Abstract
AIM: To describe the histology of the digestive tract and to investigate the occurrence of endocrine cells in Oligosarcus hepsetus (O. hepsetus).
METHODS: The digestive tract (DT) of O. hepsetus was divided into esophagus, two stomach regions (glandular and non-glandular) and two intestinal regions (anterior and posterior). These specimens were processed by routine histological techniques and stained with hematoxylin-eosin, Gomori’s trichrome, periodic acid Schiff (PAS) and Alcian blue (AB). An immunohistochemical method using avidin-biotin-peroxidase was employed.
RESULTS: The esophagus is lined with a non-keratinized stratified squamous epithelium that is reactive to PAS and AB. The stomach has a mucosa lined with a simple columnar epithelium with mucus-secreting cells that are reactive only to PAS. The intestine has a simple columnar epithelium with a brush border and goblet cells that are reactive to PAS and AB. Somatostatin, serotonin and cholecystokinin immunoreactive cells were identified throughout the DT.
CONCLUSION: This study revealed adaptations for the species’ diet and showed that the distribution and relative frequency of immunoreactive cells are similar to those of other fish.
Keywords: Fish, Esophagus, Stomach, Intestine, Endocrine cells
INTRODUCTION
The literature stresses the importance of knowledge of the anatomy of the digestive tract (DT) of fishes, because this structure is highly variable, related to the diversity of feeding habits, type of food and lifestyles[1-3].
The histological architecture of the DT includes a layer of mucus-secreting cells, observed by histochemical techniques in various studies of teleosts. The secretions vary among different fish species and also according to the location in the DT within the same species[4]. These secretions play an important role in lubricating the organ and protecting against proteolytic degeneration and pathogenic microorganisms[5].
Besides this, to control the functions of the different DT segments, endocrine cells compose a complex system disseminated among the epithelial components, with the ability to secrete physiologically active polypeptide hormones and amines[6]. According to Deveney et al[7], hormones have important functions in the overall regulation of the digestive process, such as nutrient absorption, the secretion of intestinal and associated glands, gut motility and intestinal blood flow
Oligosarcus hepsetus (O. hepsetus), known commonly as the thin dogfish, belongs to the Characidae family. It is carnivorous, with a diet basically composed of small fish. It mainly lives in rivers and reservoirs, generally at the middle to the bottom of the water column. It has a slightly rounded body with small fins and is considered a good swimmer[8]. Many articles have been published about this species’ distribution, ecology, feeding habits and diet, but no study has been published reporting the microscopic analysis of the organs of the DT of O. hepsetus. Although it does not have economic importance, it is one of the most abundant species in the reservoirs of Rio de Janeiro[9] and the Paraíba do Sul River[10], with distribution in the coastal region of south to south-eastern Brazil between Santa Catarina and Rio de Janeiro[11].
Studies involving microscopic anatomy and histochemistry provide information to characterize the organs of the digestive system, facilitating understanding of the physiology of the DT and the feeding habits of the species under investigation[12]. These studies are essential for efforts to restock native species to improve the condition of ecosystems[13].
The purpose of the present work was to describe histological and histochemical aspects of the DT of O. hepsetus and to investigate immunohistochemically the occurrence of the endocrine cells secreting somatostatin (SOM), serotonin (5-hydroxytryptamine, 5-HT), cholecystokinin (CCK), gastrin (GAS), glucagon (GLUC) and insulin (INS) in the DT of this fish, seeking to relate them to its feeding habits, in order to provide data for future studies.
MATERIALS AND METHODS
Collection area
The specimens were collected from two reservoirs: Funil (22°30’S, 44°45’W), and Ribeirão das Lajes (22°43’S, 44°46’W), as well as two points of the Paraíba do Sul River: Ilha dos Pombos (21º84’S, 42º 58’W) and Santa Cecília (22º48’S; 43º84’W), all located in Rio de Janeiro state, Brazil.
Tissue processing
Fourteen specimens were used in this study without sexual distinction, four collected in Funil Reservoir, four in Ribeirão das Lajes Reservoir, and three each from the Ilha dos Pombos and Santa Cecília sites of the Paraíba do Sul River. The fish were dissected in the field, after being anesthetized with benzocaine hydrochloride (50 mg/L) and killed rapidly by hypothermia for immediate removal of the DT. Fragments of the DT were fixed for 8 h in Bouin’s fluid and then placed in 70% alcohol. The esophagus, two stomach regions (glandular and non-glandular) and two intestinal regions (anterior and posterior) were obtained from each specimen (Figure 1). These materials were taken to the Histology and Embryology Laboratory of Rio de Janeiro Federal Rural University where they were processed by routine histological techniques, consisting of: dehydration (in a rising ethanol series - 70° GL to 100° GL), diaphanization in xylol and embedding in Histosec-Paraffin, to obtain 5 μm histological sections.
Figure 1.
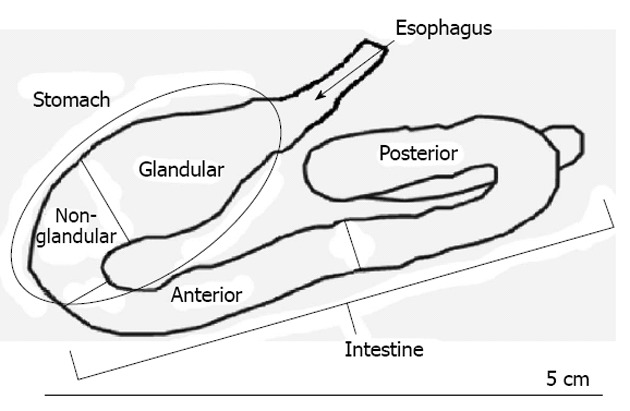
Anatomical diagram of the digestive tract of Oligosarcus hepsetus.
Histological and histochemical analysis
The sections obtained from the DT of O. hepsetus were stained with hematoxylin-eosin for analysis of the tissue architecture and with Gomori’s trichrome for differential visualization of the connective tissue and collagen fibers.
The histochemical analysis involved periodic acid Schiff (PAS) and Alcian blue (AB) pH 2.5 staining to reveal the neutral and acid glycoconjugates (GCs), respectively. Five slides from each specimen were prepared for each protocol, one from each of the five sectioned regions.
Immunohistochemistry
For the immunohistochemical procedure, 5 μm-thick sections were cut by microtome and mounted on glass slides precoated with 0.1% poly-L-lysine, after being dewaxed and dehydrated by the routine protocol. They were incubated in citrate buffer (pH 6.0-0.01 M) and placed in a microwave oven for 15 min to recover the antigen, then they were incubated with a solution of 3% H2O2 in methanol for 15 min to block any endogenous peroxidase. Subsequently, the sections were incubated at room temperature in a humid chamber with a 1:100 μL dilution of bovine serum albumin in phosphate buffered saline (PBS) solution for 30 min. The sections were first incubated overnight at 4 °C with the primary antisera against the individual gastrointestinal hormones (Table 1). The sections were then incubated with biotinylated “Universal” secondary antibody diluted to 1:200 μL for 30 min at room temperature, then with avidin-biotin-peroxidase complex, diluted at 1:200 μL for 30 min at room temperature. Subsequently, the peroxidase label was revealed by reaction with Stable DAB/Plus, prepared according to the kit’s instructions. All dilutions and thorough washes between stages were performed using PBS (pH 7.4). The sections were counterstained with Harris hematoxylin, rinsed with deionized water, dehydrated through a series of ethanol and methylcyclohexane solutions and mounted using Entellan. To investigate the specificity of the reactions, negative and positive controls were used. The negative control was prepared by replacement of the primary antibody with non-immune serum and PBS (pH 7.4). Positive controls were produced using tissue sections for each respective antiserum, as indicated in the product data sheet.
