Abstract
Establishment of sensitive methods for the detection of cellular sterols and their derivatives is a critical step in developing comprehensive lipidomics technology. We demonstrate that electrospray ionization tandem (triple quadrupole) mass spectrometry (ESI-MS/MS) is an efficient method for monitoring steryl glucosides (SGs) and acyl steryl glucosides (ASGs). Comparison of analysis of SGs and ASGs by ESI-MS/MS with analysis by gas chromatography with flame ionization detection (GC-FID) shows that the two methods yield similar molar compositions. These data demonstrate that ESI-MS/MS response per molar amount of sterol conjugate is similar among various molecular species of SGs and ASGs. Application of ESI-MS/MS to seed samples from wild-type Arabidopsis and a mutant deficient in two UDP-glucose:sterol glucosyltransferases, UGT80A2 and UGT80B1, revealed new details on the composition of sitosteryl, campesteryl and stigmasteryl glucosides and ASGs. SGs were decreased by 86% in the ugt80A2,B1 double mutant, compared to wild-type, while ASGs were reduced 96%. The results indicate that these glucosyltransferases account for much of the accumulation of the sterol conjugates in wild-type Arabidopsis seeds.
Keywords: Steryl glucosides, Acyl steryl glucosides, Sterols, Electrospray ionization tandem mass spectrometry, Gas chromatography, Arabidopsis thaliana, UDP-glucose:sterol glucosyltransferase, ugt80A2, B1, UGT80A2, UGT80B1
Introduction
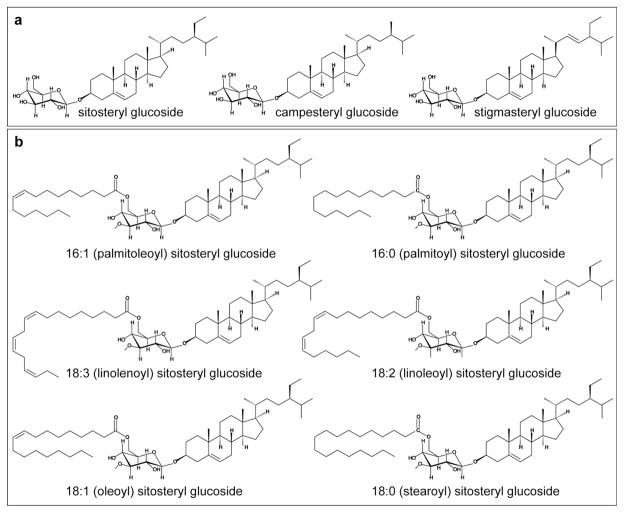
Sterols are ubiquitous constituents of eukaryotic cells. In contrast to animals and fungi, which contain primarily cholesterol and ergosterol, respectively, plants synthesize a complex mixture of sterols comprised of sitosterol, campesterol, stigmasterol and additional minor sterols that include cholesterol [1]. While sitosterol and stigmasterol are implicated in membrane functions [2, 3], campesterol is the precursor to the brassinosteroids, which are plant steroid hormones required for cell division and cell expansion [4, 5]. In plants, steryl glycosides (SG) and acyl steryl glycosides (ASG) are major derivatives of sterols [6–9]. Variation in SGs originates from differences in the type of sterol, the sugar, the configuration of the linkage, number of sugar groups, and acylation of the sugar(s). In the most common form of SG, a sugar monomer, usually the pyranose form of D-glucose, is attached to the 3β-hydroxy group on the C3 of the sterol (Fig. 1a). Acylation may occur at the C6 of the sugar moiety with fatty acids (Fig. 1b), palmitic (16:0) being most common.
Figure 1.
Molecular structures for SGs and ASGs. (a) SG structures are shown for sitosteryl, campesteryl, and stigmasteryl glucosides. The pyranose form of D-glucose is covalently attached to the 3β-hydroxy group on C3 of the A ring of the sterol. (b) ASG structures are shown for sitosteryl glucosides with various acyl chain modifications: 16:1 (palmitoleic), 16:0 (palmitic), 18:3 (linolenic), 18:2 (linoleic), 18:1 (oleic) and 18:0 (stearic) acids. The acyl modification occurs on C6 of the D-glucose monomer that is attached to the sterol.
As sterol conjugates, SGs and ASGs, which are biosynthetically interconvertible, serve as membrane components, storage forms of sterols, transporters, and/or signaling molecules in plants [6], and one report has suggested the role of SGs as primers in the synthesis of cellulose [10]. SGs are synthesized by UDP-glucose:sterol glucosyltransferases (UGTs) which catalyze the glucosylation of the 3β-hydroxy group of sterols to produce 3-β-D-glucosides [11, 12]. Arabidopsis ugt80A2,B1, double mutant for UGT80A2 and UGT80B1, was shown to have a significant reduction in SG and ASG levels in several plant tissues [13]. These mutants display an array of phenotypes in the seed, including small embryo size, transparent testa, defects in flavonoid deposition, loss of the cuticle layer, and a decrease in aliphatic suberin and cutin-like polymers. Characterization of the ugt80A2,B1 mutants has indicated a role for SGs and ASGs in trafficking of lipid polyester precursors in seeds [13].
