Abstract
Background
Clinician compliance with clinical guidelines in the treatment of patients with Hepatitis C (HCV) has been reported to be as low as 18.5%. Treatment is complex and patient compliance is often inconsistent thus, active clinician surveillance and support is essential to successful outcomes. A clinical decision support system (CDSS) embedded within an electronic health record can provide reminders, summarize key data, and facilitate coordination of care. To date, the literature is bereft of information describing the implementation and evaluation of a CDSS to support HCV treatment.
Objective
The purpose of this case report is to describe the design, implementation, and initial evaluation of an HCV-specific CDSS while piloting data collection metrics and methods to be used in a larger study across multiple practices.
Methods
The case report describes the design and implementation processes with preliminary reporting on impact of the CDSS on quality indicator completion by comparing the pre-CDSS group to the post-CDSS group.
Results
The CDSS was successfully designed and implemented using an iterative, collaborative process. Pilot testing of the clinical outcomes of the CDSS revealed high rates of quality indicator completion in both the pre- and post-CDSS; although the post-CDSS group received a higher frequency of reminders (4.25 per patient) than the pre-CDSS group (.25 per patient).
Conclusions
This case report documents the processes used to successfully design and implement an HCV CDSS. While the small sample size precludes generalizability of findings, results did positively demonstrate the feasibility of comparing quality indicator completion rates pre-CDSS and post-CDSS. It is recommended that future studies include a larger sample size across multiple providers with expanded outcomes measures related to patient outcomes, staff satisfaction with the CDSS, and time studies to evaluate efficiency and cost effectiveness of the CDSS.
Keywords: Decision support system, Hepatitis C, healthcare quality indicators, chronic core model, nurse practitioner advanced practice nursing, access and evaluation health care quality
1. Introduction
Hepatitis C (HCV) is the leading cause of death from liver disease and the most common reason for liver transplantation in the United States [1]. HCV is a virus that affects over 3.2 million people in the United States [2] and 180 million people worldwide [3]. Approximately 80% of patients exposed to HCV develop chronic infection [1].
Treatment of HCV reduces histologic progression of liver disease and increases life expectancy [4, 5]. Sustained viral response is now achieved in up to 70% of patients across 24 to 48 weeks of treatment using pegylated interferon and ribavirin (standard therapy) for all genotypes with the recent addition of a protease inhibitor (triple therapy) for patients with genotype 1 [6]. These medication regimens, however, are complicated and potentiate side effects that include depression (30%), fatigue (50%), rash (32–56%), neutropenia (20%), and anemia (30–49%) [1, 6]. Additionally, patients with HCV often have comorbid conditions such as substance abuse, diabetes, obesity, and/or psychiatric conditions [7].
Managing the various aspects of both medication side effects and comorbid conditions can create barriers to quality care due to the increased clinician cognitive load required to address complex issues in an already time-constrained setting. Coordination of care is also impeded when patient data and clinical documentation are managed across multiple information sources that segregate critical information such as laboratory results, vaccinations records, flow sheets, and office visit documentation [8].
Most electronic health records (EHRs) are designed around an acute illness model [8] and many medical practices organize care and documentation using both paper charts and/or an EHR system. An informal query of the regional liver transplantation center and surrounding hepatology practices revealed data management systems using a combination of paper and EHR formats with no integration, automation, or centralization of the HCV quality care indicators. Dorr [8] reports that this lack of integration and centralization increases the likelihood that vital components of patient care will be overlooked or arbitrarily executed with adverse effects on patient satisfaction, treatment outcomes, and quality of care.
Multiple studies have reported a lack of clinician compliance with clinical guidelines in the management of patients with HCV [9–15]. Most recently, Kanwal et al. evaluated a nationwide health insurance database and found that only 18.5% of patients with HCV received all of the Centers for Medicare & Medicaid Services (CMS) quality indicators [9]. The researchers concluded that the current processes of care and compliance with quality indicators falls short of clinical guideline recommendations [9]. These deficiencies correlate with the “quality chasm” of chronic health care in the United States described by the Institute of Medicine (IOM). The IOM postulated that improving the processes of care, proficiently translating evidence into practice, and effectively utilizing information technology could mitigate the chasm [16].
