Abstract
Leukemia inhibitory factor (LIF) and interleukin-6 (IL-6), two multifunctional cytokines lacking structural homology and binding to distinct receptors, share interesting functional similarities, which include induction of hematopoietic differentiation in normal and myeloid leukemia cells, induction of neuronal cell differentiation, and stimulation of acute-phase protein synthesis in hepatocytes. Structural information on the LIF receptor is not yet available, whereas recent cloning of the IL-6 receptor has shown it to be bipartite, with a signal-transducing subunit that lacks sequence homology to known protein kinases and produces second messengers of unknown nature. The molecular nature of the mechanisms which LIF and IL-6 use to induce cell differentiation is not known. To address this issue, we took advantage of a clone of M1 myeloblastic leukemia cells capable of being induced for terminal differentiation by both LIF and IL-6 directly activate the same set of immediate early response genes upon induction of M1 myeloid differentiation. At least two mechanisms of gene activation, one transcriptional and the other posttranscriptional, are shown to be involved. It is also shown that the LIF and IL-6 immediate early response, at suboptimal cytokine concentrations, is additive. Using a variety of protein kinase activators and inhibitors, we have shown that the intracellular signalling pathways for both LIF and IL-6 are distinct from those of known second messengers and involve protein phosphorylation, notably tyrosine phosphorylation of a 160-kDa protein, as an essential step(s) in the immediate early activation of MyD gene expression. These observations indicate that the functional similarities of LIF and IL-6 as inducers of cell differentiation prevail at the level of the complex differentiation immediate early response and implicate common mechanisms of signal transduction for LIF- and IL-6-induced differentiation.