Table 1.
Details of primary antisera used in this study
| Primary antisera | Donor | Code No. | Working dilution | Source |
| Somatostatin | Rabbit | A566 | 1:300 | Dako Corp., CA, United States |
| Serotonin | Rabbit | S5545 | 1:8.000 | Sigma-Aldrich, Inc., United States |
| Cholecystokinin | Rabbit | C2581 | 1:8.000 | Sigma-Aldrich, Inc., United States |
| Gastrin | Rabbit | G0785 | 1:1.000 | Sigma-Aldrich, Inc., United States |
| Glucagon | Mouse | G2654 | 1:2.000 | Sigma-Aldrich, Inc., United States |
| Insulin | Mouse | I2018 | 1:1.000 | Sigma-Aldrich, Inc., United States |
Observation and photomicrography
Photomicrographs of all samples from each of the fourteen specimens were obtained with a digital camera Nikon Coolpix 4300 attached to a microscope Olympus BX41. The number of immunoreactive endocrine cells to each antiserum per analyzed segment was recorded and the intensity of immunoreaction was classified: absent (-) or low (+), medium (++) and strong (+++) immunoreactivity (Table 2).
Table 2.
Regional distribution and intensity of immunoreaction in endocrine cells in the digestive tract of Oligosarcus hepsetus
| Antisera | Segment of the esophagus | Segments of the stomach | Segments of the gut | ||
| Gl | NGl | ANT | POST | ||
| Somatostatin | +++ | ++ | + | - | - |
| Serotonin | - | +++ | ++ | - | - |
| Cholecystokinin | - | - | - | - | ++ |
| Glucagon | - | - | - | - | - |
| Gastrin | - | - | - | - | - |
| Insulin | - | - | - | - | - |
Intesity of immunoreactions: (-), absent; (+), low; (++), medium; (+++), strong. Gl: Glandular; NGl: Non-glandular; ANT: Anterior; POST: Posterior.
RESULTS
Histological and histochemical study of digestive tract
The following layers were observed in the DT of O. hepsetus: mucosa, submucosa, muscular and adventitia or serosa. The muscularis mucosae is absent in this species.
Esophagus
Histological examination revealed that the mucosa of the esophagus of O. hepsetus has many longitudinal folds and is lined with a stratified epithelium with non-keratinized squamous surface cells. The majority of the mucus-secreting cells are interspersed with a smaller number of non-secretory cells (Figure 2A).
Figure 2.
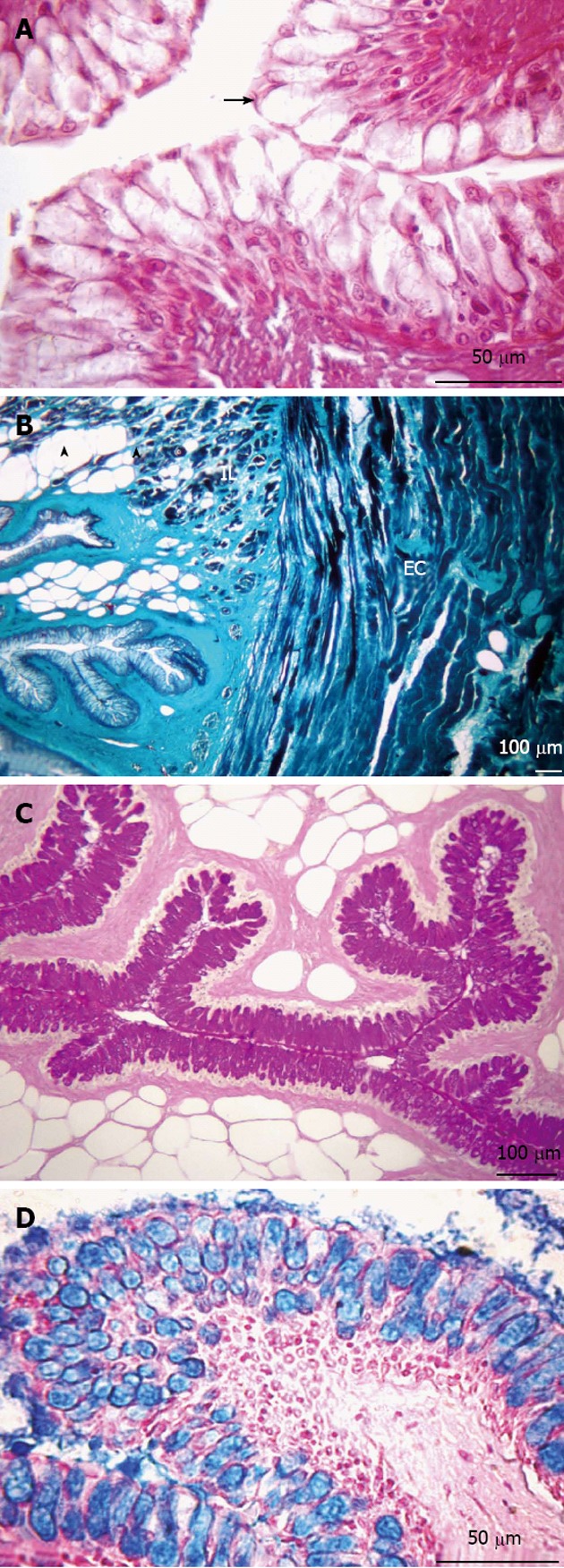
Transversal sections of the esophagus. A: Non-keratinized stratified squamous epithelium (arrow). Hematoxylin and eosin stain; B: Presence of adipose tissue (arrowheads) in the submucosa layer. Muscular layer formed by two sub-layers, internal longitudinal (IL) and external circular (EC). Gomori’s trichrome stain; C: Presence of neutral glycoconjugates (GCs). Periodic acid-Schiff stain; D: Acid GCs. Alcian blue stain.
The secretory cells reacted positively to PAS and AB staining, indicating the presence of neutral and acid GCs, respectively. The lamina propria is composed of connective tissue and does not have glands. The muscular layer is formed by two sub-layers of striated skeletal muscle, with an internal longitudinal and an external circular layer. Externally, the esophagus is enveloped by an adventitia, composed of connective tissue with some nerve fibers and blood vessels (Figure 2).