Wagner’s Chronic Care Model aligns with the IOM recommendations and provides a theoretical perspective to the design of the HCV clinical decision support system (CDSS) [17]. The Chronic Care Model proposes that the complex demands of chronic illness management are best coordinated using a centralized and integrated clinical information system that supports the ability of clinicians to see a dynamic picture of all patient data to better plan, deliver, and monitor care [17]. These features facilitate a healing relationship between patients and caregivers through the administration of meticulous coordination of care and increased attention to those with poor compliance [17].
Active CDSSs are programmed with evidence-based knowledge that is intelligently filtered to summarize patient-specific information according to disease-specific algorithms [18]. A CDSS can reduce the excessive time required to retrieve pertinent data during a clinical visit especially if it is integrated with the Electronic Health Record (EHR) database and workflow [19]. Numerous studies have documented the benefits of a CDSS integrated into an EHR to improve clinical performance related to immunization completion, guideline adherence, medication dosing, and chronic health management for conditions such as asthma, hypertension, and HIV [18–24].
As health systems move toward the Accountable Care Model, pre-determined outcomes will be increasingly measured and incentivized for compliance with the CMS quality indicators [25]. CMS has defined, eight HCV treatment quality indicators including genotype testing, offering/prescribing treatment, baseline and twelve-week viral load testing, documentation of hepatitis A and B vaccinations, alcohol abstinence counseling, and pregnancy prevention counseling [26]. A CDSS should enhance HCV treatment quality, however, no studies have been published to date that evaluate the use of a CDSS to support the completion of HCV quality indicators.
2. Objectives
The purpose of this case report is to describe the development and implementation of an HCV quality indicator-specific CDSS while piloting data collection methods to evaluate the impact of the CDSS on quality indicator completion and patient reminders as a framework for future studies.
2.1 Setting
This study was conducted at a multi-physician gastroenterology practice in the Midwest that serves as a referral center for six surrounding counties. In this practice all HCV patients are managed by a nurse practitioner with the administrative support of a medical assistant. Although the clinic has used an EHR since 2003, the EHR did not sufficiently support the complex surveillance and dynamic interaction that is required for managing HCV patients. Prior to the development of the CDSS, patient tracking was accomplished via the EHR augmented with paper and software-based spreadsheets that were not integrated into or synchronized with the EHR. The spreadsheets were scanned into separate files in various sections of the electronic record, which resulted in a segregated format for storing and documenting office visits, laboratory results, and patient communication logs. This disparate system demanded excessive clinician time and effort to access pertinent, patient data that was scattered throughout the patient’s e-record.
In recent years, several changes in the clinic contributed to the development and implementation of the CDSS:
-
1.
The volume of patients receiving treatment for HCV dramatically increased;
-
2.
The clinic implemented an electronic lab messaging system to input laboratory data directly into the EHR; and
-
3.
The FDA approved the use of protease inhibitors as a third agent for the treatment of patients with HCV genotype 1 which increased the need for medication management and laboratory surveillance [6].
Both the physician and nurse practitioner agreed that the increased demand for treatment, the ability to automatically aggregate electronic lab data, and the new treatment challenges created a sense of urgency for developing and implementing a CDSS.
2.2 Design and development of the CDSS
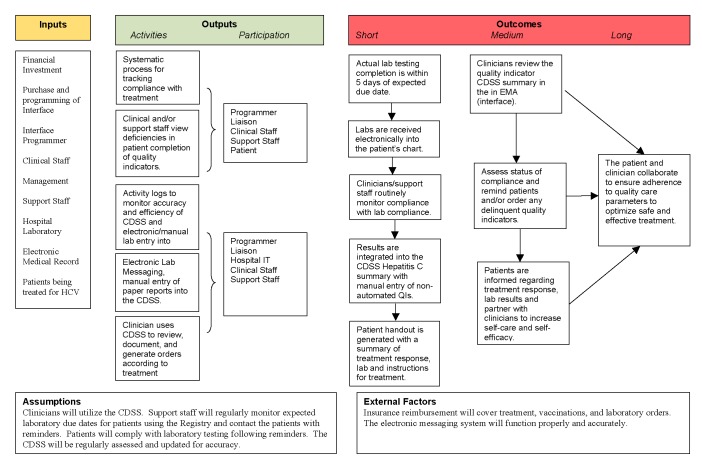
An organizational assessment of the clinic revealed gaps in the processes of care because of the lack of an integrated, sustainable system for tracking laboratory data, identifying treatment deficiencies, or reminding patients to complete quality indicator recommendations. A proactive, automated process was deemed necessary to bridge this gap [17] for the management of both individual patients and the HCV treatment population as a whole. A program logic model was developed to identify the inputs, outputs, and expected outcomes from the development and implementation of the CDSS (▶Fig. 1).
