Abstract
Multiple extracellular signals have been identified that regulate actin dynamics within motile cells, but how these instructive cues present on the cell surface exert their precise effects on the internal actin cytoskeleton is still poorly understood. One particularly interesting class of these cues is a group of extracellular proteins that negatively alter the movement of cells and their processes. Over the years, these types of events have been described using a variety of terms and herein we provide an overview of inhibitory/repulsive cellular phenomena and highlight the largest known protein family of repulsive extracellular cues, the Semaphorins. Specifically, the Semaphorins (Semas) utilize Plexin cell-surface receptors to dramatically collapse the actin cytoskeleton and we summarize what is known of the direct molecular and biochemical mechanisms of Sema-triggered actin filament (F-actin) disassembly. We also discuss new observations from our lab that reveal that the multi-domain oxidoreductase (Redox) enzyme MICAL, an important mediator of Sema/Plexin repulsion, is a novel F-actin disassembly factor. Our results indicate that MICAL triggers Sema/Plexin-mediated reorganization of the F-actin cytoskeleton and suggest a role for specific Redox signaling events in regulating actin dynamics.
Keywords: Actin Depolymerization, Signal Transduction to the Actin Cytoskeleton, Repulsive Signaling, Motility, Navigation, Axon Guidance and Cell Morphology
Actin proteins assemble together into long filaments and it is this property that underlies cellular behaviors including polarity, morphology, adhesion, motility, process elongation, navigation, and connectivity. These behaviors are precisely controlled by signals that originate from outside of cells and impinge through signal transduction pathways on the proteins that directly regulate actin dynamics. Many of these extracellular signals have now been identified including small molecules such as amino acids, metals, lipids, and cyclic nucleotides; peptides and pheromones; plant chemotropic cues; cell adhesion molecules such as CAMs and cadherins; extracellular matrix molecules such as laminin and fibronectin; growth factors such as brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF); morphogens such as hedgehog, wingless-ints (Wnts), and bone morphogenetic proteins (BMPs); cytokines including chemokines and tumor necrosis factors; and axon guidance cues including ephrins, netrins, semaphorins, and slits. Interestingly, these extracellular regulators of actin dynamics can be generally grouped into two classes based on either their positive or negative effects on cell motility. Positive signals attract cells or provide permissive/adhesive signals to induce cell movement, whereas negative signals prevent cells from moving or repel them from inappropriate areas. In this review, we will examine these negative or inhibitory effects on cell movement and highlight recent results that identify a new link between one of the largest families of these repulsive cues, the semaphorins, and the negative regulation of actin dynamics.
Contact Inhibition and Repulsive Guidance – Historical Perspectives
In the late 1800’s, Theodor Engelmann, Wilhelm Pfeffer and others working with bacteria, flagellata, leukocytes, and plant spermatozoa noticed that chemicals exhibit differences in their effects on motile processes (Reviewed in (Eisenbach 2004; Loeb 1906; Porter et al. 2011; Rosen 1962)). Those chemicals triggering movement towards themselves were termed attractants, while chemicals inducing movements in the opposite direction were called repellents. These positive and negative responses were also regarded as chemotaxis, if the chemical stimulated the oriented movement of an organism or cell, and chemotropism, if only parts of the organism or cell reoriented in response to the stimulus. Influenced by these seminal observations, the Spanish Nobel laureate Santiago Ramon y Cajal proposed his chemotactic (neurotropic) hypothesis to explain how a nerve cell develops, displaces its cell body and axonal and dendritic processes, and forms its connections (Cajal 1893; Cajal 1899). At the time, observations on nerve fiber growth after injury and transplantation also brought forth the idea that inhibitory barriers (“negative neurotropism”) might prevent axon regeneration (Figure 1a; (Cajal 1893; Cajal 1919; Lugaro 1906; Windle 1955)). It was these extensive observations on poorly regenerating axons that “suggested that there might be some form of short-range cell interaction which limited cell movement (Abercrombie 1970)” and influenced the seminal observations of Michael Abercrombie, Paul Weiss, and others working during that time (Abercrombie 1970; Harris 1974; Heaysman 1978; Loeb 1921; Weiss 1958; Wolf 1921). Specifically, Abercrombie and colleagues noted that when fibroblast cells meet each other in culture, their locomotion stops immediately in the region of contact, which is followed by a localized contraction of the cell membrane, a subsequent change in direction, and migration away from the area of contact (Figure 1b; (Abercrombie and Heaysman 1953)). Abercrombie named this cellular behavior “contact inhibition of locomotion (CIL)” and related behaviors have also been observed using a number of cell types (reviewed in (Abercrombie 1980; Harris 1974; Keynes and Cook 1995; Mayor and Carmona-Fontaine 2010; Schwab et al. 1993)).
Figure 1. Negative Regulation of Cell Shape, Motility, and Navigation.
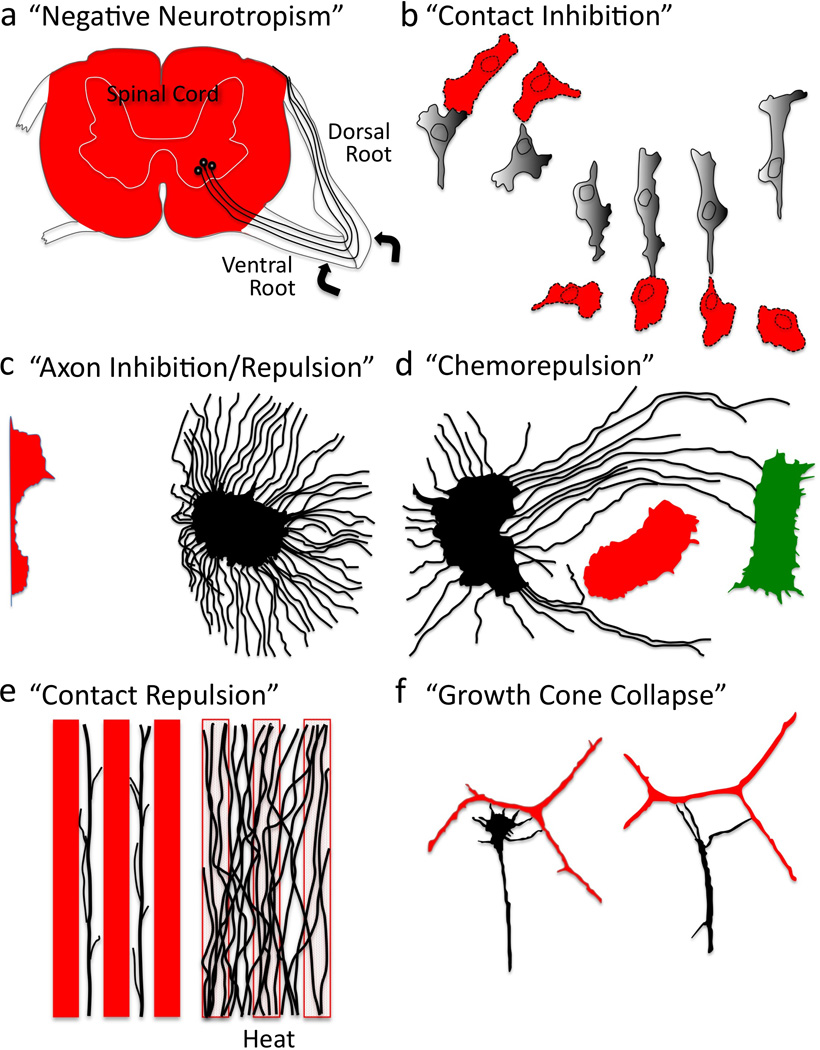
Various experimental examples of negative cellular behaviors and the terms used to describe them (See also Box 1). (a) Regenerating axons (black) grow extensively on some substrates (ventral and dorsal roots) and not others (spinal cord, red). (Lugaro 1906). (b) Cultured cells retract upon contact and change direction. (Weiss 1958). (c) Neuronal fiber extension (black) is inhibited/repelled upon co-culturing in proximity to a tissue explant (red). (Ebendal 1982). (d) Axons (black) circuitously grow away (red) or towards (green) different tissues explants. (Peterson and Crain 1982). (e) Axons (black) avoid substrates (red), a phenomenon that is abolished upon high temperature treatment (heat); revealing that axons grow on certain substrates not because of “attraction”, but because of avoidance of other substrates. (Walter et al. 1987b). (f) A neuronal growth cone (black) collapses/retracts upon contact with an unlike neuronal fiber (red). (Kapfhammer and Raper 1987a).
Over the years, these negative chemotaxic and chemotropic events have been perhaps best characterized using neurons and watching the growth of their axons. Several groups first noted that growing nerve fibers spontaneously retract both in vivo and in vitro (Burrows 1911; Speidel 1933), and further characterization revealed that these retractive events often occur when the growing tips of nerve fibers (growth cones) encounter obstructions including other cells and axons (Hughes 1953; Nakai 1964). Further analysis of these behaviors suggested that nerve fibers selectively associate according to their subtype (“selective fasciculation” (Weiss 1947)) and that when axons make contact with one another they either grow along the other fiber or they retract (Nakajima 1965). Therefore, the reaction of a nerve fiber to contact appeared to be selective – either positive or negative – and in light of the similarity of these retractive events to CIL, these negative reactions were termed “contact inhibition of extension” (Dunn 1971; Ebendal 1976). Dennis Bray, Dick Bunge and their colleagues extended these observations when they noticed that individual fibers from the same region of the nervous system tend to adhere or extend along one another in culture, while fibers cultured from different regions often retract upon contact and avoid one another (Bray et al. 1980). These results suggested that nerve fibers might use inhibitory or repulsive activities to prevent unwanted associations; immediately suggesting both a means to guide developing axons and a mechanism to explain substrate-dependent differences affecting regenerating axons (Aguayo et al. 1979; Berry 1982; David and Aguayo 1981; Reier et al. 1983; Schwab and Thoenen 1985).
Today, it is clear that these observations on axonal selectivity and inhibitory axonal responses complement those made by others documenting that axonal behaviors vary significantly depending on cell, tissue, or substrate encounters (e.g., (Bonhoeffer and Huf 1980; Keynes and Stern 1984; Lawrence 1975; Letourneau 1975; Nuttall and Zinsmeister 1983; Raper et al. 1983; Smalheiser et al. 1981; Sperry 1963; Tosney and Landmesser 1985; Wessells et al. 1980; Yoon 1979; Zinsmeister and Nuttall 1986)). Yet, it was mechanistically critical to confirm that these retractive events did not result from a lack of growth cone adhesion – as might occur when axons encounter a substrate that is simply non-permissive for elongation (Cajal 1928; Carter 1965; Keynes and Cook 1990; Letourneau 1987; Martz and Steinberg 1973). Importantly, follow-up experiments further demonstrated that axons actively avoid certain cells/tissues, often taking circuitous detours around them (Figure 1c–d; e.g., (Ebendal 1982; Peterson and Crain 1982; Pini 1993; Verna 1985)), and these avoidance responses are dependent on cell/tissue viability (Figure 1e; (Ebendal 1982; Verna 1985; Walter et al. 1987a)). As we discuss below, inhibitory and repulsive influences are now known to regulate the motility and navigation of multiple types of axons and cells (see also Box 1). Furthermore, inhibitors and repellents are not only present on cell membranes, where they induce a contact-mediated effect (i.e., contact repulsion), but they are also soluble (i.e., chemorepellents) and diffuse away from the cells that secrete them (Tessier-Lavigne and Goodman 1996).
Box 1: Contact Inhibition versus Repulsive Guidance: the same or different?
Over the years, negative influences on cell shape and movement have been termed “negative neurotropism”, “contact inhibition of locomotion”, “negative chemotaxis”, “contact inhibition of extension”, “growth cone collapse”, “chemorepulsion”, and “repulsive guidance”. For the purposes of this review, we have grouped together all of these negative celllular behaviors with the intention of differentiating adverse effects that disrupt cystoskeletal and adhesive elements from the simple inability to grow on or adhere to a particular substrate (which would not require the activation of a negative signaling cascade). Also, terms such as “repulsion” and “negative neurotropism” imply a negative response that imparts directionality to cells or their processes (i.e., pushing them away), while “inhibition” is often used to describe a negative response where only motility is prevented (i.e., without imparting any directionality) (see discussion in (Tessier-Lavigne and Goodman 1996)). However, these terms have not always been used in this manner, and the molecular commonalities and differences between these related responses remain to be fully understood. For example, contact inhibition of locomotion (CIL) and repulsive guidance may describe identical phenomena. CIL has been extensively characterized in non-neuronal cells and describes when a cell’s leading edge adheres to another cell upon contact, experiences a paralysis of motility, and then retracts. Repulsive guidance describes a similar set of behaviors. For instance, Sema-mediated growth cone collapse is reminiscent of CIL (Heaysman 1978; Kapfhammer and Raper 1987a; Luo and Raper 1994). While historically there has been resistance to grouping these phenomena together, it is likely that they are related and use similar types of molecules and mechanisms. Specifically, it has long been hypothesized that CIL occurs when a signal is passed between colliding cells and that this signal triggers each cell’s avoidance response (Abercrombie 1970). Today, although very little is known of the molecular mechanisms of CIL, cues such as ephrins and Wnts that have been identified as mediating CIL (Astin et al. 2010; Carmona-Fontaine et al. 2008; Mayor and Carmona-Fontaine 2010) are also known to repel axons and induce growth cone collapse. Likewise, factors such as Semas, which repel axons, also produce CIL-like effects on non-neuronal cells.
Extracellular Repulsive and Inhibitory Cues
To identify the mechanisms underlying these inhibitory/repulsive effects, Jonathan Raper and his colleagues returned to the assays of Bray and Bunge and in a series of groundbreaking studies better characterized negative axon behaviors (Fan et al. 1993; Fan and Raper 1995; Kapfhammer et al. 1986; Kapfhammer and Raper 1987a; Kapfhammer and Raper 1987b; Luo et al. 1993; Raper and Grunewald 1990; Raper and Kapfhammer 1990). Specifically, Raper and colleagues used time-lapse video microscopy to carefully document that neurons cultured on a uniform substrate extend growth cones at a relatively steady rate with only rare spontaneous retractions. Likewise, they observed that growth cones typically cross over other fibers without delay or retraction. Yet, they found that when different types of neurons contact one another, their growth cones rapidly decrease in size and withdraw from contact (Figure 1f). Raper and colleagues called this phenomenon “growth cone collapse” – and they also noticed that within an hour the collapsed growth cone regains its typical morphology and motility. However, they also noticed that if growth cones continue to advance into unlike fibers this collapse/retraction/recovery/extension cycle repeats itself multiple times. The researchers postulated that these events revealed the existence of specific chemical labels that negatively alter axon elongation even in environments that are permissive for outgrowth.
To determine the molecular identity of this “chemical label”, Raper and colleagues devised a simple and ingenuous bioassay in which they grew axons on a permissive substrate and then screened membrane fractions from different cell lines and tissue sources for growth cone collapse (Raper and Kapfhammer 1990). Strikingly, they identified both membrane-associated and soluble fractions that induce growth cone collapse, and their results suggested that this “collapsing activity” was induced by a protein, since it was both heat and trypsin sensitive. Using successive rounds of biochemical separation, collapsing activity tests, and enrichment, the researchers went about trying to purify this mysterious “collapsing factor” (Luo et al. 1993; Raper and Kapfhammer 1990). Their peptide sequencing results identified a specific extracellular protein that they called Collapsin that exerts negative effects on cell motility and morphology (Luo et al. 1993). Surprisingly, Collapsin was highly similar to a protein that had been identified by Alex Kolodkin, Corey Goodman and their colleagues in grasshoppers for its effects on axon guidance and named Fasciclin IV (Kolodkin et al. 1992). Today, we know that both Fasciclin IV (Sema-1a) and Collapsin (Sema3A) are the founding members of one of the largest known families of repulsive guidance cues, the Semaphorins (Figure 2a; (Kolodkin et al. 1993; Luo et al. 1995; Yazdani and Terman 2006)). Over the years, a number of additional extracellular proteins such as ephrins, netrins, slits, repulsive guidance molecules (RGMs), Wnts, BMPs, Nogo, myelin-associated glycoprotein (MAG), oligodendrocyte-myelin glycoprotein (OMgp), and tenascin have been found that exert these same types of negative effects on cell shape and motility (Figure 2b; Reviewed in (Dickson 2002; Giger et al. 2010; Huber et al. 2003; Keynes and Cook 1995; Schwab et al. 1993; Tessier-Lavigne and Goodman 1996)). Likewise, selective neurotransmitters, amino acids, peptides, proteases, lipids, and posttranslational modifications including chondroitin sulfate proteoglycans (CSPGs) have also been described as inhibitors/repellents (Reviewed in (Fields and Nelson 1994; Giger et al. 2010; Schwab et al. 1993)). As we highlight below for the Semaphorins (Semas), much has been learned about the mechanisms of action of these inhibitory/repulsive signals (Bashaw and Klein 2010; Giger et al. 2010; Huber et al. 2003), yet it still remains poorly understood how they negatively alter the actin cytoskeletal machinery necessary for cell morphology and motility.