Stomach
In the stomach of O. hepsetus, the mucosa is lined with a simple epithelium layer composed of columnar mucus-secreting cells with basal nuclei. These were reactive to PAS but not to AB, revealing the presence of only neutral GCs. The stomach epithelium forms crypts along the gastric mucosa. The mucosa layer projects toward the organ’s lumen, forming various gastric folds, arranged longitudinally. In the non-glandular region, the submucosa and muscular layers accompany the mucosa in forming these folds, making the lumen very small (Figure 3).
Figure 3.
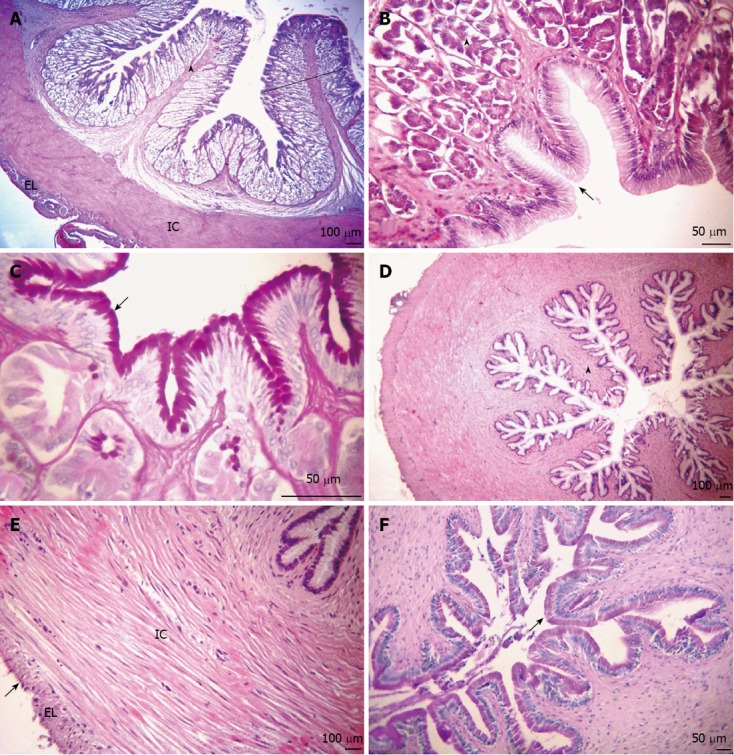
Transversal sections of the stomach. A: Mucosa layer with many high gastric folds (arrowhead), thickening of the lamina propria caused by a large number of tubular gastric glands (outline). Organization of the internal circular (IC) and external longitudinal (EL) muscular layers. Hematoxylin and eosin (HE) stain; B: The simple columnar epithelium with mucus-secreting cells forming faveola gastrica (arrow) and fundic glands composed of oxynticopeptic cells (arrowhead). HE stain; C: Presence of neutral glycoconjugates (GCs) (arrow). Periodic acid Schiff (PAS) stain; D: Mucosa layer, together with the sub-mucosa and internal muscular layers, forming large longitudinal folds (arrowhead). HE stain; E: The muscular layer of smooth muscle fibers, composed of IC and EL sub-layers and serosa (arrow). HE stain; F: Neutral GCs (arrow). PAS stain. (A-C: Glandular region; D-F: Non-glandular region).
The division of the stomach observed in the present work is in accordance with the structural characteristics of the two regions. The glandular region is characterized by having well-developed tubular gastric glands, composed of oxynticopeptic cells, occupying the entire lamina propria (Figure 3B). These are smaller and less numerous in the initial portion and increase in number and size in the direction of the non-glandular region. The non-glandular region has a well-developed muscular layer (Figure 3D and E). The submucosa layer is composed of connective tissue and blood vessels.
In the stomach, the muscular layer is composed of smooth muscle fibers arranged in two directions: internal circular and external longitudinal (Figure 3E). The glandular region contains myenteric plexuses arranged in sparse groups, composing the enteric nervous system and located between the muscular sub-layers, and there is a serosa layer which surrounds these structures.
Intestine
Just as for the stomach, the adopted division of the intestine follows specific structural patterns in relation to the mucosa layer. The histological analysis of the intestine of O. hepsetus revealed that the pattern of folds varies, characterizing two distinct regions: anterior and posterior. The anterior region has numerous thin and elongated folds with villi (Figure 4A). In contrast, in the posterior part the folds are less sinuous due to the absence of villi and are thicker, with some being leaf-shaped, and also have a greater number of goblet cells (Figure 4B).
Figure 4.

Longitudinal sections of the intestine. A-C: Anterior portion; D-F: Posterior portion; A: Overview of the anterior intestine, showing the arrangement of small folds (arrowhead) presenting villi (arrow). Organization of the internal circular (IC) and external longitudinal (EL) muscular layers. Hematoxylin and eosin (HE) stain; B: Simple columnar epithelium with brush border and goblet cells, indicating the presence of acid glycoconjugates (GCs) (arrow). Alcian blue (AB) stain; C: Neutral GCs (arrows). Periodic acid Schiff (PAS) stain; D: Mucosa layer with thick folds (arrowhead). HE stain; E: Myenteric plexus (arrow). HE stain; F: Simple columnar epithelium with striated border and goblet cells with acid GCs (arrow). AB stain; G: Neutral GCs (arrow). PAS stain.
The intestinal mucosa is lined by a simple columnar epithelium with a striated border and goblet cells, which were positive to PAS and AB stainings, with pink (PAS) and blue (AB) coloration (Figure 4), indicating the presence of neutral and acid GCs, respectively.
The limits between the lamina propria and the submucosa layer are not evident, with connective tissue and blood vessels present in both these regions. In both parts of the intestine, the muscular layer has the same organization as in the stomach, with an internal circular layer and an external longitudinal one, observed in the cross-sections, both composed of smooth muscle cells (Figure 4A). The posterior part of the intestine contains a continuous layer of nervous tissue and a myenteric plexus between the muscular sub-layers (Figure 4E). Externally there is a serosa layer.
Immunohistochemical study of digestive tract
SOM-, 5-HT- and CCK-immunorective (IR) cells were identified in the DT of O. hepsetus, but GAS-, GLUC- and INS-IR cells were not present (Table 2).
Esophagus
Somatostatin immunoreactivity: In the esophagus, SOM-IR cells were detected in the basal layer of the stratified squamous epithelium (Figure 5). Morphologically, these cells were totally colored by chromogen, making it impossible to visualize a nuclear halo. The nucleus of these cells is very small, occupying a tiny area inside them.
Figure 5.
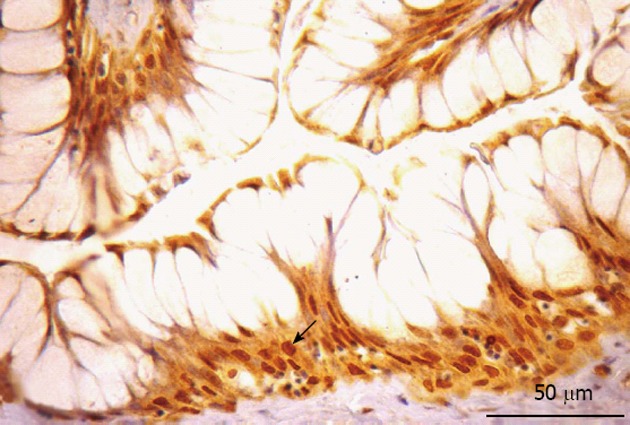
Somatostatin-immunorective cells in the esophagus. Somatostatin-immunorective cell were detected in the basal layer of the stratified squamous epithelium (arrow).
