Summary
Interplay between the immune system and renin angiotensin system is emerging as a crucial factor in the development and progression of hypertension. The current study aimed to determine the involvement of immune cells in the hypertension and renal injury produced by a non-angiotensin II-dependent form of hypertension, deoxycorticosterone acetate (DOCA)-salt induced hypertension in rats.
Male Sprague-Dawley rats underwent uninephrectomy and either received a sustained release pellet of DOCA s.c. and 0.9% NaCl (saline) to drink, or received a placebo pellet and water to drink for 21 days. Additional groups of DOCA-saline and placebo-treated rats were treated concurrently with the immune suppressant mycophenolate mofetil (MMF; 30 mg.kg−1.d−1). Rats were placed in metabolic cages for 24 h urine collection prior to and at weekly intervals during the 21-day experimental period.
MMF significantly attenuated development of hypertension in DOCA-saline rats (mean arterial pressure by telemetry of 146±7 versus 180±3 mmHg at day 18; P < 0.001), and of proteinuria (87±27 versus 305±63 mg/d at day 21) and albuminuria (51±15 versus 247±73 mg/d at day 21). Creatinine clearance was better preserved in MMF-treated DOCA-saline rats (0.74±0.07 ml/min) compared to untreated DOCA-saline rats (0.49±0.09 ml/min; P < 0.05), but was still significantly reduced compared to the placebo group (1.15±0.12 ml/min; P < 0.05). MMF treatment significantly attenuated the DOCA-salt induced rise in renal cortical T-lymphocyte and macrophage infiltration (P < 0.05).
These data indicate immune cells play a deleterious role in both the hypertension and renal injury associated with DOCA-salt hypertension.
Keywords: T-lymphocyte, macrophage, mineralocorticoid, hypertension, kidney
Introduction
It has been known for many years that the immune system contributes to hypertension, although the precise mechanisms underlying this are only now beginning to be determined. Svendsen provided one of the earliest reports of immune involvement in the progression of hypertension in 1976.1 This study found that athymic nude mice displayed blunted development of deoxycorticosterone (DOCA)-salt hypertension, but that thymus grafting conferred the ability of these mice to develop hypertension of equal severity to normal haired mice.1 Later studies by Bataillard and colleagues demonstrated that thymectomy2 or macrophage depletion3 of Lyon hypertensive rats attenuated the severity of this genetic model of hypertension, and that transfer of lymphoid cells from Lyon hypertensive rats to an F1 cross of Lyon hypertensive and normotensive rats partially conferred a hypertensive phenotype on these rats.4 More recently, Guzik et al5 elegantly demonstrated the involvement of T-lymphocytes in both angiotensin II-and DOCA-salt induced hypertension in mice, utilizing the lymphocyte deficient Rag-1 null mouse.5 Their finding of a reduced hypertensive response in the Rag-1 null mouse which can be restored by reconstitution of T- but not B-lymphocytes5 echoes data obtained from human HIV-positive patients, who are less likely to have systolic hypertension than HIV-negative patients when not being treated with anti-retroviral therapy, but display an increased prevalence of hypertension following highly active antiretroviral therapy.6 Treatment with the immunosuppressant mycophenolate mofetil (MMF) reduces the severity of hypertension in a diverse array of experimental models,7–14 as well as in patients with psoriasis and rheumatoid arthritis.15 Together, these studies confirm the widespread importance of the immune system in contributing to hypertension.
As detailed above, interference with immune function is beneficial in treating DOCA-salt hypertension.1, 5 Precisely how immune suppression attenuates hypertension in this, or indeed in other models of hypertension, is not yet clear. Much research interest to date has centered around interactions of immune cells or inflammatory cytokines with angiotensin II.5, 13, 16–18 However, the DOCA-salt model is a low renin form of hypertension, and so it is possible that mechanistic differences may exist between this and hypertensive states where angiotensin II is more prominently implicated. One common denominator in the pathogenesis of hypertension is renal dysfunction and injury. The kidney is the primary regulator of fluid and electrolyte balance and thus the long-term control of blood pressure.19 Accordingly, hypertension-induced renal injury is particularly pernicious as it further compromises the ability of the kidney to maintain salt and water balance. The role of immune cells in renal dysfunction and injury in the DOCA-salt model has thus far received little attention but may play an important underlying role in the progression of hypertension in this model. The current study therefore tested the hypothesis that immunosuppression will ameliorate the hypertension and renal dysfunction observed in the DOCA-salt model of hypertension in rats.