Fig. 1.
Project logic model for Hepatitis C clinical decision support system
A literature review clarified the design features that were essential for a successful CDSS infrastructure. Numerous studies have demonstrated that an EHR-integrated CDSS can improve patient care [18–24]. Kawamoto, Haulihan, Balas, & Lobach’s [27] review of over 70 CDSS studies reported that “68% of the systems significantly improved clinical practice” (p. 2). These studies were conducted on a variety of systems and disease processes. Kawamoto et al. synthesized four successful CDSS criteria which are that the CDSS is:
-
1.
computer-based rather than paper-based;
-
2.
seamlessly integrated into the clinician workflow;
-
3.
available at the point of care; and
-
4.
reflective of specific, evidence-based recommendations [27].
These four criteria guided the CDSS framework and functionality.
To operationalize the CDSS the clinic invested in a third-party interface (Electronic Medical Assistant, 2011) that is interoperable with the SQL database of the EHR (Medinformatix, 2011) and is amenable to customization, automation, and consolidation of HCV treatment parameters. This interface also serves as the platform for the HL7 laboratory messaging system which provides an integrated view of laboratory data across time with alerts for abnormal results or past due orders [28]. To program the CDSS, the HCV clinical guidelines and CMS quality indicators were parsed and organized according to pertinent patient-specific data (patient’s clinical dashboard) and/or HCV treatment population-specific data (registry).
The patient’s clinical dashboard includes treatment-specific order sets (defined according to clinical guidelines), automated treatment length calculations (based on genotype and response to treatment), quality indicator data, and laboratory results received electronically or entered manually. Icons indicate overdue orders and color-coding identifies whether the latest result was normal, abnormal, or critical (▶Fig. 2). Items in need of intervention can be addressed within the dashboard by messaging the staff or activating quick-click orders. The actions taken in the patient’s clinical dashboard seamlessly transfer to all office visit documentation, patient handouts, and letters to the primary care provider to facilitate communication and coordination of care [8, 17]. Additionally, the dashboard quality indicators synchronize with the content of the registry to provide a real-time data summary of all clinic patients on HCV treatment.
Fig. 2.
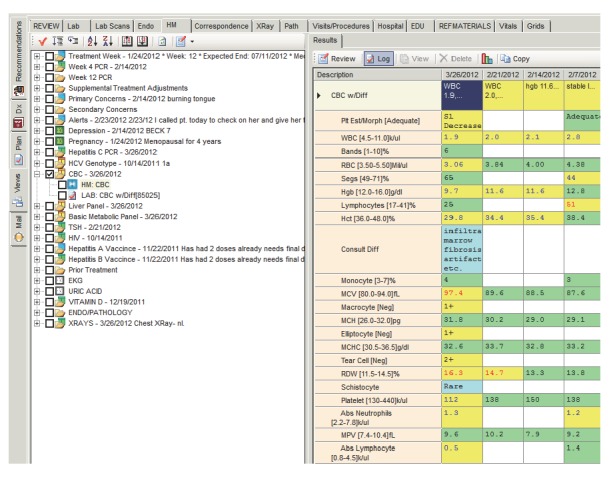
Dashboard view
Because the registry provides a summarized view of quality indicator status for the entire HCV treatment population, all members of the provider and administrative support team are able to proactively monitor and support at-risk patients from one location in the CDSS. Color-coding in the registry highlights orders due within one week (yellow) and overdue orders (red) (▶Fig. 3). The CDSS also generates a biweekly message to the medical assistant listing all patients with overdue orders to prompt patient reminders during the interval between office visits. The nurse practitioner reviews the alerts and issues reminders during patient office visits. Patient phone calls and interactions can be recorded in the registry, which synchronizes with the EHR and the patient’s dashboard to provide multiple layers of communication and seamless care coordination across the entire patient management system.
Fig. 3.