Stomach
Serotonin and somatostatin immunoreactivity: Serotonin (5-HT)-IR cells (Figure 6) and SOM-IR cells (Figure 7) were observed in the lining epithelium and glandular epithelium of the stomach. Regarding the morphology of immunoreactive cells, two types were found in this portion, namely closed-type cells and open-type cells.
Figure 6.
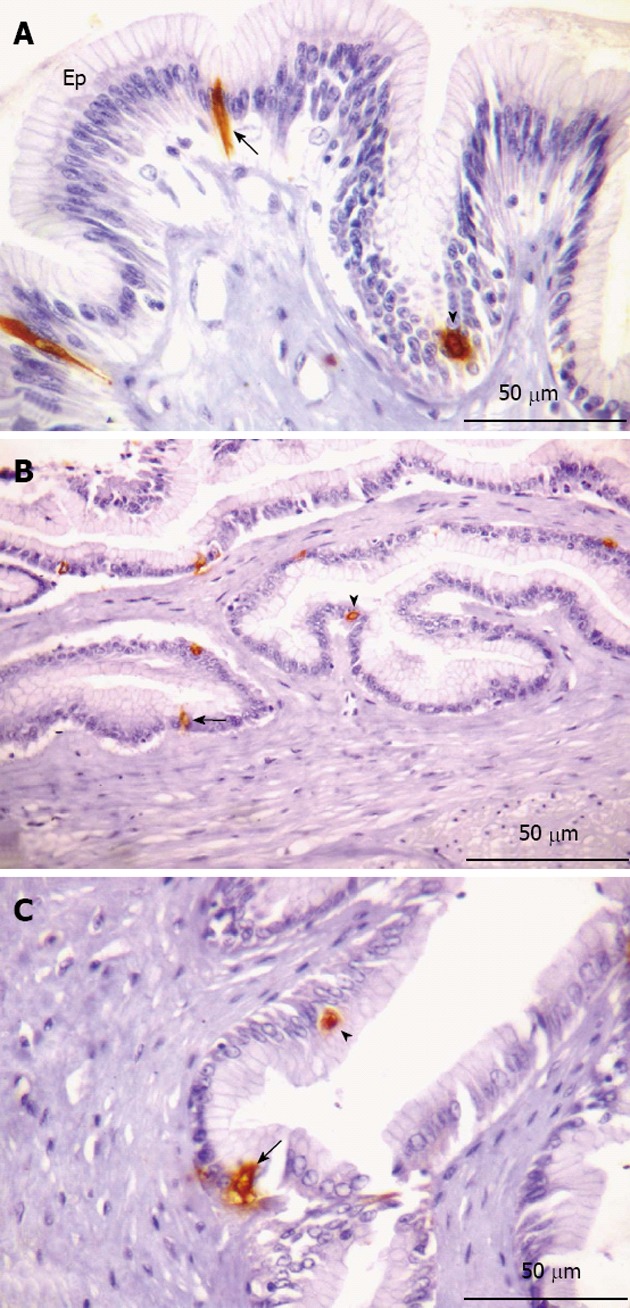
Serotonin-immunorective cells in the stomach. A-B: Glandular region; C: Non-glandular region. A: Presence of open (arrow) and closed (arrowhead) type cells interspersed in the epithelium (Ep); B: Highlight of open (arrow) and closed (arrowhead) type immunorective (IR) cells; C: Indications of open (arrow) and closed (arrowhead) IR cells.
Figure 7.
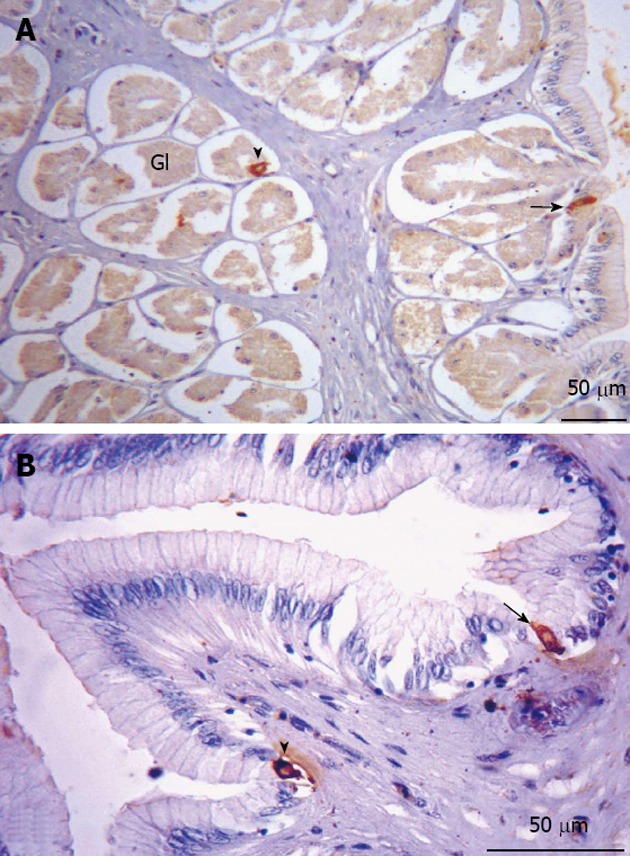
Somatostatin-immunorective cells in the stomach. A: Glandular region. Immunorective (IR) cells along the entire region, of the open (arrow) and closed (arrowhead) types, present both in the lining epithelium and the glandular epithelium; B: Non-glandular region. Indications of IR cells of the open (arrow) and closed (arrowhead) types. Gl: Glandular.
Intestine
Cholecystokinin immunoreactivity: CCK-IR cells were only observed in the lining epithelium of the posterior part of the intestine of O. hepsetus (Figure 8). Closed-type and open-type immunoreactive cells were found.
Figure 8.
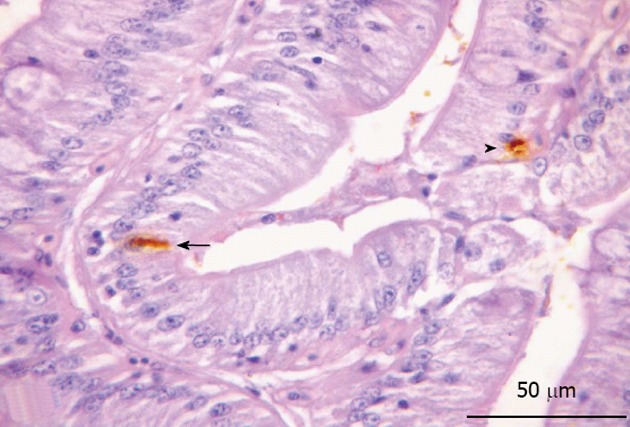
Cholecystokinin-immunorective cells in the posterior region of the intestine. Epithelial marking of open (arrow) and closed (arrowhead) immunorective cells.