Methods
Animal experiments
Experiments were performed on adult male Sprague-Dawley rats (Harlan Laboratories, Indianapolis, USA). All animals were cared for in accordance with the Guide for the Care and Use of Laboratory Animals, with procedures approved in advance by the Medical College of Georgia Institutional Animal Care and Use Committee. Rats were anesthetized with sodium pentobarbital (65 mg/kg i.p.; Abbott Laboratories, North Chicago, USA) and underwent surgery for implantation of telemetry transmitters (PA-C40, Data Sciences International, St Paul, USA) for recording of conscious blood pressure via a catheter inserted into the abdominal aorta according to the manufacturer’s specifications.20 Data were collected for 10 s every 10 min and used to calculate 24 h average mean arterial pressures (MAP). During a 7–10 day recovery period and 4-day baseline recording period, rats were maintained on regular 0.4% NaCl chow and tap water, before undergoing surgery for uninephrectomy and DOCA or placebo pellet implantation. Rats were anesthetized with isoflurane (2% by inhalation; Baxter Healthcare, Deerfield, USA), the right kidney removed via a flank incision and either a 60-day, timed-release pellet of DOCA (200 mg) or a placebo pellet (both from Innovative Research of America, Sarasota, USA) was implanted subcutaneously. Drinking water of DOCA-treated rats was then replaced with 0.9% NaCl solution (DOCA-salt treatment) whereas placebo-treated rats continued to receive tap water to drink. All rats were randomized to also receive either MMF (MMF; 30 mg.kg−1.d−1 p.o.) or vehicle (sugar-free chocolate pudding at 1 ml.kg−1) once daily. Rats were placed in metabolic cages for a 24 h acclimation period followed by a 24 h urine collection period, prior to uninephrectomy surgery (baseline) and at weekly intervals after surgery (collections on day 7, 14 and 21 of treatment). Telemetry data were not collected while rats were in metabolic cages. After 3 weeks of treatment, rats were anesthetized (sodium pentobarbital, 65 mg/kg i.p.), a terminal blood sample collected from the abdominal aorta and the left kidney immersion fixed in 10% neutral buffered formalin for histological analysis (see below).
Histological analyses
After overnight fixation, kidneys were transferred to 70% ethanol for 24 h then paraffin embedded, sectioned at 4 µm onto Superfrost plus slides and processed as described previously.21 Slides were incubated in the presence or absence of primary antibodies to ED-1 for macrophages (CD68; Serotec, Kidlington, UK) or CD3 for T-lymphocytes (Santa Cruz Biotechnology, Santa Cruz, USA) in humidity chambers at 4°C, followed by incubation for 30 min with peroxidase-conjugated goat anti-mouse IgG (Serotec, Kidlington, Oxford, UK) at room temperature. Positive cell staining was detected with diaminobenzamidine (DakoCytomation, Carpinteria, USA) and counterstained with Mayers hematoxylin. The stained sections were viewed with an Olympus BX40 microscope (Olympus America, Melville, NY) on bright-field setting fitted with a digital camera (Olympus DP70; Olympus America). For quantification of CD3 and ED-1 staining, positive cells were counted in 20 randomly selected 200×200 µm fields of kidney cortex per rat by an individual blinded to the treatment groups. Positive cells in the outer and inner medulla were likewise counted, in 20 and 10 randomly selected 200×200 µm fields for each region respectively. Results were expressed as number of positive cells per mm2. A semi-quantitative analysis of Gomori’s Trichrome-stained slides was conducted in a blinded fashion from 4 animals per group and assessed the morphology of the glomeruli and presence of proteinaceous tubular casts. Glomeruli were given a score of 0 (>90% normal), 1 (70% normal), 2 (30% normal) or 3 (<10% normal), and the presence of tubular casts throughout the kidney rated at low, moderate or high.
Assays
Urinary concentrations of sodium (ion-sensitive electrode, EasyLyte Na/K/Cl/Li Analyzer, Medica Corporation, Bedford, USA), protein (Bradford assay, Bio-Rad, Hercules, USA) and albumin (EIA, Cayman Chemical, Ann Arbor, USA) were determined at baseline and weekly during the 21-day experimental period and 24-h excretion rates calculated. Urinary and plasma creatinine concentrations were determined from samples collected at day 21 (picric acid method adapted for microtiter plates22) and creatinine clearance was calculated according to the standard formula.
Statistical Analysis
Data are presented as mean ± SE with P ≤ 0.05 deemed statistically significant. Statistical analyses were performed using Graph Pad Prism 5.0 (San Diego, USA). Data collected at multiple time points were analyzed by analysis of variance with repeated measures, testing for main effects of time and treatment group, with Bonferroni post-hoc test used to examine differences between pairs of groups at individual time points. Data collected at one time point were analyzed by one-factor analysis of variance and Newman-Keuls multiple comparison test.
Results
Data for 24-h average mean arterial pressures at the mid-point of each week is presented in Figure 1. As expected, DOCA-salt treatment induced robust hypertension, with the average mean arterial pressure at day 18 of treatment reaching 180 ± 3 mmHg (Figure 1). Treatment of DOCA-salt rats with MMF significantly attenuated the development of hypertension, with this difference being significant at days 11 and 18 and mean arterial pressure reaching 146 ± 7 mmHg at day 18 (P < 0.05 versus all other groups). Mean arterial pressure remained fairly stable and did not differ significantly during the experiment in the placebo plus vehicle and placebo plus MMF-treated groups (109 ± 4 and 107 ± 2 mmHg at day 18).