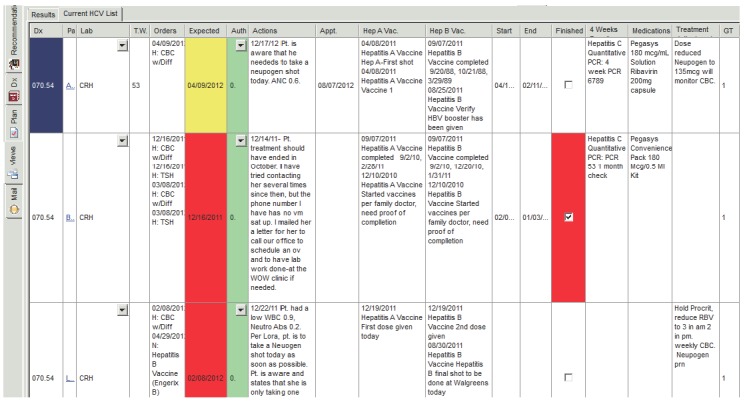
Registry view
2.3 Planning the implementation
Clinical team acceptance of the CDSS into the clinic workflow was essential. The Practical, Robust, Implementation and Sustainability Model (PRISM) provided the framework for the project implementation. PRISM synthesizes principles from the Diffusion of Innovation, Model for Improvement, RE-AIM, and the Chronic Care Model into six domains that delineate the design, development, implementation, and evaluation measures of an effective, sustainable intervention [29]. Domain-specific questions developed by Feldstein & Glasgow [29] were used to identify and mitigate performance gaps, patient barriers, staff readiness, workflow integration, and organizational support to increase the effectiveness of the implementation of the CDSS.
PRISM specifically guided the implementation process through the emphasis on close collaboration between the clinical staff, nurse practitioner, and the CDSS technical programmer. All changes were communicated to the entire provider and administrative team prior to full implementation. Iterative feedback from the clinical staff guided the refinement of both the content and format of the CDSS to ensure optimal performance at the point-of-care. Small tests of change were implemented with each new CDSS feature to ensure workflow was not interrupted and that the CDSS maintained accuracy.
3. Methods of evaluation
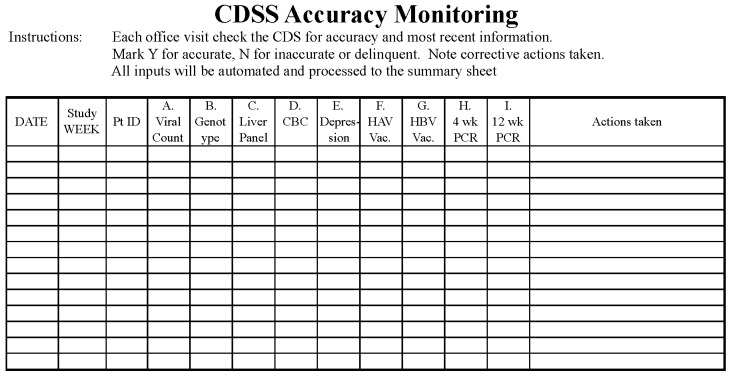
A quality control plan was instituted using a checklist in order to ensure the accuracy of the CDSS prior to each patient’s office visit with the benchmark set at 80% of items checked and verified (▶Fig. 4). This control plan not only validated the accuracy of the automated information retrieval from the EHR database, but also prompted the medical assistant to request reports from laboratory test sites that were not electronically imported to the EHR for manual entry. Verification of accuracy or remediation of inaccurate data prior to an office visit maximized the clinician’s ability to provide efficient point-of-care patient management.
Fig. 4.
Control plan spreadsheet used throughout the study period to ensure the CDSS data was current and accurate
All patients age 18 and older that began HCV treatment in the clinic were considered for the case report. Following the CDSS implementation, a ten-month case report period (April 2011 through January 2012) was defined as the “post-CDSS” period for comparison to a randomly selected similar number of patients that met the same inclusion criteria who were treated for HCV during the “pre-CDSS” study period (prior to decision to design the CDSS in December 2010). For purposes of this case report, the nurse practitioner was also the primary investigator however additional staff members were utilized to verify accuracy across all aspects of data collection.