DISCUSSION
The stratification of the wall of the DT of O. hepsetus has the same organization observed in the majority of other teleosts, with some modifications associated with the species’ feeding habits. We observed four layers: mucosa, submucosa, muscular and serosa. The muscularis mucosae is not present in the examined areas of the DT, unlike that observed in Pimelodus maculates (P. maculatus)[14] and Semaprochilodus insignis[15]. These authors assumed that the existence of muscular tissue between the lamina propria and submucosa aids in the elimination of the substances produced by the glands.
The very large longitudinal folds of the mucosa layer of the esophagus of O. hepsetus substantially increases the organ’s capacity for distension, an effect described by other researchers[16,17]. The digestive capacity is related to the volume of the folds in the mucosa, with a greater number of folds implying more efficient digestion.
The lining of the mucosa by a stratified squamous epithelium, according to Hunbert et al[18], acts to protect the fish against mechanical aggression and invasive bacteria. The same pattern was found in Prionotus carolinus by Blake[19], but was not observed in Salmo trutta by Burnstock[20] or in P. maculatus by Santos et al[14]. The submucosa contains bundles of adipose tissue and blood vessels; the same was found in Dentex dentex (D. dentex)[21].
The positive reaction of the epithelium to PAS and AB staining revealed the production of neutral and acid GCs, respectively, in the esophagus. The first type of mucus has low viscosity and is important to assure laminar flow during the lubrication and treatment of particles, to enable digestion to be conducted by the esophagus until the upper region of the stomach. In turn, acid GCs have high viscosity and are fundamental to trap particles[22]. The presence of these GCs was also reported in Anguilla anguilla[23], D. dentex[21], P. maculatus[14], Cynoscion guatucupa (C. guatucupa)[24], Pelteobagrus fulvidraco (P. fulvidraco)[25] and Hyphessobrycon anisitsi (H. anisitsi)[26].
The transition from the esophagus to the stomach is characterized by an abrupt change in the lining epithelium, which starts to present a single layer of columnar cells secreting mucus. This type of stomach lining epithelium has been observed in the majority of other teleosts as well[4,14,21,23,24], but in Plecostomus plecostomus[27] and Epinephelus marginatus[28], the epithelium described at the beginning of the stomach was of the squamous and cubic type, respectively, becoming simple columnar in the posterior regions. The mucus-secreting cells were only reactive to PAS in the two stomach regions; the same was found in C. guatucupa[24], P. fulvidraco[25] and H. anisitsi[26], but was not like that observed in Anguilla anguilla (A. anguilla)[23] and Chanos chanos[29]. As mentioned, the probable function of this mucus is to promote a flow able to carry the food bolus to the intestine. Besides this, since cells producing hydrochloric acid (HCL), essential for digestion, were identified in the stomach region, this mucus also functions as a layer to protect the epithelial cells.
The invaginations formed by the lining epithelium are called gastric crypts, which in the glandular region communicate with well-developed and branched tubular glands, as also observed by Díaz et al[24] in C. guatucupa and by Domeneghini et al[23] in A. anguilla. These are common characteristics of carnivorous fish species[30]. The gastric glands are composed of oxynticopeptic cells, which play a role similar to that of the principal and parietal cells in mammals, by synthesizing HCL and pepsinogen. In this case, we believe the glandular region has digestive functions while the non-glandular region only acts to carry the food to the gut with the epithelial secretions, with the help of the muscular layer, which in this region is thicker[14]. As seen in the esophagus, the stomach regions also contain well-developed longitudinal folds, whose function is to allow expansion of the organ’s diameter to store a large volume of food[16,17], another common characteristic of carnivorous fish species[30].
The intestine of O. hepsetus has two distinct parts, the same as in Tilapia spp.[31]. The anterior part is characterized by a larger number of thin and elongated longitudinal folds, which branch out to form villi, making the organ’s lumen very small. The posterior region, endowed with thicker and less sinuous longitudinal folds, contains a larger number of goblet cells, similar to the pattern observed in the large intestine of mammals. This pattern of ample folds of the mucosa is common in carnivores, acting to expand the surface absorption area, since carnivores have a relatively short intestine compared to animals with other feeding habits[30]. Sections of the lining epithelium of both regions, when submitted to the PAS and AB histochemical protocols, reacted positively, showing cells with pink (PAS) and blue (AB) coloring, indicating the presence of neutral and acid GCs, respectively, as also observed by Cinar et al[32] in Pseudophoxinus antalyae, by Carrassón et al[21] in D. dentex and by Leknes[26] in H. anisitsi, but unlike that observed in P. fulvidraco[25] and Chanos chanos[29].
This allows the hindgut to lubricate the tube and to trap particles to be eliminated, permitting the food bolus to reach this region in dehydrated form[33].
The organization of the muscular layer along the entire DT of O. hepsetus is the same as observed in P. maculatus[14] and P. fulvidraco[25], except in the esophagus, where the pattern resembled that found in mammals. The function of this layer is to promote motility in the DT, carrying and mixing food with the digestive secretions. This motility and also the release of these secretions are favored by the existence of a myenteric plexus between the muscular tissue sub-layers, observed in the glandular region of the stomach as well as in the posterior intestine. Unlike in mammals, the myenteric plexus does not have the form of ganglia, but rather appears in sparse form or in continuous layers, as seen in Pimelodus maculatus[34], but unlike that observed in Salmo trutta[20] and P. fulvidraco[25].
The results obtained in this study demonstrate that the DT of O. hepsetus has three types (5-HT-, SOM- and CCK) of endocrine cells, but three other types (GAS-, GLUC- and INS-IR cells) were not present. However, these cells have been observed in other fish, such as: GLU-IR cells in the gastric mucosa of cartilaginous fishes[35]; GAS-IR cells in the stomach pyloric region of Oncorhynchus mykiss; and INS-IR cells in the stomach pyloric region of Monopterus albus (M. albus) and the stomach cardiac and pyloric regions of Pelteobagrus fulvidraco[36]. The reason for the absence of these endocrine cells in the DT of O. hepsetus may be related to its digestive histophysiology, but further studies should be conducted to confirm this relationship.
The peptide SOM is a component responsible for inhibiting many substances, such as GAS, CCK, GLUC, INS, secretin, motilin and gastric acid[37]. In mammals, it also controls the absorption of amino acids and glucose[38]. It is thus essential in the DT, since it participates in basic mechanisms for efficient food processing. Ku et al[39] identified the production of this hormone along the DT of the reptile Trachemys scripta elegans, including in the esophagus, where we found SOM-IR cells in O. hepsetus. Other studies investigating the presence of this hormone in the DT have been performed by Lee et al[40] and Pan et al[36], the latter analyzing the presence of endocrine cells in eight fish species: P. fulvidraco, M. albus, Siniperca chuatsi (S. chuatsi), Colossoma brachypomum and Tilapia nilotica, all of which presented SOM-IR cells in the gastric mucosa, as observed in O. hepsetus. Although we did not visualize this hormone in the intestinal parts of the thin dogfish, it was reported in the gut of the spiny dogfish Squalus acanthias[41], Oncorhynchus mykiss[42], P. fulvidraco, M. albus, S. chuatsi[36], Zacco platypus[43] and Coreoperca herzi (C. herzi)[44].