After isolation of SGs and ASGs from tissues, gas chromatography (GC) can be used to quantify these lipids. To perform the analysis, the SGs and ASGs are each hydrolyzed to yield sterols and trans-esterified to yield fatty acid methyl esters. Gas chromatography with flame ionization (GC-FID) is quantitative, but the classes need to be isolated and hydrolyzed/transesterified. In this report we demonstrate the detection of underivatized SG and ASG conjugates of sitosterol, campesterol, and stigmasterol from seed samples using electrospray ionization tandem (triple quadrupole) mass spectrometry (ESI-MS/MS). Mass spectrometry methods, exemplified by ESI-MS/MS, have enabled high sensitivity and high mass resolution specificity for the identification and quantification of lipids from diverse biological samples [14]. Previously it was shown that ESI-MS/MS can be utilized to efficiently quantify phospholipids and polar glycerolipids from Arabidopsis [15, 16].
In the present study we show that ESI-MS/MS is a simple and rapid detection method that is advantageous over GC methods for the analysis of steryl glycolipids, since there is no need for derivatization, and quantitative information about the specific SG and ASG molecular species is readily obtained. Using seed samples from ugt80A2,B1 mutants and wild-type, ESI-MS/MS analysis has revealed new details about SG and ASG molecular species, adding to our understanding of the roles of the UGT80 glucosyltransferases in Arabidopsis seeds.
Experimental Procedure
Plant materials and growth conditions
Wild-type and mutant Arabidopsis thaliana lines were in the Wassilewskija (WS)-O background. ugt80A2,B1, double mutant for UGT80A2 (At3g07020) and UGT80B1 (At1g43620), was previously described [13]. Plants were grown on soil comprised of 7:3:4 Metro-Mix 380:Vermiculite (Therm-O-Rock #10-2200):Perlite (Therm-O-Rock #10-1123) (Hummert International, Topeka, KS) under continuous light at 23°C and 70% humidity in a standard growth chamber. Dry seeds were harvested at eight weeks. For the comparison of ESI-MS/MS to GC-FID analysis, mixtures of isolated soybean-derived SGs (catalog #1117: 54% sitosteryl glucoside, 27% campesteryl glucoside, 17% stigmasteryl glucoside, 1% Δ5-avenasteryl glucoside) and ASGs (catalog #1118: esterified steryl glucosides, fatty acid composition: 34% 16:0, 8% 18:0, 8% 18:1, 36% 18:2, 4% 18:3, 1% 20:0, 4% 22:0, 2% 23:0, 2% 24:0 and 1% others; According to the Matreya LLC 2011-2012 catalog, “actual composition may vary according to dietary history and condition of the source”) were purchased from Matreya LLC, Pleasant Gap, PA.
Lipid extraction from seeds
Seeds were extracted by a modification of the method of Bligh and Dyer [17]. 100 Arabidopsis seeds were added to 2.0 ml isopropanol with 0.01% butylated hydroxytoluene at 75°C; the heating at 75°C was continued for 15 min, before cooling to room temperature. To the seed-solvent mixture precise amounts of internal standards: 10 nmol of di12:0 PtdGro and 10 nmol of di20:0 (diphytanoyl) PtdGro (Avanti Polar Lipids, Alabaster, AL) were added. The mixture was then transferred to a Dounce homogenizer, homogenized to allow for complete and consistent extraction of lipids, and transferred to a glass tube. The homogenizer was rinsed with 2.0 ml each of chloroform and methanol to fully recover the sample, and the rinse was combined with the isopropanol-seed mixture. 1.6 ml water was added and the one-phase mixture was shaken. An additional 1.0 ml each of chloroform and water were added, followed by shaking and centrifugation to form two phases. The lower layer was removed. 2.0 ml of chloroform were added, the tube was shaken and centrifuged, and the lower layer was removed. This was repeated and the three lower layers were combined. 1.0 ml of 1 M KCl was added, the mixture shaken and centrifuged for 10 min, and the upper layer was removed and discarded. A second wash was performed with 1.0 ml water, followed by evaporation of the solvent. Samples were dissolved in 1 ml chloroform and 5–15 μl of each of these samples were added to 1.2 ml chloroform/methanol/300 mM ammonium acetate in water (300/665/35) for ESI-MS/MS analysis.
Gas chromatography
The SG mixture (#1117, Matreya LLC, Pleasant Gap, PA) and the ASG mixture (#1118, Matreya LLC, Pleasant Gap, PA), both isolated from soybean by the commercial provider, were prepared for sterol analysis according to Rintoul et al. [18]. SGs (40–100 μg) were hydrolyzed by heating at 70°C for 1 h in 2.5 ml 2 M sodium hydroxide in methanol/water (5:1). 2.0 ml water was added and the sample was extracted twice with pentane. Combined pentane extracts were dried with sodium sulfate. The pentane was evaporated, and the samples were redissolved in 100 μl pentane, of which 1 μl was analyzed. Sterols were analyzed on a Hewlett Packard 5890A gas chromatograph on a 15-m, 0.25 mm inner diameter, Rtx-65TG column (#17005 Restek; distributed by Alltech, State College, PA). Injection was in the splitless mode, with helium as the carrier gas with a head pressure of 125 kPa and a flow of 54 ml/min. The oven temperature was maintained isothermally at 240°C and the injector and detector temperatures were at 300°C. Total analysis time was 12 min with the major plant sterols eluting between 8 and 11 min. Detection was by flame ionization. Peak areas were integrated with a Beckmann 427 integrator. The areas were divided by the molecular weights before determining the mole percentage of each sterol.