During the post-CDSS period, a total of 11 patients began treatment. The records from these 11 patients were de-identified and then compared to 11 de-identified medical records of patients that were randomly selected from the database of all patients who received HCV treatment in the pre-CDSS period. Medical records of patients that did not pursue HCV treatment were excluded.
Outcomes of interest included not only the quality control plan measures previously described, but also the initial evaluation of the effectiveness of the CDSS for improving clinician compliance with documentation of quality indicators for each patient in the post-CDSS period compared to the pre-CDSS period. The case report outcomes focused on the degree of compliance with documentation for six of the treatment-related quality indicators that are all required by CMS to receive quality care incentives and described in the 2010 Physician Quality Reporting Initiative [26]. The indicators include:
-
1.
pre-treatment HCV genotype testing;
-
2.
baseline viral load testing;
-
3.
counseling regarding alcohol abstinence;
-
4.
counseling for pregnancy prevention;
-
5.
documentation of at least one dose of the hepatitis A; and
-
6.
hepatitis B vaccine (or documentation of serologic immunity) for all patients that began therapy during the study period.
Two other CMS quality indicators, the 12-week viral load testing and the percentage of all patients that were prescribed treatment, were not evaluated due to the time limits of the study [26].
The proportion of quality indicator completion was calculated according to whether each quality indicator was documented for each patient in the pre-CDSS and post-CDSS groups. In addition, the proportion of patients that had documentation for all six-quality indicators was also measured in the pre-CDSS and the post-CDSS groups. The number of reminders issued by the medical assistant and/ or the nurse practitioner was tracked in both study periods according to the documentation in the EHR.
4. Results
All data were entered into Excel and exported into SPSS 19.0.0.1 (IBM, Chicago, IL) for analysis. In terms of the key process measure, the quality control plan for reviewing the accuracy of the CDSS prior to each office visit was completed 100% of the time.
The pre- and post-CDSS groups were tested for significant differences in key sample characteristics using chi-square and were found to be homogeneous for age, genotype and presence of cirrhosis, but heterogeneous for gender, government vs. private insurance, and prescribed triple therapy (▶Table 1). The pre-CDSS group was 73% male and 73% with private insurance compared to the post-CDSS group which was 18% male with 27% privately insured. No patients in either group had co-infection with HIV or hepatitis B and one patient in the pre-CDSS had compensated cirrhosis. All of the pre-CDSS patients received standard therapy but in the post-CDSS group 45% received triple therapy (first available June 2011).
Table. 1.
Patient characteristics.
| Demographics | Pre-CDSS | Post-CDSS | P |
|---|---|---|---|
| N | 11 | 11 | |
| Mean age (SD) | 49 (7) | 42 (13) | = 0.14 |
| Male | 8 (73%) | 2(18%) | < 0.01* |
| Insurance | = 0.03* | ||
| Government | 3 (27%) | 8 (73%) | |
| Private | 8 (73%) | 3 (27%) | |
| Genotype | = 0.81 | ||
| 1 | 7 (64%) | 8 (73%) | |
| 2/3 | 4 (36%) | 3 (27%) | |
| Cirrhosis | 1 (9%) | 0 | = 1.00 |
| Triple Therapy | 0 | 5 (45%) | = 0.035* |
The proportions of patients with each completed quality indicator as well as the proportion in each group with all six quality indicators completed were similar across both study groups (▶Table 2). Both study groups had four patients that were lacking one or more vaccinations, therefore these patients needed reminders. Although the number of patients needing reminders was the same in both groups, the pre-CDSS group had an average of 0.25 reminders issued per patient compared to the post-CDSS group average of 4.25 reminders per patient which was significantly different, t(6) = 11.31, p<0.000.
Table. 2.
Proportion of quality indicator documentation.
| Quality Indicators Documented | Pre-CDSS | Post-CDSS |
|---|---|---|
| Genotype | 11 (100%) | 11 (100%) |
| Baseline Viral Test | 11 (100%) | 11 (100%) |
| Alcohol Counseling | 11 (100%) | 11 (100%) |
| Pregnancy Counseling | 9 (82%) | 11 (100%) |
| Hepatitis A Vaccine | 9 (82%) | 7 (64%) |
| Hepatitis B Vaccine | 8 (73%) | 8 (73%) |
| All 6 Quality Indicators | 6 (55%) | 7 (64%) |
5. Discussion
This case report presents the methods used to develop and implement an HCV-specific CDSS while piloting data collection methods for evaluating the impact on quality indicator completion following the CDSS implementation. The strengths of this study lie in the innovative, automated, and synchronous design of the CDSS that aligns with the Chronic Care Model and seamlessly integrates into the clinic following a collaborative design and implementation process guided by the PRISM framework.
