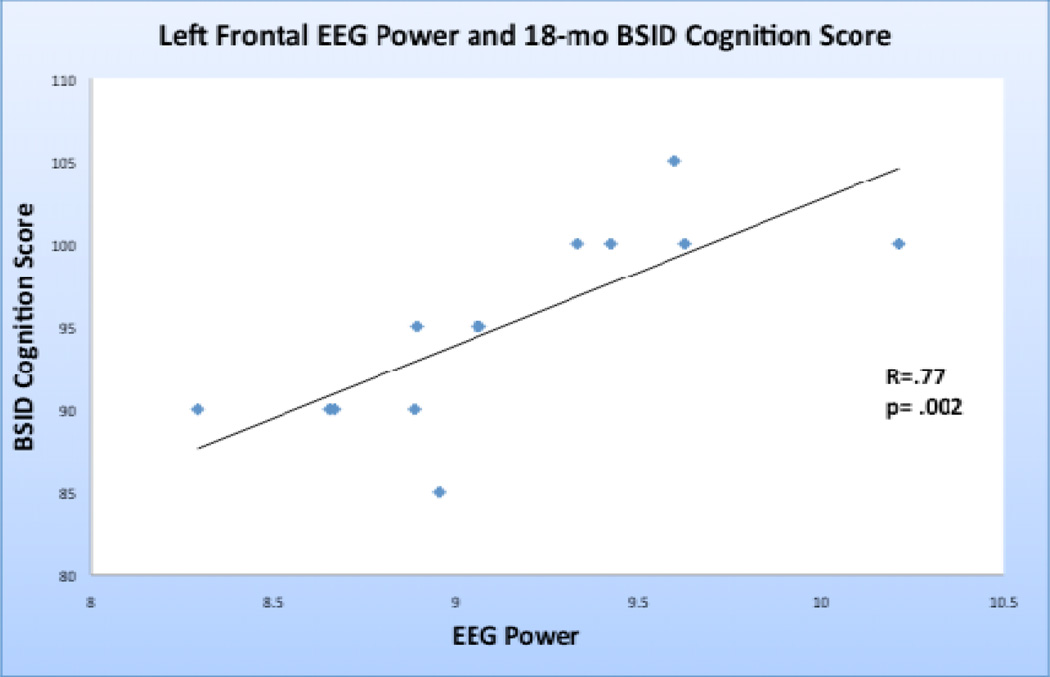
The mean gestational age at birth of the cohort was 38.8 weeks and mean postconceptional age at the time of EEG assessment was 40.4 weeks. Neonatal EEG left frontal polar (r=.583, p=.037) and left frontal (r=.769,p=.002) beta power correlated with BSID cognitive scores. [Figure 4] Beta interhemispheric (left frontal polar to right frontal polar, r=0.5), and intrahemispheric (left frontal polar to left occipital, r=0.45) coherence correlated highly with BSID cognition scores, however, in this small sample these coherence-related correlations were not statistically significant following Bonferroni correction. [Table 2]
Figure 4.
EEG left frontal beta power versus BSID cognition scores
Table 2.
EEG beta coherence and power correlations with BSID-III cognition scores
| EEG Beta Power | Pearson Correlation | P value |
|---|---|---|
| Left Frontal | 0.796 | 0.002 |
| Right Frontal | 0.375 | 0.207 |
| Left Frontal Polar | 0.583 | 0.037 |
| Right Frontal Polar | 0.492 | 0.088 |
| EEG Beta Coherence | Pearson Correlation | P value |
| Left Frontal-Right Frontal | 0.304 | 0.312 |
| Left Frontal Polar-Right Frontal Polar | 0.499 | 0.083 |
| Left Frontal Polar-Left Occipital | 0.462 | 0.112 |
| Right Frontal Polar-Right Occipital | 0.4 | 0.176 |
In secondary analyses, EEG power in the delta, alpha, low gamma, and gamma frequency bands also were correlated with BSID cognitive scores and associations with other brain regions were explored. Both low gamma and gamma showed significant correlations between left frontal power and BSID cognition score, similar to that demonstrated in the beta frequency band. In the delta frequency band, significant correlations between EEG power and BSID cognition score were not limited to the frontal polar region but were also demonstrated in the left parietal and right occipital regions. In addition, we found significant correlations between delta frequency band power and BSID Language scores in the left frontal, left temporal, left parietal, left occipital, right parietal, and right occipital regions. There were no significant correlations seen in the alpha or theta frequency bands. Other than the associations between delta global power and BSID Language scores, all the significant correlations were limited to the Cognitive scores. No correlations were seen between neonatal EEG measures and BSID motor scores.