Full text
PDF


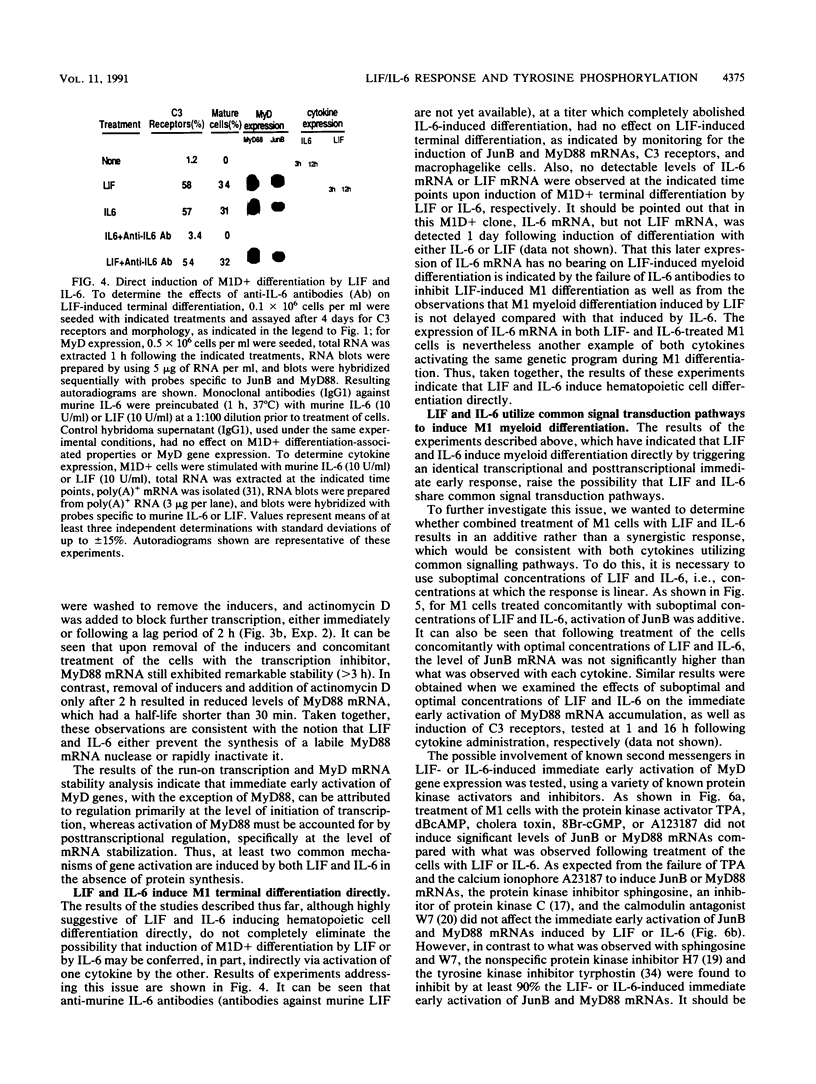
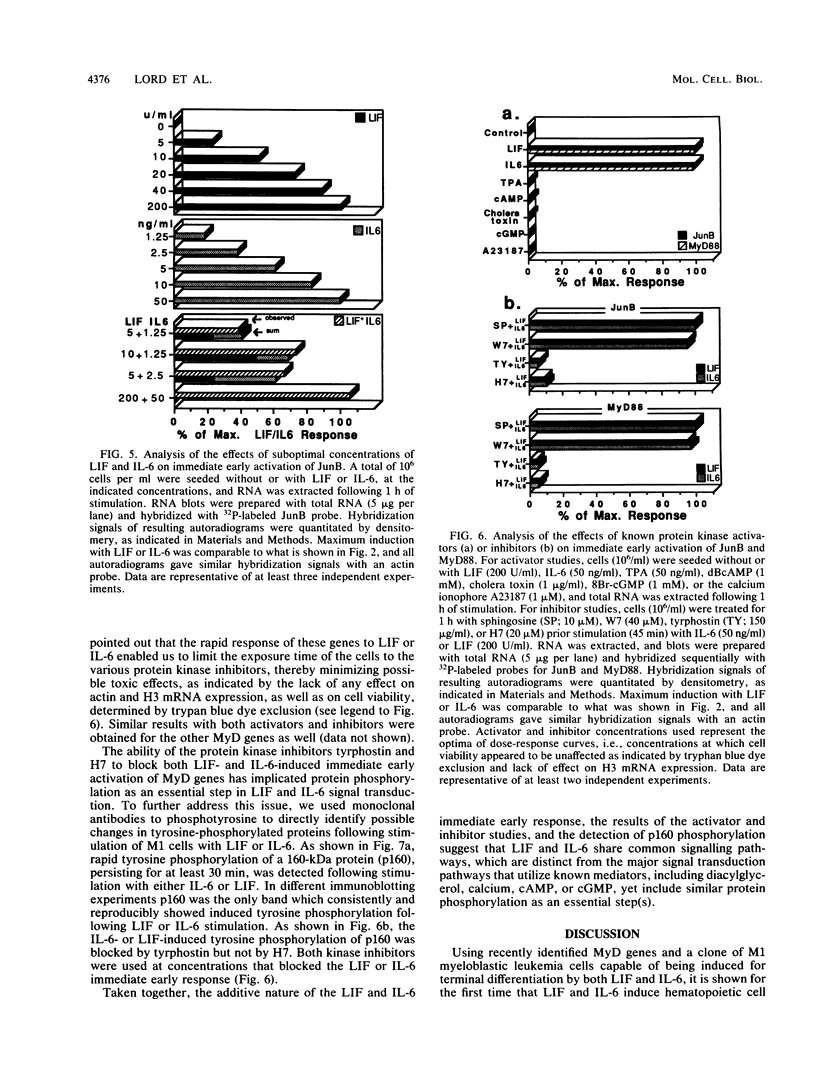
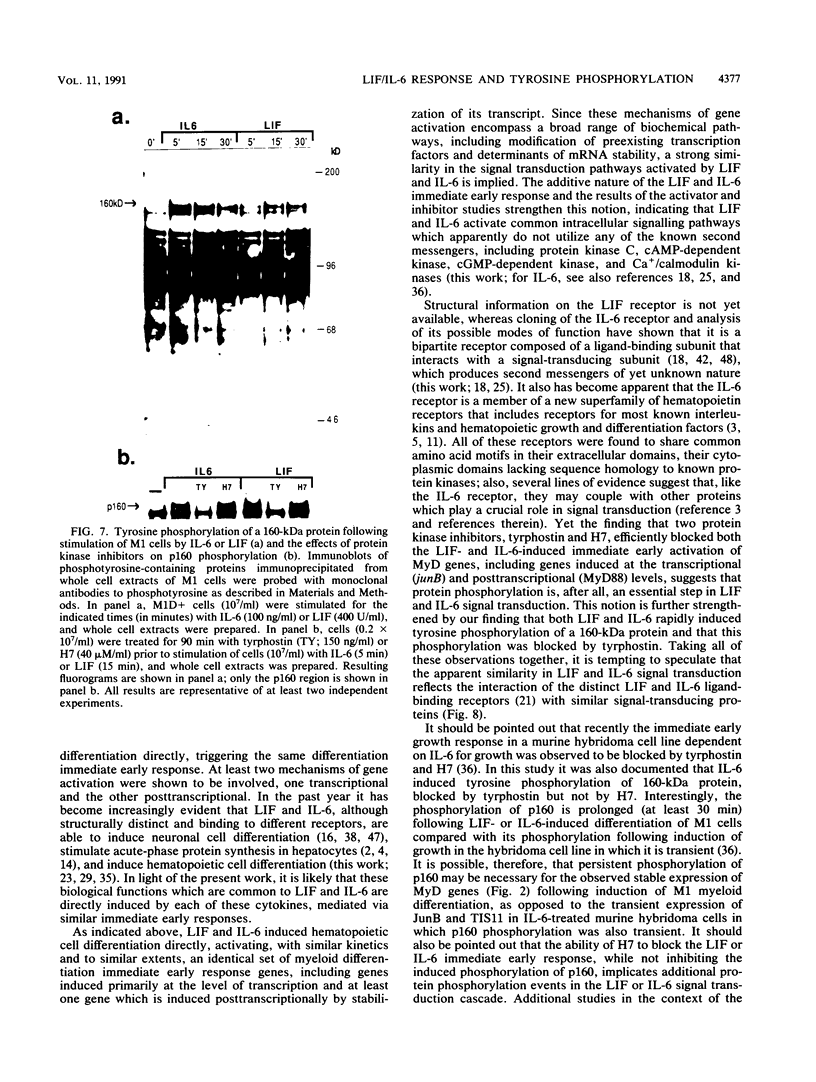
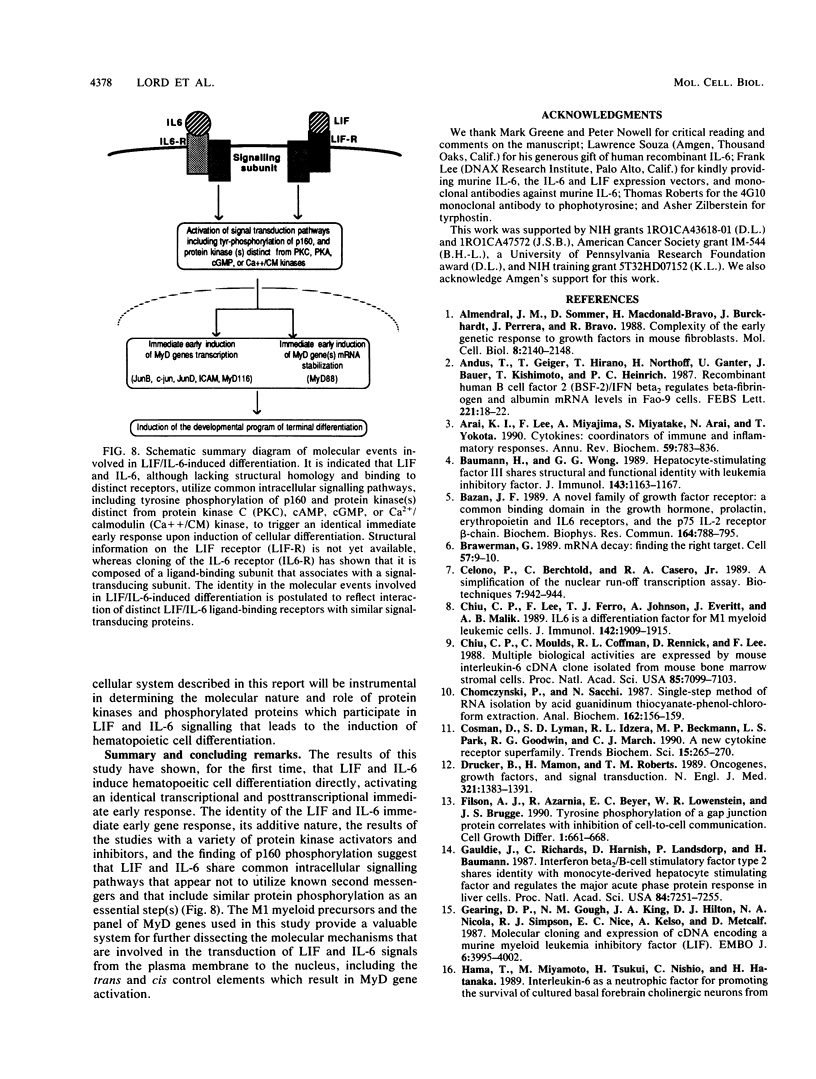

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andus T., Geiger T., Hirano T., Northoff H., Ganter U., Bauer J., Kishimoto T., Heinrich P. C. Recombinant human B cell stimulatory factor 2 (BSF-2/IFN-beta 2) regulates beta-fibrinogen and albumin mRNA levels in Fao-9 cells. FEBS Lett. 1987 Aug 31;221(1):18–22. doi: 10.1016/0014-5793(87)80344-7. [DOI] [PubMed] [Google Scholar]
- Arai K. I., Lee F., Miyajima A., Miyatake S., Arai N., Yokota T. Cytokines: coordinators of immune and inflammatory responses. Annu Rev Biochem. 1990;59:783–836. doi: 10.1146/annurev.bi.59.070190.004031. [DOI] [PubMed] [Google Scholar]
- Baumann H., Wong G. G. Hepatocyte-stimulating factor III shares structural and functional identity with leukemia-inhibitory factor. J Immunol. 1989 Aug 15;143(4):1163–1167. [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Brawerman G. mRNA decay: finding the right targets. Cell. 1989 Apr 7;57(1):9–10. doi: 10.1016/0092-8674(89)90166-9. [DOI] [PubMed] [Google Scholar]
- Celano P., Berchtold C., Casero R. A., Jr A simplification of the nuclear run-off transcription assay. Biotechniques. 1989 Oct;7(9):942–944. [PubMed] [Google Scholar]
- Chiu C. P., Lee F. IL-6 is a differentiation factor for M1 and WEHI-3B myeloid leukemic cells. J Immunol. 1989 Mar 15;142(6):1909–1915. [PubMed] [Google Scholar]
- Chiu C. P., Moulds C., Coffman R. L., Rennick D., Lee F. Multiple biological activities are expressed by a mouse interleukin 6 cDNA clone isolated from bone marrow stromal cells. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7099–7103. doi: 10.1073/pnas.85.19.7099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cosman D., Lyman S. D., Idzerda R. L., Beckmann M. P., Park L. S., Goodwin R. G., March C. J. A new cytokine receptor superfamily. Trends Biochem Sci. 1990 Jul;15(7):265–270. doi: 10.1016/0968-0004(90)90051-c. [DOI] [PubMed] [Google Scholar]