The ASG mixture (#1118, Matreya LLC, Pleasant Gap, PA) was prepared for analysis of fatty acid composition by converting the acyl groups to fatty acid methyl esters. ASG (~200 μg) in 1.0 ml 3 M methanolic HCl was heated under nitrogen at 78°C for 1 h. 2.0 ml water was added, followed by two extractions with pentane. Water was removed from the combined extracts with sodium sulfate, pentane was evaporated, and the sample was dissolved in ~30 μl of carbon disulfide, of which 1 μl was analyzed. Fatty acid methyl esters were analyzed on a Hewlett Packard 5890A gas chromatograph, on a 30-m SP2380 column with a 0.32 mm inner diameter (#2-4115, Supelco, Bellefonte, PA). Injection was in the splitless mode with helium as the carrier gas with a head pressure of 50 kPa and a flow of 52 ml/min. The oven temperature was maintained isothermally at 160°C, and the injector and detector temperatures were 220°C. Detection was by flame ionization. Total analysis time was 25 min with the fatty acid methyl esters of interest eluting between 13 and 21 min. Peak areas were integrated with a Beckmann 427 integrator. The areas were divided by the molecular weights before determining the mole percentage of each fatty acid methyl ester. To calculate mole % of SGs and ASGs for the spike-in experiment, the signals (in weight %) were adjusted for the molecular weights of the free sterols and fatty acid methyl esters, and a random distribution of fatty acids on the various sterols in ASGs was assumed.
Electrospray ionization tandem mass spectrometry
Purified SGs or ASGs (Matreya LLC, Pleasant Gap, PA) or unfractionated lipid extracts were introduced by continuous infusion into the ESI source on a triple quadrupole MS/MS (4000 QTrap, Applied Biosystems, Foster City, CA). 5–15 μl of Arabidopsis extracts or 1–20 nmol of purified SG or ASG in 1.2 ml of chloroform/methanol/300 mM ammonium acetate in water (300/665/35) were introduced by an autosampler (LC Mini PAL, CTC Analytics AG, Zwingen, Switzerland) fitted with the required injection loop for the acquisition time and presented to the ESI needle at 30 μl/min. Targeted methods for analysis of internal standards, SG and ASG lipid species were employed. In the Arabidopsis samples, the internal standards (di12:0 PtdGro and di20:0 PtdGro) were detected with a scan for neutral loss of the head group moiety, NL 189.04 (C3H9O6P + NH3) in positive mode. In all samples, SGs were revealed with a scan for neutral loss of the hexose moiety, NL 197.09 (C6H12O6 + NH3) in the positive mode, and ASG lipid species were detected with the following neutral loss scans for the hexose moiety acylated with a particular fatty acid: 16:1, NL 433.30 (C22H40O7 + NH3); 16:0, NL 435.32 (C22H42O7 + NH3); 18:3, NL 457.30 (C24H40O7 + NH3); 18:2, NL 459.32 (C24H42O7 + NH3); 18:1, NL 461.34 (C24H44O7 + NH3); and 18:0, NL 463.35 (C24H46O7 + NH3). While adducts other than the [M + NH4]+ can be formed by ESI, the addition of ammonium acetate to the lipid mixture enhances the formation of [M + NH4]+, and scanning for NL of the ammonia-containing hexose or acylated hexose fragments is critical to assure that [M + NH4]+ adducts are the detected precursors.
The collision gas pressure was set at 2 (arbitrary units). Collision energies, with nitrogen in the collision cell, were +21 V for SGs and +30 V for ASGs. Declustering potentials were +65 V for SGs and +100 V for ASGs. Entrance potentials were +10 V for SGs and +14 V for ASGs. Exit potentials were +10 V for SGs and +14 V for ASGs. The mass analyzers were adjusted to a resolution of 0.7 u full width at half height. For each spectrum, 400 continuum scans were averaged in multiple channel analyzer (MCA) mode. The source temperature (heated nebulizer) was 100°C, the interface heater was on, +5.5 kV was applied to the electrospray capillary, the curtain gas was set at 20 (arbitrary units), and the ion source gases were set at 45 (GS1) and 45 (GS2) (arbitrary units). Mass spectra were detected by fragment ion counting.
For each spectrum, background subtraction, data smoothing and peak area integration were performed using a custom script and Applied Biosystems Analyst software. Within each spectra, the data were isotopically deconvoluted based on the sterol portion of SGs and ASGs. For purified SG and ASG samples, the signals for containing each indicated sterol or fatty acid group were summed and divided by the total signal to obtain mol fractions. For Arabidopsis samples, the sterol derivatives in each class were quantified in comparison to the two internal standards (di12:0 PtdGro and di20:0 PtdGro) using LipidomeDB Data Calculation Environment at the website http://lipidome.bcf.ku.edu:9000/Lipidomics/ [19]. The SG and ASG signals were normalized such that a signal of 1.0 represents a signal equal to the average signal of 1.0 nmol of di12:0 PtdGro and di20:0 PtdGro, and were additionally corrected for isotopic overlap between head groups (NL fragments).
