Abstract
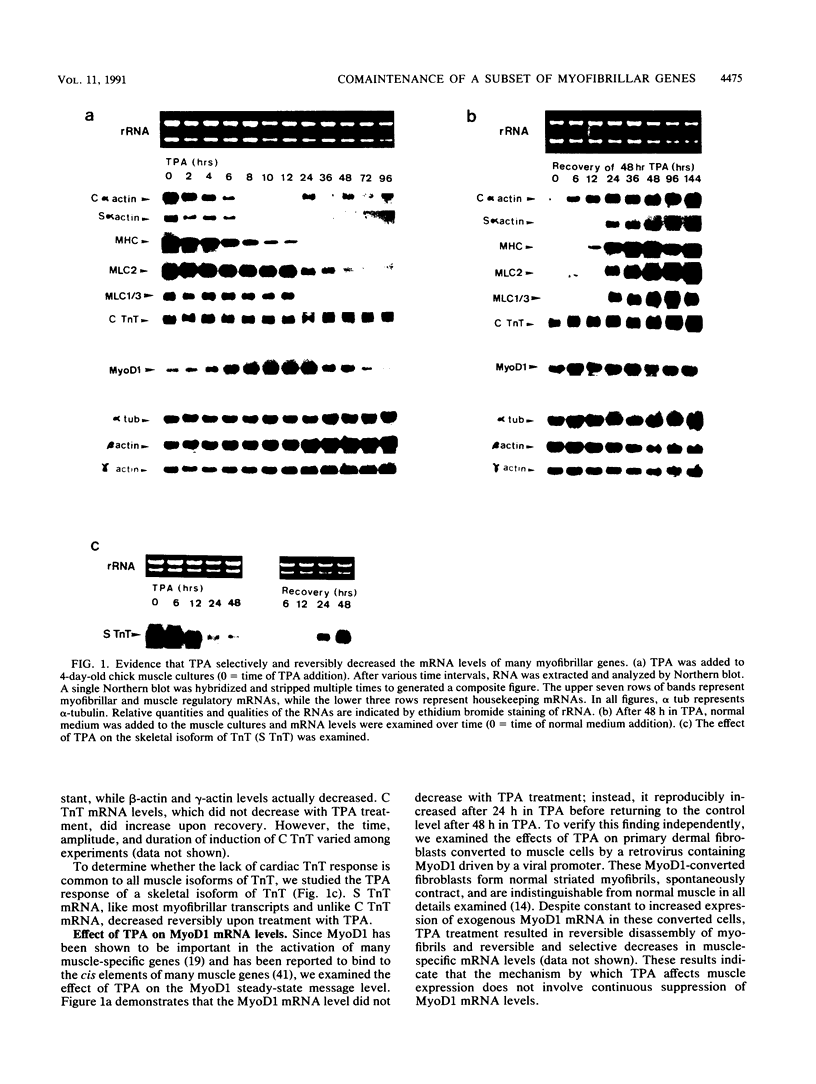
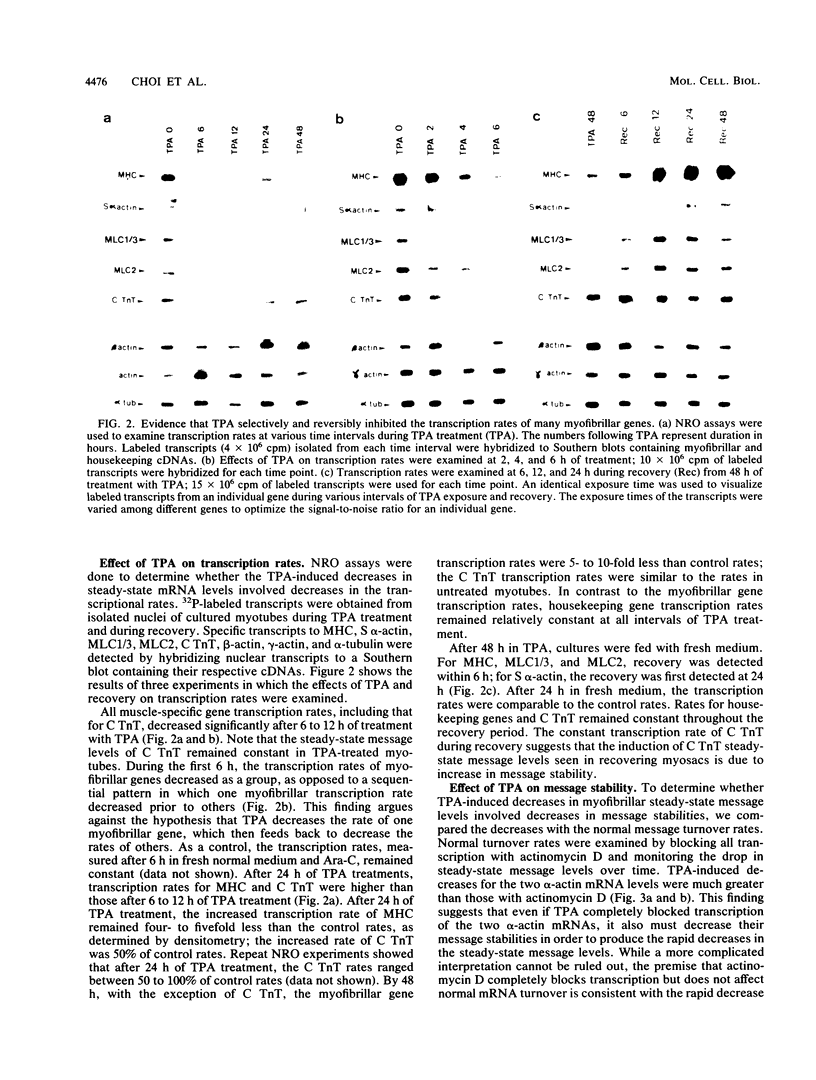
Phorbol esters selectively and reversibly disassemble the contractile apparatus of cultured skeletal muscle as well as inhibit the synthesis of many contractile proteins without inhibiting that of housekeeping proteins. We now demonstrate that phorbol esters reversibly decrease the mRNA levels of at least six myofibrillar genes: myosin heavy chain, myosin light chain 1/3, myosin light chain 2, cardiac and skeletal alpha-actin, and skeletal troponin T. The steady-state message levels decrease 50- to 100-fold after 48 h of exposure to phorbol esters. These decreases can be attributed at least in part to decreases in transcription rates. For at least two genes, cardiac and skeletal alpha-actin, some of the decreases are the result of increased mRNA turnover. In contrast, the cardiac troponin T steady-state message level does not change, and its transcription rate decreases only transiently upon exposure to phorbol esters. Phorbol esters do not decrease the expression of the housekeeping genes, alpha-tubulin, beta-actin, and gamma-actin. Phorbol esters do not decrease the steady-state message levels of MyoD1, a gene known to be important in the activation of many skeletal muscle-specific genes. Cycloheximide blocks the phorbol ester-induced decreases in transcription, message stability, and the resulting steady-state message level but does not block the tetradecanoyl phorbol acetate-induced rapid disassembly of the I-Z-I complexes. These results suggests a common mechanism for the regulation of many myofibrillar genes independent of MyoD1 mRNA levels, independent of housekeeping genes, but dependent on protein synthesis.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Bentley D. L., Groudine M. A block to elongation is largely responsible for decreased transcription of c-myc in differentiated HL60 cells. Nature. 1986 Jun 12;321(6071):702–706. doi: 10.1038/321702a0. [DOI] [PubMed] [Google Scholar]
- Billeter R., Quitschke W., Paterson B. M. Approximately 1 kilobase of sequence 5' to the two myosin light-chain 1f/3f gene cap sites is sufficient for differentiation-dependent expression. Mol Cell Biol. 1988 Mar;8(3):1361–1365. doi: 10.1128/mcb.8.3.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Bober E., Winter B., Rosenthal N., Arnold H. H. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO J. 1990 Mar;9(3):821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher E. A., Maisonpierre P. C., Konieczny S. F., Emerson C. P., Jr Expression of the troponin complex genes: transcriptional coactivation during myoblast differentiation and independent control in heart and skeletal muscles. Mol Cell Biol. 1988 Oct;8(10):4134–4142. doi: 10.1128/mcb.8.10.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buskin J. N., Hauschka S. D. Identification of a myocyte nuclear factor that binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989 Jun;9(6):2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang K. S., Zimmer W. E., Jr, Bergsma D. J., Dodgson J. B., Schwartz R. J. Isolation and characterization of six different chicken actin genes. Mol Cell Biol. 1984 Nov;4(11):2498–2508. doi: 10.1128/mcb.4.11.