Figure 2. Semas and Repulsive/Inhibitory Extracellular Cues.
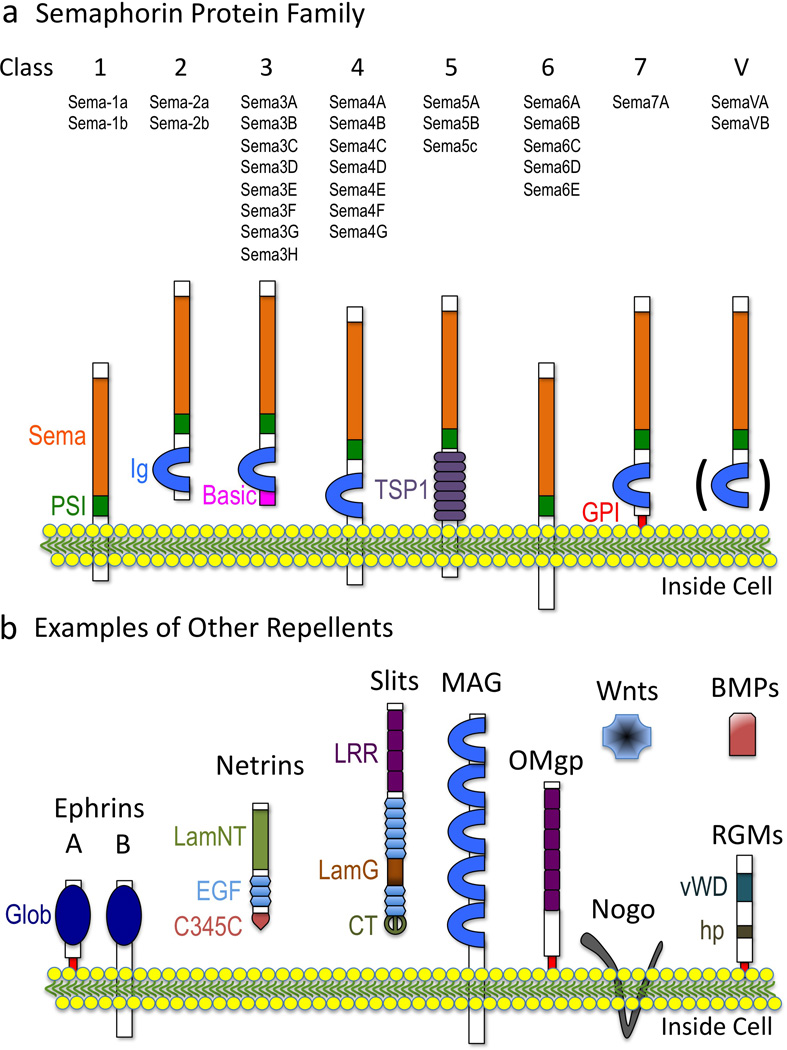
(a) Semas. (b) Other well-known repulsive/inhibitory cues. Domain names are from SMART (http://smart.embl-heidelberg.de) except Glob, globular; hp, hydrophobic. The ( ) in (a) refers to the observation that Ig domains are present in some Class V (viral) Semas.
Semaphorins Negatively Regulate Cell Motility and Cytoskeletal Organization
Since the discovery of Sema-1a and Sema3A, over twenty Semas have been reported in organisms as diverse as nematode worms, insects, crustaceans, vertebrates, and viruses. Semas exist as secreted, transmembrane, and glycosylphosphatidylinositol (GPI)-linked proteins and for these reasons and the presence of other conserved protein domains, they are grouped into eight classes (Figure 2a; (Tran et al. 2007; Yazdani and Terman 2006)). Semas serve not only diverse functions in the nervous system but also specify the immune, cardiovascular, and musculoskeletal systems (Melani and Weinstein 2010; Suzuki et al. 2008; Tran et al. 2007; Yazdani and Terman 2006). Likewise, Semas have been linked to a number of pathologies including different cancers, musculoskeletal disorders, neurodegeneration, and heart disease (Mann et al. 2007; Neufeld and Kessler 2008; Yazdani and Terman 2006). Yet, much of our understanding of the cellular effects of Semas has come through characterizing Sema-mediated growth cone collapse. Norman Wessells, Kenneth Yamada, and their colleagues first identified that the periphery of growth cones, including their dynamic filopodia, are highly enriched in F-actin (Yamada et al. 1970; Yamada et al. 1971). Their observations also revealed that disrupting the actin network in axons using the actin depolymerization drug Cytochalasin B causes growth cones to “round-up” (i.e., collapse) (Figure 3a; (Wessells et al. 1971; Yamada et al. 1970)). In hindsight, these observations with actin depolymerization drugs suggested that there might exist specific “repulsive” signaling cascades that locally disassemble F-actin and negatively regulate motility. However, such an effector was unknown until Raper, Phillip Gordon-Weeks, and their colleagues observed that contact with the extracellular cue Sema3A dramatically decreases F-actin in the growth cone periphery and its filopodia (Figure 3b; (Fan et al. 1993)). Multiple Semas have now been found to induce growth cone collapse in vivo and in vitro and negatively regulate actin dynamics (e.g., reviewed in (Yazdani and Terman 2006)). Indeed, the picture that has emerged is that Semas collapse growth cones by locally destabilizing F-actin (Brown and Bridgman 2009; Fan and Raper 1995) – events that are characterized by a loss of peripheral F-actin, a rapid decrease in F-actin bundles, and a reduced ability to polymerize new F-actin (Brown and Bridgman 2009; Dent et al. 2004; Fan et al. 1993; Fournier et al. 2000; Fritsche et al. 1999; Gallo 2006; Mikule et al. 2002; Roche et al. 2009).
Figure 3. F-actin Disassembly Underlies Sema-mediated Repulsion.
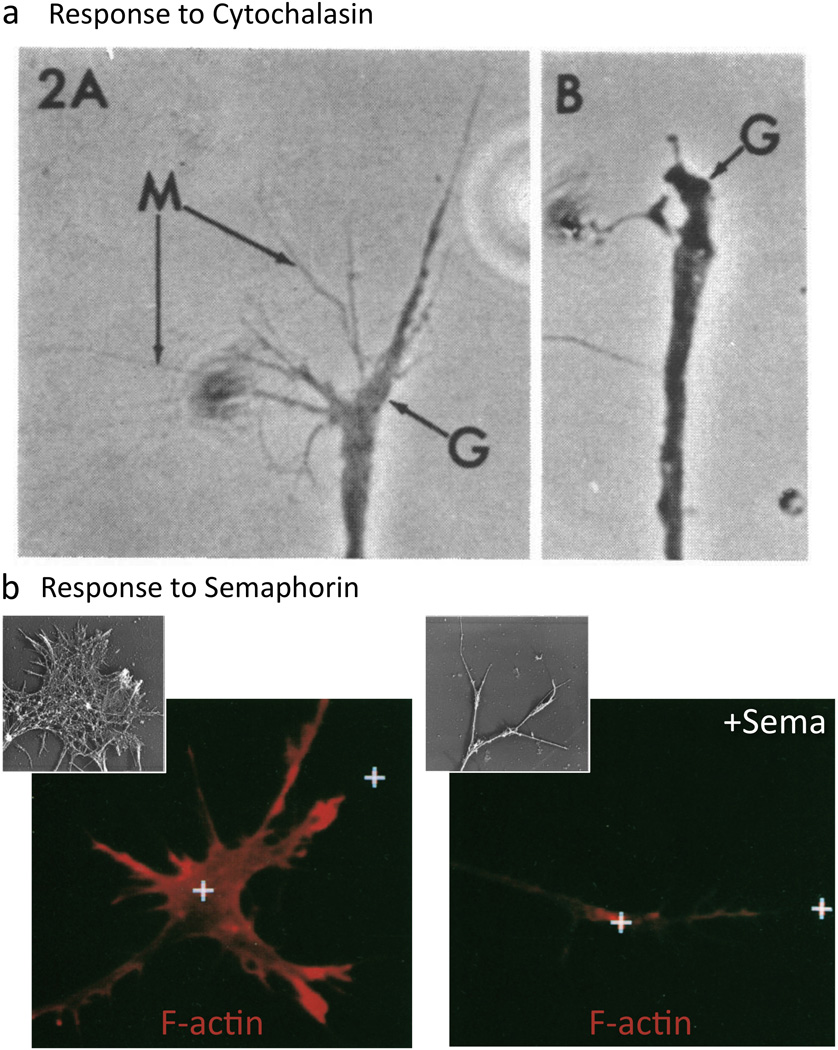
(a) F-actin disassembly collapses growth cones (Yamada et al. 1970). A growth cone (G) exhibits extensive filopodia (M) until cytochalasin treatment (B; 6 minute treatment) disrupts actin polymerization, disassembles F-actin, and induces collapse. (b) Sema treatment results in loss of F-actin. Rhodamine phalloidin staining reveals F-actin present in control (left) and Sema-treated growth cones (right; 5 min treatment). (Fan et al. 1993). (Insets) Rotary shadow EM shows the growth cone F-actin before (left) and after Sema application (right; 30 min). Reprinted with permission of John Wiley & Sons, Inc. (Brown and Bridgman 2009).
Importantly, these cellular effects of Semas are not just a peculiarity of neuronal growth cones, but Semas also exert these same types of negative effects on multiple different cell types including glia, endothelial cells, platelets, different types of immune cells, mechanosensory cells, and a number of cancer cell lines (Barberis et al. 2004; Bielenberg et al. 2008; Eickholt et al. 1999; Guttmann-Raviv et al. 2007; Hung et al. 2010; Kashiwagi et al. 2005; Lepelletier et al. 2006; Li and Lee 2010; Miao et al. 1999; Shimizu et al. 2008; Takahashi et al. 1999; Torres-Vazquez et al. 2004; Tran-Van et al. 2011; Turner and Hall 2006; Varshavsky et al. 2008; Walzer et al. 2005; Xu et al. 2000; Yukawa et al. 2005). In many ways, the cellular response to Semas is similar to that seen after cytochalasin treatment, yet differences have been noted including that growth cones continue to advance after treatment with cytochalasin, but not Sema3A (e.g., (Fan et al. 1993; Yamada et al. 1970)). It is thought that these differences result because Semas not only impair actin dynamics, but also negatively regulate microtubule stability (Dent et al. 2004; Fan et al. 1993; Fritsche et al. 1999) and cell-substrate interactions (Barberis et al. 2004; Gatlin et al. 2006; Kashiwagi et al. 2005; Mikule et al. 2002; Serini et al. 2003; Walzer et al. 2005; Woo and Gomez 2006). Interestingly, these effects on microtubules and adhesion are separable from Sema-mediated F-actin disassembly (discussed in (Gallo and Letourneau 2004)) and indicate that Semas are likely to independently regulate F-actin, microtubules, and adhesion.
Searching for the Molecular and Biochemical Basis of Sema-mediated F-actin Disassembly
Since the initial observations of Raper and colleagues over 25 years ago, the molecular mechanisms of Sema-mediated repulsion have been a question of considerable interest. Major insights into these mechanisms have come with the discovery that large, cell-surface proteins in the Plexin family are Sema receptors (Figure 4a; (Comeau et al. 1998; Takahashi et al. 1999; Tamagnone et al. 1999; Winberg et al. 1998)). Interestingly, the cytoplasmic portion of Plexins directly bind Rho and Ras family GTPases, providing a direct link between Semas/Plexins and small GTP-binding proteins (reviewed in (Negishi et al. 2005; Puschel 2007)). Small GTPases, although not themselves direct actin regulatory proteins, are key regulators of cytoskeletal dynamics and cell adhesion (Hall 1998; Hall and Lalli 2010). Moreover, Plexin receptors contain a GTPase activating protein (GAP) domain and Plexins are GAPs for specific Ras superfamily GTPases (Figure 4a; reviewed in (Gay et al. 2011)). Extensive work now indicates that Plexins utilize their GAP activity to inactivate Ras family GTPases and thereby turn-off RasGTP-integrin-dependent cell-substrate adhesion (Figure 4b; reviewed in (Gay et al. 2011)). Progress has also been made in characterizing the means by which Sema/Plexins alter microtubule organization. The current model is that Semas differentially regulate the activities of two microtubule regulatory proteins, tau and Collapsin Response Mediator Protein (CRMP), and thereby alter microtubule dynamics (Figure 4c; (reviewed in (Hou et al. 2008; Schmidt and Strittmatter 2007)). In contrast, much less is known of how Semas/Plexins directly connect and disassemble F-actin. Complicating these investigations are results indicating that Plexins have class-specific differences in their intracellular binding partners (reviewed in (Yazdani and Terman 2006; Zhou et al. 2008), and so they may differ in how they induce F-actin disassembly. Likewise, an understanding of Sema/Plexin F-actin disassembly has been confounded by oberservations that Semas also induce positive effects on motility, guidance, and adhesion (reviewed in (Roth et al. 2009)) and at least some Semas can initiate “reverse signaling” through their own cytoplasmic regions or by coupling with transmembrane proteins on the same cell (reviewed in (Yazdani and Terman 2006; Zhou et al. 2008)). So, although numerous proteins including kinases, phosphatases, and regulators of G protein signaling play important roles in Sema-mediated repulsion (for discussion and review, see (Bechara et al. 2007; Franco and Tamagnone 2008; Potiron et al. 2007; Yazdani and Terman 2006; Zhou et al. 2008)), only a few of these proteins are known to directly associate with actin.
Figure 4. Semas Employ Plexins to Direct Repulsion.
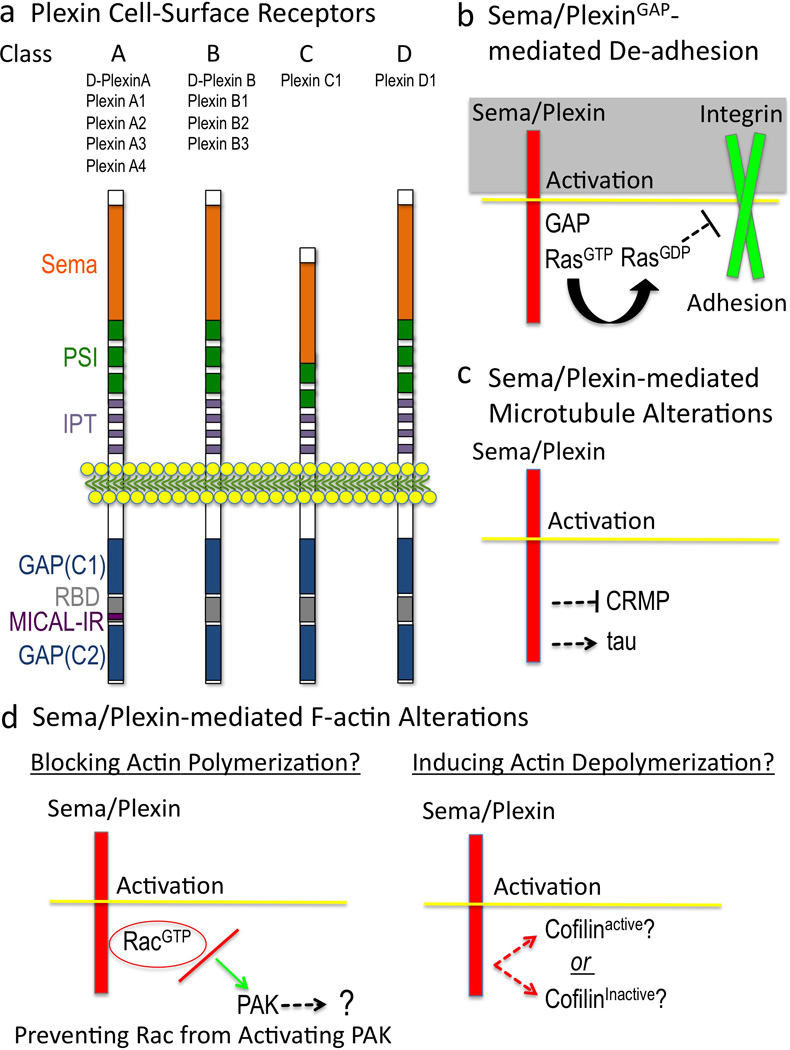
(a) Plexins. Domain names are from SMART (http://smart.embl-heidelberg.de) except GAP, GTPase activating protein; C1, conserved 1; C2, conserved 2; RBD, Rho GTPase binding domain; MICAL-IR, MICAL interacting region. (b) Sema/Plex-mediated effects on cell adhesion. The current model is that 1) the Plexin GAP is activated by binding of both Sema and a Rac GTPase to the Plexin extracellular and intracellular regions, respectively, 2) the Plexin GAP activity locally enriches for the GDP-bound form of Ras family GTPases, which 3) inactivates (through “inside-out” signaling) integrin-extracellular matrix-mediated adhesion. (c) Sema effects on microtubules. The current model is that Semas “turn-on” tau-mediated microtubule alterations and “turn-off” CRMP-mediated tubulin assembly. (d) Current models suggest that Sema-mediated F-actin alterations occur by limiting actin polymerization (left) and/or by inducing actin depolymerization by regulating the levels of active cofilin (right). A few other actin-associated proteins including myosin II and ERM are involved in Sema-mediated repulsion (Gallo 2008; Mintz et al. 2008; Schlatter et al. 2008), but their effects appear secondary to directly inducing F-actin disassembly (Brown et al. 2009; Gallo 2006; Takamatsu et al. 2010).