Figure 1.
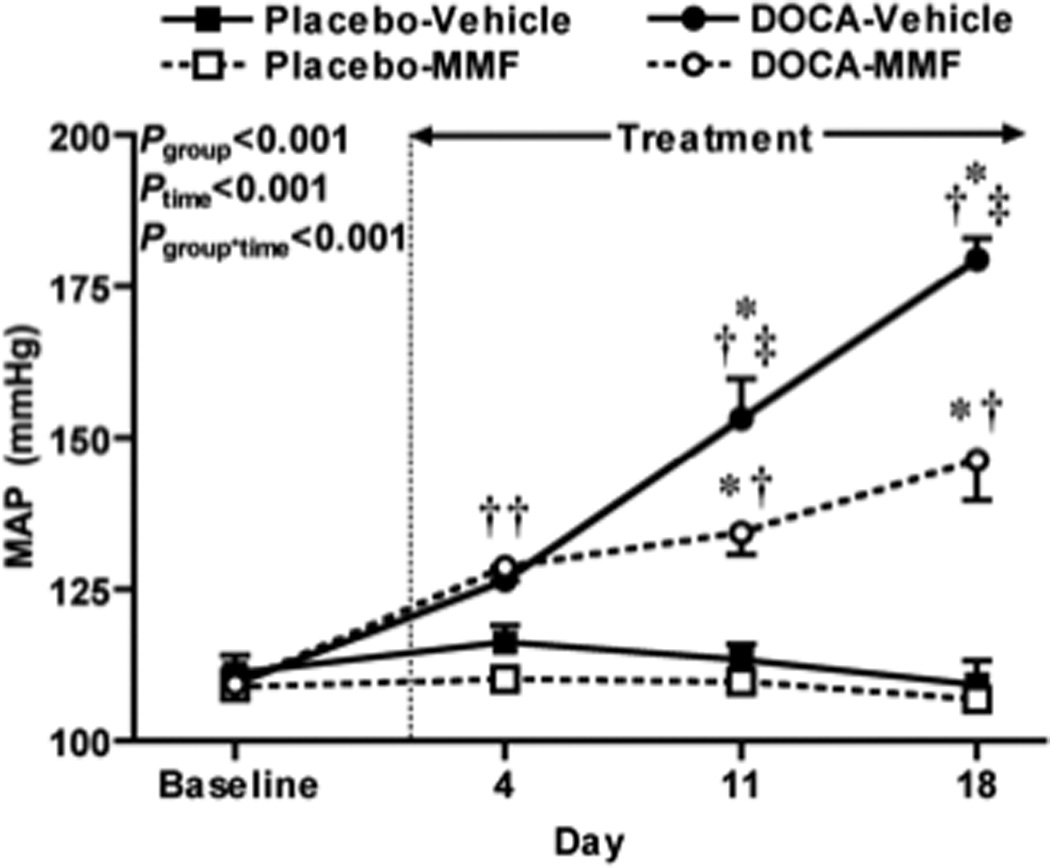
24-h average mean arterial pressures (MAP) at the mid-point of each week before (baseline) and during DOCA-salt or placebo treatment. Data shown are mean ± SE for n = 7 in all groups except for DOCA-salt plus MMF (n = 9). MAP were compared by two-way ANOVA with repeated measures, with P values for main effects of group and time, and the interaction between group and time (Pg*t) as shown. Symbols indicate P values obtained by Bonferroni post-hoc tests as follows: * P < 0.05 versus placebo-vehicle group; † P < 0.05 versus placebo-MMF group; ‡ P < 0.05 for DOCA-salt vehicle versus DOCA-salt MMF groups.
Treatment with DOCA-salt led to marked increases in Na+ intake, water intake, Na+ excretion and urine flow as expected (Figure 2). Although DOCA-MMF treated rats displayed attenuated rises in Na+ and water intake relative to DOCA-vehicle treated rats at days 7 and 14 (P < 0.05), there was no significant effect of treatment with MMF on these variables as assessed at day 21 (Figure 2). There were no significant differences in these variables between placebo-vehicle and placebo-MMF treated rats at any time point studied (Figure 2).
Figure 2.

Salt and water intake and excretion before and during DOCA-salt or placebo treatment. (a) Na+ intake, (b) Water intake, (c) Na+ excretion rate and (d) urine flow are presented as mean ± SE for n = 6 – 9. Statistical analyses are as per Figure 1.