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi J. C., Rubinstein N., Strahs K., Holtzer H. Synthesis of myosin heavy and light chains in muscle cultures. J Cell Biol. 1975 Dec;67(3):523–537. doi: 10.1083/jcb.67.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Imagawa M., Imbra R. J., Bockoven J. R., Karin M. Multiple cis- and trans-acting elements mediate the transcriptional response to phorbol esters. Nature. 1987 Oct 15;329(6140):648–651. doi: 10.1038/329648a0. [DOI] [PubMed] [Google Scholar]
- Choi J., Costa M. L., Mermelstein C. S., Chagas C., Holtzer S., Holtzer H. MyoD converts primary dermal fibroblasts, chondroblasts, smooth muscle, and retinal pigmented epithelial cells into striated mononucleated myoblasts and multinucleated myotubes. Proc Natl Acad Sci U S A. 1990 Oct;87(20):7988–7992. doi: 10.1073/pnas.87.20.7988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cooper T. A., Ordahl C. P. A single troponin T gene regulated by different programs in cardiac and skeletal muscle development. Science. 1984 Nov 23;226(4677):979–982. doi: 10.1126/science.6095446. [DOI] [PubMed] [Google Scholar]
- Cossu G., Ranaldi G., Senni M. I., Molinaro M., Vivarelli E. 'Early' mammalian myoblasts are resistant to phorbol ester-induced block of differentiation. Development. 1988 Jan;102(1):65–69. doi: 10.1242/dev.102.1.65. [DOI] [PubMed] [Google Scholar]
- Croop J., Dubyak G., Toyama Y., Dlugosz A., Scarpa A., Holtzer H. Effects of 12-O-tetradecanoyl-phorbol-13-acetate on Myofibril integrity and Ca2+ content in developing myotubes. Dev Biol. 1982 Feb;89(2):460–474. doi: 10.1016/0012-1606(82)90334-7. [DOI] [PubMed] [Google Scholar]
- Davis R. L., Weintraub H., Lassar A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987 Dec 24;51(6):987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate accumulation of contractile protein mRNAs during myoblast differentiation. Dev Biol. 1979 Mar;69(1):202–216. doi: 10.1016/0012-1606(79)90286-0. [DOI] [PubMed] [Google Scholar]
- Duncan C. J., Jackson M. J. Different mechanisms mediate structural changes and intracellular enzyme efflux following damage to skeletal muscle. J Cell Sci. 1987 Feb;87(Pt 1):183–188. doi: 10.1242/jcs.87.1.183. [DOI] [PubMed] [Google Scholar]
- Eldridge J., Zehner Z., Paterson B. M. Nucleotide sequence of the chicken cardiac alpha actin gene: absence of strong homologies in the promoter and 3'-untranslated regions with the skeletal alpha actin sequence. Gene. 1985;36(1-2):55–63. doi: 10.1016/0378-1119(85)90069-1. [DOI] [PubMed] [Google Scholar]