One hypothesis proposes that Sema “turns-off” actin polymerization. Specifically, Plexins associate with GTP-bound Rac, and B class Plexins prevent RacGTP from associating with its downstream effector, p21-activated kinase (PAK) (Figure 4d; (Driessens et al. 2001; Hu et al. 2001; Vikis et al. 2002)). Since PAK localizes to regions of cytoskeletal assembly and plays important roles in cell motility (Bokoch 2003), Sema-mediated repulsion/loss of motility may be accomplished by preventing Rac from activating PAK (Figure 4d). Yet, PAK has multiple targets (Bokoch 2003; Hofmann et al. 2004) and it is unknown which pathway and direct actin regulatory proteins it is important to keep PAK away from in response to Semas. Moreover, in contrast to B class Plexins, Plexin A-induced repulsion does not appear to “sequester active Rac from PAK” since Plexin A requires active Rac for “collapse” (e.g., (Dalpe et al. 2004; Jin and Strittmatter 1997; Kuhn et al. 1999; Toyofuku et al. 2005; Turner et al. 2004; Vastrik et al. 1999)). Furthermore, Plexin A - RacGTP interactions regulate Plexin GAP-mediated negative effects on adhesion (Toyofuku et al. 2005), further complicating this “sequestering” model to explain how F-actin disassembles upon Sema treatment. Indeed, Plexin A - RacGTP signaling activates an endocytic pathway that accompanies Sema-mediated growth cone collapse but is not a part of the initial F-actin disassembly process (Fournier et al. 2000; Jurney et al. 2002).
Another hypothesis first raised by Raper and colleagues (Fan et al. 1993) is that “‥‥actin polymerizes normally in collapsing growth cones but is depolymerized at an accelerated rate‥‥…[which] could occur if the collapsing activity induced a filament severing and capping activity or a modification of actin that facilitates depolymerization”. Along these lines, a key player implicated in Sema-mediated F-actin disassembly are the actin depolymerizing factor (ADF)/Cofilins, a family of phylogenetically conserved proteins that depolymerize F-actin (Bamburg 1999; Oser and Condeelis 2009). However, while F-actin depolymerization is a hallmark of Sema-mediated negative motility, depolymerization of F-actin is also required for the turnover and recycling of actin that is needed for locomotion (Oser and Condeelis 2009; Pollard and Borisy 2003). Therefore, F-actin depolymerization induced by cofilin often favors motility, since it generates more free actin and barbed ends for polymerization (Oser and Condeelis 2009). These positive effects on actin assembly complicate any models employing cofilin and not surprisingly, both cofilin activation (Fritsche et al. 1999; Hu et al. 2001; Kashiwagi et al. 2005; Nukazuka et al. 2008; Shimizu et al. 2008; Vikis et al. 2002) and inactivation (Aizawa et al. 2001; Scott et al. 2009; Walzer et al. 2005) have been suggested to underlie Sema-mediated repulsion (Figure 4d). Likewise, cofilin only poorly depolymerizes bundled F-actin (e.g., (Schmoller et al. 2011)), which makes up the leading edge of cells and the filopodia of growth cones, has different effects on actin as its concentration changes (e.g., (Andrianantoandro and Pollard 2006)), and neither cofilin activation nor inactivation is always associated with or sufficient for Sema/Plexin-mediated F-actin disassembly. Thus, further analysis of the levels, spatiotemporal activation, and regulation of cofilin (e.g., (Andrianantoandro and Pollard 2006; Bernstein and Bamburg 2010)) is necessary to fully understand its role in Sema/Plexin-mediated repulsion.
MICALs are Plexin–interacting Proteins that Negatively Regulate Cell Shape and Motility
To identify new molecules underlying Sema-mediated repulsion, we and others have conducted unbiased genetic, molecular, and biochemical screens in model organisms. These screens have identified new Sema signaling components including a family of unusual proteins, the MICALs (Terman et al. 2002). The first MICAL (Human MICAL-1) was identified by Hisamaru Hirai and colleagues and named for its interaction with the SH3 domain-containing protein, CasL (Molecule Interacting with CasL; (Suzuki et al. 2002)). Cas proteins regulate integrin-mediated cell adhesion and cytoskeletal organization (Cabodi et al. 2010) and although the functional significance of this MICAL-CasL interaction remains to be determined, these biochemical observations suggested that MICAL-1 might alter cell morphology and motility. In parallel experiments, Terman, Kolodkin, and colleagues identified Drosophila Mical in a screen for Plexin A-interacting proteins and based on similarity, found two additional mammalian MICAL family members (MICAL-2 and MICAL-3) (Figure 5a and Box 2; (Terman et al. 2002)). Subsequent work revealed that vertebrate MICALs also associate with A class Plexins; MICAL-1 immunoprecipitates with both Plexin A1 and Plexin A3, while MICAL-2 interacts with Plexin A4 (Schmidt et al. 2008; Terman et al. 2002). Interestingly, MICALs may not directly associate with B, C, or D Class Plexins (Terman et al. 2002), but the significance of these class-specific binding preferences are unclear since at least some different classes of Plexins join together into complexes (Ayoob et al. 2006; Turner et al. 2004; Usui et al. 2003), substitute for one another (Ayoob et al. 2006), and work with MICALs in vivo ((Ayoob et al. 2006); see below).
Figure 5. MICALs are Multi-domain Cytosolic Redox Enzymes.
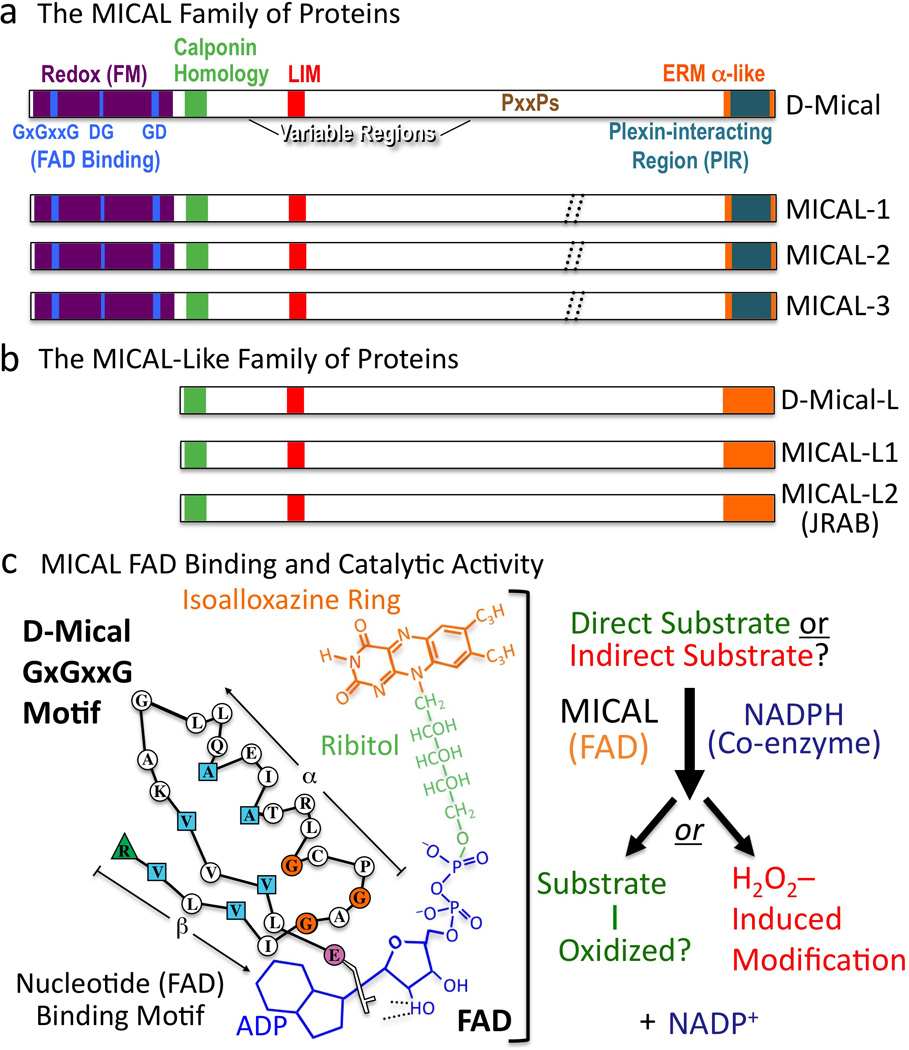
(a) MICAL family proteins are characterized by their flavoprotein monooxygenase (Redox [FM]), calponin homology, LIM, and ERM alpha-like domains. Variable regions differ in length and continuity among family members (dotted //; Box 3). To conform with Drosophila nomenclature guidelines, lowercase lettering is now used when describing invertebrate MICALs (Mical), and all capitals are used when describing the vertebrate MICALs (and the MICAL family of proteins). (b) MICAL-Like proteins are similar to MICALs in domain organization except they lack the Redox domain. (c) FAD binding and MICAL enzymatic activity. (Left) The MICAL cofactor FAD is composed of ADP (including a pyrophosphate group), ribitol, and an isoalloxazine ring. To bind to the ADP region of FAD, Mical relies on its GxGxxG region where the critical residues are colored (Modified from (Wierenga et al. 1986)). To bind to the pyrophosphate and ribitol moieties of FAD, Mical relies on its DG and GD regions, respectively. (Right) Results indicate that the Redox domain of MICAL binds FAD, consumes NADPH, and generates H2O2. MICAL substrate/s are unknown. Alternatively, MICAL may have no direct substrate and may simply generate H2O2.
Box 2 MICAL and MICAL-Like Proteins.
There is a protein family that is similar to MICALs but their members lack the Redox domain (Terman et al. 2002). These proteins have been called MICAL-Likes (MICAL-L) with one Drosophila Mical-L gene and two mammalian MICAL-Ls (Figure 5b; (Terman et al. 2002)). It is unknown if they interact with Plexin or function in Sema/Plexin repulsive signaling, but it is unlikely that MICAL-L proteins function in a similar manner to MICAL since they lack the critical Redox domain that is required for MICAL to associate and induce F-actin disassembly. However, MICAL-L proteins have been extensively linked with regulating the actin cytoskeleton. For instance, the MICAL-Ls are targeted to the membrane and colocalize with F-actin in stress fibers, actin cables, lamellipodia, and at cell-cell contact sites (Kanda et al. 2008; Sharma et al. 2009; Terai et al. 2006). Likewise, MICAL-Ls regulate neurite outgrowth and guidance (Sakane et al. 2010) and they are involved in repulsive events including inhibiting axon extension and regulating epithelial cell scattering (Kanda et al. 2008; Nishimura and Sasaki 2008; Sakane et al. 2010; Terai et al. 2006). In addition, both MICAL-L1 and MICAL-L2 (also known as JRAB) control the formation of cell-junctions including both tight and adherens junctions (Nakatsuji et al. 2008; Yamamura et al. 2008). Similar to the MICALs (Hung et al. 2010; Schmidt et al. 2008), the MICAL-Ls also exist in an autoinhibited state (Sakane et al. 2010). Furthermore, MICAL-Ls have been found to interact with several proteins including Rab8, Rab13, CRMP2, C-terminal Eps15 homology domain (EHD) proteins, and the F-actin bundling protein actinin-4 to regulate the recycling of membrane associated proteins including cell adhesion molecules, cadherins, and integrins (Nakatsuji et al. 2008; Rahajeng et al. 2010; Sakane et al. 2010; Sharma et al. 2009; Terai et al. 2006; Yamamura et al. 2008).
MICALs are broadly expressed in many tissues and this expression varies considerably with age. For example, MICALs are highly expressed throughout the embryonic, postnatal and adult nervous systems in a variety of neuronal cell types and glia (Ashida et al. 2006; Bron et al. 2007; Fischer et al. 2005; Kirilly et al. 2009; Morinaka et al. 2011; Pasterkamp et al. 2006; Terman et al. 2002; Weide et al. 2003; Xue et al. 2010). MICALs are also highly expressed in non-neuronal tissues including within skeletal muscle (Ashida et al. 2006; Beuchle et al. 2007; Fischer et al. 2005; Terman et al. 2002; Xue et al. 2010), heart (Ashida et al. 2006; Fischer et al. 2005; Xue et al. 2010), fibroblasts (Hochman et al. 2006), lung (Ashida et al. 2006; Fischer et al. 2005; Suzuki et al. 2002), kidney (Ashida et al. 2006; Fischer et al. 2005; Suzuki et al. 2002), bone marrow (Ashida et al. 2006), thymus (Ashida et al. 2006; Suzuki et al. 2002), spleen (Suzuki et al. 2002), liver (Ashida et al. 2006; Fischer et al. 2005), testis (Ashida et al. 2006; Miura and Imaki 2008b; Suzuki et al. 2002)), and hematopoietic and fibroblast cell lines (Hochman et al. 2006; Suzuki et al. 2002). MICAL is also upregulated in several different cancer cell lines (Ashida et al. 2006) and in glia and meningeal fibroblasts after neural trauma (Pasterkamp et al. 2006). Decreased MICAL expression is also associated with neurological diseases including epilepsy (Luo et al. 2011). Subcellularly, the MICALs are associated with the cytoskeletal fraction and reside within axons, dendrites, and growth cones, along pre- and post-synaptic terminals of neuronal synapses, at the plasma membrane of muscle attachment sites, and within the growing tips of actin-rich mechanosensory bristle processes in close association with F-actin (Beuchle et al. 2007; Fischer et al. 2005; Hung et al. 2010; Kirilly et al. 2009; Morinaka et al. 2011; Suzuki et al. 2002; Terman et al. 2002; Weide et al. 2003).
The physiological functions of the MICALs have been best characterized in Drosophila where both loss-of-function (“knockout) and gain-of-function (overexpression) studies have revealed that Sema-1a and its receptor Plexin A (PlexA) repel axons (Winberg et al. 1998; Yu et al. 1998). Likewise, in vivo results indicate that Mical functions with Sema-1a/PlexA to mediate axon-axon repulsion (Figure 6a; (Ayoob et al. 2004; Beuchle et al. 2007; Hung et al. 2010; Terman et al. 2002)) and Mical also has roles in Sema2/PlexB-mediated repulsion (Ayoob et al. 2006). Genetic screens have also revealed that the synaptic terminals of neurons do not spread and increase in size in Mical mutants (Figure 6b; (Beuchle et al. 2007)), and that Mical is required to prune developing neuronal dendrites (Figure 6c; (Kirilly et al. 2009)). Drosophila Mical is also necessary for muscle organization, larval movements, and flight (Figure 6d; (Beuchle et al. 2007; Langer et al. 2010)). Complementing these observations with Drosophila Mical, vertebrate MICALs also have been found to have neuronal as well as non-neuronal functions. MICAL-1 decreases cell size, axon outgrowth, and cell death in culture and mediates Semas effects on axon length and growth cone collapse (Morinaka et al. 2011; Schmidt et al. 2008; Zhou et al. 2011). Knockdown approaches have also indicated that MICAL-3 is required for Sema-mediated growth cone collapse in vitro, and together with Semas/Plexins, controls motor neuron migration in vivo (Bron et al. 2007; Morinaka et al. 2011). MICAL-2, in contrast, has not been examined in neuronal contexts, but is highly expressed in prostate cancer cells where its knockdown reduces cancer cell viability (Ashida et al. 2006).
Figure 6. MICAL is Necessary and Sufficient to Regulate F-actin Organization In Vivo.