- Danska J. S., McIntyre B. W., McDevitt H. O., Weissman I. L. Structural similarity between a primitive chordate membrane heterodimer and lymphocyte antigen receptors. Int Immunol. 1990;2(9):795–802. doi: 10.1093/intimm/2.9.795. [DOI] [PubMed] [Google Scholar]
- Druker B. J., Mamon H. J., Roberts T. M. Oncogenes, growth factors, and signal transduction. N Engl J Med. 1989 Nov 16;321(20):1383–1391. doi: 10.1056/NEJM198911163212007. [DOI] [PubMed] [Google Scholar]
- Filson A. J., Azarnia R., Beyer E. C., Loewenstein W. R., Brugge J. S. Tyrosine phosphorylation of a gap junction protein correlates with inhibition of cell-to-cell communication. Cell Growth Differ. 1990 Dec;1(12):661–668. [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing D. P., Gough N. M., King J. A., Hilton D. J., Nicola N. A., Simpson R. J., Nice E. C., Kelso A., Metcalf D. Molecular cloning and expression of cDNA encoding a murine myeloid leukaemia inhibitory factor (LIF). EMBO J. 1987 Dec 20;6(13):3995–4002. doi: 10.1002/j.1460-2075.1987.tb02742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hibi M., Murakami M., Saito M., Hirano T., Taga T., Kishimoto T. Molecular cloning and expression of an IL-6 signal transducer, gp130. Cell. 1990 Dec 21;63(6):1149–1157. doi: 10.1016/0092-8674(90)90411-7. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton D. J., Nicola N. A., Metcalf D. Specific binding of murine leukemia inhibitory factor to normal and leukemic monocytic cells. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5971–5975. doi: 10.1073/pnas.85.16.5971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Yasukawa K., Harada H., Taga T., Watanabe Y., Matsuda T., Kashiwamura S., Nakajima K., Koyama K., Iwamatsu A. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov 6;324(6092):73–76. doi: 10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- Ishibashi T., Kimura H., Uchida T., Kariyone S., Friese P., Burstein S. A. Human interleukin 6 is a direct promoter of maturation of megakaryocytes in vitro. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5953–5957. doi: 10.1073/pnas.86.15.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee F., Chiu C. P., Wideman J., Hodgkin P., Hudak S., Troutt L., Ng T., Moulds C., Coffman R., Zlotnik A. Interleukin-6. A multifunctional regulator of growth and differentiation. Ann N Y Acad Sci. 1989;557:215-28, discussion 228-9. [PubMed] [Google Scholar]
- Liebermann D. A., Hoffman-Liebermann B. Proto-oncogene expression and dissection of the myeloid growth to differentiation developmental cascade. Oncogene. 1989 May;4(5):583–592. [PubMed] [Google Scholar]
- Liebermann D., Hoffman-Liebermann B., Sachs L. Regulation and role of different macrophage-and granulocyte-inducing proteins in normal and leukemic myeloid cells. Int J Cancer. 1982 Feb 15;29(2):159–161. doi: 10.1002/ijc.2910290208. [DOI] [PubMed] [Google Scholar]
- Liebermann D., Sachs L. Co-regulation of type C RNA virus production and cell differentiation in myeloid leukemic cells. Cell. 1978 Nov;15(3):823–835. doi: 10.1016/0092-8674(78)90267-2. [DOI] [PubMed] [Google Scholar]
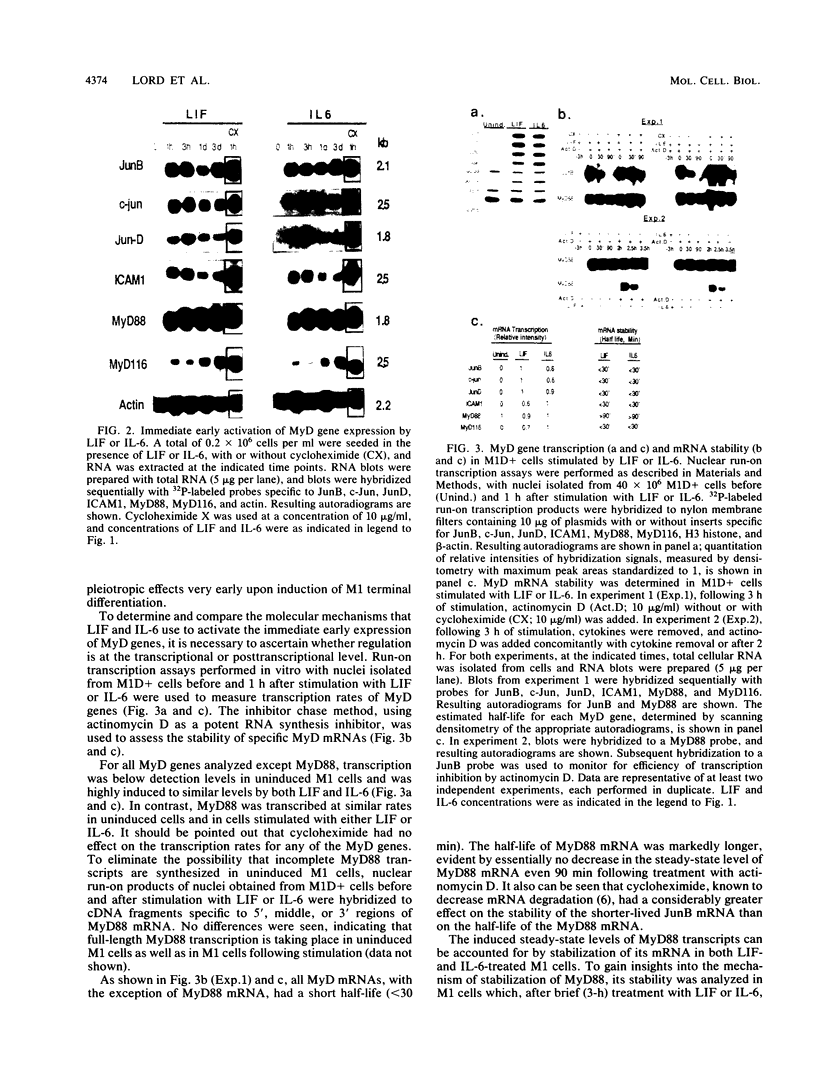
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Complexity of the immediate early response of myeloid cells to terminal differentiation and growth arrest includes ICAM-1, Jun-B and histone variants. Oncogene. 1990 Mar;5(3):387–396. [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Nucleotide sequence and expression of a cDNA encoding MyD88, a novel myeloid differentiation primary response gene induced by IL6. Oncogene. 1990 Jul;5(7):1095–1097. [PubMed] [Google Scholar]