Effective and efficient workflow processes are of utmost importance in a demanding health care environment [27, 30]. One of the lessons learned was that the iterative tests of change throughout the CDSS development and early implementation processes created an environment of innovation and continual improvement within the practice. The staff responded to the development and implementation processes with active engagement as they discovered that the CDSS improved their ability to efficiently manage patients. Another lesson was that new processes are more readily accepted if the new system solves problems important to clinic providers and administrative staff. Prior to the CDSS, there was a generalized sense of frustration because the coordination of patient’s receiving HCV treatment required labor-intensive processes involving manual entry of laboratory results and quality care indicators across segregated paper flowcharts, software spreadsheets, and the EHR.
The implementation of the CDSS provided the staff with a dynamic “birds-eye view” of the treatment population (registry) and a patient-specific data summary (dashboard). As a result, the CDSS quickly became the centralized and well-accepted tool for addressing any HCV-related issue. Communication between the both the provider and administrative staff and patients improved because of the synchronous nature of the documentation between the patient registry, the patient’s EHR, and the dashboard. Additionally, as the post-CDSS findings demonstrate, reminders were more readily issued by providers and administrative staff for delinquent quality indicators as the systems of care were transformed by the automated, structured, and streamlined format of the CDSS. The limitations inherent in this case report preclude generalizability of findings, but the study did pilot both measures and methods that will be helpful to a larger, multi-site study.
5.1 Future research recommendations
This project lays the groundwork for a larger scale investigation of the efficacy of an HCV CDSS to improve quality care and patient outcomes. Because HCV treatment spans 24–48 weeks, a study period that parallels the entire treatment period would facilitate measurement of all HCV quality indicators to benchmark clinician performance against published studies [9]. Additionally, translating the HCV CDSS design and implementation principles across multiple clinics and providers, under the direction of an independent investigator with a research design that minimizes potential Hawthorne effect, would allow for expanded, rigorous evaluation of the functionality, generalizability, and efficacy of the CDSS in relation to clinician satisfaction, performance, and patient outcomes. Although the CDSS was implemented in a clinic that had a well-established EHR system, the data collection methods and measures would also be applicable for measuring efficacy following the transition from a paper charting system to an EHR integrated HCV CDSS system.
In addition to the process and outcome measures included in this study, it is also recommended that future studies include quantitative measurements of staff satisfaction both pre- and post-CDSS implementation. These surveys can analyze the impact on staff workflow and perceived benefit of the CDSS. Time studies would also be beneficial to determine the financial impact of the CDSS in relation to the potential for patient management and care coordination efficiencies.
6. Conclusion
The purpose of this case report is to describe the development and implementation of an HCV quality indicator-specific CDSS while piloting data collection methods to evaluate the impact of the CDSS on quality indicator completion and patient reminders as a framework for a larger, multi-practice study. Through close, iterative collaboration between the provider and administrative support team and EHR programmers, we improved both the clinical care reminder functionality and the number of patients with completion of all six quality indicators through the development of an EHR-integrated CDSS with patient dashboard and population registry components. This single clinic case report demonstrated improved outcomes; however an expanded study with larger sample sizes across multiple providers and settings should be performed to determine if our results are generalizable.
Clinical Relevance Statement
This case report describes the design and implementation processes used to develop and implement an EHR-integrated HCV CDSS while piloting data collection methods and measures to evaluate the CDSS impact on quality indicator completion with subsequent recommendations for future research.
Conflict of Interest
The authors declare that there were no conflicts of interest in the research.
Human Subjects Protections
The study received approval from the university’s institutional review board as an exempt study. The review board’s guidelines are in compliance with the World Medical Association Declaration of Helsinki on Ethical Principles for Medical Research Involving Human Subjects.