Fetal Cerebral Doppler Measures and Neonatal EEG
Fetal MCA PI z-score correlated with neonatal EEG left frontal polar (r=.596, p=.04) and left frontal (r=.598, p=.04) beta power. Similarly, compared with fetuses with normal CPR, those with CPR<1(N=7) had lower left frontal polar (t=2.36, p=.038) and leftfrontal (t=2.85, p=.016) beta power.
A multivariable linear regression model including both fetal MCA PI z-score and EEG left frontal beta power was significant (p=0.017) and accounted for 59% of the variance in 18-month Bayley Cognitive Score, however only EEG remained a significant predictor (β=7.1, p=0.044).
DISCUSSION
Infants with congenital heart disease are at high risk for neurobehavioral difficulties later in life. In this pilot study, we have isolated two markers, one fetal and one neonatal, of early childhood neurodevelopmental outcome. Early identification of the high-risk patient would be beneficial for multiple reasons. Knowledge of risk status would enable more informative prenatal and neonatal counseling of families. Medical and surgical care strategies may be tailored according to predicted outcome, including implementation of early intervention strategies in those at highest risk prior to the overt display of developmental delays.
This is the first prospective investigation to link altered fetal cerebral Doppler measures to neurologic functional outcomes in the CHD population. We found that, similar to cases with IUGR, evidence of decreased cerebrovascular resistance is associated with poorer cognitive performance at 18 months of age. While results of our study are correlative, we hypothesize a mechanism for this association: the autoregulatory decrease in cerebrovascular resistance tied to diminished oxygen delivery is not sufficient to meet cerebral metabolic demands and is easily overwhelmed in the setting of CHD. Therefore, discovery of regulatory changes that promote brainsparing, is an ominous finding.
In our study, we found that only those cerebral Doppler measures obtained early in pregnancy, less than 26 weeks GA, were informative. Underlying mechanisms for this association are speculative. Evidence of brain-sparing early in pregnancy may demonstrate a supply-demand mismatch that is detrimental to fetal cerebral development in a manner that deficiency later in gestation is not.
Neonatal EEG can assess cortical development in at-risk populations such as preterm infants,17, 18 and infants exposed to maternal alcohol consumption,19 and can provide a marker of developmental outcomes.20, 21 Grieve et al. reported differences in regional coherence between preterm and term infants that may explain variation in neurodevelopmental outcomes.7 In this study, we demonstrated that neonatal EEG beta power in the left frontal and left frontal polar regions correlated highly with 18-month cognitive developmental score among children with CHD. None of the coherence related measures assessed in this study achieved statistical significance, though this may be due to limited sample size.
Neonatal EEG reveals alterations in brain function associated with structural abnormalities detected on MRI, such as periventricular leukomalacia.22 Studies have shown white matter lesions including periventricular leukomalacia exist pre-operatively in infants with CHD.23 Evidence of relationships between these MRI findings and ultimate neurodevelopmental outcome is lacking however. Compared with MRI, highdensity bedside electroencephalograms are relatively inexpensive, obviate the need for time-consuming individual electrode placement, do not require sedation, and provide a real-time assessment of brain activity and functional connectivity. Whether EEG will become the neonatal tool of choice to evaluate brain function in high risk populations remains to be seen. Longitudinal investigations investigating the associations of both neonatal EEG and MRI on neurodevelopmental outcome may be illuminating in this regard.
In our analysis, while both fetal cerebrovascular resistance and neonatal EEG measures correlated with 18-month cognitive score, in a multivariable equation, only neonatal EEG beta power in the left frontal region remained an independent predictor. One explanation for this finding is that fetal cerebrovascular resistance accounts for the same portion of variance in cognitive score as EEG beta power, i.e., these factors are highly intercorrelated. As our primary objective was to identify markers of neurologic outcome, we can conclude that both neonatal EEG and fetal cerebrovascular resistance are useful predictors even though they are not independent of one another. Though neonatal high-density EEG is readily obtainable in a cost-effective and non-invasive manner, Doppler assessment of the middle cerebral artery is a standard component of the fetal echocardiogram, and can relay prognostic information prenatally, which in itself offers unique benefit for family and medical caregivers.