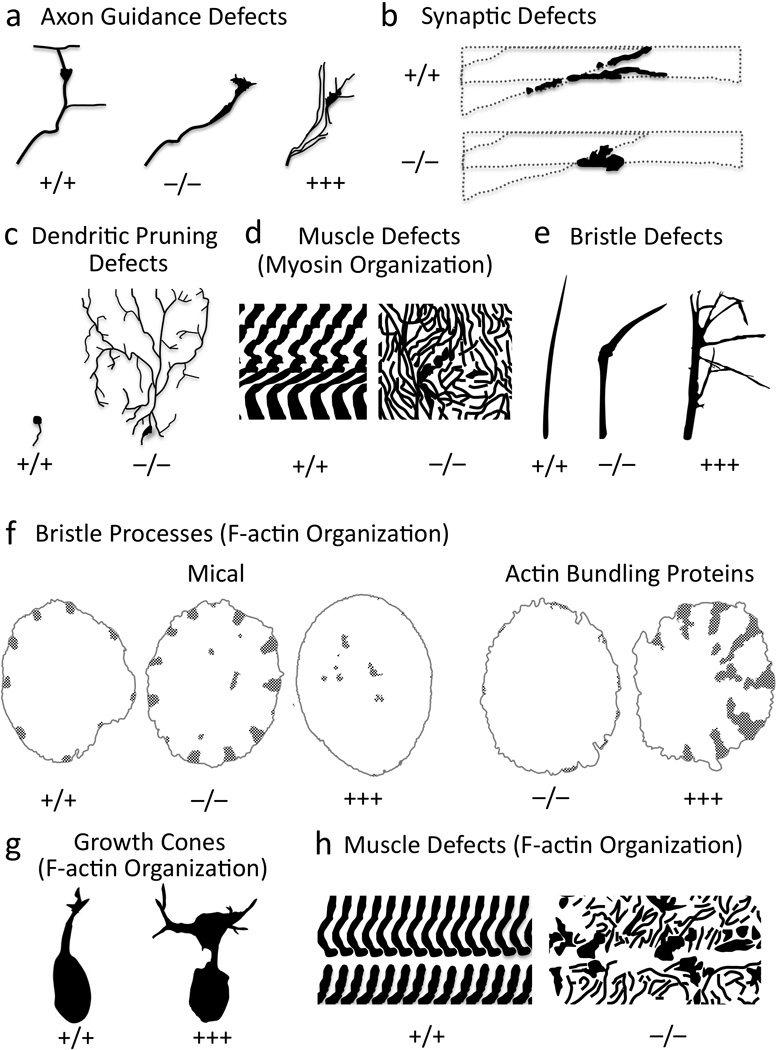
(a–e) Mical is required for axon guidance (a), neuronal synapse formation (b), dendrite pruning (c), muscle formation (d), and bristle shape (e). (f-h) Altering Mical levels in vivo generate abnormal F-actin organization in bristles (f), growth cones (g), and muscles (h). In bristles, F-actin organization (stippling) in Mical “knockouts” is similar to overexpression of actin bundling proteins, while Mical overexpression mimics loss of bundling proteins. +/+, wild-type; −/−, “knockout”; +++, overexpression. Modified from (Beuchle et al. 2007; Hung et al. 2010; Kirilly et al. 2009; Tilney et al. 1998; Tilney et al. 1995; Yoon and Terman submitted).
MICALs are Multi-domain Oxidoreductase (Redox) Enzymes
MICALs are large proteins (some of which are >300kDa) with a phylogenetically conserved domain organization that undergoes alternative splicing (Figure 5a; Box 3). At their C-termini, MICALs contain their plexin-interacting region, which is a coiled-coil motif that shows similarity to the alpha-helical region present in Ezrin, Radixin, and Moesin (ERM) proteins (Figure 5a; (Terman et al. 2002)). Interestingly, this plexin-interacting region autoinhibits MICAL function, and it has been proposed that Sema-induced Plexin-MICAL interactions relieve this autoinhibition and activate MICAL (Hung et al. 2010; Schmidt et al. 2008). Adjacent to the ERM alpha-like portion is a region enriched in proline residues, including multiple PxxP motifs, which are ligands for SH3 domain–containing proteins including CasL (Figure 5a; (Suzuki et al. 2002; Terman et al. 2002)). This C-terminal portion of MICAL also interacts with vimentin (Suzuki et al. 2002), Rab small GTPases including Rabs 1, 8, 10, 13, 15, 35, and 36 (Fischer et al. 2005; Fukuda et al. 2008; Grigoriev et al. 2011; Weide et al. 2003), and the Rab6-interacting coiled-coil protein ELKS (Grigoriev et al. 2011). At least some of these MICAL-Rab interactions regulate vesicle fusion at the plasma membrane (Grigoriev et al. 2011). Preceding this proline-rich region is a LIM domain (Figure 5a), which is a zinc binding region that mediates protein interactions in a variety of proteins including cytoskeletal-associated proteins (Kadrmas and Beckerle 2004). The LIM domain of MICAL-1 is important for interactions with CRMP (Schmidt et al. 2008), and these interactions regulate both MICAL-mediated repulsion (Schmidt et al. 2008) and CRMP-mediated effects on microtubules (Morinaka et al. 2011). This region, together with the C-terminal region, of MICAL-1 also associates with NDR/Tricornered serine-threonine kinases and regulates their activation and effects on cell survival (Zhou et al. 2011). N-terminal to the LIM domain, MICALs contain a single calponin homology (CH) domain (Figure 5a). CH domains typically bind F-actin when they are present in tandem (Gimona et al. 2002), but Mical requires its single CH domain for proper localization in vivo (Hung et al. 2010) and not for F-actin binding (Hung et al. 2010; Sun et al. 2006). At their N termini, MICALs contain a nucleotide-binding motif that is distinct from mononucleotide and cyclic nucleotide binding motifs. In particular, MICALs have a Rossmann fold (GxGxxG motif) that matches the consensus for binding the adenosine diphosphate (ADP)-moiety of flavin adenine dinucleotide (FAD) (Figure 5a, c; (Terman et al. 2002; Wierenga et al. 1986)). Proteins use FAD to catalyze oxidation-reduction (Redox) reactions and MICALs contain three separate motifs (GxGxxG, GD, DG) found in flavoprotein monooxygenases (also called hydroxylases), a subclass of oxidoreductase (Redox) enzymes (Figure 5a, c; (Eppink et al. 1997; Terman et al. 2002)). Flavoprotein monooxygenases (FMs) insert one atom of molecular oxygen directly into their substrate using nucleotides such as nicotinamide adenine dinucleotide phosphate (NADPH) as electron donors. Therefore, the presence of this FM domain suggested that MICAL is a Redox enzyme. Yet, each FM has distinct substrates so predicting MICAL substrates based on FM sequence is not possible (Terman et al. 2002).
Box 3 MICALs and Alternative Splicing.
MICALs appear to undergo extensive splicing and/or posttranslational modifications. Based on cDNA sequence, the single Mical gene is annotated as producing 18 different transcripts (FlyBase). Some of these cDNAs differ in the untranslated regions or within two variable regions of MICAL (Figure 5a;(Terman et al. 2002)), but other cDNAs suggest that different truncated versions of Mical (without some domains) may also exist. Northern analysis using probes to both the N and C terminus reveal similar large Drosophila Mical transcripts (Terman et al. 2002), but Western analysis with a Mical-specific antibody indicates many forms/sizes of Mical (Terman et al. 2002). Multiple different vertebrate MICAL isoforms also appear to exist (Ashida et al. 2006; Fischer et al. 2005; Friedberg 2009; Lin et al. 2009; Pasterkamp et al. 2006; Suzuki et al. 2002; Terman et al. 2002; Weide et al. 2003). Interestingly, some of these isoforms correspond to only the N-terminal portion of MICALs, while some of them specify only the C-terminal portion. These multiple splice forms have even led to the naming of a MICAL C-terminal-like protein (also called EBITEIN1) (Miura 2008; Miura and Imaki 2008a; Miura and Imaki 2008b), which is a portion of MICAL-2 (Terman et al. 2002). These splice forms could differentially regulate MICAL-mediated actin dynamics and Sema/Plexin signaling. For instance, our results point to the necessity and sufficiency of the Redox domain for MICAL-mediated F-actin disassembly and that the other regions of MICAL (including the Plexin-interacting region) properly localize and regulate this Redox activity (Hung et al. 2010). Yet, there might also be roles for the C-terminal portion of MICAL apart from these functions. It is interesting, however, that when we utilize transgenic strains of Drosophila that contain inducible promoters inserted within the MICAL genomic locus (i.e., overexpress “endogenous” MICAL), we generate F-actin rearrangements that mimic the expression of the full-length Mical cDNA, and not some truncated cDNA (Hung et al. 2010).
MICALs are unique among FMs in that they contain additional domains besides their Redox domain, including their CH and LIM domains that are also present in cytoskeletal-associated proteins. Likewise, the MICALs represent a new class of FMs because they contain additional motifs through which they directly associate with transmembrane proteins such as Plexins. However, the large size of the MICALs has made characterizing their biochemical function difficult. Therefore, Terman and colleagues expressed and purified recombinant protein containing only the FM domain of Mical and found that Mical proteins exhibit the yellow color characteristic of FAD binding proteins (Terman et al. 2002). Flavins, such as FAD, contain an isoalloxazine ring (Figure 5c) that absorbs light in the ultraviolet (UV) and visible spectral range (Chapman and Reid 1999) and is responsible for the yellow appearance of proteins that bind flavins (flavoproteins). MICAL proteins also exhibit flavoprotein-like UV-Visible absorption spectra (Terman et al. 2002), and structural analysis reveals that MICAL is an FAD binding protein whose closest structural relative is the FM p-hydroxybenzoate hydroxylase (PHBH) (Nadella et al. 2005; Siebold et al. 2005). Furthermore, purified protein corresponding to the FM domain of MICAL is able to consume NADPH, indicating that like PHBH, MICAL uses NADPH as a co-enzyme (Hung et al. 2010; Nadella et al. 2005; Schmidt et al. 2008). Interestingly, MICAL is also able to produce hydrogen peroxide (H2O2; (Morinaka et al. 2011; Nadella et al. 2005; Schmidt et al. 2008)). Thus, H2O2 may be the physiological signal generated by MICAL. However, FMs such as MICAL typically directly associate and catalyze the addition of oxygen to specific substrates and produce small amounts of H2O2 in the absence of a substrate (Massey 1995; Terman et al. 2002). In this way, FMs are different from oxidases, which simply produce H2O2. Therefore, the current model for MICALs catalytic function is that it binds and employs the cofactor/prosthetic group FAD, utilizes the coenzyme NADPH, and either directly oxidizes a specific substrate/s and/or produces H2O2 (Figure 5c).
MICAL Links Sema/Plexins to F-actin Disassembly
So what is the specific cellular role of these MICAL Redox enzymes? Our laboratory recently gained new insights into this question when we noticed defective hairs (bristles) on the bodies of Mical mutant adult flies (Hung et al. 2010). Drosophila bristles have long served as a simple, high-resolution single cell model to characterize molecules and mechanisms that regulate actin dynamics (Sutherland and Witke 1999; Tilney and DeRosier 2005). Specifically, Lewis Tilney and his colleagues extensively described that bristle process elongation, like neuronal process extension, is actin-dependent such that a bristle cell extends an unbranched, slightly curved, actin-rich process (Figure 6e; (Hung et al. 2010; Tilney and DeRosier 2005)). Utilizing this in vivo model system for studying actin dynamics, we determined that Mical mutant bristles are abnormally shaped – with bends and tip alterations (Figure 6e) – and restoring Mical expression in bristle cells rescues these defects. In contrast, we found no defects in bristle cell numbers or positioning, indicating that Mical has specific effects on cell morphology. Closer examination revealed that the normal parallel organization of F-actin in bristles is disrupted in Mical−/− mutants, and F-actin bundles are unusually thick and abnormally localized (Figure 6f). These Mical “knockout” bristles resemble those generated by overexpressing F-actin-stabilizing proteins such as actin crosslinking/bundling proteins, indicating that Mical controls the size, abundance and bundling of F-actin (Figure 6f). Consistent with this hypothesis, elevating Mical levels (overexpression) specifically in bristles generates thinner, fewer, and shorter arrays of F-actin and F-actin bundles (Figure 6f); again, defects that also appear when F-actin is destabilized in bristles using either cytochalasin treatment or loss-of-function mutations in actin bundling proteins (Figure 6f). Therefore, Mical is a critical inducer of F-actin instability in vivo.
The effects of the MICALs on the actin cytoskeleton are not simply a curiosity of bristle processes. Elevating Mical activity in neuronal growth cones in vivo also results in F-actin remodeling (Figure 6g; (Hung et al. 2010)). Likewise, Mical mutants exhibit localized defects in actin organization in skeletal muscle, and F-actin abnormally accumulates at postsynaptic regions of the neuromuscular junction (Figure 6h;(Beuchle et al. 2007)). Mammalian MICALs also regulate the F-actin dependent “collapse” of cells and growth cones in culture (Morinaka et al. 2011; Schmidt et al. 2008). Moreover, this MICAL-mediated actin remodeling occurs in response to Sema signaling (Hung et al. 2010; Morinaka et al. 2011; Schmidt et al. 2008). For instance, most Drosophila bristle cells are mechanosensors that are similar to the hair cells of the vertebrate inner ear that detect sound, gravity and acceleration (Muller and Littlewood-Evans 2001). Neuronal dendrites extend alongside elongating bristle processes and specify their actin arrangement and curved shape (Hung et al. 2010; Tilney and DeRosier 2005). We found that Class 1 Semas localize along these bristle-innervating dendrites whereas PlexA and Mical co-localize in bristles at sites of Mical-mediated F-actin disassembly (Figure 7a; (Hung et al. 2010)). Altering Sema/PlexA levels generates Mical-like morphological and F-actin defects, and also regulates the extent of Mical-mediated F-actin disassembly (Hung et al. 2010). Likewise, we also found that Mical “knockout” mutations dramatically suppress/eliminate PlexA-dependent F-actin remodeling in bristle processes in vivo (Figure 7b; (Hung et al. 2010)). Thus, Mical is both activated and required for Sema-induced F-actin disassembly in vivo (Figure 7i).
Figure 7. MICAL Disassembles F-actin and Directly Links Sema/Plexin Repulsion and F-actin Collapse.
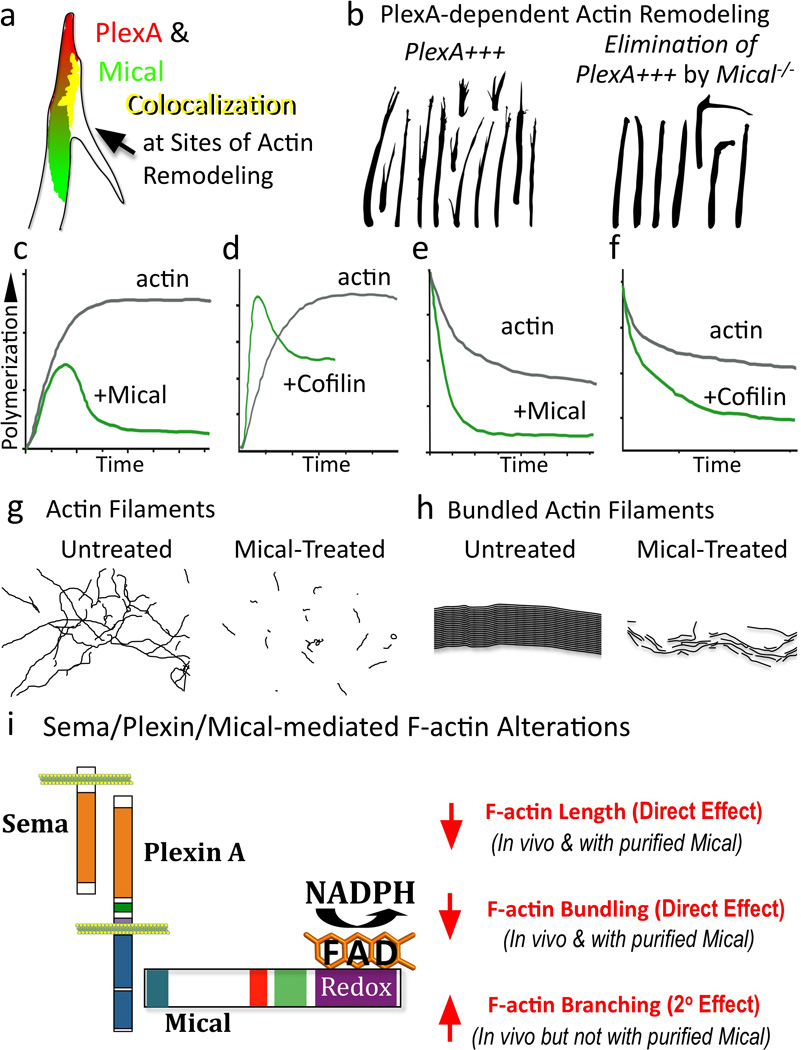
(a–b) Mical colocalizes with and is required by PlexA for F-actin reorganization. (c–f) Mical directly disassembles F-actin in a manner that is distinct from cofilin. (c) Actin polymerization slows-down in the presence of Mical (and its coenzyme NADPH). (d) In the presence of cofilin, polymerization initially speeds-up, a result of the diverse properties of cofilin (e.g., (Andrianantoandro and Pollard 2006; Bernstein and Bamburg 2010)) including that it generates more free ends for polymerization. (e–f) Mical and cofilin affect actin in a similar manner in an actin depolymerization assay. Modified from (Du and Frieden 1998; Hung et al. 2010; Moriyama and Yahara 2002). (g–h) Mical directly decreases F-actin length (g) and the length and width of Fascin-bundled actin filaments (h). (i) Sema/Plexin/Mical/Actin signaling pathway and its effects on F-actin.