Acknowledgements
Southeastern Indiana Gastroenterology is acknowledged for their financial support of the third-party interface and programming fees for the CDSS development. Thanks also to Boris Katz, President of Integrated Business Intelligence Solutions, who provided valuable insight and support to the project by customizing the CDSS specifically to meet our clinic and patient needs. Dr. Steven Pletcher provided ongoing design and content support to the CDSS. Medical Assistant Candace Poindexter used the CDSS to coordinate HCV patients while providing essential feedback for CDSS refinement. Julie Abraham performed repeated measurement of the data to ensure the integrity of the data collection. Dr. Michael Weaver, Professor at Indiana University School of Nursing provided helpful statistical guidance.
References
- 1.Ghany MG, Strader DB, Thomas DL, Seef LB. AASLD practice guidelines: diagnosis, management, and treatment of hepatitis C an update. Hepatology 2009; 49(4): 1335–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control Hepatitis C information for health professionals [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2011[updated 2008 July 28; cited 2012 Jan 12]. Available from:http://www.cdc.gov/hepatitis/hcv/hcvfaq.htm [Google Scholar]
- 3.Barnes E.Initiatives for vaccine research [Internet]. United Kingdom: World Health Organization; 2010[updated 2010 Feb 8; cited 2012 Jan 12]. Available from:http://www.who.int/vaccine_research/disease/viral_cancers/en/index2.html [Google Scholar]
- 4.Shiratori Y, Ito Y, Yokosuka O, Imazeki F, Nakata R, Tanaka N, Arakawa Y, Hashimoto E, Hirota K, Yoshida H, Ohashi Y, Omata M, Antiviral therapy for cirrhotic hepatitis C: association with reduced hepatocellular carcinoma development and improved survival. Ann Intern Med 2005; 142(2): 105–114 [DOI] [PubMed] [Google Scholar]
- 5.Yoshida H, Arakawa Y, Sata M, Nishiguchi S, Yano M, Fujiyama S, Yamada G, Yokosuka O, Shiratori Y, Omata M. Interferon therapy prolonged life expectancy among chronic hepatitis C patients. Gastroenterology 2002; 123(2): 483–491 [DOI] [PubMed] [Google Scholar]
- 6.Ghany MG, Nelson DR, Strader DB, Thomas DL, Seef LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American association for the study of liver diseases. Hepatology 2011; 54(4): 1433–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basseri B, Yamini D, Chee G, Enayati P, Tran T, Poordad F. Comorbidities associated with the increasing burden of hepatitis C infection. Liver International 2010; 30(7): 1012–1018 [DOI] [PubMed] [Google Scholar]
- 8.Dorr D. Primary care managers supported by information technology systems improve outcomes, reduce costs for patients with complex conditions [Internet]. Rockville (MD): AHRQ Health Care Innovations Exchange; 2008[updated 2011 Jul 20; cited 2012 Jan 15]. Available from: http://www.innovations.ahrq.gov/content.aspx?id=264 [Google Scholar]
- 9.Kanwal F, Schnitzler MS, Bacon BR, Hoang T, Buchanan PM, Asch SM. Quality of care in patients with chronic hepatitis C virus infection: a cohort study. Ann Intern Med 2010; 153(4): 231–239 [DOI] [PubMed] [Google Scholar]
- 10.Hernandez B, Hasson NK, Cheung R. Hepatitis C performance measure on hepatitis A and B vaccination: missed opportunities? Am J Gastroenterol 2009; 104(8): 1961–1967 [DOI] [PubMed] [Google Scholar]
- 11.Hachem CY, Kramer JR, Kanwal F, El-Serag HB. Hepatitis vaccination in patients with hepatitis C: practice and validation of codes at a large veterans administration medical center. Aliment Pharm Therap 2008; 28(9): 1078–1087 [DOI] [PubMed] [Google Scholar]
- 12.Shim M, Khaykis I, Park J, Bini EJ. Susceptibility to hepatitis A in patients with chronic liver disease due to hepatitis C virus infection: missed opportunities for vaccination. Hepatology 2005; 42(3): 688–695 [DOI] [PubMed] [Google Scholar]