Total phosphatidylcholine (PtdCho) and phosphatidylethanolamine (PtdEtn) of the wild-type and ugt80A2,B1 seed extracts were analyzed using 10 μl of extract and the mass spectral conditions described in Devaiah et al. [16], considering the amounts for all the PtdCho species in that publication except minor species PtdCho(36:1) and all the PtdEtns, except minor species PtdEtn(34:1), PtdEtn(36:1), PtdEtn(38:6), and PtdEtn(42:4). The PtdCho and PtdEtn mass spectral signals were quantified in relation to the same PtdGro internal standards used for the SG and ASG analysis.
Spike-in experiment
Varying quantities of soybean SGs containing various known amounts of each glucoside (as determined by GC analysis of derived sterols) in varying quantities were spiked into 5 μl from 1 ml of extract from 100 wild-type seeds and into 5 μl of 1 ml of extract from 100 mutant seeds (each containing 50 pmol di12:0 and di20:0 PtdGro internal standards). ASGs containing known concentrations of 18:2 and 16:0 ASGs (as determined by GC analysis of fatty acids and sterols and assuming random combinations into ASGs) in varying quantitities were spiked into 15 μl of 1 ml of extract from 100 wild-type seeds and 15 μl of 1 ml extract from 100 mutant seeds (each containing 150 pmol di12:0 and di20:0 PtdGro internal standards). The samples were analyzed by ESI-MS/MS and the data processed as described above, except 939 to 1000 continuum scans for each analysis were averaged in multiple channel analyzer (MCA) mode. Five biological replicate analyses were performed for SGs and each ASG. Signals for SG and ASG analytes, normalized to the average signal for the internal standards in the analyzed sample, are shown.
Results
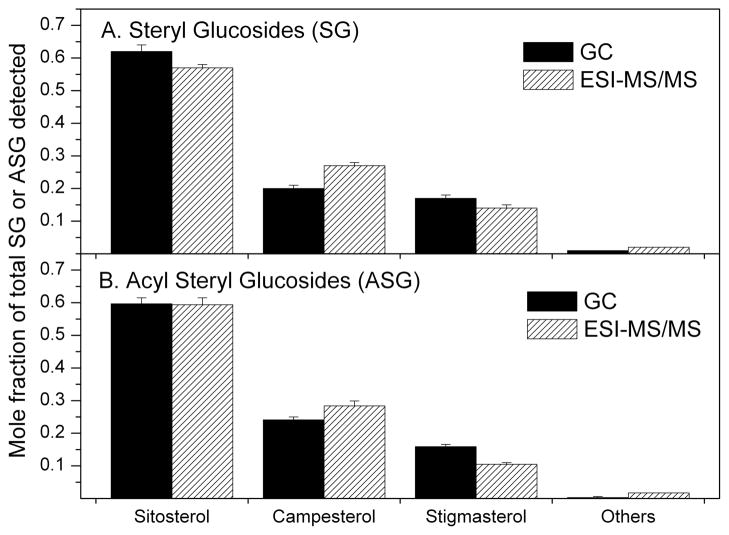
A direct infusion ESI-MS/MS approach was used to detect and quantify SGs and ASGs containing sitosterol, campesterol and stigmasterol (Fig. 1). A major advantage of this approach is that it is not necessary to separate SGs and ASGs from other compounds prior to analysis. Both SGs and ASGs can be analyzed as [M + NH4]+ ions, and detected in positive mode by a series of neutral loss scans specific for the hexose or acyl hexose moieties. SGs and ASGs with greater than one sugar are very rare [7] and we did not target these compounds in our analysis. Table 1 shows the mass spectral scan values that were used to detect SG and ASG molecules. A previous report on analysis of cholesteryl esters using ESI-MS/MS indicated that mass spectral response varied considerably among steryl esters with different acyl groups [20]. To determine whether various SG and ASG molecular species have variable mass spectral responses, isolated soybean-derived SG and ASG mixtures from a commercial source were separately analyzed by GC-FID and the results were compared with those obtained by ESI-MS/MS. The data indicate that, although there was a slight tendency of the mass spectrometer to be more sensitive to SGs and ASGs containing campesterol relative to those containing the sitosterol and stigmasterol, the mole fractions of each sterol in the SG and ASG mixtures detected by the two methods were very similar (Fig. 2). Sitosterol was the most prominent sterol found in both commercially-obtained soybean SGs and ASGs. Campesterol and stigmasterol were the other major sterols, while minor sterols comprised ~2% or less.
Table 1.
Mass spectral scan values for steryl glucosides and acyl steryl glucosides.
| Class | Formula [M] | Scan mode | Mass of intact ion analyzed [M+NH4]+ (m/z) |
|---|---|---|---|
| Steryl glucoside (SG) | |||
| Campesteryl | C34H58O6 | +NL 197.09 | 580.46 |
| Stigmasteryl | C35H58O6 | +NL 197.09 | 592.46 |
| Sitosteryl | C35H60O6 | +NL 197.09 | 594.47 |
| Acyl steryl glucoside (ASG) | |||
| 16:1 campesteryl | C50H86O7 | +NL 433.30 | 816.67 |
| 16:1 stigmasteryl | C51H86O7 | +NL 433.30 | 828.67 |
| 16:1 sitosteryl | C51H88O7 | +NL 433.30 | 830.69 |
| 16:0 campesteryl | C50H88O7 | +NL 435.32 | 818.69 |
| 16:0 stigmasteryl | C51H88O7 | +NL 435.32 | 830.69 |
| 16:0 sitosteryl | C51H90O7 | +NL 435.32 | 832.70 |
| 18:3 campesteryl | C52H86O7 | +NL 457.30 | 840.67 |
| 18:3 stigmasteryl | C53H86O7 | +NL 457.30 | 852.67 |
| 18:3 sitosteryl | C53H88O7 | +NL 457.30 | 854.69 |
| 18:2 campesteryl | C52H88O7 | +NL 459.32 | 842.69 |
| 18:2 stigmasteryl | C53H88O7 | +NL 459.32 | 854.69 |
| 18:2 sitosteryl | C53H90O7 | +NL 459.32 | 856.70 |
| 18:1 campesteryl | C52H90O7 | +NL 461.34 | 844.70 |
| 18:1 stigmasteryl | C53H90O7 | +NL 461.34 | 856.70 |
| 18:1 sitosteryl | C53H92O7 | +NL 461.34 | 858.72 |
| 18:0 campesteryl | C52H92O7 | +NL 463.35 | 846.72 |
| 18:0 stigmasteryl | C53H92O7 | +NL 463.35 | 858.72 |
| 18:0 sitosteryl | C53H94O7 | +NL 463.35 | 860.73 |
Figure 2.