MICALs are a New Class of F-Actin Disassembly Factors
Critical studies have revealed that these MICAL-mediated effects on repulsive axon guidance, synaptogenesis, cell “collapse”, exocytosis, and muscle organization require an intact Redox domain (Beuchle et al. 2007; Grigoriev et al. 2011; Hung et al. 2010; Morinaka et al. 2011; Schmidt et al. 2008; Terman et al. 2002). Namely, different mutations selectively disrupting the FAD binding motifs generate MICAL loss-of-function-like defects, eliminate MICAL-mediated gain-of-function effects, and suppress Sema-dependent repulsion. Likewise, our results utilizing many of these reagents indicate that the Redox activity of MICAL is necessary in vivo for both normal F-actin organization and Mical-induced F-actin disassembly (Hung et al. 2010). Interestingly, we also found by examining different truncated forms of the Mical protein that Mical-mediated F-actin disassembly only occurs in vivo when Mical co-localizes with F-actin in specific regions of the cell (Hung et al. 2010). These observations suggested that Mical might be directly associating with F-actin or an F-actin regulatory protein, and thereby locally targeting the F-actin cytoskeleton for Redox-dependent disassembly. To directly test this possibility, we moved to in vitro assays with purified Mical protein and found using several F-actin binding assays that different forms of purified recombinant Mical protein containing the Redox domain directly associate with in vitro-generated actin filaments (Hung et al. 2010). Strikingly, we also observed that purified Mical substantially decreases the rate, extent, and steady-state level of actin polymerization when it was activated with its co-enzyme NADPH (Figure 7c;(Hung et al. 2010)). In contrast, activated Mical does not directly induce actin branching, alter actin-bundling protein activity, or regulate the dynamics of in vitro-generated microtubules (Hung et al. 2010). Thus, Mical directly and specifically alters actin filament assembly. Indeed, follow-up analysis using pyrene actin assays, co-sedimentation, gel filtration, and electron microscopy (EM) revealed that activated Mical directly disassembles actin filaments (Figures 7e, g;(Hung et al. 2010)). Moreover, using purified proteins to perform in vitro assays that closely resemble the bundled organization of F-actin present within bristles and growth cone filopodia revealed that activated Mical also directly disassembles bundled actin filaments, decreasing both their length and width (Figure 7h;(Hung et al. 2010)).
Redox-dependent Effects on the Actin Cytoskeleton?
There are several families of actin depolymerization factors including members of the ADF/Cofilin family (Bugyi and Carlier 2010; Ono 2007) and our results identify Mical as a novel actin depolymerization factor that directly disassembles both individual and bundled F-actin. Interestingly, Mical has actin regulatory properties unlike any previously characterized protein indicating that Mical is likely to be mediating disassembly in a novel way. In particular, Mical’s activity in different actin polymerization and depolymerization assays indicates that Mical does not mediate its destabilizing effects on F-actin by strictly functioning as a monomer binding/sequestering protein, a barbed (+) -end capping protein, a pointed (−) -end capping protein, or even a severing protein such as cofilin (Cooper 1992; Hertzog and Carlier 2005). Interestingly, Mical, unlike cofilin, does not enhance polymerization, but solely decreases the rate and extent of actin polymerization (compare Figures 7c–d and e–f). In addition, the enzymatic moiety of Mical is required in vivo and sufficient in vitro for F-actin disassembly, suggesting that Mical induces F-actin disassembly through a catalytic process (Hung et al. 2010). In support of this hypothesis, the co-enzyme NADPH is required for Mical-mediated F-actin disassembly (Hung et al. 2010). Likewise, the rate and extent of Mical-mediated F-actin disassembly changes with NADPH concentration (Hung et al. 2010). Moreover, Mical-mediated F-actin disassembly occurs even with lower (substoichiometric) levels of purified Mical in comparison to actin (Hung et al. 2010), further suggesting that Mical is an actin regulatory enzyme.
Although the nature of this enzymatic activity and its effect on actin have not been determined, the observed Redox activity of the MICALs suggests a novel role for specific Redox signaling events in altering actin dynamics. Interestingly, previous results have suggested that actin is susceptible to Redox regulation, but no specific enzymes have been identified that perform this function. For instance, it has long been reported that oxidation alters the physicochemical properties of actin (Bailey and Perry 1947; Balin and Barany 1967; Feuer et al. 1948; Ishiwata 1976; Katz and Mommaerts 1962; Martonosi and Gouvea 1961) and the reducing agent dithiothreitol (DTT) is employed to “protect” stored actin (Cooper 1992; Ishiwata 1976; Tang et al. 1999). Moreover, numerous reports have indicated that oxidants including H2O2 adversely affect actin polymerization (e.g., (Balin and Barany 1967; Dalle-Donne et al. 2001; DalleDonne et al. 1995; Guan et al. 2005; Hinshaw et al. 1986; Lassing et al. 2007; Milzani et al. 1997; Mirabelli et al. 1988; Moldovan et al. 1999)), providing a precedent for oxidative events altering actin dynamics. Therefore, we wondered if the MICALs, since they produce H2O2, simply had non-specific effects on actin. However, Mical’s effects on actin dynamics are substantially more robust than lethal doses of H2O2, which do not disassemble F-actin and only modestly alter actin polymerization under the conditions we employed with Mical (Hung et al. 2010). Likewise, PHBH, which also releases H2O2, has no effect on actin dynamics (Hung et al. 2010). Indeed, our observations that the Mical Redox region requires specific localization in vivo also support the hypothesis that Mical does not indirectly alter actin dynamics (e.g., through general effects on the Redox state of the cell or global production of H2O2) but selectively targets F-actin for disassembly.
Conclusions and Perspectives
Our recent results now provide new insights into the molecular logic by which the largest family of repulsive guidance cues negatively alters actin dynamics in a spatiotemporal manner. In our model, Semas directly bind cell-surface Plexin receptors and activate them to directly interact via their cytoplasmic regions with the C-terminus of Mical (Figure 7i; (Hung et al. 2010)). Upon association with Sema-bound Plexin, Mical is activated to directly and locally disassemble both individual and bundled F-actin in a Redox-dependent manner (Figure 7i; (Hung et al. 2010)). Our observations also provide a more complete understanding of the roles of repellents in vivo. In particular, we find that Sema/Plexin/Mical-mediated F-actin disassembly, like cytochalasin treatment, is accompanied by F-actin–rich branch formation in vivo (Figures 6e, g; (Hung et al. 2010; Yoon and Terman submitted)). This branching appears to result from the Mical-induced “transformation” of parallel-arranged bundles of F-actin, that are a hallmark of bristles and growth cone filopodia, into branched meshwork arrays of F-actin reminiscent of that seen in lamellipodia (Hung et al. 2010). Since we find no evidence that purified Mical directly performs actin branching in vitro (Hung et al. 2010) and continuing to raise the levels of Mical in vivo eventually serves to decrease this branching (Yoon and Terman submitted), we have concluded that Sema/Plexin/Mical-dependent increases in actin branching are secondary to Mical-mediated F-actin disassembly (Figure 7i). This would suggest that following Sema/Plexin/Mical-mediated F-actin disassembly, other actin regulatory proteins including actin nucleating factors reorganize the actin cytoskeleton and generate new actin branches (Chhabra and Higgs 2007). Interestingly, it has long been noted that Sema-mediated F-actin collapse generates more complex branched growth cones and processes (Campbell et al. 2001; Fenstermaker et al. 2004; Kapfhammer and Raper 1987a; Liu and Halloran 2005; Sakai and Halloran 2006). Likewise, other repellents including slits and ephrins induce both collapse and branching (e.g., (Castellani et al. 1998; Davenport et al. 1999; Wang et al. 1999)). These may be two distinct activities but in light of our observations with Sema/Plexin/MICAL, we have proposed a model that repellents such as Semas disassemble or ‘prune back’ the actin network in vivo, and this pruning process at times initiates secondary events that serve to enhance cellular complexity/plasticity. This Sema/Plexin/Mical-triggered change in morphology, in combination with Sema/Plexin-dependent effects on microtubules and substrate adhesion, would help cells and their processes locate more permissive substrates for outgrowth and underlie the directional changes induced by repellents. Interestingly, when growth cones reach choice points in vivo where they make a directional decision, they become complex and extend multiple filopodia that select a new pathway (Broadie et al. 1993; Godement et al. 1994; Harris et al. 1987; Murray et al. 1998; Tosney and Landmesser 1985). In this manner, these negative and positive effectors of cell motility work “hand-in-hand” to provide instructions to cells and their processes.
Our findings also raise important new questions related to Sema/Plexin/MICAL-mediated F-actin remodeling. Many of these questions center on Mical’s effects on actin. How does Mical induce F-actin disassembly? Does this occur through severing, depolymerization, and/or some other means? Does Mical mediate its effects by releasing small molecules or directly altering its substrate/s? How is this activity regulated? Do mammalian MICALs exert similar effects on actin dynamics? It is also critical to further characterize the role of the other domains of MICAL, which appear to localize and regulate MICAL activity. Likewise, it is important to determine the physiological and biochemical roles that other MICAL-interacting proteins such as CasL, CRMP, NDR/Tricornered kinases, vimentin, and Rabs play in Sema/Plexin/MICAL-dependent F-actin disassembly. In light of these protein associations, the MICALs may provide some intermediary between the actin, microtubule, and intermediate filament cytoskeletons. It might also be possible to identify specific small molecule inhibitors of MICAL function. At present, general inhibitors of FMs including the green tea polyphenols epigallocatechin gallate (EGCG) and epicatechin (EC) block Sema-mediated axon repulsion and growth cone collapse (Hochman et al. 2006; Pasterkamp et al. 2006; Terman et al. 2002), but although EGCG is known to diminish the activity of purified MICAL (Nadella et al. 2005), EGCG has many known (and unknown) targets and it is unclear if EGCG alters MICAL action in cells. Finally, it will also be interesting to determine if there are cell biological roles for MICAL either independent of Sema/Plexin signaling or with other repellents. Likewise, as noted above, MICAL appears to work in combination with other actin regulatory proteins to remodel the actin cytoskeleton, and Mical may also work with other F-actin disassembly proteins such as cofilin. For example, in addition to common roles in Sema/Plexin-mediated F-actin disassembly where Mical and cofilin may work in concert for the disassembly of actin filaments, F-actin bundles, and the recycling of subunits, it is interesting to note that cofilin-mediated F-actin depolymerization is susceptible to oxidation-dependent regulation (Bernstein and Bamburg 2010). Regardless, it will be important to determine the extent of the MICALs effects on the multitude of actin-dependent processes that occur throughout the body.
Acknowledgments
We thank Jonathan Raper, Weichun Lin, and Chris Spaeth for comments on our manuscript and Terman Lab members for discussions. Supported by a Cancer Prevention Research Institute of Texas (CPRIT) Pre-doctoral fellowship to R.-J.H and NIH/NINDS (NS073968) and Welch Foundation (I-1749) grants to J.R.T.
References
- Abercrombie M. Contact inhibition in tissue culture. In Vitro. 1970;6:128–142. doi: 10.1007/BF02616114. [DOI] [PubMed] [Google Scholar]
- Abercrombie M. The crawling movement of metazoan cells. Proc R Soc Lond B Biol Sci. 1980;207(1167):129–147. [Google Scholar]
- Abercrombie M, Heaysman JE. Observations on the social behaviour of cells in tissue culture. I. Speed of movement of chick heart fibroblasts in relation to their mutual contacts. Exp Cell Res. 1953;5(1):111–131. doi: 10.1016/0014-4827(53)90098-6. [DOI] [PubMed] [Google Scholar]
- Aguayo AJ, Bray GM, Perkins CS, Duncan ID. Axon-sheath cell interactions in peripheral and central nervous system transplants. Society for neuroscience symposia: aspects of developmental neurobiology. 1979;4:361–383. [Google Scholar]
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, et al. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A- induced growth cone collapse. Nat Neurosci. 2001;4(4):367–373. doi: 10.1038/86011. [DOI] [PubMed] [Google Scholar]
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24(1):13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Ashida S, Furihata M, Katagiri T, Tamura K, Anazawa Y, Yoshioka H, Miki T, Fujioka T, Shuin T, Nakamura Y, et al. Expression of novel molecules, MICAL2-PV (MICAL2 prostate cancer variants), increases with high Gleason score and prostate cancer progression. Clin Cancer Res. 2006;12(9):2767–2773. doi: 10.1158/1078-0432.CCR-05-1995. [DOI] [PubMed] [Google Scholar]
- Astin JW, Batson J, Kadir S, Charlet J, Persad RA, Gillatt D, Oxley JD, Nobes CD. Competition amongst Eph receptors regulates contact inhibition of locomotion and invasiveness in prostate cancer cells. Nat Cell Biol. 2010;12(12):1194–204. doi: 10.1038/ncb2122. [DOI] [PubMed] [Google Scholar]
- Ayoob JC, Terman JR, Kolodkin AL. Drosophila Plexin B is a Sema-2a receptor required for axon guidance. Development. 2006;133(11):2125–2135. doi: 10.1242/dev.02380. [DOI] [PubMed] [Google Scholar]
- Ayoob JC, Yu HH, Terman JR, Kolodkin AL. The Drosophila receptor guanylyl cyclase Gyc76C is required for semaphorin-1a–plexin A-mediated axonal repulsion. J Neurosci. 2004;24(30):6639–6649. doi: 10.1523/JNEUROSCI.1104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K, Perry SV. The role of SH groups in the myosin-actin interaction. Biochem J. 1947;41(2):xxii. doi: 10.1016/0006-3002(47)90167-4. [DOI] [PubMed] [Google Scholar]
- Balin G, Barany M. Studies on actin-actin and actin-myosin interaction. Biochim Biophys Acta. 1967;140:208–221. doi: 10.1016/0005-2795(67)90461-8. [DOI] [PubMed] [Google Scholar]
- Bamburg JR. Proteins of the ADF/cofilin family: essential regulators of actin dynamics. Annu Rev Cell Dev Biol. 1999;15:185–230. doi: 10.1146/annurev.cellbio.15.1.185. [DOI] [PubMed] [Google Scholar]
- Barberis D, Artigiani S, Casazza A, Corso S, Giordano S, Love CA, Jones EY, Comoglio PM, Tamagnone L. Plexin signaling hampers integrin-based adhesion, leading to Rho-kinase independent cell rounding, and inhibiting lamellipodia extension and cell motility. Faseb J. 2004;18(3):592–594. doi: 10.1096/fj.03-0957fje. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2(5):001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Falk J, Moret F, Castellani V. Modulation of semaphorin signaling by Ig superfamily cell adhesion molecules. Adv Exp Med Biol. 2007;600:61–72. doi: 10.1007/978-0-387-70956-7_6. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20(4):187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. Post-injury myelin-breakdown products inhibit axonal growth: an hypothesis to explain the failure of axonal regeneration in the mammalian central nervous system. Bibl Anat. 1982;(23):1–11. [PubMed] [Google Scholar]