- Lord K. A., Hoffman-Liebermann B., Liebermann D. A. Sequence of MyD116 cDNA: a novel myeloid differentiation primary response gene induced by IL6. Nucleic Acids Res. 1990 May 11;18(9):2823–2823. doi: 10.1093/nar/18.9.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyall R. M., Zilberstein A., Gazit A., Gilon C., Levitzki A., Schlessinger J. Tyrphostins inhibit epidermal growth factor (EGF)-receptor tyrosine kinase activity in living cells and EGF-stimulated cell proliferation. J Biol Chem. 1989 Aug 25;264(24):14503–14509. [PubMed] [Google Scholar]
- Metcalf D., Nicola N. A., Gearing D. P. Effects of injected leukemia inhibitory factor on hematopoietic and other tissues in mice. Blood. 1990 Jul 1;76(1):50–56. [PubMed] [Google Scholar]
- Nakajima K., Wall R. Interleukin-6 signals activating junB and TIS11 gene transcription in a B-cell hybridoma. Mol Cell Biol. 1991 Mar;11(3):1409–1418. doi: 10.1128/mcb.11.3.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponte P., Gunning P., Blau H., Kedes L. Human actin genes are single copy for alpha-skeletal and alpha-cardiac actin but multicopy for beta- and gamma-cytoskeletal genes: 3' untranslated regions are isotype specific but are conserved in evolution. Mol Cell Biol. 1983 Oct;3(10):1783–1791. doi: 10.1128/mcb.3.10.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh T., Nakamura S., Taga T., Matsuda T., Hirano T., Kishimoto T., Kaziro Y. Induction of neuronal differentiation in PC12 cells by B-cell stimulatory factor 2/interleukin 6. Mol Cell Biol. 1988 Aug;8(8):3546–3549. doi: 10.1128/mcb.8.8.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B. Interleukin-6: molecular pathophysiology. J Invest Dermatol. 1990 Jun;94(6 Suppl):2S–6S. doi: 10.1111/1523-1747.ep12874963. [DOI] [PubMed] [Google Scholar]