5-HT-IR cells have been detected in the DT of various vertebrates: fish[44], amphibians[45], reptiles[46], birds[47] and mammals[48,49]. Researchers state that all vertebrates have this type of endocrine cell in the DT, assuming that these cells’ location is based on the evolution of these higher life forms[50]. It is known that in fish, serotonin promotes gastrointestinal motility[51] and blood flow, in the latter case by triggering vasoconstriction[52,53]. In O. hepsetus, 5-HT-IR cells were observed only in the regions of the stomach, both in the lining epithelium and the glandular epithelium, as also observed in C. herzi by Lee et al[44].
As was described for the fish species Oncorhynchus mykiss[54], Salmo trutta[55], Odontesthes bonariensis[56] and Rhamdia quelen[57], in O. hepsetus we observed CCK-IR cells in the intestine; in this case only in the posterior part, while in O. bonariensis[56] these cells were observed throughout the gut, but with greater concentration in the hindgut. There were no CCK-IR cells in the other regions of the DT of O. hepsetus. This hormone controls intestinal motility, by stimulating the release of pancreatic juice and inhibiting gastric emptying[58,59]. The existence of these cells has been verified in fish[35,42], reptiles[39], birds[60] and mammals[61].
In conclusion, our histological and histochemical study of the DT of O. hepsetus revealed adaptation for the species’ feeding habits, to protect the tract and increase the absorptive processes. The immunohistochemical study showed that the DT of this fish species contains different types of endocrine cells similar to those found in other vertebrate species. This study will help comprehension of the digestive physiology of this species and provide a basis for diagnosing diseases that affect the digestive tract of carnivorous teleosts.
ACKNOWLEDGMENTS
We thank Ilza Lucas Coelho Meirelles for her technical assistance.
COMMENTS
Background
Studies indicate the importance of knowledge of the morphology of the digestive tract (DT) because of the different physiological conditions in which animals live and their varied eating habits, which are manifested by adaptations and modifications in the digestive system. Information about the morphological aspects of the DT provides support for research on physiology and nutrition, aiming to improve the diet and management of livestock and support activities for restocking and restoration of natural ecosystems, besides being important for various research areas, including biological systems and conservation.
Research frontiers
The integration of the motor, secretory and absorptive phenomena of the DT is fundamental for the reduction of food into simpler particles that can be absorbed. This association is achieved by actions and interactions of the nervous and endocrine systems, which in the digestive system are represented by the endocrine cells. The diffuse neuroendocrine system (DNS) acts to control the motility and transit speed of the ingesta, the various secretions of the DT, the absorption of nutrients and the blood flow, to assure the activation and action of enzymes at the proper moment. Therefore, the products of the digestive system can be absorbed by the organism and reach the blood and lymphatic circulation systems.
Innovations and breakthroughs
The avidin-biotin-peroxidase complex method was applied to study the endocrine cells in the DT of Oligosarcus hepsetus (O. hepsetus). This method involves the use of three reagents: the primary antibody, which binds to the receptor of the specific hormone of interest; the secondary antibody, which is produced linked to a molecule of the vitamin biotin (C) and binds to receptors of the primary antibody; and the glycoprotein-avidin complex, produced from biotin and peroxidase, with joins with the previous reagent, the secondary antibody.
Applications
The immunohistochemical study showed that the DT of O. hepsetus contains different types of endocrine cells similar to those found in other vertebrate species. This study will help comprehension of the digestive physiology of this species and provide a basis for diagnosing diseases that affect the digestive tube of carnivorous teleosts.
Terminology
The gastrointestinal epithelium is permeated by a set of cells, originating from the DNS, called endocrine cells. The secretions of these cells control the digestion of food to ensure it is efficient, by regulating the digestive processes. Besides controlling the absorption of nutrients, they play an important role in determining secretions from the gut and associated glands and in regulating the intestinal blood flow.
Peer review
In this study, the authors describe the microscopic anatomy of the DT of a carnivorous fish species and analyze the functional components that aid the digestion of food. The results are relevant by enabling comparison with other fish species, contributing to phylogenetic studies.
Footnotes
Supported by Federal Rural University of Rio de Janeiro for the Research Grant under the Internal Scientific Initiation Program, to Vieira-Lopes DA
P- Reviewer Cinar K S- Editor Gou SX L- Editor Logan S E- Editor Li JY
References
- 1.Bicca DF, Queirol E, Braccini MC. Aspectos morfológicos e histológicos do estômago de Acestrorhynchus pantaneiro (Menezes, 1992) (Teleostei, Acestrorhynchidae) na Bacia do Rio Uruguai médio. Biodivers Pampeana. 2006;4:5–10. [Google Scholar]
- 2.Winemiller KO. Ontogenetic diet shifts and resource partitioning among piscivorous fishes in the Venezuelan ilanos. Environ Biol Fishes. 1989;26:177–199. [Google Scholar]
- 3.Wainwright PC, Richard BA. Predicting patterns of prey use from morphology with fishes. Environ Biol Fishes. 1995;44:97–113. [Google Scholar]
- 4.Díaz AO, García AM, Devincenti CV, Goldemberg AL. Morphological and histochemical characterization of the mucosa of the digestive tract in Engraulis anchoita. Anat Histol Embryol. 2003;32:341–346. doi: 10.1111/j.1439-0264.2003.00490.x. [DOI] [PubMed] [Google Scholar]
- 5.Reid PE, Volz D, Cho KY, Owen DA. A new method for the histochemical demonstration of O-acyl sugars in human colonic epithelial glycoproteins. Histochem J. 1988;20:510–518. doi: 10.1007/BF01002649. [DOI] [PubMed] [Google Scholar]