Sterols from purified soybean SGs and ASGs as mole fraction of total detected sterol compounds. After hydrolysis of the SGs or ASGs, sterols were determined by GC-FID, or the SGs and ASGs were analyzed intact by ESI-MS/MS. In the ESI-MS/MS data, the signals for various ASG molecular species with each sterol component were summed. (A) SGs. (B) ASGs. Error bars represent standard deviations (sd) for GC (n = 3 for the number of independent samples) and ESI-MS/MS (n = 12 for the number of independent samples).
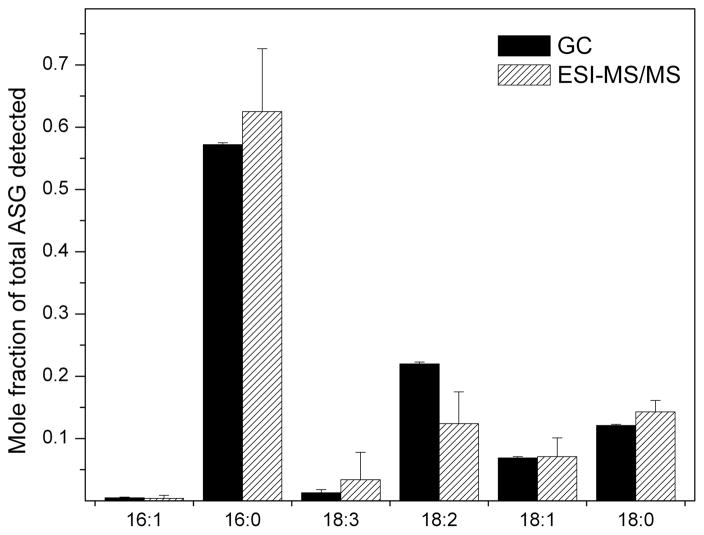
Analysis of the purified ASG mixture by GC-FID and ESI-MS/MS showed a tendency of the mass spectrometer to be less sensitive to 18:2 (linoleic acid)-containing ASGs than to other fatty-acyl SGs, but the fatty acid compositions of the purified ASGs determined by the two methods were similar overall (Fig. 3). The major fatty acid found in purified commercially-obtained soybean ASGs was 16:0 (palmitic acid), comprising greater than 50% of the total, followed by 18:2 (linoleic), 18:0 (stearic), 18:1 (oleic), 18:3 (linolenic) and 16:1 (palmitoleic) acids. Taken together, the data indicate that the composition of SG and ASG determined by ESI-MS/MS of the isolated compounds is very close to that determined by GC-FID, indicating that various SG and ASG molecular species have similar molar mass spectral responses. In addition, our data are comparable to the percentile compositions provided by Matreya LLC, the commercial source of the SG and ASG mixtures (see Experimental Procedure).
Figure 3.
Fatty acids of purified soybean ASG as a mole fraction of total detected fatty acids. Fatty acids were determined by GC-FID after formation of methyl esters, or the ASGs were analyzed intact by ESI-MS/MS. In the ESI-MS/MS data, the signals for various ASG molecular species with each fatty acid component were summed. Error bars represent standard deviations (sd) for GC (n = 3 for the number of independent samples) and ESI-MS/MS (n = 12 for the number of independent samples).
