INTRODUCTION
The eggs of Xenopus laevis have been widely used in studies investigating a variety of aspects of biology, such as control of the cell cycle, RNA processing, and the cytoskeleton. The Western clawed frog Xenopus tropicalis is likely to prove useful for such studies in the future, because of the potential to combine traditional experimental approaches with genetic analysis and the available genome sequence. The eggs of X. tropicalis are also a key starting material for transgenesis. Here, we describe a method for the routine collection of eggs from X. tropicalis, together with a method for in vitro fertilization. Very large numbers of eggs, often more than 2000, can be obtained from a single X. tropicalis female. In vitro fertilization is a valuable alternative to natural mating for generating embryos. It is particularly useful for microinjection experiments and when collecting embryos at a series of defined developmental stages (e.g., for studies of gene expression), because it produces embryos that develop synchronously during early embryonic development. The typical yield of embryos ranges from several hundred to more than 1000 per fertilization.
RELATED INFORMATION
A protocol for Natural Mating and Tadpole Husbandry in the Western Clawed Frog Xenopus tropicalis (Showell and Conlon 2009a) is also available. Embryos should be staged according to criteria described by Nieuwkoop and Faber (1967). See The Western Clawed Frog (Xenopus tropicalis): An Emerging Vertebrate Model for Developmental Genetics and Environmental Toxicology (Showell and Conlon 2009b) for an introduction to X. tropicalis as a model organism.
MATERIALS
CAUTIONS AND RECIPES: Please see Appendices for appropriate handling of materials marked with <!>, and recipes for reagents marked with <R>.
Reagents
Aquatic system water, chlorine-free (pH 6.5–7.0; conductivity 600–800 µS)
- <!>Cysteine hydrochloride dejellying solution (2%, w/v)Prepare a 2% (w/v) cysteine hydrochloride dejellying solution by dissolving L-cysteine hydrochloride monohydrate (reagent grade, ≥98%) in distilled H2O and adjusting the pH to 8.0 with 10 M NaOH.
- Ethyl 4-aminobenzoate (benzocaine)Store as a 10% (w/v) stock solution in ethanol. Dilute 200X with H2O before use.
- Human chorionic gonadotropin (hCG)Dissolve the contents of the vial (Sigma CG10; 10,000 U) in 10 mL of sterile H2O to produce a 1000 U/mL stock solution. Store at 4°C. (A further dilution is required to produce the 100 U/mL solution used in Step 4.)
- Leibovitz L-15 medium supplemented with 0.3 g/L L-glutamine (Sigma)Add bovine calf serum (defined, iron-supplemented, sterile-filtered; e.g., Hyclone) to the medium to a final concentration of 10% (v/v) before use.
<R>Marc’s modified Ringer’s (MMR) (10X, pH 7.4), diluted to 1X in distilled H2O before use
<R>Modified Barth’s saline (MBS) (1X, pH 7.8), diluted to 0.1X in distilled H2O before use
- <!>Tricaine (ethyl 3-aminobenzoate methanesulfonate salt)Store as a 2.5% (w/v) stock solution in sterile H2O at 4°C.
X. tropicalis frogs (male and female)
Equipment
Dissecting scissors (straight and sharp-pointed)
Dissection microscope (optional; see Step 16)
Forceps (jeweler’s microforceps, straight and smooth)
Housing tanks (plastic; 9 1/8 in. × 6 in. × 6 5/8 in.) (e.g., Kritter Keeper, Lee’s Aquarium and Pet Products)
Kitchen shears
Needles (25 gauge, 5/8 in.)
Pasteur pipettes (glass)
Petri dishes (10 cm)
Incubator (refrigerated; preset to 16°C; see Step 11)
Sheet to cover tank (optional; see Step 10)
Syringe (1 mL)
Transfer pipettes (plastic)
METHOD
In Steps 1–10, do not expose frogs to temperatures below 23°C (73.4°F), because this can lead to death. Ideally, water temperatures should be maintained between 25°C (77.0°F) and 28°C (82.4°F). For successful routine harvesting of eggs, it is usually necessary to prime four or more frogs. In our experience, an average of one in three females produce healthy eggs using the method described, although this is critically dependent upon the general state of health of the frogs used. When embryos are required for microinjection or other purposes, more than one male should be primed with hCG to improve the likelihood of successful in vitro fertilization.