DOCA-salt treatment induced a steady and significant increase in urinary protein (Figure 3a) and albumin excretion (Figure 3b), such that at day 21 of DOCA-salt treatment these were 305 ± 63 and 247 ± 73 mg/day respectively in the DOCA-salt plus vehicle-treated group compared to 43 ± 18 and 39 ± 29 mg/day in the vehicle-treated placebo group (P < 0.05 in both cases). This effect of DOCA-salt treatment on proteinuria was ameliorated by treatment with MMF, with protein excretion being significantly lower in MMF-treated DOCA salt rats at days 14 and 21 compared to DOCA-salt plus vehicle-treated rats (P < 0.05), and not significantly different to either placebo group at any time point studied (Figure 3a). Similarly, post-hoc testing indicated that albuminuria was significantly attenuated by MMF in DOCA-salt rats at day 21 (51 ± 15 mg/day, P < 0.05 versus DOCA-salt plus vehicle), with there being no significant difference in albumin excretion between placebo-vehicle, placebo-MMF or DOCA-salt plus MMF treated rats at day 21 (Figure 3b). Creatinine clearance, measured at day 21 of treatment, was significantly reduced in both DOCA-salt treated groups compared to placebo groups, although this effect was significantly attenuated by treatment with MMF (Table 1). Both DOCA-salt groups displayed increased renal hypertrophy compared to the placebo groups as determined by raw kidney weight and kidney to body weight ratio (P < 0.001), however treatment with MMF did not alter this effect (Table 1).
Figure 3.

Proteinuria and albuminuria before and during DOCA-salt or placebo treatment. Data are mean ± SE for (a) urinary protein excretion rate (n = 7 – 9), and (b) urinary albumin excretion rate (n = 4 – 6). Statistical analyses are as per Figure 1.
Table 1.
Body weight, kidney weight and creatinine clearance at day 21 of DOCA or placebo treatment.
| Placebo-Vehicle | Placebo-MMF | DOCA-vehicle | DOCA-MMF | |
|---|---|---|---|---|
| Creatinine Clearance (ml/min) | 1.15 ± 0.12 | 0.92 ± 0.06 | 0.49 ± 0.09*† | 0.74 ± 0.07*‡ |
| Creatinine Clearance (ml/min/kg) | 3.05 ± 0.32 | 2.68 ± 0.26 | 1.44 ± 0.23*† | 2.21 ± 0.22‡ |
| Body Weight (g) | 379 ± 9 | 352 ± 16 | 330 ± 20 | 336 ± 8 |
| Kidney Weight (g) | 1.73 ± 0.05 | 1.55 ± 0.05 | 2.08 ± 0.13*† | 2.04 ± 0.08* |
| Kidney : Body Weight (g/kg) | 4.6 ± 0.2 | 4.3 ± 0.1 | 6.6 ± 0.5*† | 6.2 ± 0.3*† |
| n | 7 | 7 | 7 | 9 |
Values are mean ± SE for the number of observations (n) given, except for kidney weight and kidney-to-body weight ratio, where n = 6, 4, 6 and 7. Post-hoc analyses were performed when P < 0.05:
P < 0.05 versus placebo-vehicle group;
P < 0.05 versus placebo-MMF group;
P < 0.05 for DOCA-salt vehicle versus DOCA-salt MMF groups.
Representative photomicrographs of renal cortical T-lymphocyte and macrophage staining are shown in Figure 4. Renal cortical, outer and inner medullary T-lymphocyte infiltration was significantly increased in DOCA-salt treated rats compared to placebo-treated rats, and this was markedly attenuated by MMF treatment such that T-lymphocyte numbers in the DOCA-MMF group were not significantly different to either placebo group (Figure 5). Renal cortical macrophage numbers were significantly increased by DOCA-salt treatment, and this effect was significantly attenuated by treatment with MMF (Figure 5b). While a similar trend was observed in outer and inner medullary macrophage numbers, the data were more variable and there were no significant differences found between groups (P = 0.06 by one-way ANOVA for both outer and inner medullary macrophage numbers; Figure 5). All DOCA-salt plus vehicle and 3 out of 4 DOCA-salt plus MMF rats displayed moderate to severe levels of glomerular injury, with only one DOCA-salt plus MMF-treated rat showing >90% normal glomeruli. All DOCA-salt plus vehicle-treated rats displayed high levels of proteinaceous casts, whereas only one DOCA-salt plus MMF-treated rat displaying high levels and 3 out of 4 rats displaying low levels of proteinaceous casts.
Figure 4.