- Beuchle D, Schwarz H, Langegger M, Koch I, Aberle H. Drosophila MICAL regulates myofilament organization and synaptic structure. Mech Dev. 2007;124(5):390–406. doi: 10.1016/j.mod.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Shimizu A, Klagsbrun M. Semaphorin-induced cytoskeletal collapse and repulsion of endothelial cells. Methods Enzymol. 2008;443:299–314. doi: 10.1016/S0076-6879(08)02015-6. [DOI] [PubMed] [Google Scholar]
- Bokoch GM. Biology of the p21-activated kinases. Annu Rev Biochem. 2003;72:743–781. doi: 10.1146/annurev.biochem.72.121801.161742. [DOI] [PubMed] [Google Scholar]
- Bonhoeffer F, Huf J. Recognition of cell types by axonal growth cones in vitro. Nature. 1980;288(5787):162–164. doi: 10.1038/288162a0. [DOI] [PubMed] [Google Scholar]
- Bray D, Wood P, Bunge RP. Selective fasciculation of nerve fibers in culture. Exp. Cell Res. 1980;130:241–250. doi: 10.1016/0014-4827(80)90060-9. [DOI] [PubMed] [Google Scholar]
- Broadie K, Sink H, Van Vactor D, Fambrough D, Whitington PM, Bate M, Goodman CS. From growth cone to synapse: the life history of the RP3 motoneuron. Development Supplement. 1993:227–238. [PubMed] [Google Scholar]
- Bron R, Vermeren M, Kokot N, Andrews W, Little GE, Mitchell KJ, Cohen J. Boundary cap cells constrain spinal motor neuron somal migration at motor exit points by a semaphorin-plexin mechanism. Neural Dev. 2007;2:21. doi: 10.1186/1749-8104-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Bridgman PC. Disruption of the cytoskeleton during Semaphorin 3A induced growth cone collapse correlates with differences in actin organization and associated binding proteins. Dev Neurobiol. 2009;69(10):633–646. doi: 10.1002/dneu.20732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Wysolmerski RB, Bridgman PC. Dorsal root ganglion neurons react to semaphorin 3A application through a biphasic response that requires multiple myosin II isoforms. Mol Biol Cell. 2009;20(4):1167–1179. doi: 10.1091/mbc.E08-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugyi B, Carlier MF. Control of actin filament treadmilling in cell motility. Annu Rev Biophys. 2010;39:449–470. doi: 10.1146/annurev-biophys-051309-103849. [DOI] [PubMed] [Google Scholar]
- Burrows MT. The growth of tissues of the chick embryo outside the animal body, with special reference to the nervous system. J. Exp. Zool. 1911;10(1):63–84. [Google Scholar]
- Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, Defilippi P. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer. 2010;10(12):858–870. doi: 10.1038/nrc2967. [DOI] [PubMed] [Google Scholar]
- Cajal S. La rétine des Vertébrés. La cellule. 1893;9:120–255. [Google Scholar]
- Cajal S. Textura del Sistema Nervioso del Hombre y de los Vertebrados. Madrid: mprenta y Librería de Nicolás Moya. 1899 [Google Scholar]
- Cajal S. La acción neurotrópica de los epitelios. Trab Lab Invest Biol Univ Madrid. 1919;17:181–228. [Google Scholar]
- Cajal S. Degeneration and regeneration of the nervous system. New York: Hafner; 1928. [Google Scholar]
- Campbell DS, Regan AG, Lopez JS, Tannahill D, Harris WA, Holt CE. Semaphorin 3A elicits stage-dependent collapse, turning, and branching in Xenopus retinal growth cones. J Neurosci. 2001;21(21):8538–8547. doi: 10.1523/JNEUROSCI.21-21-08538.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmona-Fontaine C, Matthews HK, Kuriyama S, Moreno M, Dunn GA, Parsons M, Stern CD, Mayor R. Contact inhibition of locomotion in vivo controls neural crest directional migration. Nature. 2008;456(7224):957–961. doi: 10.1038/nature07441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SB. Principles of cell motility: the direction of cell movement and cancer invasion. Nature. 1965;208(5016):1183–1187. doi: 10.1038/2081183a0. [DOI] [PubMed] [Google Scholar]
- Castellani V, Yue Y, Gao PP, Zhou R, Bolz J. Dual action of a ligand for Eph receptor tyrosine kinases on specific populations of axons during the development of cortical circuits. J Neurosci. 1998;18(12):4663–4672. doi: 10.1523/JNEUROSCI.18-12-04663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SK, Reid GA, editors. Flavoprotein Protocols. Totowa, NJ: Humana Press; 1999. p. 256. [Google Scholar]
- Chhabra ES, Higgs HN. The many faces of actin: matching assembly factors with cellular structures. Nat Cell Biol. 2007;9(10):1110–1121. doi: 10.1038/ncb1007-1110. [DOI] [PubMed] [Google Scholar]
- Comeau MR, Johnson R, DuBose RF, Peterson M, Gearing P, VandenBos T, Park L, Farrah T, Buller RM, Cohen JI, et al. A poxvirus-encoded semaphorin induces cytokine production from monocytes and binds to a novel cellular semaphorin receptor, VESPR. Immunity. 1998;8:473–482. doi: 10.1016/s1074-7613(00)80552-x. [DOI] [PubMed] [Google Scholar]
- Cooper JA. Actin filament assembly and organization in vitro. In: Carraway KL, Carraway CAC, editors. The Cytoskeleton: A Practical Approach. New York: Oxford University Press; 1992. pp. 47–71. [Google Scholar]
- Dalle-Donne I, Rossi R, Milzani A, Di Simplicio P, Colombo R. The actin cytoskeleton response to oxidants: from small heat shock protein phosphorylation to changes in the redox state of actin itself. Free Radic Biol Med. 2001;31(12):1624–1632. doi: 10.1016/s0891-5849(01)00749-3. [DOI] [PubMed] [Google Scholar]
- DalleDonne I, Milzani A, Colombo R. H2O2-treated actin: assembly and polymer interactions with cross- linking proteins. Biophys J. 1995;69(6):2710–2719. doi: 10.1016/S0006-3495(95)80142-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalpe G, Zhang LW, Zheng H, Culotti JG. Conversion of cell movement responses to Semaphorin-1 and Plexin-1 from attraction to repulsion by lowered levels of specific RAC GTPases in C. elegans. Development. 2004;131(9):2073–2088. doi: 10.1242/dev.01063. [DOI] [PubMed] [Google Scholar]
- Davenport RW, Thies E, Cohen ML. Neuronal growth cone collapse triggers lateral extensions along trailing axons. Nature Neurosci. 1999;(2):254–259. doi: 10.1038/6360. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 1981;214(4523):931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Dent EW, Barnes AM, Tang F, Kalil K. Netrin-1 and semaphorin 3A promote or inhibit cortical axon branching, respectively, by reorganization of the cytoskeleton. J Neurosci. 2004;24(12):3002–3012. doi: 10.1523/JNEUROSCI.4963-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298(5600):1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- Driessens MH, Hu H, Nobes CD, Self A, Jordens I, Goodman CS, Hall A. Plexin-B semaphorin receptors interact directly with active Rac and regulate the actin cytoskeleton by activating Rho. Curr Biol. 2001;11(5):339–344. doi: 10.1016/s0960-9822(01)00092-6. [DOI] [PubMed] [Google Scholar]
- Du J, Frieden C. Kinetic studies on the effect of yeast cofilin on yeast actin polymerization. Biochemistry. 1998;37(38):13276–13284. doi: 10.1021/bi981117r. [DOI] [PubMed] [Google Scholar]
- Dunn GA. Mutual contact inhibition of extension of chick sensory nerve fibres in vitro. J Comp Neurol. 1971;143(4):491–507. doi: 10.1002/cne.901430406. [DOI] [PubMed] [Google Scholar]
- Ebendal T. The relative roles of contact inhibition and contact guidance in orientation of axons extending on aligned collagen fibrils in vitro. Exp Cell Res. 1976;98(1):159–169. doi: 10.1016/0014-4827(76)90475-4. [DOI] [PubMed] [Google Scholar]
- Ebendal T. Orientational behavior of extending neurites. In: Bellairs R, Curtis A, Dunn G, editors. Cell Behavior. A tribute to Michael Abercrombie. Cambridge: Cambridge University Press; 1982. pp. 281–297. [Google Scholar]
- Eickholt BJ, Mackenzie SL, Grahm A, Walsh FS, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development. 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]
- Eisenbach M. Chemotaxis. London: Imperial College Press; 2004. [Google Scholar]
- Eppink MH, Schreuder HA, Van Berkel WJ. Identification of a novel conserved sequence motif in flavoprotein hydroxylases with a putative dual function in FAD/NAD(P)H binding. Protein Sci. 1997;6(11):2454–2458. doi: 10.1002/pro.5560061119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Mansfield SG, Redman T, Phillip R, Gordon-Weeks PR, Raper JA. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J. Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Raper JA. Localized collapsing cues can steer growth cones without inducing their full collapse. Neuron. 1995;14:263–274. doi: 10.1016/0896-6273(95)90284-8. [DOI] [PubMed] [Google Scholar]
- Fenstermaker V, Chen Y, Ghosh A, Yuste R. Regulation of dendritic length and branching by semaphorin 3A. J Neurobiol. 2004;58(3):403–412. doi: 10.1002/neu.10304. [DOI] [PubMed] [Google Scholar]
- Feuer G, Molnar F, Pettko E, Straub FB. Studies on the composition and polymerization of actin. Hung. Acta Physiol. 1948;1:150–163. [PubMed] [Google Scholar]
- Fields RD, Nelson PG. Growth cone collapse in response to electrical activity, cell contact and diffusible factors. Physiol Chem Phys Med NMR. 1994;26(1):27–53. [PubMed] [Google Scholar]
- Fischer J, Weide T, Barnekow A. The MICAL proteins and rab1: a possible link to the cytoskeleton? Biochem Biophys Res Commun. 2005;328(2):415–423. doi: 10.1016/j.bbrc.2004.12.182. [DOI] [PubMed] [Google Scholar]
- Fournier AE, Nakamura F, Kawamoto S, Goshima Y, Kalb RG, Strittmatter SM. Semaphorin3A enhances endocytosis at sites of receptor-F-actin colocalization during growth cone collapse. J Cell Biol. 2000;149(2):411–422. doi: 10.1083/jcb.149.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco M, Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: shifting into overdrive. EMBO Rep. 2008;9(9):865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg F. Alternative splicing for members of human mosaic domain superfamilies. I. The CH and LIM domains containing group of proteins. Mol Biol Rep. 2009;36(5):1059–1081. doi: 10.1007/s11033-008-9281-9. [DOI] [PubMed] [Google Scholar]
- Fritsche J, Reber BF, Schindelholz B, Bandtlow CE. Differential Cytoskeletal Changes during Growth Cone Collapse in Response to hSema III and Thrombin. Mol Cell Neurosci. 1999;14(4/5):398–418. doi: 10.1006/mcne.1999.0777. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel rab effectors reveals unexpected broad Rab binding specificity. Mol Cell Proteomics. 2008;7(6):1031–1042. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- Gallo G. RhoA-kinase coordinates F-actin organization and myosin II activity during semaphorin-3A–induced axon retraction. J Cell Sci. 2006;119(Pt 16):3413–3423. doi: 10.1242/jcs.03084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo G. Semaphorin 3A inhibits ERM protein phosphorylation in growth cone filopodia through inactivation of PI3K. Dev Neurobiol. 2008;68(7):926–933. doi: 10.1002/dneu.20631. [DOI] [PubMed] [Google Scholar]
- Gallo G, Letourneau PC. Regulation of growth cone actin filaments by guidance cues. J Neurobiol. 2004;58(1):92–102. doi: 10.1002/neu.10282. [DOI] [PubMed] [Google Scholar]
- Gatlin JC, Estrada-Bernal A, Sanford SD, Pfenninger KH. Myristoylated, alanine-rich C-kinase substrate phosphorylation regulates growth cone adhesion and pathfinding. Mol Biol Cell. 2006;17(12):5115–5130. doi: 10.1091/mbc.E05-12-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CM, Zygmunt T, Torres-Vazquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Dev Biol. 2011;349(1):1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger RJ, Hollis ER, 2nd, Tuszynski MH. Guidance molecules in axon regeneration. Cold Spring Harb Perspect Biol. 2010;2(7):a001867. doi: 10.1101/cshperspect.a001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimona M, Djinovic-Carugo K, Kranewitter WJ, Winder SJ. Functional plasticity of CH domains. FEBS Lett. 2002;513(1):98–106. doi: 10.1016/s0014-5793(01)03240-9. [DOI] [PubMed] [Google Scholar]
- Godement P, Wang LC, Mason CA. Retinal axon divergence in the optic chiasm: dynamics of growth cone behavior at the midline. J Neurosci. 1994;14(11 Pt 2):7024–7039. doi: 10.1523/JNEUROSCI.14-11-07024.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoriev I, Yu KL, Martinez-Sanchez E, Serra-Marques A, Smal I, Meijering E, Demmers J, Peranen J, Pasterkamp RJ, van der Sluijs P, et al. Rab6, Rab8, and MICAL3 Cooperate in Controlling Docking and Fusion of Exocytotic Carriers. Curr Biol. 2011;21(11):967–974. doi: 10.1016/j.cub.2011.04.030. [DOI] [PubMed] [Google Scholar]
- Guan JQ, Takamoto K, Almo SC, Reisler E, Chance MR. Structure and dynamics of the actin filament. Biochemistry. 2005;44(9):3166–3175. doi: 10.1021/bi048021j. [DOI] [PubMed] [Google Scholar]
- Guttmann-Raviv N, Shraga-Heled N, Varshavsky A, Guimaraes-Sternberg C, Kessler O, Neufeld G. Semaphorin-3A and semaphorin-3F work together to repel endothelial cells and to inhibit their survival by induction of apoptosis. J Biol Chem. 2007;282(36):26294–26305. doi: 10.1074/jbc.M609711200. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279(5350):509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hall A, Lalli G. Rho and Ras GTPases in axon growth, guidance, and branching. Cold Spring Harb Perspect Biol. 2010;2(2):a001818. doi: 10.1101/cshperspect.a001818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AK. Contact inhibition of cell locomotion. In: RP Cox., editor. Cell Communication. New York: John Wiley; 1974. pp. 147–185. [Google Scholar]
- Harris WA, Holt CE, Bonhoeffer F. Retinal axons with and without their somata, growing to and arborizing in the tectum of Xenopus embryos: a time-lapse video study of single fibres in vivo. Development. 1987;101(1):123–133. doi: 10.1242/dev.101.1.123. [DOI] [PubMed] [Google Scholar]
- Heaysman JE. Contact inhibition of locomotion: a reappraisal. Int Rev Cytol. 1978;55:49–66. doi: 10.1016/s0074-7696(08)61886-0. [DOI] [PubMed] [Google Scholar]
- Hertzog M, Carlier MF. Functional Characterization of Proteins Regulating Actin Assembly. Current Protocols in Cell Biology. 2005:13.6.1–13.6.23. doi: 10.1002/0471143030.cb1306s26. [DOI] [PubMed] [Google Scholar]
- Hinshaw DB, Sklar LA, Bohl B, Schraufstatter IU, Hyslop PA, Rossi MW, Spragg RG, Cochrane CG. Cytoskeletal and morphologic impact of cellular oxidant injury. Am J Pathol. 1986;123(3):454–464. [PMC free article] [PubMed] [Google Scholar]
- Hochman E, Castiel A, Jacob-Hirsch J, Amariglio N, Izraeli S. Molecular pathways regulating pro-migratory effects of Hedgehog signaling. J Biol Chem. 2006;281(45):33860–33870. doi: 10.1074/jbc.M605905200. [DOI] [PubMed] [Google Scholar]
- Hofmann C, Shepelev M, Chernoff J. The genetics of Pak. J Cell Sci. 2004;117(Pt 19):4343–4354. doi: 10.1242/jcs.01392. [DOI] [PubMed] [Google Scholar]
- Hou ST, Jiang SX, Smith RA. Permissive and repulsive cues and signalling pathways of axonal outgrowth and regeneration. Int Rev Cell Mol Biol. 2008;267:125–181. doi: 10.1016/S1937-6448(08)00603-5. [DOI] [PubMed] [Google Scholar]
- Hu H, Marton TF, Goodman CS. Plexin B mediates axon guidance in Drosophila by simultaneously inhibiting active Rac and enhancing RhoA signaling. Neuron. 2001;32(1):39–51. doi: 10.1016/s0896-6273(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Huber AB, Kolodkin AL, Ginty DD, Cloutier JF. Signaling at the growth cone: ligand-receptor complexes and the control of axon growth and guidance. Annu Rev Neurosci. 2003;26:509–563. doi: 10.1146/annurev.neuro.26.010302.081139. [DOI] [PubMed] [Google Scholar]
- Hughes A. The growth of embryonic neurites; a study of cultures of chick neural tissues. J Anat. 1953;87(2):150–162. [PMC free article] [PubMed] [Google Scholar]
- Hung RJ, Yazdani U, Yoon J, Wu H, Yang T, Gupta N, Huang Z, van Berkel WJ, Terman JR. Mical links semaphorins to F-actin disassembly. Nature. 2010;463(7282):823–827. doi: 10.1038/nature08724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiwata S. Freezing of actin. Reversible oxidation of a sulfhydryl group and structural change. J Biochem. 1976;80(3):595–609. doi: 10.1093/oxfordjournals.jbchem.a131315. [DOI] [PubMed] [Google Scholar]
- Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapse. J. Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurney WM, Gallo G, Letourneau PC, McLoon SC. Rac1-mediated endocytosis during ephrin-A2- and semaphorin 3A–induced growth cone collapse. J Neurosci. 2002;22(14):6019–6028. doi: 10.1523/JNEUROSCI.22-14-06019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadrmas JL, Beckerle MC. The LIM domain: from the cytoskeleton to the nucleus. Nat Rev Mol Cell Biol. 2004;5(11):920–931. doi: 10.1038/nrm1499. [DOI] [PubMed] [Google Scholar]
- Kanda I, Nishimura N, Nakatsuji H, Yamamura R, Nakanishi H, Sasaki T. Involvement of Rab13 and JRAB/MICAL-L2 in epithelial cell scattering. Oncogene. 2008;27(12):1687–1695. doi: 10.1038/sj.onc.1210812. [DOI] [PubMed] [Google Scholar]
- Kapfhammer JP, Grunewald BE, Raper JA. The selective inhibition of growth cone extension by specific neurites in culture. J. Neurosci. 1986;6:2527–2534. doi: 10.1523/JNEUROSCI.06-09-02527.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer JP, Raper JA. Collapse of growth cone structure on contact with specific neurites in culture. J. Neurosci. 1987a;7:201–212. doi: 10.1523/JNEUROSCI.07-01-00201.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapfhammer JP, Raper JA. Interactions between growth cones and neurites growing from different neural tissues in culture. J. Neurosci. 1987b;7:1595–1600. doi: 10.1523/JNEUROSCI.07-05-01595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi H, Shiraga M, Kato H, Kamae T, Yamamoto N, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Negative regulation of platelet function by a secreted cell repulsive protein, semaphorin 3A. Blood. 2005;106(3):913–921. doi: 10.1182/blood-2004-10-4092. [DOI] [PubMed] [Google Scholar]
- Katz AM, Mommaerts WF. The sulfhydryl groups of actin. Biochim Biophys Acta. 1962;65:82–92. doi: 10.1016/0006-3002(62)90151-8. [DOI] [PubMed] [Google Scholar]
- Keynes R, Cook G. Cell-cell repulsion: clues from the growth cone? Cell. 1990;62(4):609–610. doi: 10.1016/0092-8674(90)90103-l. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Cook GMW. Repulsive and inhibitory signals. Curr. Opin. Neurobiol. 1995;5:75–78. doi: 10.1016/0959-4388(95)80090-5. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Stern CD. Segmentation in the vertebrate nervous system. Nature. 1984;310:220–223. doi: 10.1038/310786a0. [DOI] [PubMed] [Google Scholar]
- Kirilly D, Gu Y, Huang Y, Wu Z, Bashirullah A, Low BC, Kolodkin AL, Wang H, Yu F. A genetic pathway composed of Sox14 and Mical governs severing of dendrites during pruning. Nat Neurosci. 2009 doi: 10.1038/nn.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes D, Goodman CS. The semaphorin genes encode a family of transmembrane and secreted growth cone guidance molecules. Cell. 1993;75:1389–1399. doi: 10.1016/0092-8674(93)90625-z. [DOI] [PubMed] [Google Scholar]
- Kolodkin AL, Matthes D, O’Connor T, Patel NH, Admon A, Bentley D, Goodman CS. Fasciclin IV: Sequence, expression, and function during growth cone guidance in the grasshopper embryo. Neuron. 1992;9:831–835. doi: 10.1016/0896-6273(92)90237-8. [DOI] [PubMed] [Google Scholar]
- Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: Inhibition of collapse by opposing mutants of Rac1. J. Neurosci. 1999;19:1965–1975. doi: 10.1523/JNEUROSCI.19-06-01965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer CC, Ejsmont RK, Schonbauer C, Schnorrer F, Tomancak P. In vivo RNAi rescue in Drosophila melanogaster with genomic transgenes from Drosophila pseudoobscura. PLoS One. 2010;5(1):e8928. doi: 10.1371/journal.pone.0008928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassing I, Schmitzberger F, Bjornstedt M, Holmgren A, Nordlund P, Schutt CE, Lindberg U. Molecular and structural basis for redox regulation of beta-actin. J Mol Biol. 2007;370(2):331–348. doi: 10.1016/j.jmb.2007.04.056. [DOI] [PubMed] [Google Scholar]
- Lawrence PA. The structure and properties of a compartment border: the intersegmental boundary in Oncopeltus. Ciba Found Symp. 1975;0(29):3–23. doi: 10.1002/9780470720110.ch2. [DOI] [PubMed] [Google Scholar]
- Lepelletier Y, Moura IC, Hadj-Slimane R, Renand A, Fiorentino S, Baude C, Shirvan A, Barzilai A, Hermine O. Immunosuppressive role of semaphorin-3A on T cell proliferation is mediated by inhibition of actin cytoskeleton reorganization. Eur J Immunol. 2006;36(7):1782–1793. doi: 10.1002/eji.200535601. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. Possible roles for cell-to-substratum adhesion in neuronal morphogenesis. Dev Biol. 1975;44(1):77–91. doi: 10.1016/0012-1606(75)90378-4. [DOI] [PubMed] [Google Scholar]
- Letourneau PC. What happens when growth cones meet neurites: attraction or repulsion? Trends Neurosci. 1987;10(10):390–393. [Google Scholar]
- Li X, Lee AY. Semaphorin 5A and plexin-B3 inhibit human glioma cell motility through RhoGDIalpha-mediated inactivation of Rac1 GTPase. J Biol Chem. 2010;285(42):32436–32445. doi: 10.1074/jbc.M110.120451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L, Jiang P, Shen S, Sato S, Davidson BL, Xing Y. Large-scale analysis of exonized mammalian-wide interspersed repeats in primate genomes. Hum Mol Genet. 2009;18(12):2204–2214. doi: 10.1093/hmg/ddp152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Halloran MC. Central and peripheral axon branches from one neuron are guided differentially by Semaphorin3D and transient axonal glycoprotein-1. J Neurosci. 2005;25(45):10556–10563. doi: 10.1523/JNEUROSCI.2710-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb J. Chemotropism and related phenomena. The Dynamics of Living Matter. New York: The Columbia University Press; 1906. pp. 152–155. [Google Scholar]
- Loeb L. Amoeboid movement, tissue formation and consistency of protoplasm. Am. J Physiol. 1921;56:140–167. doi: 10.1126/science.53.1368.261. [DOI] [PubMed] [Google Scholar]
- Lugaro E. Sul neurotropismo e sui trapianti dei nervi. Riv. di Patol Nerv Ment. 1906;11:320–327. [Google Scholar]
- Luo J, Xu Y, Zhu Q, Zhao F, Zhang Y, Peng X, Wang W, Wang X. Expression pattern of Mical-1 in the temporal neocortex of patients with intractable temporal epilepsy and Pilocarpine-induced rat model. Synapse. 2011 doi: 10.1002/syn.20961. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raible D, Raper JA. Collapsin: a protein in brain that induces the collapse and paralysis of neuronal growth cones. Cell. 1993;75:217–227. doi: 10.1016/0092-8674(93)80064-l. [DOI] [PubMed] [Google Scholar]
- Luo Y, Raper JA. Inhibitory factors controlling growth cone motility and guidance. Curr Opin Neurobiol. 1994;4(5):648–654. doi: 10.1016/0959-4388(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Luo Y, Shepherd I, Li J, Renzi MJ, Chang S, Raper J. A family of molecules related to collapsin in the embryonic chick nervous system. Neuron. 1995;14:1131–1140. doi: 10.1016/0896-6273(95)90261-9. [DOI] [PubMed] [Google Scholar]
- Mann F, Chauvet S, Rougon G. Semaphorins in development and adult brain: Implication for neurological diseases. Prog Neurobiol. 2007;82(2):57–79. doi: 10.1016/j.pneurobio.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Martonosi A, Gouvea MA. Studies on actin. V. Chemical modification of actin. J Biol Chem. 1961;236:1338–1344. [PubMed] [Google Scholar]
- Martz E, Steinberg MS. Contact inhibition of what? An analytical review. J Cell Physiol. 1973;81(1):25–37. doi: 10.1002/jcp.1040810104. [DOI] [PubMed] [Google Scholar]
- Massey V. Introduction: flavoprotein structure and mechanism. Faseb J. 1995;9(7):473–475. doi: 10.1096/fasebj.9.7.7737454. [DOI] [PubMed] [Google Scholar]
- Mayor R, Carmona-Fontaine C. Keeping in touch with contact inhibition of locomotion. Trends Cell Biol. 2010;20(6):319–328. doi: 10.1016/j.tcb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melani M, Weinstein BM. Common factors regulating patterning of the nervous and vascular systems. Annu Rev Cell Dev Biol. 2010;26:639–665. doi: 10.1146/annurev.cellbio.093008.093324. [DOI] [PubMed] [Google Scholar]
- Miao H-Q, Soker S, Feiner L, Alonso JL, Raper JA, Klagsbrun M. Neuropilin-1 mediates collapsin-1/semaphorin III inhibition of endothelial cell motility: Functional competition of collapsin-1 and vascular endothelial growth factor-165. J. Cell Biol. 1999;146:233–241. doi: 10.1083/jcb.146.1.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikule K, Gatlin JC, de la Houssaye BA, Pfenninger KH. Growth cone collapse induced by semaphorin 3A requires 12/15- lipoxygenase. J Neurosci. 2002;22(12):4932–4941. doi: 10.1523/JNEUROSCI.22-12-04932.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milzani A, DalleDonne I, Colombo R. Prolonged oxidative stress on actin. Arch Biochem Biophys. 1997;339(2):267–274. doi: 10.1006/abbi.1996.9847. [DOI] [PubMed] [Google Scholar]
- Mintz CD, Carcea I, McNickle DG, Dickson TC, Ge Y, Salton SR, Benson DL. ERM proteins regulate growth cone responses to Sema3A. J Comp Neurol. 2008;510(4):351–366. doi: 10.1002/cne.21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirabelli F, Salis A, Marinoni V, Finardi G, Bellomo G, Thor H, Orrenius S. Menadione-induced bleb formation in hepatocytes is associated with the oxidation of thiol groups in actin. Arch Biochem Biophys. 1988;264(1):261–269. doi: 10.1016/0003-9861(88)90593-0. [DOI] [PubMed] [Google Scholar]
- Miura K. ERK2-binding domain is required for phosphorylation of EBITEIN1, a potential downstream interactor of ERK2. Biochem Biophys Res Commun. 2008;375(3):367–371. doi: 10.1016/j.bbrc.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Miura K, Imaki J. Identification of ERK2-binding domain of EBITEIN1, a novel ERK2-binding protein. Biochim Biophys Acta. 2008a;1784(9):1319–1325. doi: 10.1016/j.bbapap.2008.05.015. [DOI] [PubMed] [Google Scholar]
- Miura K, Imaki J. Molecular cloning of Ebitein1: a novel extracellular signal-regulated kinase 2-binding protein in testis. Biochem Biophys Res Commun. 2008b;368(2):336–342. doi: 10.1016/j.bbrc.2008.01.078. [DOI] [PubMed] [Google Scholar]
- Moldovan L, Irani K, Moldovan NI, Finkel T, Goldschmidt-Clermont PJ. The actin cytoskeleton reorganization induced by Rac1 requires the production of superoxide. Antioxid Redox Signal. 1999;1(1):29–43. doi: 10.1089/ars.1999.1.1-29. [DOI] [PubMed] [Google Scholar]
- Morinaka A, Yamada M, Itofusa R, Funato Y, Yoshimura Y, Nakamura F, Yoshimura T, Kaibuchi K, Goshima Y, Hoshino M, et al. Thioredoxin Mediates Oxidation-Dependent Phosphorylation of CRMP2 and Growth Cone Collapse. Sci Signal. 2011;4(170):ra26. doi: 10.1126/scisignal.2001127. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Yahara I. The actin-severing activity of cofilin is exerted by the interplay of three distinct sites on cofilin and essential for cell viability. Biochem J. 2002;365(Pt 1):147–155. doi: 10.1042/bj20020231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Littlewood-Evans A. Mechanisms that regulate mechanosensory hair cell differentiation. Trends Cell Biol. 2001;11(8):334–342. doi: 10.1016/s0962-8924(01)02046-3. [DOI] [PubMed] [Google Scholar]
- Murray MJ, Merritt DJ, Brand AH, Whitington PM. In vivo dynamics of axon pathfinding in the Drosophilia CNS: a time-lapse study of an identified motorneuron. J Neurobiol. 1998;37(4):607–621. [PubMed] [Google Scholar]
- Nadella M, Bianchet MA, Gabelli SB, Barrila J, Amzel LM. Structure and activity of the axon guidance protein MICAL. Proc Natl Acad Sci U S A. 2005;102(46):16830–16835. doi: 10.1073/pnas.0504838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai J. The movement of neurons in tissue culture. In: Allen RD, Kamija N, editors. Primitive motile systems in cell biology. New York: Academic Press; 1964. pp. 377–385. [Google Scholar]
- Nakajima S. Selectivity in fasciculation of of nerve fibers in vitro. J Comp Neurol. 1965;125(2):193–195. doi: 10.1002/cne.901250204. [DOI] [PubMed] [Google Scholar]
- Nakatsuji H, Nishimura N, Yamamura R, Kanayama HO, Sasaki T. Involvement of actinin-4 in the recruitment of JRAB/MICAL-L2 to cell-cell junctions and the formation of functional tight junctions. Mol Cell Biol. 2008;28(10):3324–3335. doi: 10.1128/MCB.00144-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Oinuma I, Katoh H. R-Ras as a key player for signaling pathway of Plexins. Mol Neurobiol. 2005;32:217–222. doi: 10.1385/MN:32:3:217. [DOI] [PubMed] [Google Scholar]
- Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8(8):632–645. doi: 10.1038/nrc2404. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sasaki T. Regulation of epithelial cell adhesion and repulsion: role of endocytic recycling. J Med Invest. 2008;55(1–2):9–16. doi: 10.2152/jmi.55.9. [DOI] [PubMed] [Google Scholar]
- Nukazuka A, Fujisawa H, Inada T, Oda Y, Takagi S. Semaphorin controls epidermal morphogenesis by stimulating mRNA translation via eIF2alpha in Caenorhabditis elegans. Genes Dev. 2008;22(8):1025–1036. doi: 10.1101/gad.1644008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuttall RP, Zinsmeister PP. Differential response to contact during embryonic nerve-nonnerve cell interactions. Cell Motil. 1983;3(4):307–320. doi: 10.1002/cm.970030403. [DOI] [PubMed] [Google Scholar]
- Ono S. Mechanism of depolymerization and severing of actin filaments and its significance in cytoskeletal dynamics. Int Rev Cytol. 2007;258:1–82. doi: 10.1016/S0074-7696(07)58001-0. [DOI] [PubMed] [Google Scholar]
- Oser M, Condeelis J. The cofilin activity cycle in lamellipodia and invadopodia. J Cell Biochem. 2009;108(6):1252–1262. doi: 10.1002/jcb.22372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Dai HN, Terman JR, Wahlin KJ, Kim B, Bregman BS, Popovich PG, Kolodkin AL. MICAL flavoprotein monooxygenases: Expression during neural development and following spinal cord injuries in the rat. Mol Cell Neurosci. 2006;31:52–69. doi: 10.1016/j.mcn.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Peterson ER, Crain SM. Preferential growth of neurites from isolated fetal mouse dorsal root ganglia in relation to specific regions of co-cultured spinal cord explants. Devl Brain Res. 1982;2:363–382. doi: 10.1016/0165-3806(81)90044-4. [DOI] [PubMed] [Google Scholar]
- Pini A. Chemorepulsion of axons in the developing mammalian central nervous system. Science. 1993;261:95–98. doi: 10.1126/science.8316861. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Borisy GG. Cellular motility driven by assembly and disassembly of actin filaments. Cell. 2003;112(4):453–465. doi: 10.1016/s0092-8674(03)00120-x. [DOI] [PubMed] [Google Scholar]
- Porter SL, Wadhams GH, Armitage JP. Signal processing in complex chemotaxis pathways. Nat Rev Microbiol. 2011;9(3):153–165. doi: 10.1038/nrmicro2505. [DOI] [PubMed] [Google Scholar]
- Potiron V, Nasarre P, Roche J, Healy C, Boumsell L. Semaphorin signaling in the immune system. Adv Exp Med Biol. 2007;600:132–144. doi: 10.1007/978-0-387-70956-7_11. [DOI] [PubMed] [Google Scholar]
- Puschel AW. GTPases in semaphorin signaling. Adv Exp Med Biol. 2007;600:12–23. doi: 10.1007/978-0-387-70956-7_2. [DOI] [PubMed] [Google Scholar]
- Rahajeng J, Giridharan SS, Naslavsky N, Caplan S. Collapsin response mediator protein-2 (Crmp2) regulates trafficking by linking endocytic regulatory proteins to dynein motors. J Biol Chem. 2010;285(42):31918–31922. doi: 10.1074/jbc.C110.166066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA, Bastiani M, Goodman CS. Pathfinding by neuronal growth cones in grasshopper embryos. II. Selective fasciculation onto specific axonal pathways. J Neurosci. 1983;3(1):31–41. doi: 10.1523/JNEUROSCI.03-01-00031.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper JA, Grunewald EB. Temporal retinal growth cones collapse on contact with naasal retinal axons. Exp. Neurol. 1990;109:70–74. doi: 10.1016/s0014-4886(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Raper JA, Kapfhammer JP. The enrichment of a neuronal growth cone collapsing activity from embryonic chick brain. Neuron. 1990;4:21–29. doi: 10.1016/0896-6273(90)90440-q. [DOI] [PubMed] [Google Scholar]