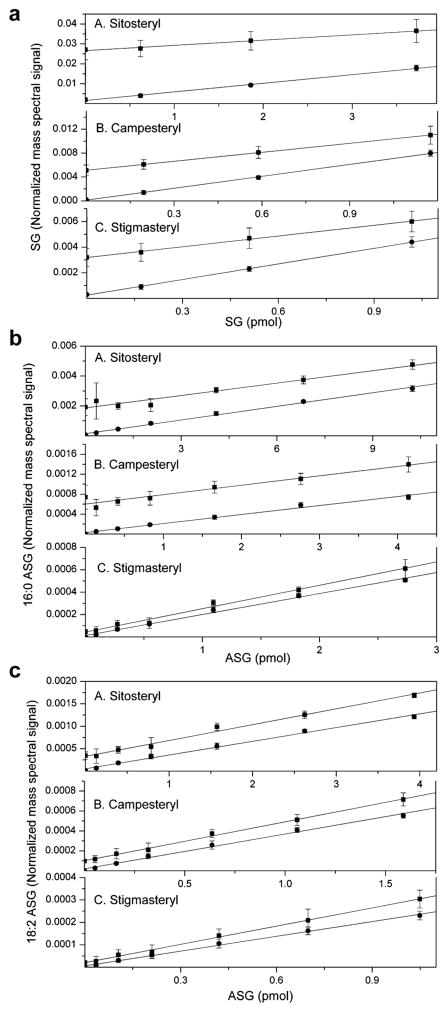
We next applied ESI-MS/MS to seed samples from wild-type Arabidopsis and the ugt80A2,B1 mutant using a spike-in experiment to verify linearity of response of the normalized mass spectral signals. SGs and ASGs were analyzed by direct infusion of unseparated seed extracts, and known amounts of soybean SGs and ASGs were spiked-in to wild-type and mutant samples to determine the linearity of their ESI-MS/MS response in the presence of other seed lipids. The linearity of SG and ASG response was good over the range in which the mutant and wild-type are compared, and the mutant and wild-type spike-in data form parallel lines (Fig. 4). The data show that wild-type values fall within the linear range of the spike-in to the mutant samples, indicating that the amounts of SGs and ASGs in the wild-type samples are linearly related to the amounts in the mutant samples. These data indicate that the ESI-MS/MS method is useful for comparison of biological samples.
Figure 4.
Spike-in experiments indicate linear responses in mass spectral signals from ESI-MS/MS analysis of SGs and ASGs. Normalized mass spectral signals are shown for compounds from Arabidopsis seeds of wild-type (squares) and the ugt80A2,B1 mutant (circles) for (a) SGs, (b) 16:0 ASGs, and (c) 18:2 ASGs in three panels from top to bottom. Each panel is further sub-divided to indicate quantification of (A) sitosteryl, (B) campesteryl, and (C) stigmasteryl SGs, 16:0 ASGs or 18:2 ASGs, respectively. A normalized signal of 1.0 for SGs and ASGs is the same amount of signal as the average signal produced by 1.0 nmol of the internal standards, di12:0 and di20:0 PtdGro. Error bars represent standard deviations (sd) for n = 5 biological replicates.
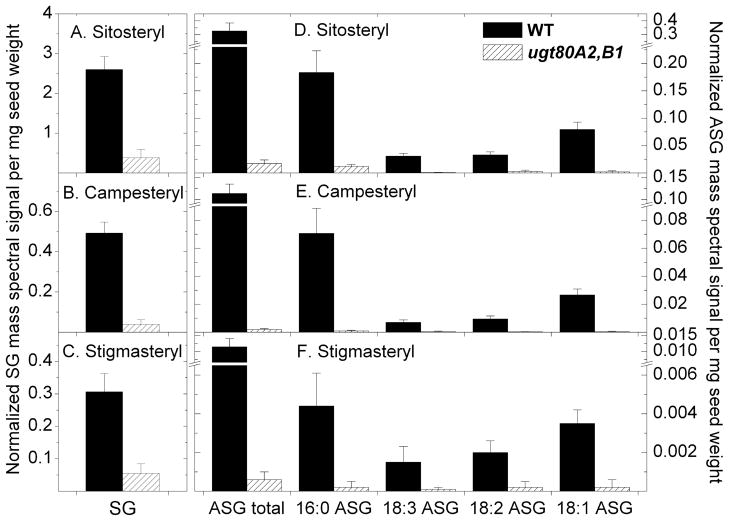
Levels of SGs and ASGs in Arabidopsis seeds of ugt80A2,B1 mutants and wild-type are shown in Fig. 5 (Table S1) with individual molecular species of SGs and ASGs indicated. The masses detected were consistent with identification of sitosterol, campesterol, and stigmasterol as the three major sterols in Arabidopsis seed SGs and ASGs. Minor sterols, with masses consistent with identifications of cholesterol and brassicasterol, were also detected by ESI-MS/MS as ~2% or less of the total SGs (data not shown). The data show that sitosteryl, campesteryl, and stigmasteryl glucosides were significantly reduced by 85%, 92%, and 82% (7-, 13- and 6-fold), respectively, in ugt80A2,B1 in comparison to wild-type seeds, and total SGs were reduced by 86% (7-fold) (Fig. 5; Table S1). Although SG levels were reduced in ugt80A2,B1, the relative proportions of SGs that contained sitosterol, campesterol and stigmasterol were similar to those in wild-type. In addition, we observed that the levels of phospholipids PtdCho and PtdEtn were very similar in the mutant and wild-type (Table S2), indicating that the ugt80A2,B1 mutant is specifically deficient in SGs and ASGs.
Figure 5.
SG and ASG levels are reduced in seeds from ugt80A2,B1 mutants of Arabidopsis. ESI-MS/MS was applied to analyze SGs and ASGs from seeds of wild-type (WT) (solid bars) and ugt80A2,B1 (stippled bars). Normalized mass spectral signals per mg seed weight are indicated for each compound: (A-C) SGs and (D-F) 16:0, 18:3, 18:2, and 18:1 ASGs. (A) SGs and (D) ASGs containing sitosterol. (B) SGs and (E) ASGs containing campesterol. (C) SGs and (F) ASGs containing stigmasterol. The total ASG amount was calculated by adding the normalized mass spectral signal of molecular lipid species of that group. Error bars represent standard deviations (sd) for n = 5 biological replicates.