- 6.Carvalheira AF, Welsch U, Pearse AG. Cytochemical and ultrastructural observations on the argentaffin and argyrophil cells of the gastro-intestinal tract in mammals, and their place in the APUD series of polypeptide-secreting cells. Histochemie. 1968;14:33–46. doi: 10.1007/BF00268031. [DOI] [PubMed] [Google Scholar]
- 7.Deveney CW, Way LW. Regulatory peptides of gut. In: Greenspan FS, Forsham PH, editors. Basic and Clinical Endocrinology. Singapore: Maruzen; 1983. pp. 479–499. [Google Scholar]
- 8.Szpilman M. Peixes marinhos do Brasil. Guia prático de identificação. Rio de Janeiro: Mauad; 2000. [Google Scholar]
- 9.Araújo FG, Santos LN. Distribution of fish assemblages in Lajes reservoir, Rio de Janeiro. Braz J Biol. 2001;61:563–576. doi: 10.1590/s1519-69842001000400006. [DOI] [PubMed] [Google Scholar]
- 10.Teixeira TP, Terra BF, Estiliano EO, Gracia D, Pinto BCT, Araújo FG. Distribuição da ictiofauna em locais impactados no Rio Paraíba do Sul. Rev Univ Rural Ser Cien Vida. 2004;24:167–174. [Google Scholar]
- 11.Menezes NA. Três espécies novas de Oligosarcus Gunther, 1864 e redefinição taxonômica das demais espécies do gênero (Osteichthyes, Teleostei, Characidae) Bolm Zool. 1987;11:1–39. [Google Scholar]
- 12.Fugi R, Hahn NS. Espectro alimentar e relações morfológicas com o aparelho digestivo de três espécies de peixes comedores de fundo do Rio Paraná, Brasil. Rev Bras Biol. 1991;51:873–879. [Google Scholar]
- 13.Castro EF, Fonseca CC, Menin E, Neves MTD. Caracterização histológica e detecção de células endócrinas no estômago de peixes de água doce, com diferentes hábitos alimentares. Biotemas. 2003;16:105–130. [Google Scholar]
- 14.Santos CM, Duarte S, Souza TGL, Ribeiro TP, Sales A, Araújo FG. Histologia e caracterização histoquímica do tubo gastrintestinal de Pimelodus maculatus (Pimelodidae, Siluriformes) no reservatório de Funil, Rio de Janeiro, Brasil. Iheringia Ser Zool. 2007;97:411–417. [Google Scholar]
- 15.Chaves PTC, Vazzoler C. Aspectos biológicos de peixes anatômicos. III. Anatomia microscopica do esôfago, estômago e cecos pilóricos de Semaprochilodus insignis (Characiformes: Prochilodontidae) Acta Amaz. 1984;14:343–353. [Google Scholar]
- 16.Legler JM. Morphology and physiology of the Chelonia. In: Glasby CJ, Ross GJB, Beesley PL, editors. Caberra: Fauna of Australia; 1993. pp. 108–119. [Google Scholar]
- 17.Zug GR, Vitt LJ, Caldwell JP. Herpetology: An introduction biology of amphibians and reptiles. 2nd ed. Florida: Academic Press; 2001. [Google Scholar]
- 18.Hunbert W, Kirsch R, Meister MF. Scanning electron microscopic study of the oesophageal mucous layer in the eel, Anguilla anguilla. J Fish Biol. 1984;25:117–122. [Google Scholar]
- 19.Blake IH. Studies on the comparative histology of the digestive tube of certain teleost fishes. II. A bottom feeding fish, the sea-robin (Prionotus carolinus) J Morphol. 1936;60:77–102. [Google Scholar]
- 20.Burnstock G. The morphology of the gut of the Brown trout (Salmo trutta) Q J Microsc Sci. 1959;100:183–198. [Google Scholar]
- 21.Carrassón M, Grau A, Dopazo LR, Crespo S. A histological, histochemical and ultrastructural study of the digestive tract of Dentex dentex (Pisces, Sparidae) Histol Histopathol. 2006;21:579–593. doi: 10.14670/HH-21.579. [DOI] [PubMed] [Google Scholar]
- 22.Sibbing FA, Uribe R. Regional specialization in the oro-pharyngeal wall and food processing in carp (Cyprinus carpio L) Neth J Zool. 1985;35:377–422. [Google Scholar]
- 23.Domeneghini C, Arrighi S, Radaelli G, Bosi G, Veggetti A. Histochemical analysis of glycoconjugate secretion in the alimentary canal of Anguilla anguilla L. Acta Histochem. 2005;106:477–487. doi: 10.1016/j.acthis.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 24.Díaz AO, García AM, Goldemberg AL. Glycoconjugates in the mucosa of the digestive tract of Cynoscion guatucupa: a histochemical study. Acta Histochem. 2008;110:76–85. doi: 10.1016/j.acthis.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Cao XJ, Wang WM. Histology and mucin histochemistry of the digestive tract of yellow catfish, Pelteobagrus fulvidraco. Anat Histol Embryol. 2009;38:254–261. doi: 10.1111/j.1439-0264.2009.00932.x. [DOI] [PubMed] [Google Scholar]
- 26.Leknes IL. Histochemical studies on mucin-rich cells in the digestive tract of a teleost, the Buenos Aires tetra (Hyphessobrycon anisitsi) Acta Histochem. 2011;113:353–357. doi: 10.1016/j.acthis.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 27.Petrini LM. Sobre a presença de capilares intra-epiteliais na mucosa do estômago do cascudo (Plecostomus plecostomus Linneu) Cien Cult. 1961;13:175. [Google Scholar]
- 28.Borges JCS, Sanches EG, Oliveira MS, Silva JRMC. Anatomia e histologia gastrintestinal da garoupa-verdadeira Epinephelus marginatus (Lowe, 1834) (Teleostei, Serranidae) Acta Sci Biol Sci. 2010;32:407–414. [Google Scholar]
- 29.Ferraris RP, Tan JD, De la Cruz MC. Development of the digestive tract of milkfish, Chanos chanos (Forsskal): Histology and histochemistry. Aquaculture. 1987;61:241–257. [Google Scholar]
- 30.Rotta MA. Aspectos gerais da fisiologia e estrutura do sistema digestivo dos peixes relacionados à piscicultura. Corumbá: Embrapa Pantanal; 2003. [Google Scholar]
- 31.Gargiulo AM, Ceccarelli P, Dall’Aglio C, Pedini V. Histology and ultrastructure of the gut of the tilapia (Tilapia spp.), a hybrid teleost. Anat Histol Embryol. 1998;27:89–94. doi: 10.1111/j.1439-0264.1998.tb00162.x. [DOI] [PubMed] [Google Scholar]
- 32.Cinar K, Senol N. Histological and histochemical characterization of the mucosa of the digestive tract in flower fish (Pseudophoxinus antalyae) Anat Histol Embryol. 2006;35:147–151. doi: 10.1111/j.1439-0264.2005.00629.x. [DOI] [PubMed] [Google Scholar]
- 33.Young B, Heath J W. Histologia functional. Rio de Janeiro: Guanabara Koogan; 2000. [Google Scholar]
- 34.de Souza RR, Ferri S, Ferraz de Carvalho CA, Paranhos GS. Myenteric plexus in a freshwater teleost intestine. I. Quantitative study of nerve cells. Anat Anz. 1982;152:359–362. [PubMed] [Google Scholar]
- 35.Tagliafierro G, Farina L, Faraldi G, Rossi GG, Vacchi M. Distribution of somatostatin and glucagon immunoreactive cells in the gastric mucosa of some cartilaginous fishes. Gen Comp Endocrinol. 1989;75:1–9. doi: 10.1016/0016-6480(89)90001-4. [DOI] [PubMed] [Google Scholar]