- Fornwald J. A., Kuncio G., Peng I., Ordahl C. P. The complete nucleotide sequence of the chick a-actin gene and its evolutionary relationship to the actin gene family. Nucleic Acids Res. 1982 Jul 10;10(13):3861–3876. doi: 10.1093/nar/10.13.3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossett L. A., Zhang W., Olson E. N. Dexamethasone-dependent inhibition of differentiation of C2 myoblasts bearing steroid-inducible N-ras oncogenes. J Cell Biol. 1988 Jun;106(6):2127–2137. doi: 10.1083/jcb.106.6.2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P., Hardeman E., Wade R., Ponte P., Bains W., Blau H. M., Kedes L. Differential patterns of transcript accumulation during human myogenesis. Mol Cell Biol. 1987 Nov;7(11):4100–4114. doi: 10.1128/mcb.7.11.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings K. E., Bucher E. A., Emerson C. P., Jr Generation of troponin T isoforms by alternative RNA splicing in avian skeletal muscle. Conserved and divergent features in birds and mammals. J Biol Chem. 1985 Nov 5;260(25):13699–13703. [PubMed] [Google Scholar]
- Hayward L. J., Schwartz R. J. Sequential expression of chicken actin genes during myogenesis. J Cell Biol. 1986 Apr;102(4):1485–1493. doi: 10.1083/jcb.102.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzer H., Rubinstein N., Fellini S., Yeoh G., Chi J., Birnbaum J., Okayama M. Lineages, quantal cell cycles, and the generation of cell diversity. Q Rev Biophys. 1975 Nov;8(4):523–557. doi: 10.1017/s0033583500001980. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Strahs K., Biehl J., Somlyo A. P., Ishikawa H. Thick and thin filaments in postmitotic, mononucleated myoblasts. Science. 1975 May 30;188(4191):943–945. doi: 10.1126/science.1138363. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986 Feb 7;231(4738):597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Julius D., Livelli T. J., Jessell T. M., Axel R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science. 1989 Jun 2;244(4908):1057–1062. doi: 10.1126/science.2727693. [DOI] [PubMed] [Google Scholar]
- Kaneko H., Okamoto M., Goshima K. Structural change of myofibrils during mitosis of newt embryonic myocardial cells in culture. Exp Cell Res. 1984 Aug;153(2):483–498. doi: 10.1016/0014-4827(84)90615-3. [DOI] [PubMed] [Google Scholar]
- Klein S., Gerster T., Picard D., Radbruch A., Schaffner W. Evidence for transient requirement of the IgH enhancer. Nucleic Acids Res. 1985 Dec 20;13(24):8901–8912. doi: 10.1093/nar/13.24.8901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Buskin J. N., Lockshon D., Davis R. L., Apone S., Hauschka S. D., Weintraub H. MyoD is a sequence-specific DNA binding protein requiring a region of myc homology to bind to the muscle creatine kinase enhancer. Cell. 1989 Sep 8;58(5):823–831. doi: 10.1016/0092-8674(89)90935-5. [DOI] [PubMed] [Google Scholar]