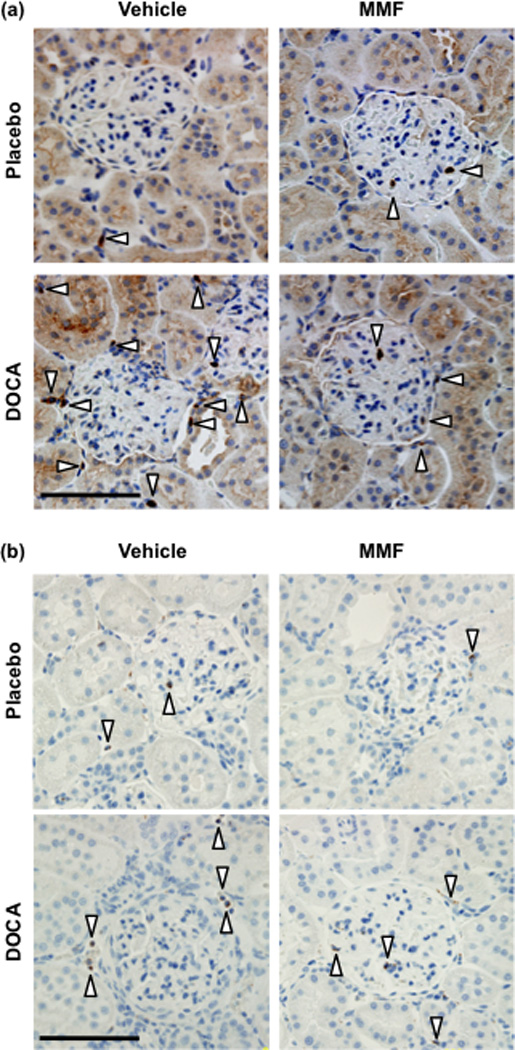
Representative images showing immunohistochemical localization of (a) T-lymphocytes (CD3 positive cells) and (b) monocytes/macrophages (ED-1 positive cells) in the renal cortex. White arrowheads indicate positive cells. Scale bar, 100 µm.
Figure 5.

Immune cell infiltration of the kidney. Cells staining positive for CD3 (T-lymphocytes) or ED-1 (monocytes/macrophages) were quantified per mm2 in histological sections of renal cortex (a and b), outer medulla (c and d) and inner medulla (e and f). Data shown are mean ± SE for n = 7 in all groups except for DOCA-salt plus MMF, where n = 9. Abbreviations: P-V, placebo-vehicle; P-MMF, placebo-MMF; D-V, DOCA salt plus vehicle; D-MMF, DOCA-salt plus MMF. Post-hoc analyses were performed when P < 0.05: * P < 0.05 versus placebo-vehicle group; † P < 0.05 versus placebo-MMF group; ‡ P < 0.05 for DOCA-salt vehicle versus DOCA-salt MMF groups.
Discussion
Immunosuppression, either with MMF or by other means, ameliorates hypertension in many different animal models1–3, 5, 7–14 and also in some patient populations.6, 15 The mechanisms underlying the involvement of the immune system in the development of hypertension are only beginning to be determined, although alterations in renal function would seem a likely possibility given the central role of the kidney in long-term blood pressure control, evidence of immune cell infiltration of the kidney in many hypertensive models, and amelioration of hypertension and renal damage following immunosuppression in this and many other studies.7, 10–12, 14, 23 Whether activation of the immune system constitutes a primary event in the genesis of essential or experimental hypertension, or occurs secondarily to exposure to elements found within the hypertensive milieu is currently unknown and under active investigation.
Although there has recently been a bourgeoning interest in the role of the immune system in angiotensin II-dependent hypertension, the potential for aldosterone to interact with the immune system has received much less attention. The importance of specifically examining mineralocorticoid-dependent contributions to hypertension has been highlighted by findings that mineralocorticoid receptor antagonism with eplerenone has similar or greater antihypertensive efficacy to AT1 receptor blockade in humans,24 and can further reduce blood pressure in patients who do not have blood pressure adequately controlled by renin-angiotensin system blockade.25 Moreover, primary aldosteronism, the syndrome of excessive aldosterone secretion secondary to adrenal adenoma, hyperplasia or a select number of other causes, is a significant cause of hypertension, and is thought to account for as much as 5–10% of cases of hypertension in general, and perhaps 20% of cases of severe or resistant hypertension.26 Accordingly, we chose to investigate the role of the immune system in the DOCA-salt model of hypertension.
There is growing evidence that the immune system may constitute a non-classical mediator of mineralocorticoid action in hypertension. A variety of immune cells, including both T- and B- lymphocytes,27 macrophages28, 29 and dendritic cells30 express mineralocorticoid receptors (MR). However, relatively little appears to be currently known regarding the direct actions of mineralocorticoid receptor activation in these cells in either physiological or pathophysiological conditions. DOCA-salt hypertension is less severe in mice lacking T- and B-lymphocytes (Rag-1 −/− mice) compared to wild-type mice.5 It is not clear whether mineralocorticoids exert direct actions on T-lymphocytes, or if T-lymphocyte involvement in mineralocorticoid-dependent hypertension comes about by a less direct route. Results of a recent study30 suggest that the activation of MR on dendritic cells promotes activation of CD8+ T-lymphocytes and induces a pro-inflammatory Th17 phenotype in CD4+ T-lymphocytes.30 Interestingly, Guzik et al5 reported increased CD4+ and CD8+ cell accumulation in the aortic adventitia and periadventitial fat of angiotensin II-infused mice whereas Herrada and colleagues30 were unable to detect significant MR expression on CD4+ and CD8+ T-lymphocytes in their study. Thus aldosterone may promote T-lymphocyte activation via effects on antigen presenting cells, rather than by direct action on T-lymphocytes themselves. A recent study using targeted deletion of the MR from macrophages revealed that MR signaling in macrophages contributes to both the hypertension and cardiac fibrosis in DOCA-salt hypertension.29 Thus by reducing renal T-lymphocyte and macrophage infiltration in the kidneys of DOCA-salt rats, MMF treatment may have removed cells that are direct targets of DOCA itself, thereby ameliorating part of the deleterious processes that may be independent of activation of MR on renal tubular cells.