- Shabo Y., Lotem J., Rubinstein M., Revel M., Clark S. C., Wolf S. F., Kamen R., Sachs L. The myeloid blood cell differentiation-inducing protein MGI-2A is interleukin-6. Blood. 1988 Dec;72(6):2070–2073. [PubMed] [Google Scholar]
- Shabo Y., Sachs L. Inhibition of differentiation and affinity purification with a monoclonal antibody to a myeloid cell differentiation-inducing protein. Blood. 1988 Nov;72(5):1543–1549. [PubMed] [Google Scholar]
- Taga T., Hibi M., Hirata Y., Yamasaki K., Yasukawa K., Matsuda T., Hirano T., Kishimoto T. Interleukin-6 triggers the association of its receptor with a possible signal transducer, gp130. Cell. 1989 Aug 11;58(3):573–581. doi: 10.1016/0092-8674(89)90438-8. [DOI] [PubMed] [Google Scholar]
- Takai Y., Wong G. G., Clark S. C., Burakoff S. J., Herrmann S. H. B cell stimulatory factor-2 is involved in the differentiation of cytotoxic T lymphocytes. J Immunol. 1988 Jan 15;140(2):508–512. [PubMed] [Google Scholar]
- Uyttenhove C., Coulie P. G., Van Snick J. T cell growth and differentiation induced by interleukin-HP1/IL-6, the murine hybridoma/plasmacytoma growth factor. J Exp Med. 1988 Apr 1;167(4):1417–1427. doi: 10.1084/jem.167.4.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Localization of tyrosine kinase-coding region in v-abl oncogene by the expression of v-abl-encoded proteins in bacteria. J Biol Chem. 1985 Jan 10;260(1):64–71. [PubMed] [Google Scholar]
- Yamamori T., Fukada K., Aebersold R., Korsching S., Fann M. J., Patterson P. H. The cholinergic neuronal differentiation factor from heart cells is identical to leukemia inhibitory factor. Science. 1989 Dec 15;246(4936):1412–1416. doi: 10.1126/science.2512641. [DOI] [PubMed] [Google Scholar]
- Yamasaki K., Taga T., Hirata Y., Yawata H., Kawanishi Y., Seed B., Taniguchi T., Hirano T., Kishimoto T. Cloning and expression of the human interleukin-6 (BSF-2/IFN beta 2) receptor. Science. 1988 Aug 12;241(4867):825–828. doi: 10.1126/science.3136546. [DOI] [PubMed] [Google Scholar]