- 13.Chandra T, Reyes M, Nguyen H, Borum M. Frequency of alcohol and smoking cessation counseling in hepatitis C patients among internists and gastroenterologists. World J Gastroentero 2009; 15(47): 6010–6011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rongey CA, Kanwal F, Hoang T, Gifford AL, Asch SM. Viral RNA testing in hepatitis C antibody-positive veterans. Am J Prev Med 2009; 36(3): 235–238 [DOI] [PubMed] [Google Scholar]
- 15.Wong V, Wreghitt TG, Alexander GJ. Prospective study of hepatitis B vaccination in patients with chronic hepatitis C. Brit Med J 1996; 312(7042): 1336–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute of Medicine Crossing the quality chasm: a new health system for the 21st Century. Washington (DC): National Academy Press; 2001 [PubMed] [Google Scholar]
- 17.Wagner EH, Austin BT, Davis C, Hindmarsh M, Schaefer J, Bonomi A. Improving chronic illness care: translating evidence into action. Health Affair 2001; 20(6): 64–78 [DOI] [PubMed] [Google Scholar]
- 18.Pestotnik SL. Expert clinical decision support systems to enhance antimicrobial stewardship programs: insights from the Society of Infectious Disease Pharmacists. Pharmacotherapy 2005; 25(8): 1116–1125 [DOI] [PubMed] [Google Scholar]
- 19.Smith SA, Murphy ME, Huschka TR, Dinneen SF, Gorman CA, Zimmerman BR, Rizza RA, Naessens JM. Impact of a diabetes electronic management system on the care of patients seen in a subspecialty diabetes clinic. Diabetes Care 1998; 21(6): 972–976 [DOI] [PubMed] [Google Scholar]
- 20.Fiks AG, Grundmeier RW, Biggs LM, Localio AR, Alessandrini EA. Impact of clinical alerts within an electronic health record on routine childhood immunization in an urban pediatric population. Pediatrics 2007; 120(4): 707–714 [DOI] [PubMed] [Google Scholar]
- 21.Montgomery AA, Fahey T, Peters TJ, MacIntosh C, Sharp DJ. Evaluation of computer based clinical decision support system and risk chart for management of hypertension in primary care: randomised controlled trial.Brit Med J 2000; 320(7236): 686–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goud R, de Keizer NF, ter Reit G, Wyatt JC, Hasman A, Hellemans IM, Peek N. Effect of guideline based computerised decision support on decision making of multidisciplinary teams: cluster randomised trial in cardiac rehabilitation. Brit Med J 2009; 338: bl880 Available from:http://www.bmj.com/content/338/bmj.bl880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kitahata MM, Dillingham PW, Chaiyakunapruk N, Buskin SE, Jones JL, Harrington RD, Hooton TM, Holmes KK. Electronic human immunodeficiency virus (HIV) clinical reminder system improves adherence to practice guidelines among the University of Washington HIV study cohort. Clin Infect Dis 2003; 36(6): 803–811 [DOI] [PubMed] [Google Scholar]
- 24.Bell LM, Grundmeier R, Localio R, Zorc J, Fiks AG, Zhang X, Stephens TB, Swietlik M, Guevara JP.Electronic health record based decision support to improve asthma care: a cluster-randomized trial. Pediatrics 2010; 125(4): 770–777 [DOI] [PubMed] [Google Scholar]
- 25.Reynolds J, Roble D. The financial implications of ACOs for providers. Healthcare Financial Management. 2011; 65(10): 76–82 [PubMed] [Google Scholar]
- 26.CMS.gov [Internet] Baltimore (MD): 2010 PQRI measures list; 2009 Nov 13 [cited 2012 Jan 16]. Available from:https://www.cms.gov/PQRS/Downloads/2010_PQRI_MeasuresList_l11309.pdf
- 27.Kawamoto K, Houlihan CA, Balas EA, Lobach DF. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. Brit Med J 2005; 2(330). Available from: 10.1136/bmj.38398.500764.8F [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDonald CJ, Overhage JM, Barnes M, Schadow G, Blevins L, Dexter PR, Mamlin B.The Indiana network for patient care: A working local health information infrastructure. Health Affairs 2005; 24:1214–1220 [DOI] [PubMed] [Google Scholar]
- 29.Feldstein AC, Glasgow RE. A practical, robust implementation and sustainability model (PRISM) for integrating research findings into practice. Joint Comm J Qual Im 2008; 34(4): 228–243 [DOI] [PubMed] [Google Scholar]
- 30.Denekamp Y. Clinical decision support systems for addressing information needs of physicians. Israel Med Assoc J 2007; 9(11): 771–776 [PubMed] [Google Scholar]