Macrophages and T-lymphocytes may exert a number of deleterious effects on the kidney in DOCA-salt hypertension. It has been reported that MR stimulation increases production of inflammatory cytokines,31 reactive oxygen species32 and angiotensin converting enzyme expression and activity32 by monocytes/macrophages. T-lymphocytes are also capable of generating reactive oxygen species, releasing inflammatory cytokines implicated in hypertension such as TNF-α, and possess an endogenous renin-angiotensin system.5, 18 Little is known, however, regarding T-lymphocyte function specifically in the context of mineralocorticoid-induced hypertension. A recent study in another “low renin” model of hypertension, the Dahl Salt-Sensitive rat, suggested that renal T-lymphocytes may contribute to elevated intra-renal levels of AngII, hypertension and renal injury.33 Whether T-lymphocyte mediated increases in intrarenal AngII plays a role in the renal injury induced by DOCA-salt hypertension is not known, but this could help explain the beneficial effects of renin-angiotensin system blockade on renal injury in the DOCA-salt model.34
We chose to treat rats with the immunosuppressant MMF, which is best known for its inhibitory effects on T- and B-lymphocyte proliferation but also has inhibitory and anti-inflammatory effects on other immune cell types including monocytes/macrophages and dendritic cells.35 This approach was taken rather than targeting a specific immune cell type since previous studies suggest that both T-lymphocytes5 and macrophages29, 36 may contribute to the hypertension and end organ damage observed in DOCA-salt hypertension. A recent study by Machnik et al37 suggests that macrophages or related phagocytic cells actually serve a protective function against salt-sensitive hypertension by promoting lymphatic vessel growth, buffering the effect on blood pressure of increases in Na+ retention. Whether inhibition of this mechanism by MMF occurs is currently unknown but would potentially limit the therapeutic potential of MMF in treating salt-sensitive hypertension. As discussed above, findings of a recent study suggest that dendritic cells are also well positioned to contribute to DOCA-salt hypertension via their interactions with T-lymphocytes.30 MMF did not affect water intake of placebo-treated rats, but MMF-treated DOCA-salt rats displayed reduced saline intake compared to vehicle-treated DOCA-salt rats at days 7 and 14, but not on day 21 (Figure 2b). Thus although MMF does not appear to directly affect thirst under conditions of normal salt intake, we are unable to rule out some complex effect on thirst or salt appetite in the early phases of DOCA-salt hypertension.
The interrelationship between the immune system, blood pressure and renal injury is complex. In the current study we observed that MMF treatment reduced albuminuria and proteinuria, and attenuated impairment of creatinine clearance in the DOCA-salt model of hypertension. MMF treatment also appeared to have a beneficial effect in reducing the severity of proteinaceous cast formation. While it could be argued that the renoprotective effects of MMF observed in our study may be due to the concurrent attenuation of hypertension, data from other studies suggest the protective effect of MMF might not be explicable on the basis of blood pressure lowering alone. Crowley et al13 reported that treatment of AngII-infused mice with MMF reduced renal injury, without significantly attenuating the hypertension. In a previous publication from our group, endothelin ETA receptor blockade produced an approximately 40 mmHg attenuation of DOCA-salt hypertension in rats but did not prevent the decline in creatinine clearance.22 As our experiments were not designed to determine if the renoprotective effects of immune suppression are secondary to reductions of arterial pressure, this remains a matter for future investigation.
In summary, our results demonstrate the important contribution of the immune system to the development of hypertension, proteinuria and albuminuria, and decline in renal function (reduced creatinine clearance) induced by DOCA-salt treatment in rats. Together with recent data indicating a role for MR expressed by macrophages in mediating DOCA-salt induced hypertension and cardiac fibrosis,29 and evidence that aldosterone can influence T-lymphocyte activation and phenotype,30 these findings strongly indicate that interactions between mineralocortcoids and the immune system may play a key role in mediating cardiovascular and renal disease.
Acknowledgements
The authors wish to thank Hiram Ocasio, Heather Socha, Janet Hobbs and Carolyn Rhoden for their technical assistance with these studies. This work was supported by The American Heart Association (E.I.B. and D.L.W.), and NIH Grants HL74167 (D.M.P.) and HL60653 (J.S.P).