The major ASG molecular species, 16:0 (palmitic), 18:1 (oleic), 18:2 (linoleic), and 18:3 (linolenic) acids were reduced to a slightly greater extent than SGs in ugt80A2,B1 compared to wild-type seeds. Total ASG levels were reduced 96% (23-fold) in the double mutant (Fig. 5; Table S1). Among the ASGs detected in seeds, 16:0 (palmitic acid) was the predominant acyl group accounting for 57% and 66% of the total ASG signal in wild-type and mutant, respectively. Strikingly, campesterol-containing ASGs were reduced 98% (61-fold), while decreases in sitosterol- and stigmasterol-containing ASGs were reduced to a lesser extent. Campesterol-containing ASGs accounted for only 10% of the ASGs in ugt80A2,B1 in comparison to 25% in wild-type. In wild-type, the signal from SGs accounted for 88% of the total glucosylated sterol signal (SGs + ASGs), and in the ugt80A2,B1 mutant the signal from SGs accounted for 96% percent of the total glucosylated sterols. Overall, there was a reduction of 87% (8-fold) in SG + ASG content in the mutant in comparison to wild-type.
Discussion
The development of a high throughput platform for the detection of sterol conjugates will enhance our ability to characterize mutations that affect the levels of these compounds. Here we present a first step towards including SG and ASG analysis in a comprehensive lipidomics platform. We show that ESI-MS/MS, with a direct injection non-chromatographic approach, allows us to analyze seed extracts directly without purification of the SG and ASG classes from the lipid extract. This approach is simple compared to the traditional GC-FID method since derivatization and chromatographic separation of products is not required. Our spike-in data reveal the mass spectral signals behave linearly to spiked-in standards for a given matrix (Fig. 4). The levels of SGs and ASGs were comparable to those reported for tobacco seeds [21] and various Arabidopsis tissues [13, 22].
While this article was in review and revision, another article describing the mass spectral analysis of SGs and ASGs was published [22]. Wewer and coworkers analyzed SGs and ASGs by direct infusion, but used quadrupole time-of-flight mass spectrometry in contrast to the triple quadrupole method that was applied in our work. In agreement with our data, these authors determined that naturally-occurring sterol derivatives had similar response factors in ESI-MS/MS analysis. Sample preparation by Wewer et al. [22] included a solid phase extraction step followed by direct infusion of samples to the mass spectrometer. Addition of this step yielded increased sensitivity and reduced matrix effects during ionization, which the authors deemed critical for the development of a comprehensive lipidomics platform encompassing linear responses of mass spectral signals from multiple lipid classes. In the current work, care was taken to keep the matrix relatively constant during analysis. Our results, like those of Wewer et al. [22], indicate that ESI mass spectrometry methods are very sensitive for SGs and ASGs from biological samples. In particular, the use of the triple quadrupole mass spectrometer in our work allowed detection of changes in SGs and ASGs at sub-picomole levels.
Application of ESI-MS/MS to seed samples from wild-type Arabidopsis and a mutant in two UGT enzymes (UGT80A2 and UGT80B1) has revealed new details on the composition of SGs and ASGs in the ugt80A2,B1 mutant. A previous study, using GC-FID analysis, showed that combined SG + ASG content was decreased 5-, 21-, and 22-fold in leaf, stem, and inflorescence + silique tissues of ugt80A2,B1 mutants in comparison to wild-type [13]. In our work, analyses of dry seeds from ugt80A2,B1 and wild-type indicate that total SG + ASG levels were reduced similarly, 87% or about 8-fold, in ugt80A2,B1 seeds. The ESI-MS/MS analysis revealed that the reduction is slightly greater in ASG than in SG levels. In addition, the lower levels of acyl campesteryl glucosides in the mutant, compared to other ASGs, may reflect a preference of the acylating enzymes for sitosteryl and stigmasteryl glucosides, or possibly a different subcellular localization of the various SGs and/or acylating enzymes. Our results demonstrate that mass spectrometry methods such as ESI-MS/MS are poised to accelerate research in the detection of SG and ASG lipids whose biological roles are just beginning to be uncovered.
Supplementary Material
Acknowledgments
K.S. was supported by National Research Initiative Competitive Grants Program grant no. 2007-35304-18453 from the United States Department of Agriculture National Institute of Food and Agriculture and by the National Science Foundation (MCB 0517758). Equipment acquisition and method development at the Kansas Lipidomics Research Center were funded by the National Science Foundation (EPS 0236913, MCB 0455318 and 0920663, DBI 0521587), Kansas Technology Enterprise Corporation, Kansas IDeA Network of Biomedical Research Excellence (K-INBRE) of National Institutes of Health (P20RR16475), and Kansas State University. This is contribution no. 11-374-J from the Kansas Agricultural Experiment Station.