Limitations
This study must be viewed in light of its limitations. As a single center pilot study, the sample was small, with a predominance of males, and we did not have statistical power to discern differences among anatomic cardiac categories. Due to sample size restrictions and since we were interested in identifying early markers of risk and not causal factors, we did not control for other variables known to influence neurodevelopmental outcome, such as socio-economic status and measures of illness severity after birth. While controls with normal cardiac anatomy were enrolled for fetal measurements, the majority of the control group in this study were patients referred from affiliated hospitals who were not available for follow-up after birth. Therefore postnatal comparisons between groups were limited as was our ability to make definitive conclusions regarding our findings. Nonetheless, we present this preliminary data for the purposes of hypothesis generation and data replication. Finally, as stated before, our data allow us to show association, not causation.
Conclusions
In conclusion, in this prospective, pilot study of fetuses with hypoplastic left heart syndrome, tetralogy of Fallot, and transposition of the great arteries, diminished cerebrovascular resistance was associated with poorer cognitive performance at 18-months of age. Lower cerebrovascular resistance correlated with lower neonatal EEG beta power in the left frontal brain regions, which in turn was predictive of 18-month cognitive score. Both fetal cerebral Doppler measures as well as neonatal EEG may prove to be valuable very early markers of neurologic outcome. Further prospective investigation is on-going.
Supplementary Material
ACKNOWLEDGMENT
Dr. Williams would like to acknowledge Dr. Charles S. Kleinman, whose superb mentorship and preliminary work in this area served as the impetus for this study.
Grant Disclosure:
I.A. Williams received support from Grant No. 1K23HD061601 from the NICHD/NIH as well as from Grant Number KL2 RR024157 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at the NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from the NIH Roadmap website. W.P. Fifer and M.M. Myers received support from Grant Number 5R37HD32774 from the NICHD.
REFERENCES
- 1.Wernovsky G, Shillingford AJ, Gaynor JW. Central nervous system outcomes in children with complex congenital heart disease. Curr Op Cardiol. 2005;20(2):94–99. doi: 10.1097/01.hco.0000153451.68212.68. [DOI] [PubMed] [Google Scholar]
- 2.Mahle WT, Clancy RR, Moss EM, Gerdes M, Jobes DR, Wernovsky G. Neurodevelopmental outcome and lifestyle assessment in school-aged and adolescent children with hypoplastic left heart syndrome. Pediatrics. 2000;105(5):1082–1089. doi: 10.1542/peds.105.5.1082. [DOI] [PubMed] [Google Scholar]
- 3.Donofrio MT, Bremer YA, Schieken RM, Gennings C, Morton LD, Eidem BW, Cetta F, Falkensammer CB, Huhta JC, Kleinman CS. Autoregulation of cerebral blood flow in fetuses with congenital heart disease: The brain sparing effect. Pediatr Cardiol. 2003;24:436–443. doi: 10.1007/s00246-002-0404-0. 2003. [DOI] [PubMed] [Google Scholar]
- 4.Kaltman JR, Di H, Tian Z, Rychik J. Impact of congenital heart disease on cerebrovascular blood flow dynamics in the fetus. Ultrasound Obstet Gynecol. 2005;25(1):32–36. doi: 10.1002/uog.1785. [DOI] [PubMed] [Google Scholar]
- 5.Dubiel M, Gunnarsson GO, Gudmundsson S. Blood redistribution in the fetal brain during chronic hypoxia. Ultrasound Obstet Gynecol. 2002;20(2):117–121. doi: 10.1046/j.1469-0705.2002.00758.x. [DOI] [PubMed] [Google Scholar]
- 6.Arduini D, Rizzo G. Normal values of Pulsatility Index from fetal vessels: a cross-sectional study on 1556 healthy fetuses. J Perinat Med. 1990;18(3):165–172. doi: 10.1515/jpme.1990.18.3.165. [DOI] [PubMed] [Google Scholar]
- 7.Biagioni E, Frisone MF, Laroche S, Kapetanakis BA, Ricci D, Adeyi-Obe M, Lewis H, Kennea N, Cioni G, Cowan F, Rutherford M, Azzopardi D, Mercuri E. Maturation of cerebral electrical activity and development of cortical folding in young very preterm infants. Clin Neurophysiol. 2007 Jan;118(1):53–59. doi: 10.1016/j.clinph.2006.09.018. Epub 2006 Nov 13. [DOI] [PubMed] [Google Scholar]