References
- 1.Svendsen UG. Evidence for an initial, thymus independent and a chronic, thymus dependent phase of DOCA and salt hypertension in mice. Acta Pathol. Microbiol. Scand. A. 1976;84:523–528. doi: 10.1111/j.1699-0463.1976.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 2.Bataillard A, Freiche JC, Vincent M, Sassard J, Touraine JL. Antihypertensive effect of neonatal thymectomy in the genetically hypertensive LH rat. Thymus. 1986;8:321–330. [PubMed] [Google Scholar]
- 3.Bataillard A, Renaudin C, Sassard J. Silica attenuates hypertension in Lyon hypertensive rats. J. Hypertens. 1995;13:1581–1584. [PubMed] [Google Scholar]
- 4.Renaudin C, Bataillard A, Sassard J. Partial transfer of genetic hypertension by lymphoid cells in Lyon rats. J. Hypertens. 1995;13:1589–1592. [PubMed] [Google Scholar]
- 5.Guzik TJ, Hoch NE, Brown KA, et al. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J. Exp. Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seaberg EC, Munoz A, Lu M, et al. Association between highly active antiretroviral therapy and hypertension in a large cohort of men followed from 1984 to 2003. AIDS. 2005;19:953–960. doi: 10.1097/01.aids.0000171410.76607.f8. [DOI] [PubMed] [Google Scholar]
- 7.Rodriguez-Iturbe B, Quiroz Y, Nava M, et al. Reduction of renal immune cell infiltration results in blood pressure control in genetically hypertensive rats. Am. J. Physiol. Renal Physiol. 2002;282:F191–F201. doi: 10.1152/ajprenal.0197.2001. [DOI] [PubMed] [Google Scholar]
- 8.Alvarez V, Quiroz Y, Nava M, Pons H, Rodriguez-Iturbe B. Overload proteinuria is followed by salt-sensitive hypertension caused by renal infiltration of immune cells. Am. J. Physiol. Renal Physiol. 2002;283:F1132–F1141. doi: 10.1152/ajprenal.00199.2002. [DOI] [PubMed] [Google Scholar]
- 9.Vanegas V, Ferrebuz A, Quiroz Y, Rodriguez-Iturbe B. Hypertension in Page (cellophane-wrapped) kidney is due to interstitial nephritis. Kidney Int. 2005;68:1161–1170. doi: 10.1111/j.1523-1755.2005.00508.x. [DOI] [PubMed] [Google Scholar]
- 10.Stewart T, Jung FF, Manning J, Vehaskari VM. Kidney immune cell infiltration and oxidative stress contribute to prenatally programmed hypertension. Kidney Int. 2005;68:2180–2188. doi: 10.1111/j.1523-1755.2005.00674.x. [DOI] [PubMed] [Google Scholar]
- 11.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2006;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 12.Tian N, Gu JW, Jordan S, Rose RA, Hughson MD, Manning RD., Jr Immune suppression prevents renal damage and dysfunction and reduces arterial pressure in salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1018–H1025. doi: 10.1152/ajpheart.00487.2006. [DOI] [PubMed] [Google Scholar]
- 13.Crowley SD, Frey CW, Gould SK, et al. Stimulation of lymphocyte responses by angiotensin II promotes kidney injury in hypertension. Am. J. Physiol. Renal Physiol. 2008;295:F515–F524. doi: 10.1152/ajprenal.00527.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pechman KR, Basile DP, Lund H, Mattson DL. Immune suppression blocks sodium-sensitive hypertension following recovery from ischemic acute renal failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008;294:R1234–R1239. doi: 10.1152/ajpregu.00821.2007. [DOI] [PubMed] [Google Scholar]
- 15.Herrera J, Ferrebuz A, MacGregor EG, Rodriguez-Iturbe B. Mycophenolate mofetil treatment improves hypertension in patients with psoriasis and rheumatoid arthritis. J. Am. Soc. Nephrol. 2006;17:S218–S225. doi: 10.1681/ASN.2006080918. [DOI] [PubMed] [Google Scholar]
- 16.Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor alpha blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–562. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- 17.Lee DL, Sturgis LC, Labazi H, et al. Angiotensin II hypertension is attenuated in interleukin-6 knockout mice. Am. J. Physiol. Heart Circ. Physiol. 2006;290:H935–H940. doi: 10.1152/ajpheart.00708.2005. [DOI] [PubMed] [Google Scholar]
- 18.Hoch NE, Guzik TJ, Chen W, et al. Regulation of T-cell function by endogenously produced angiotensin II. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009;296:R208–R216. doi: 10.1152/ajpregu.90521.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guyton AC, Coleman TG, Cowley AV, Jr, Scheel KW, Manning RD, Jr, Norman RA., Jr Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am. J. Med. 1972;52:584–594. doi: 10.1016/0002-9343(72)90050-2. [DOI] [PubMed] [Google Scholar]