- Reier PJ, Stensaas LJ, Guth L. The astrocytic scar as an impediment to regeneration in the central nervous system. In: CC Kao, Bunge RP, Reier PJ., editors. Spinal Cord Reconstruction. New York: Raven Press; 1983. pp. 163–195. [Google Scholar]
- Roche FK, Marsick BM, Letourneau PC. Protein synthesis in distal axons is not required for growth cone responses to guidance cues. J Neurosci. 2009;29(3):638–652. doi: 10.1523/JNEUROSCI.3845-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen WG. Cellular chemotropism and chemotaxis. Q Rev Biol. 1962;37:242–259. doi: 10.1086/403657. [DOI] [PubMed] [Google Scholar]
- Roth L, Koncina E, Satkauskas S, Cremel G, Aunis D, Bagnard D. The many faces of semaphorins: from development to pathology. Cell Mol Life Sci. 2009;66(4):649–666. doi: 10.1007/s00018-008-8518-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai JA, Halloran MC. Semaphorin 3d guides laterality of retinal ganglion cell projections in zebrafish. Development. 2006;133(6):1035–1044. doi: 10.1242/dev.02272. [DOI] [PubMed] [Google Scholar]
- Sakane A, Honda K, Sasaki T. Rab13 regulates neurite outgrowth in PC12 cells through its effector protein, JRAB/MICAL-L2. Mol Cell Biol. 2010;30(4):1077–1087. doi: 10.1128/MCB.01067-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlatter MC, Buhusi M, Wright AG, Maness PF. CHL1 promotes Sema3A–induced growth cone collapse and neurite elaboration through a motif required for recruitment of ERM proteins to the plasma membrane. J Neurochem. 2008;104(3):731–744. doi: 10.1111/j.1471-4159.2007.05013.x. [DOI] [PubMed] [Google Scholar]
- Schmidt EF, Shim SO, Strittmatter SM. Release of MICAL autoinhibition by semaphorin-plexin signaling promotes interaction with collapsin response mediator protein. J Neurosci. 2008;28(9):2287–2297. doi: 10.1523/JNEUROSCI.5646-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Strittmatter SM. The CRMP family of proteins and their role in Sema3A signaling. Adv Exp Med Biol. 2007;600:1–11. doi: 10.1007/978-0-387-70956-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmoller KM, Semmrich C, Bausch AR. Slow down of actin depolymerization by cross-linking molecules. J Struct Biol. 2011;173(2):350–357. doi: 10.1016/j.jsb.2010.09.003. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Kapfhammer CE, Bandtlow CE. Inhibitors of neurite growth. Annu. Rev. Neurosci. 1993;16:565–595. doi: 10.1146/annurev.ne.16.030193.003025. [DOI] [PubMed] [Google Scholar]
- Schwab ME, Thoenen H. Dissociated neurons regenerate into sciatic but not optic nerve explants in culture irrespective of neurotrophic factors. J Neurosci. 1985;5(9):2415–2423. doi: 10.1523/JNEUROSCI.05-09-02415.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GA, McClelland LA, Fricke AF, Fender A. Plexin C1, a receptor for semaphorin 7a, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol. 2009;129(4):954–963. doi: 10.1038/jid.2008.329. [DOI] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424(6947):391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Sharma M, Giridharan SS, Rahajeng J, Naslavsky N, Caplan S. MICAL-L1 links EHD1 to tubular recycling endosomes and regulates receptor recycling. Mol Biol Cell. 2009;20(24):5181–5194. doi: 10.1091/mbc.E09-06-0535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A, Mammoto A, Italiano JE, Jr, Pravda E, Dudley AC, Ingber DE, Klagsbrun M. ABL2/ARG tyrosine kinase mediates SEMA3F–induced RhoA inactivation and cytoskeleton collapse in human glioma cells. J Biol Chem. 2008;283(40):27230–27238. doi: 10.1074/jbc.M804520200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebold C, Berrow N, Walter TS, Harlos K, Owens RJ, Stuart DI, Terman JR, Kolodkin AL, Pasterkamp RJ, Jones EY. High-resolution structure of the catalytic region of MICAL (molecule interacting with CasL), a multidomain flavoenzyme-signaling molecule. Proc Natl Acad Sci U S A. 2005;102(46):16836–16841. doi: 10.1073/pnas.0504997102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser NR, Peterson ER, Crain SM. Neurites from mouse retina and dorsal root ganglion explants show specific behavior within co-cultured tectum or spinal cord. Brain Res. 1981;208(2):499–505. doi: 10.1016/0006-8993(81)90584-9. [DOI] [PubMed] [Google Scholar]
- Speidel CC. Studies of living nerves. II. Activities of ameboid growth cones, sheath cells, and myelin segments, as revealed by prolonged observation of individual nerve fibers in frog tadpoles. Am J Anat. 1933;52(1):1–79. [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl. Acad. Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Dai H, Zhang J, Jin X, Xiong S, Xu J, Wu J, Shi Y. Solution structure of calponin homology domain of Human MICAL-1. J Biomol NMR. 2006;36(4):295–300. doi: 10.1007/s10858-006-9062-5. [DOI] [PubMed] [Google Scholar]
- Sutherland JD, Witke W. Molecular genetic approaches to understanding the actin cytoskeleton. Curr Opin Cell Biol. 1999;11(1):142–151. doi: 10.1016/s0955-0674(99)80018-0. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Kumanogoh A, Kikutani H. Semaphorins and their receptors in immune cell interactions. Nat Immunol. 2008;9(1):17–23. doi: 10.1038/ni1553. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Nakamoto T, Ogawa S, Seo S, Matsumura T, Tachibana K, Morimoto C, Hirai H. MICAL, a novel CasL interacting molecule, associates with vimentin. J Biol Chem. 2002;277(17):14933–14941. doi: 10.1074/jbc.M111842200. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Fournier A, Nakamura F, Wang L-H, Murakami Y, Kalb RG, Fujisawa H, Strittmatter SM. Plexin-neuropilin-1 complexes form functional semaphorin-3A receptors. Cell. 1999;99:59–69. doi: 10.1016/s0092-8674(00)80062-8. [DOI] [PubMed] [Google Scholar]
- Takamatsu H, Takegahara N, Nakagawa Y, Tomura M, Taniguchi M, Friedel RH, Rayburn H, Tessier-Lavigne M, Yoshida Y, Okuno T, et al. Semaphorins guide the entry of dendritic cells into the lymphatics by activating myosin II. Nat Immunol. 2010;11(7):594–600. doi: 10.1038/ni.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagnone L, Artigiani S, Chen H, He Z, Ming G-L, Song H-J, Chedotal A, Winberg ML, Goodman CS, Poo M-M, et al. Plexins are a large family of receptors for transmembrane, secreted, and GPI-anchored semaphorins in vertebrates. Cell. 1999;99:71–80. doi: 10.1016/s0092-8674(00)80063-x. [DOI] [PubMed] [Google Scholar]
- Tang JX, Janmey PA, Stossel TP, Ito T. Thiol oxidation of actin produces dimers that enhance the elasticity of the F-actin network. Biophys J. 1999;76(4):2208–2215. doi: 10.1016/S0006-3495(99)77376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terai T, Nishimura N, Kanda I, Yasui N, Sasaki T. JRAB/MICAL-L2 is a junctional Rab13-binding protein mediating the endocytic recycling of occludin. Mol Biol Cell. 2006;17(5):2465–2475. doi: 10.1091/mbc.E05-09-0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terman JR, Mao T, Pasterkamp RJ, Yu HH, Kolodkin AL. MICALs, a family of conserved flavoprotein oxidoreductases, function in plexin-mediated axonal repulsion. Cell. 2002;109(7):887–900. doi: 10.1016/s0092-8674(02)00794-8. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Connelly PS, Vranich KA, Shaw MK, Guild GM. Why are two different cross-linkers necessary for actin bundle formation in vivo and what does each cross-link contribute? J Cell Biol. 1998;143(1):121–133. doi: 10.1083/jcb.143.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, DeRosier DJ. How to make a curved Drosophila bristle using straight actin bundles. Proc Natl Acad Sci U S A. 2005;102(52):18785–18792. doi: 10.1073/pnas.0509437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Guild GM. F actin bundles in Drosophila bristles. I. Two filament cross-links are involved in bundling. J Cell Biol. 1995;130(3):629–638. doi: 10.1083/jcb.130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7(1):117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Growth cone morphology and trajectory in the lumbosacral region of the chick embryo. J Neurosci. 1985;5(9):2345–2358. doi: 10.1523/JNEUROSCI.05-09-02345.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. FARP2 triggers signals for Sema3A–mediated axonal repulsion. Nat Neurosci. 2005;8(12):1712–1719. doi: 10.1038/nn1596. [DOI] [PubMed] [Google Scholar]
- Tran TS, Kolodkin AL, Bharadwaj R. Semaphorin regulation of cellular morphology. Annu Rev Cell Dev Biol. 2007;23:263–292. doi: 10.1146/annurev.cellbio.22.010605.093554. [DOI] [PubMed] [Google Scholar]
- Tran-Van H, Avota E, Bortlein C, Mueller N, Schneider-Schaulies S. Measles virus modulates dendritic cell/T-cell communication at the level of plexinA1/neuropilin-1 recruitment and activity. Eur J Immunol. 2011;41(1):151–163. doi: 10.1002/eji.201040847. [DOI] [PubMed] [Google Scholar]
- Turner LJ, Hall A. Plexin-induced collapse assay in COS cells. Methods Enzymol. 2006;406:665–676. doi: 10.1016/S0076-6879(06)06052-6. [DOI] [PubMed] [Google Scholar]
- Turner LJ, Nicholls S, Hall A. The activity of the plexin-A1 receptor is regulated by Rac. J Biol Chem. 2004;279(32):33199–33205. doi: 10.1074/jbc.M402943200. [DOI] [PubMed] [Google Scholar]
- Usui H, Taniguchi M, Yokomizo T, Shimizu T. Plexin-A1 and plexin-B1 specifically interact at their cytoplasmic domains. Biochem Biophys Res Commun. 2003;300(4):927–931. doi: 10.1016/s0006-291x(02)02966-2. [DOI] [PubMed] [Google Scholar]
- Varshavsky A, Kessler O, Abramovitch S, Kigel B, Zaffryar S, Akiri G, Neufeld G. Semaphorin-3B is an angiogenesis inhibitor that is inactivated by furin-like pro-protein convertases. Cancer Res. 2008;68(17):6922–6931. doi: 10.1158/0008-5472.CAN-07-5408. [DOI] [PubMed] [Google Scholar]
- Vastrik I, Eickholt BJ, Walsh FS, Ridley A, Doherty P. Sema3A–induced growth-cone collapse is mediated by Rac1 amino acids 17- 32. Curr Biol. 1999;9(18):991–998. doi: 10.1016/s0960-9822(99)80447-3. [DOI] [PubMed] [Google Scholar]
- Verna J-M. In vitro analysis of interactions between sensory neurons and skin: evidence for selective innervation of dermis and epidermis. J Embryol Exp Morphol. 1985;86:53–70. [PubMed] [Google Scholar]
- Vikis HG, Li W, Guan KL. The Plexin-B1/Rac interaction inhibits PAK activation and enhances Sema4D ligand binding. Genes Dev. 2002;16(7):836–845. doi: 10.1101/gad.966402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter J, Henke-Fahle S, Bonhoeffer F. Avoidance of posterior tectal membranes by temporal retinal axons. Development. 1987a;101:909–913. doi: 10.1242/dev.101.4.909. [DOI] [PubMed] [Google Scholar]
- Walter J, Kern-Veits R, Huf J, Stolze B, Bonhoeffer F. Recognition of position-specific properties of tectal cell membranes by retinal axons in vitro. Development. 1987b;101:685–696. doi: 10.1242/dev.101.4.685. [DOI] [PubMed] [Google Scholar]
- Walzer T, Galibert L, Comeau MR, De Smedt T. Plexin C1 engagement on mouse dendritic cells by viral semaphorin A39R induces actin cytoskeleton rearrangement and inhibits integrin-mediated adhesion and chemokine-induced migration. J Immunol. 2005;174(1):51–59. doi: 10.4049/jimmunol.174.1.51. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D, Kidd T, Goodman CS, Henzel W, Tessier-Lavigne M. Biochemical purification of a mammalian Slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Weide T, Teuber J, Bayer M, Barnekow A. MICAL-1 isoforms, novel rab1 interacting proteins. Biochem Biophys Res Commun. 2003;306(1):79–86. doi: 10.1016/s0006-291x(03)00918-5. [DOI] [PubMed] [Google Scholar]
- Weiss P. The problem of specificity in growth and development. Yale J Biol Med. 1947;19(3):235–278. [PMC free article] [PubMed] [Google Scholar]
- Weiss P. Cell contact. Int Rev Cytol. 1958;7:391–423. [Google Scholar]
- Wessells NK, Letourneau PC, Nuttall RP, Luduena-Anderson M, Geiduschek JM. Responses to cell contacts between growth cones, neurites and ganglionic non-neuronal cells. J Neurocytol. 1980;9(5):647–664. doi: 10.1007/BF01205031. [DOI] [PubMed] [Google Scholar]
- Wessells NK, Spooner BS, Ash JF, Bradley MO, Luduena MA, Taylor EL, Wrenn JT, Yamaa K. Microfilaments in cellular and developmental processes. Science. 1971;171(967):135–143. doi: 10.1126/science.171.3967.135. [DOI] [PubMed] [Google Scholar]
- Wierenga RK, Terpstra P, Hol WG. Prediction of the occurrence of the ADP-binding beta alpha beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187(1):101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- Winberg ML, Noordermeer JN, Tamagnone L, Comoglio PM, Spriggs MK, Tessier-Lavigne M, Goodman CS. Plexin A is a neuronal semaphorin receptor that controls axon guidance. Cell. 1998;95:903–916. doi: 10.1016/s0092-8674(00)81715-8. [DOI] [PubMed] [Google Scholar]
- Windle WF. In: Regeneration in the central nervous system. Windle WF, editor. Springfield: Charles C. Thomas; 1955. [Google Scholar]
- Wolf EP. Experimental Studies on Inflammation : I. The Influence of Chemicals Upon the Chemotaxis of Leucocytes in Vitro. J Exp Med. 1921;34(4):375–396. doi: 10.1084/jem.34.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci. 2006;26(5):1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu XM, Fisher DA, Zhou L, White FA, Ng S, Snider WD, Luo Y. The transmembrane protein semaphorin 6A repels embryonic sympathetic axons. J Neurosci. 2000;20(7):2638–48. doi: 10.1523/JNEUROSCI.20-07-02638.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Kuok C, Xiao A, Zhu Z, Lin S, Zhang B. Identification and expression analysis of mical family genes in zebrafish. J Genet Genomics. 2010;37(10):685–693. doi: 10.1016/S1673-8527(09)60086-2. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Spooner BS, Wessells NK. Axon Growth: Roles of microfilaments and microtubules. Proc Natl Acad Sci U S A. 1970;66:1206–1212. doi: 10.1073/pnas.66.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Spooner BS, Wessells NK. Ultrastructure and function of growth cones and axons of cultured nerve cells. J Cell Biol. 1971;49(3):614–635. doi: 10.1083/jcb.49.3.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamura R, Nishimura N, Nakatsuji H, Arase S, Sasaki T. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Mol Biol Cell. 2008;19(3):971–983. doi: 10.1091/mbc.E07-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdani U, Terman JR. The Semaphorins. Genome Biol. 2006;7:211–225. doi: 10.1186/gb-2006-7-3-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon J, Terman JR. Disassembling F-actin networks in vivo through manipulations of Mical and actin bundling proteins. submitted. [Google Scholar]
- Yoon MG. Reciprocal transplantations between the optic tectum and the cerebellum in adult goldfish. J Physiol. 1979;288:211–225. [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Araj HH, Ralls SA, Kolodkin AL. The transmembrane Semaphorin Sema I is required in Drosophila for embryonic motor and CNS axon guidance. Neuron. 1998;20(2):207–220. doi: 10.1016/s0896-6273(00)80450-x. [DOI] [PubMed] [Google Scholar]
- Yukawa K, Tanaka T, Bai T, Ueyama T, Owada-Makabe K, Tsubota Y, Maeda M, Suzuki K, Kikutani H, Kumanogoh A. Semaphorin 4A induces growth cone collapse of hippocampal neurons in a Rho/Rho-kinase-dependent manner. Int J Mol Med. 2005;16(1):115–118. [PubMed] [Google Scholar]
- Zhou Y, Adolfs Y, Pijnappel WW, Fuller SJ, Van der Schors RC, Li KW, Sugden PH, Smit AB, Hergovich A, Pasterkamp RJ. MICAL-1 is a Negative Regulator of MST-NDR Kinase Signaling and Apoptosis. Mol Cell Biol. 2011 doi: 10.1128/MCB.01389-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Gunput RA, Pasterkamp RJ. Semaphorin signaling: progress made and promises ahead. Trends Biochem Sci. 2008;33(4):161–170. doi: 10.1016/j.tibs.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Zinsmeister PP, Nuttall RP. Age and cell type influences on nerve--non-nerve contact interactions. Devl. Brain Res. 1986;25(1):143–146. doi: 10.1016/0165-3806(86)90162-8. [DOI] [PubMed] [Google Scholar]