Anesthesia
-
1.
Place male and female X. tropicalis frogs (Fig. 1) into tanks that contain aquatic system water at a depth of ~2 in.
-
2.
Add the tricaine solution to a final concentration of 0.025% (w/v).
-
3.Allow ~25–30 min for the anesthetic to take effect. Do not leave the frogs in the anesthetic for longer than is necessary.It is not necessary or desirable to fully anesthetize the frog during this procedure. Anesthetization is only intended to minimize the risk of distress and injury to the frogs while administering the hormone injection. When properly anesthetized, the frogs should put up minimal resistance to handling.
FIGURE 1.
Adult X. tropicalis. Female frogs (left) are typically larger than males (right) and have noticeably pearshaped bodies and a prominent cloaca. Males often have dark nuptial pads on the inner surfaces of their forelimbs (not shown).
Prepriming
-
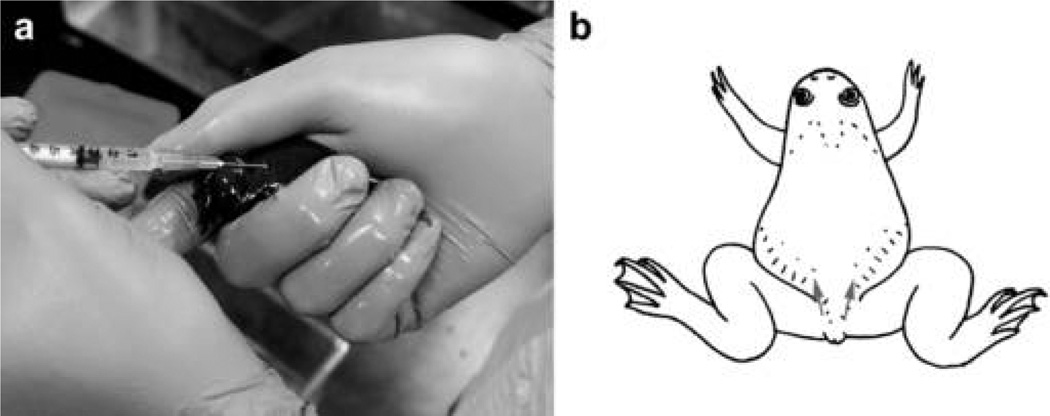
4.
Inject each frog with 0.1 mL of a 100 U/mL solution of hCG. Make the injection into a dorsal lymph sac using a 1-mL syringe and a 25-gauge needle (Fig. 2).
-
5.
Once each injection is complete, allow the frog to recover from anesthesia for 30–60 min. in shallow water (not >2 in. deep).
-
6.
Return active frogs to normal housing conditions for 20–24 h.
FIGURE 2.
Priming frogs. (a) The technique for restraining X. tropicalis for hormone injection. (b) The sites at which frogs may be injected to administer the priming dose of hCG into either dorsal lymph sac are indicated (arrows).
Priming and Egg Collection
-
7.
Anesthetize the frogs as described in Steps 1–3.
-
8.
Inject each frog with 0.2 mL of a 1000 U/mL stock solution of hCG. Make the injection into a dorsal lymph sac (Fig. 2).
-
9.
Allow the frogs to recover as described in Step 5.
-
10.Transfer individual female frogs to tanks containing 1X MMR at a depth of ~2 in., and allow them to lay eggs.Tanks should be situated in a quiet place if possible, and covered with a sheet to prevent any disturbance to the frogs during egg-laying. Females typically begin laying eggs ~2 h after hCG injection and continue for several hours.See Troubleshooting.
Testis Collection and In Vitro Fertilization
-
11.Collect testes as follows:
- Kill a male frog by immersing it in 0.05% (w/v) benzocaine for a minimum of 30 min.
- Decapitate the frog with kitchen shears.
- Rapidly pith the central nervous system, both rostrally and caudally, using forceps.
- Dissect the testes (small, white, bean-shaped organs attached to the fat bodies on either side of the midline) using forceps and dissecting scissors.
- Place the testes in Leibovitz L-15 medium containing 10% calf serum.