- Lin Z. X., Eshelman J. R., Forry-Schaudies S., Duran S., Lessard J. L., Holtzer H. Sequential disassembly of myofibrils induced by myristate acetate in cultured myotubes. J Cell Biol. 1987 Sep;105(3):1365–1376. doi: 10.1083/jcb.105.3.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. X., Eshleman J., Grund C., Fischman D. A., Masaki T., Franke W. W., Holtzer H. Differential response of myofibrillar and cytoskeletal proteins in cells treated with phorbol myristate acetate. J Cell Biol. 1989 Mar;108(3):1079–1091. doi: 10.1083/jcb.108.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Z. Y., Dechesne C. A., Eldridge J., Paterson B. M. An avian muscle factor related to MyoD1 activates muscle-specific promoters in nonmuscle cells of different germ-layer origin and in BrdU-treated myoblasts. Genes Dev. 1989 Jul;3(7):986–996. doi: 10.1101/gad.3.7.986. [DOI] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Long C. S., Ordahl C. P. Transcriptional repression of an embryo-specific muscle gene. Dev Biol. 1988 May;127(1):228–234. doi: 10.1016/0012-1606(88)90205-9. [DOI] [PubMed] [Google Scholar]
- Mar J. H., Ordahl C. P. M-CAT binding factor, a novel trans-acting factor governing muscle-specific transcription. Mol Cell Biol. 1990 Aug;10(8):4271–4283. doi: 10.1128/mcb.10.8.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford R. M., Nguyen H. T., Nadal-Ginard B. Transcriptional and cell cycle-mediated regulation of myosin heavy chain gene expression during muscle cell differentiation. J Biol Chem. 1983 Sep 25;258(18):11063–11073. [PubMed] [Google Scholar]
- Miller S. C., Ito H., Blau H. M., Torti F. M. Tumor necrosis factor inhibits human myogenesis in vitro. Mol Cell Biol. 1988 Jun;8(6):2295–2301. doi: 10.1128/mcb.8.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner J. H., Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minty A., Blau H., Kedes L. Two-level regulation of cardiac actin gene transcription: muscle-specific modulating factors can accumulate before gene activation. Mol Cell Biol. 1986 Jun;6(6):2137–2148. doi: 10.1128/mcb.6.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narindrasorasak S., Brickenden A., Ball E., Sanwal B. D. Regulation of protein kinase C by cyclic adenosine 3':5'-monophosphate and a tumor promoter in skeletal myoblasts. J Biol Chem. 1987 Aug 5;262(22):10497–10501. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Holtzer H. An analysis of myogenesis in vitro using fluorescein-labeled antimyosin. J Histochem Cytochem. 1965 Nov-Dec;13(8):726–739. doi: 10.1177/13.8.726. [DOI] [PubMed] [Google Scholar]
- Olwin B. B., Hauschka S. D. Cell surface fibroblast growth factor and epidermal growth factor receptors are permanently lost during skeletal muscle terminal differentiation in culture. J Cell Biol. 1988 Aug;107(2):761–769. doi: 10.1083/jcb.107.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Eldridge J. D. alpha-Cardiac actin is the major sarcomeric isoform expressed in embryonic avian skeletal muscle. Science. 1984 Jun 29;224(4656):1436–1438. doi: 10.1126/science.6729461. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Pearson-White S. H., Konieczny S. F., Latham K. E., Emerson C. P., Jr Myogenic lineage determination and differentiation: evidence for a regulatory gene pathway. Cell. 1988 Jun 3;53(5):781–793. doi: 10.1016/0092-8674(88)90095-5. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Sambucetti L. C., Curran T., Distel R. J., Spiegelman B. M. Common DNA binding site for Fos protein complexes and transcription factor AP-1. Cell. 1988 Feb 12;52(3):471–480. doi: 10.1016/s0092-8674(88)80039-4. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Fischman D. A. Recombinant DNA approach for defining the primary structure of monoclonal antibody epitopes. The analysis of a conformation-specific antibody to myosin light chain 2. J Mol Biol. 1985 Feb 5;181(3):411–422. doi: 10.1016/0022-2836(85)90229-3. [DOI] [PubMed] [Google Scholar]
- Rhodes S. J., Konieczny S. F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Rosenthal N. Muscle cell differentiation. Curr Opin Cell Biol. 1989 Dec;1(6):1094–1101. doi: 10.1016/s0955-0674(89)80056-0. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Induction of differentiation in human promyelocytic leukemia cells by tumor promoters. Science. 1979 May 25;204(4395):868–870. doi: 10.1126/science.286421. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Nakamura T., Porter K. R., Euteneuer U. A tumor promoter induces rapid and coordinated reorganization of actin and vinculin in cultured cells. J Cell Biol. 1984 Sep;99(3):1045–1059. doi: 10.1083/jcb.99.3.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer M. J., Tapscott S. J., Davis R. L., Wright W. E., Lassar A. B., Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989 Jul 28;58(2):241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Umeda P. K., Kavinsky C. J., Sinha A. M., Hsu H. J., Jakovcic S., Rabinowitz M. Cloned mRNA sequences for two types of embryonic myosin heavy chains from chick skeletal muscle. II. Expression during development using S1 nuclease mapping. J Biol Chem. 1983 Apr 25;258(8):5206–5214. [PubMed] [Google Scholar]
- Wang X. F., Calame K. SV40 enhancer-binding factors are required at the establishment but not the maintenance step of enhancer-dependent transcriptional activation. Cell. 1986 Oct 24;47(2):241–247. doi: 10.1016/0092-8674(86)90446-0. [DOI] [PubMed] [Google Scholar]
- Watanabe K., Sasaki F. Ultrastructural changes in the tail muscles of anuran tadpoles during metamorphosis. Cell Tissue Res. 1974;155(3):321–336. doi: 10.1007/BF00222809. [DOI] [PubMed] [Google Scholar]
- Weintraub H., Tapscott S. J., Davis R. L., Thayer M. J., Adam M. A., Lassar A. B., Miller A. D. Activation of muscle-specific genes in pigment, nerve, fat, liver, and fibroblast cell lines by forced expression of MyoD. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5434–5438. doi: 10.1073/pnas.86.14.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weydert A., Barton P., Harris A. J., Pinset C., Buckingham M. Developmental pattern of mouse skeletal myosin heavy chain gene transcripts in vivo and in vitro. Cell. 1987 Apr 10;49(1):121–129. doi: 10.1016/0092-8674(87)90762-8. [DOI] [PubMed] [Google Scholar]
- Wright W. E., Sassoon D. A., Lin V. K. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD. Cell. 1989 Feb 24;56(4):607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]