Abbreviations and Symbols
- SG
steryl glucosides
- ASG
acyl steryl glucosides
- ESI-MS/MS
electrospray ionization tandem (triple quadrupole) mass spectrometry
- GC
gas chromatography
- FID
flame ionization detection
References
- 1.Benveniste P. Biosynthesis and accumulation of sterols. Annu Rev Plant Biol. 2004;55:429–457. doi: 10.1146/annurev.arplant.55.031903.141616. [DOI] [PubMed] [Google Scholar]
- 2.Grandmougin-Ferjani A, Schuler-Muller I, Hartmann MA. Sterol modulation of the plasma membrane H+-ATPase activity from corn roots reconstituted into soybean lipids. Plant Physiol. 1997;113:163–174. doi: 10.1104/pp.113.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuler I, Milon A, Nakatani Y, Ourisson G, Albrecht AM, Benveniste P, Hartmann MA. Differential effects of plant sterols on water permeability and on acyl chain ordering of soybean phosphophatidylcholine bilayers. Proc Natl Acad Sci USA. 1991;88:6926–6930. doi: 10.1073/pnas.88.16.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asami T, Nakano T, Fujioka S. Plant brassinosteroid hormones. Plant Horm. 2005;72:479–504. doi: 10.1016/S0083-6729(05)72014-8. [DOI] [PubMed] [Google Scholar]
- 5.Kim TW, Wang ZY. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- 6.Grille S, Zaslawski A, Thiele S, Plat J, Warnecke D. The functions of steryl glycosides come to those who wait: Recent advances in plants, fungi, bacteria and animals. Prog Lipid Res. 2010;49:262–288. doi: 10.1016/j.plipres.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 7.Kovganko NV, Kashkan ZN. Sterol glycosides and acylglycosides. Chemistry of Natural Compounds. 1999;35:479–497. [Google Scholar]
- 8.Heinz E. Plant glycolipids: Structure, isolation and analysis. In: Christie WW, editor. Advances in Lipid Methodology - 3. Dundee: The Oily Press; 1996. pp. 211–332. [Google Scholar]
- 9.Wojciechowski ZA. Biochemistry of phytosterol conjugates. In: Patterson GW, Nes WD, editors. Physiology and Biochemistry of Sterols. American Oil Chemists Society; Champaign, IL: 1991. pp. 361–395. [Google Scholar]
- 10.Peng L, Kawagoe Y, Hogan P, Delmer D. Sitosterol-beta-glucoside as a primer for cellulose synthesis in plants. Science. 2002;295:147–150. doi: 10.1126/science.1064281. [DOI] [PubMed] [Google Scholar]
- 11.Ury A, Benveniste P, Bouvier-Navé P. Phospholipid-dependence of plant UDP-glucose sterol beta-d-glucosyl transferase: IV. Reconstitution into small unilamellar vesicles. Plant Physiol. 1989;91:567–573. doi: 10.1104/pp.91.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warnecke DC, Baltrusch M, Buck F, Wolter FP, Heinz E. UDP-glucose:sterol glucosyltransferase: cloning and functional expression in Escherichia coli. Plant Mol Biol. 1997;35:597–603. doi: 10.1023/a:1005806119807. [DOI] [PubMed] [Google Scholar]
- 13.DeBolt S, Scheible WR, Schrick K, Auer M, Beisson F, Bischoff V, Bouvier-Navé P, Carroll A, Hematy K, Li Y, Milne J, Nair M, Schaller H, Zemla M, Somerville C. Mutations in UDP-glucose:sterol glucosyltransferase in Arabidopsis cause transparent testa phenotype and suberization defect in seeds. Plant Physiol. 2009;151:78–87. doi: 10.1104/pp.109.140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wenk MR. Lipidomics: new tools and applications. Cell. 2010;143:888–895. doi: 10.1016/j.cell.2010.11.033. [DOI] [PubMed] [Google Scholar]
- 15.Welti R, Li W, Li M, Sang Y, Biesiada H, Zhou HE, Rajashekar CB, Williams TD, Wang X. Profiling membrane lipids in plant stress response: Role of phospholipase D alpha in freezing-induced lipid changes in Arabidopsis. J Biol Chem. 2002;277:31994–32002. doi: 10.1074/jbc.M205375200. [DOI] [PubMed] [Google Scholar]
- 16.Devaiah SP, Roth MR, Baughman E, Li M, Tamura P, Jeannotte R, Welti R, Wang X. Quantitative profiling of polar glycerolipid species from organs of wild-type Arabidopsis and a phospholipase Dalpha1 knockout mutant. Phytochemistry. 2006;67:1907–1924. doi: 10.1016/j.phytochem.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 18.Rintoul DA, Chou SM, Silbert DF. Physical characterization of sterol-depleted LM-cell plasma membranes. J Biol Chem. 1979;254:10070–10077. [PubMed] [Google Scholar]
- 19.Zhou Z, Marepally SR, Nune DS, Pallakollu P, Ragan G, Roth MR, Wang L, Lushington GH, Visvanathan M, Welti R. LipidomeDB Data Calculation Environment: Online processing of direct-infusion mass spectral data for lipid profiles. Lipids. 2011 doi: 10.1007/s11745-011-3575-8. (In press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebisch G, Binder M, Shifferer R, Langmann T, Schulz B, Schmitz G. High throughput quantification of cholesterol and cholesteryl ester by electrospray ionization tandem mass spectrometry (ESI-MS/MS) Biochim Biophys Acta. 2006;1761:121–128. doi: 10.1016/j.bbalip.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 21.Bush PB, Grunwald C. Sterol changes during germination of Nicotiana tabacum seeds. Plant Physiol. 1972;50:69–72. doi: 10.1104/pp.50.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wewer V, Dombrink I, Vom Dorp K, Dörmann P. Quantification of sterol lipids in plants by quadrupole time-of-flight mass spectrometry. J Lipids Res. 2011;52:1039–1054. doi: 10.1194/jlr.D013987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.