- 8.Biagioni E, Bartalena L, Biver P, Pieri R, Cioni G. Electroencephalographic dysmaturity in preterm infants: a prognostic tool in the early postnatal period. Neuropediatrics. 1996 Dec;27(6):311–316. doi: 10.1055/s-2007-973800. [DOI] [PubMed] [Google Scholar]
- 9.D’Angiulli A, Grunau P, Maggi S, Herdman A. Electroencephalographic correlates of prenatal exposure to alcohol in infants and children: a review of findings and implications for neurocognitive development. Alcohol. 2006;40(2):127–133. doi: 10.1016/j.alcohol.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Silberstein RB. Dynamic sculpting of brain functional connectivity and mental rotation aptitude. Prog Brain Res. 2006;159:63–76. doi: 10.1016/S0079-6123(06)59005-3. Review. [DOI] [PubMed] [Google Scholar]
- 11.Thatcher RW, North D, Biver C. EEG and intelligence: relations between EEG coherence, EEG phase delay and power. Clin Neurophysiol. 2005 Sep;116(9):2129–2141. doi: 10.1016/j.clinph.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 12.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004 Jun 25;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 13.Grieve PG, Isler JR, Izraelit A, Peterson BS, Fifer WP, Myers MM, Stark RI. Clin Neurophysiol. EEG functional connectivity in term age extremely low birth weight infants. 2008;119(12):2712–2720. doi: 10.1016/j.clinph.2008.09.020. Epub 2008 Nov 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher MS, Steppe DA, Banks DL. Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatr Neurol. 1996 Feb;14(2):137–144. doi: 10.1016/0887-8994(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 15.Bayley N. Bayley scales of infant and toddler development. third edition. San Antonio, TX: Pearson Education, Inc; 2006. [Google Scholar]
- 16.Martín-Loeches M, Muñoz-Ruata J, Martínez-Lebrusant L, Gómez-Jarabo G. Electrophysiology and intelligence: the electrophysiology of intellectual functions in intellectual disability. J Intellect Disabil Res. 2001 Feb;45(Pt 1):63–75. doi: 10.1046/j.1365-2788.2001.00292.x. [DOI] [PubMed] [Google Scholar]
- 17.Biagioni E, Frisone MF, Laroche S, Kapetanakis BA, Ricci D, Adeyi-Obe M, Lewis H, Kennea N, Cioni G, Cowan F, Rutherford M, Azzopardi D, Mercuri E. Maturation of cerebral electrical activity and development of cortical folding in young very preterm infants. Clin Neurophysiol. 2007 Jan;118(1):53–59. doi: 10.1016/j.clinph.2006.09.018. Epub 2006 Nov 13. [DOI] [PubMed] [Google Scholar]
- 18.Biagioni E, Bartalena L, Biver P, Pieri R, Cioni G. Electroencephalographic dysmaturity in preterm infants: a prognostic tool in the early postnatal period. Neuropediatrics. 1996 Dec;27(6):311–316. doi: 10.1055/s-2007-973800. [DOI] [PubMed] [Google Scholar]
- 19.D’Angiulli A, Grunau P, Maggi S, Herdman A. Electroencephalographic correlates of prenatal exposure to alcohol in infants and children: a review of findings and implications for neurocognitive development. Alcohol. 2006;40(2):127–133. doi: 10.1016/j.alcohol.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 20.Richards JE, Parmelee AH, Jr, Beckwith L. Spectral analysis of infant EEG and behavioral outcome at age five. Electroencephalogr Clin Neurophysiol. 1986 Jul;64(1):1–11. doi: 10.1016/0013-4694(86)90037-4. [DOI] [PubMed] [Google Scholar]
- 21.Scher MS, Steppe DA, Banks DL. Prediction of lower developmental performances of healthy neonates by neonatal EEG-sleep measures. Pediatr Neurol. 1996 Feb;14(2):137–144. doi: 10.1016/0887-8994(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 22.Kidokoro H, Okumura A, Watanabe K. Abnormal brushes in preterm infants with periventricular leukomalacia. Neuropediatrics. 2006;37(5):265–268. doi: 10.1055/s-2006-924614. [DOI] [PubMed] [Google Scholar]
- 23.Miller SP, McQuillen PS, Hamrick S, Xu D, Glidden DV, Charlton N, Karl T, Azakie A, Ferriero DM, Barkovich AJ, Vigneron DB. Abnormal brain development in newborns with congenital heart disease. N Engl J Med. 2007 Nov 8;357(19):1928–1938. doi: 10.1056/NEJMoa067393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.