- 36.Pan QS, Fang ZP, Huang FJ. Identification, localization and morphology of APUD cells in gastroenteropancreatic system of stomach-containing teleosts. World J Gastroenterol. 2000;6:842–847. doi: 10.3748/wjg.v6.i6.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kong HS, Lee JH, Park KD, Ku SK, Lee HS. Immunohistochemical study of the endocrine cells in the pancreas of the carp, Cyprinus carpio (Cyprinidae) J Vet Sci. 2002;3:303–314. [PubMed] [Google Scholar]
- 38.Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 39.Ku SK, Lee HS, Lee JH, Park KD. An immunohistochemical study on the endocrine cells in the alimentary tract of the red-eared slider (Trachemys scripta elegans) Anat Histol Embryol. 2001;30:33–39. doi: 10.1046/j.1439-0264.2001.00284.x. [DOI] [PubMed] [Google Scholar]
- 40.Lee HS, Ku SK. An immunohistochemical study of endocrine cells in the alimentary tract of the grass lizard, Takydromus wolteri Fischer (Laceridae) Acta Histochem. 2004;106:171–178. doi: 10.1016/j.acthis.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 41.El-Salhy M. Immunocytochemical investigation of the gastro-entero-pancreatic (GEP) neurohormonal peptides in the pancreas and gastrointestinal tract of the dogfish Squalus acanthias. Histochemistry. 1984;80:193–205. doi: 10.1007/BF00679996. [DOI] [PubMed] [Google Scholar]
- 42.Barrenechea MA, López J, Martínez A. Regulatory peptides in gastric endocrine cells of the rainbow trout Oncorhynchus mykiss: general distribution and colocalizations. Tissue Cell. 1994;26:309–321. doi: 10.1016/0040-8166(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 43.Ku SK, Lee JH, Lee HS. Immunohistochemical study on the endocrine cells in gut of the stomachless teleost, Zacco platypus (Cyprinidae) Anat Histol Embryol. 2004;33:212–219. doi: 10.1111/j.1439-0264.2004.00539.x. [DOI] [PubMed] [Google Scholar]
- 44.Lee JH, Ku SK, Park KD, Lee HS. Immunohistochemical study of the gastrointestinal endocrine cells in the Korean aucha perch. J Fish Biol. 2004;65:170–181. [Google Scholar]
- 45.Pelli-Martins AA, Machado-Santos C, Sales A, Brito-Gitirana L. Histochemical, immunohistochemical, and ultrastructural observations of the esophagus morphology of Rinnella icterica (Anuran, Bufonidae) Acta Zool. 2011;93:373–380. [Google Scholar]
- 46.Machado-Santos C, Zeca SG, Abidu-Figueiredo M, Ribeiro ICA, Sales A. The esophagus of the crocodilian Caiman latirostris (Reptilia, Crocodylia): histological, histochemical and immunohistochemical study. J Morphol Sci. 2011;28:113–119. [Google Scholar]
- 47.Yamanaka Y, Yamada J, Kitamura N, Yamashita T. An immunohistochemical study on the distribution of endocrine cells in the chicken gastrointestinal tract. Z Mikrosk Anat Forsch. 1989;103:437–446. [PubMed] [Google Scholar]
- 48.Agungpriyono S, Macdonald AA, Leus KY, Kitamura N, Adnyane IK, Goodall GP, Hondo E, Yamada J. Immunohistochemical study on the distribution of endocrine cells in the gastrointestinal tract of the babirusa, Babyrousa babyrussa (Suidae) Anat Histol Embryol. 2000;29:173–178. doi: 10.1046/j.1439-0264.2000.00258.x. [DOI] [PubMed] [Google Scholar]
- 49.Adnyane IK, Zuki AB, Noordin MM, Agungpriyono S. Immunohistochemical study of endocrine cells in the gastrointestinal tract of the barking deer, Muntiacus muntjak. Anat Histol Embryol. 2011;40:365–374. doi: 10.1111/j.1439-0264.2011.01081.x. [DOI] [PubMed] [Google Scholar]
- 50.El-Salhy M, Winder E, Lundqvist M. Comparative studies of serotonin-like immunoreactive cells in the digestive tract of vertebrates. Biomed Res. 1985;6:371–375. [Google Scholar]
- 51.Olsson C, Holmgren S. The control of gut motility. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:481–503. doi: 10.1016/s1095-6433(00)00330-5. [DOI] [PubMed] [Google Scholar]
- 52.Sundin L, Davison W, Forster M, Axelsson M. A role of 5-HT2 receptors in the gill vasculature of the antarctic fish Pagothenia borchgrevinki. J Exp Biol. 1998;201:2129–2138. doi: 10.1242/jeb.201.14.2129. [DOI] [PubMed] [Google Scholar]
- 53.Egginton S, Forster ME, Davison W. Control of vascular tone in notothenioid fishes is determined by phylogeny, not environmental temperature. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1197–R1205. doi: 10.1152/ajpregu.2001.280.4.R1197. [DOI] [PubMed] [Google Scholar]
- 54.Jensen H, Rourke IJ, Møller M, Jønson L, Johnsen AH. Identification and distribution of CCK-related peptides and mRNAs in the rainbow trout, Oncorhynchus mykiss. Biochim Biophys Acta. 2001;1517:190–201. doi: 10.1016/s0167-4781(00)00263-3. [DOI] [PubMed] [Google Scholar]
- 55.Bosi G, Di Giancamillo A, Arrighi S, Domeneghini C. An immunohistochemical study on the neuroendocrine system in the alimentary canal of the brown trout, Salmo trutta, L., 1758. Gen Comp Endocrinol. 2004;138:166–181. doi: 10.1016/j.ygcen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Vigliano FA, Muñoz L, Hernández D, Cerutti P, Bermúdez R, Quiroga MI. An immunohistochemical study of the gut neuroendocrine system in juvenile pejerrey Odontesthes bonariensis (Valenciennes) J Fish Biol. 2011;78:901–911. doi: 10.1111/j.1095-8649.2011.02912.x. [DOI] [PubMed] [Google Scholar]
- 57.Hernández DR, Vigliano FA, Sánchez S, Bermúdez R, Domitrovic HA, Quiroga MI. Neuroendocrine system of the digestive tract in Rhamdia quelen juvenile: an immunohistochemical study. Tissue Cell. 2012;44:220–226. doi: 10.1016/j.tice.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 58.Aldman G, Jönsson AC, Jensen J, Holmgren S. Gastrin/CCK-like peptides in the spiny dogfish, Squalus acanthias; concentrations and actions in the gut. Comp Biochem Physiol C. 1989;92:103–108. doi: 10.1016/0742-8413(89)90210-7. [DOI] [PubMed] [Google Scholar]
- 59.Olsson C, Aldman G, Larsson A, Holmgren S. Cholecystokinin affects gastric emptying and stomach motility in the rainbow trout Oncorhynchus mykiss. J Exp Biol. 1999;202:161–170. doi: 10.1242/jeb.202.2.161. [DOI] [PubMed] [Google Scholar]
- 60.Rawdon BB, Andrew A. An immunocytochemical survey of endocrine cells in the gastrointestinal tract of chicks at hatching. Cell Tissue Res. 1981;220:279–292. doi: 10.1007/BF00210509. [DOI] [PubMed] [Google Scholar]
- 61.Machado-Santos C, Nascimento AA, Peracchi AL, Mikalauskas JS, Rocha PA, Sales A. Distributions of the endocrine cells in the gastrointestinal tract of nectarivorous and sanguivorous bats: a comparative immunocytochemical study. Tissue Cell. 2009;41:222–229. doi: 10.1016/j.tice.2008.11.004. [DOI] [PubMed] [Google Scholar]