- 20.Pollock DM, Pollock JS. Evidence for endothelin involvement in the response to high salt. Am. J. Physiol. Renal Physiol. 2001;281:F144–F150. doi: 10.1152/ajprenal.2001.281.1.F144. [DOI] [PubMed] [Google Scholar]
- 21.Ishii N, Patel KP, Lane PH, et al. Nitric oxide synthesis and oxidative stress in the renal cortex of rats with diabetes mellitus. J. Am. Soc. Nephrol. 2001;12:1630–1639. doi: 10.1681/ASN.V1281630. [DOI] [PubMed] [Google Scholar]
- 22.Allcock GH, Venema RC, Pollock DM. ETA receptor blockade attenuates the hypertension but not renal dysfunction in DOCA-salt rats. Am. J. Physiol. 1998;275:R245–R252. doi: 10.1152/ajpregu.1998.275.1.R245. [DOI] [PubMed] [Google Scholar]
- 23.Franco M, Martinez F, Quiroz Y, et al. Renal angiotensin II concentration and interstitial infiltration of immune cells are correlated with blood pressure levels in salt-sensitive hypertension. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;293:R251–R256. doi: 10.1152/ajpregu.00645.2006. [DOI] [PubMed] [Google Scholar]
- 24.Flack JM, Oparil S, Pratt JH, et al. Efficacy and tolerability of eplerenone and losartan in hypertensive black and white patients. J. Am. Coll. Cardiol. 2003;41:1148–1155. doi: 10.1016/s0735-1097(03)00054-8. [DOI] [PubMed] [Google Scholar]
- 25.Krum H, Nolly H, Workman D, et al. Efficacy of eplerenone added to renin-angiotensin blockade in hypertensive patients. Hypertension. 2002;40:117–123. doi: 10.1161/01.hyp.0000025146.19104.fe. [DOI] [PubMed] [Google Scholar]
- 26.Gaddam KK, Pimenta E, Husain S, Calhoun DA. Aldosterone and cardiovascular disease. Curr. Probl. Cardiol. 2009;34:51–84. doi: 10.1016/j.cpcardiol.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Armanini D, Endres S, Kuhnle U, Weber PC. Parallel determination of mineralocorticoid and glucocorticoid receptors in T- and B-lymphocytes of human spleen. Acta Endocrinol. (Copenh) 1988;118:479–482. doi: 10.1530/acta.0.1180479. [DOI] [PubMed] [Google Scholar]
- 28.Armanini D, Strasser T, Weber PC. Binding of agonists and antagonists to mineralocorticoid receptors in human peripheral mononuclear leucocytes. J. Hypertens. Suppl. 1985;3:S157–S159. [PubMed] [Google Scholar]
- 29.Rickard AJ, Morgan J, Tesch G, Funder JW, Fuller PJ, Young MJ. Deletion of mineralocorticoid receptors from macrophages protects against deoxycorticosterone/salt-induced cardiac fibrosis and increased blood pressure. Hypertension. 2009;54:537–543. doi: 10.1161/HYPERTENSIONAHA.109.131110. [DOI] [PubMed] [Google Scholar]
- 30.Herrada AA, Contreras FJ, Marini NP, et al. Aldosterone promotes autoimmune damage by enhancing Th17-mediated immunity. J. Immunol. 2010;184:191–202. doi: 10.4049/jimmunol.0802886. [DOI] [PubMed] [Google Scholar]
- 31.Miura R, Nakamura K, Miura D, et al. Aldosterone synthesis and cytokine production in human peripheral blood mononuclear cells. J. Pharmacol. Sci. 2006;102:288–295. doi: 10.1254/jphs.fp0060801. [DOI] [PubMed] [Google Scholar]
- 32.Keidar S, Kaplan M, Pavlotzky E, et al. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: a possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation. 2004;109:2213–2220. doi: 10.1161/01.CIR.0000127949.05756.9D. [DOI] [PubMed] [Google Scholar]
- 33.De Miguel C, Das S, Lund H, Mattson DL. T-lymphocytes mediate hypertension and kidney damage in Dahl salt-sensitive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010;298:R1136–R1142. doi: 10.1152/ajpregu.00298.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wada T, Kanagawa R, Ishimura Y, Inada Y, Nishikawa K. Role of angiotensin II in cerebrovascular and renal damage in deoxycorticosterone acetate-salt hypertensive rats. J. Hypertens. 1995;13:113–122. [PubMed] [Google Scholar]
- 35.Allison AC, Eugui EM. Mechanisms of action of mycophenolate mofetil in preventing acute and chronic allograft rejection. Transplantation. 2005;80:S181–S190. doi: 10.1097/01.tp.0000186390.10150.66. [DOI] [PubMed] [Google Scholar]
- 36.Ko EA, Amiri F, Pandey NR, et al. Resistance artery remodeling in deoxycorticosterone acetate-salt hypertension is dependent on vascular inflammation: evidence from m-CSF-deficient mice. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H1789–H1795. doi: 10.1152/ajpheart.01118.2006. [DOI] [PubMed] [Google Scholar]
- 37.Machnik A, Neuhofer W, Jantsch J, et al. Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat. Med. 2009;15:545–552. doi: 10.1038/nm.1960. [DOI] [PubMed] [Google Scholar]