- Store the testes at 16°C in a refrigerated incubator until Step 13.Following anesthesia, the male frog should be unresponsive to being turned onto its back and should exhibit no neuromuscular response to other stimuli. For further information on the euthanasia of amphibians, see the American Veterinary Medical Association report “AVMA Guidelines on Euthanasia” (http://www.avma.org/issues/animal_welfare/euthanasia.pdf).
-
12.Collect the eggs from the tanks using a plastic transfer pipette that has been precoated with Leibovitz L-15 medium containing 10% calf serum, and transfer them to a Petri dish. Remove as much of the buffer as possible, allowing the eggs to form a monolayer in the dish.As many as 1000 eggs can be fertilized per dish. Larger numbers can lead to a reduction in the efficiency of fertilization.
-
13.
Transfer both testes to the Petri dish, place them alongside the eggs, and macerate them using forceps to release the spermatozoa.
-
14.
Holding the macerated testis tissue with forceps, pass it over the eggs systematically. Ensure that all eggs come into direct contact with the testis tissue.
-
15.Allow the eggs to sit for exactly 4 min before flooding the dish with 0.1X MBS.A brief contraction of the dark pigment at the animal pole of the zygote, an outward sign of fertilization, should be visible within 15 min of adding the 0.1X MBS. X. tropicalis embryos often do not undergo the rotation under gravity that is seen when fertilization occurs in X. laevis, so pigment contraction is often the earliest reliable indication of successful fertilization.See Troubleshooting.
-
16.After fertilization, remove the jelly coat surrounding the embryos by discarding the buffer and replacing it with 25 mL of 2% (w/v) cysteine hydrochloride dejellying solution. Gently agitate the embryos to ensure even exposure to the dejellying solution.Dejellying should take 3–5 min. The clear separation between abutting embryos, which results from the presence of the outer jelly coat, will disappear when the dejellying process is complete. Embryos in dejellying solution must be monitored continuously by eye or by dissection microscope to avoid lethal overexposure.See Troubleshooting.
-
17.Thoroughly rinse the embryos five times with 0.1X MBS to remove all dejellying solution.See Troubleshooting.
-
18.
Remove any unfertilized or abnormal embryos using a glass Pasteur pipette.
-
19.Culture the embryos in 0.1X MBS at 25°C–28°C (77.0–82.4°F), changing 80%–100% of the buffer daily.See Troubleshooting.The embryos may be harvested for analysis at the desired developmental stage. They should be staged according to criteria described by Nieuwkoop and Faber (1967). For rearing the embryos through the tadpole, metamorph, and froglet stages, see Steps 18–20 of the protocol presented in Natural Mating and Tadpole Husbandry in the Western Clawed Frog Xenopus tropicalis (Showell and Conlon 2009a).
TROUBLESHOOTING
-
Problem: The females do not lay eggs.
[Step 10]
Solution: Make sure that the hormone solution does not leak from the injection site at Steps 4 and 8.
-
Problem: The eggs are not fertilized.
[Step 15]
Solution: Try to select males that have dark nuptial pads on their inner forelimbs, because this is a sign of sexual maturity.
-
Problem: Embryos die during/immediately after the dejellying treatment.
[Steps 16 and 17]
Solution: Consider the following:- Check the concentration and pH of the 2% (w/v) cysteine hydrochloride solution.
- Minimize the treatment time in Step 16.
- Increase the number of rinses to ensure removal of all dejellying solution in Step 17.
-
Problem: The embryos die during culturing.
[Step 19]
Solution: Reduce the number of embryos cultured per dish.
REFERENCES
- Nieuwkoop PD, Faber J, editors. Normal table of Xenopus laevis (Daudin): A systematical and chronological survey of the development from the fertilized egg till the end of metamorphosis. 2nd ed. North-Holland, Amsterdam: 1967. [Google Scholar]
- Showell C, Conlon FL. Natural mating and tadpole husbandry in the Western clawed frog Xenopus tropicalis. Cold Spring Harb Protoc. 2009a doi: 10.1101/pdb.prot5292. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell C, Conlon FL. The Western clawed frog (Xenopus tropicalis): An emerging vertebrate model for developmental genetics and environmental toxicology. Cold Spring Harb Protoc. 2009b doi: 10.1101/pdb.emo131. (this issue). [DOI] [PMC free article] [PubMed] [Google Scholar]