Abstract
The purpose is to evaluate sensitivity of basal-like breast cancer to treatment with anti-DR5 alone and in combination with chemotherapy. Cytotoxicity of TRA-8 anti-DR5 alone and in combination with doxorubicin or paclitaxel was examined. The role of a DR5-associated molecule (DDX3) in the regulation of apoptosis by recruitment of cIAP1 to the DR5/DDX3 complex was studied. SUM159 and 2LMP orthotopic xenografts were treated with TRA-8 alone and in combination with Abraxane or doxorubicin, and tumor growth inhibition determined. Diffusion-weighted magnetic resonance imaging was used to monitor early tumor response. The majority (12/15) of basal-like cell lines were very sensitive to TRA-8-induced cytotoxicity (IC50 values of 1.0–49 ng/ml). In contrast, 8/11 luminal or HER2-positive cell lines were resistant (IC50 > 1,000 ng/ml). Enhanced killing of basal-like cell lines was produced by combination treatment with TRA-8 and doxorubicin. Majority of basal cell lines expressed lower levels of DR5-associated DDX3 and cIAP1 than luminal and HER2-positive cell lines. TRA-8 inhibited growth of basal xenografts and produced 20% complete 2LMP tumor regressions. TRA-8 and chemotherapy produced greater 2LMP growth inhibition than either alone. An increase in apparent diffusion coefficient in 2LMP tumors was measured in a week of therapy with TRA-8 and Abraxane. Basal-like cell lines were more sensitive to TRA-8-mediated cytotoxicity than HER2-over-expressing and luminal cell lines, and chemotherapy enhanced cytotoxicity. High sensitivity of basal cells to TRA-8 correlated with low expression of DR5/DDX3/cIAP1 complex. Treatment with TRA-8 and chemotherapy may be an effective therapy for basal-like breast cancer.
Keywords: Basal-like breast cancer, Anti-DR5 antibody, Chemotherapy
Introduction
Breast cancer is the leading cause of cancer and second in cancer caused mortality in American women with an estimated 209,000 cases diagnosed and 40,000 deaths in 2010 [1]. The disease is heterogeneous, and treatment strategies are increasingly subtype specific. Gene expression profiles were used to characterize luminal, HER2-amplified, basal-like, and normal-like breast cancer subtypes [2–6]. In general, luminal subtypes are ER positive (PR variable), HER2-amplified may be ER positive or negative, and basal-like are primarily ER/PR/HER2 negative (triple-negative breast cancer, TNBC) [7]. The term “targeted” therapy includes the use of anti-estrogen therapy in luminal breast cancer and anti-HER2 therapy in HER2-amplified tumors. Unfortunately, patients with basal-like or TNBC have no successful “targeted” treatment strategy and represent a priority for novel therapies [8].
TRAIL is a cytokine that binds to pro-apoptotic cell surface death receptors DR4 and DR5, anti-apoptotic decoy receptors DcR1 and DcR2, and soluble osteoprotegerin [9]. TRAIL preferentially induces apoptosis in many human tumor cell lines compared with normal cells, and agonistic monoclonal antibodies targeting DR4 or DR5 also induce apoptosis in a variety of human cancer cell lines [10]. The potential utility of TRAIL or death receptor antibodies is limited by intrinsic resistance of many tumor cell lines and tumors to these agents [11], and clinical development of TRAIL or antibodies targeting DR4 or DR5 would be facilitated by the identification of patients most likely to respond to these agents [12].
It was reported that basal B breast cancer cell lines have substantial in vitro sensitivity to TRAIL-mediated cytotoxicity in contrast to TRAIL resistance by basal A, luminal, and HER2-amplified cell lines [13]. Our previous report [14] of monoclonal anti-DR5 (TRA-8)-mediated cytotoxicity to breast cancer cell lines included sensitivity by three basal-like and resistance by five HER2-amplified or luminal cell lines. Combining chemotherapy agents (doxorubicin or paclitaxel) with anti-DR5 enhanced in vitro cytotoxicity and in vivo antitumor efficacy in a sub-cutaneous 2LMP (basal B) xenograft model [14].
The present studies examined monoclonal anti-DR5-mediated cytotoxicity alone and combined with chemotherapy in a large panel of breast cancer cell lines (n = 26) and two basal-like orthotopic xenograft murine models and included mechanistic studies of these in vitro and in vivo observations.
Materials and methods
Cells and reagents
Details on breast cancer cell lines and culture conditions are presented in the Supplementary material. TRA-8 antibody (mouse IgG1 isotype) was prepared at the University of Alabama at Birmingham [15]. Doxorubicin was obtained from Polymed Therapeutics (Houston, TX). Paclitaxel was from Sigma Aldrich Chemical Co. (St. Louis, MO). Abraxane was from the University of Alabama at Birmingham Hospital Pharmacy.
Cell viability assays using ATPLite
Cell viability assays were performed as described previously [14, 16]. Briefly, cell viability was assessed after 24 h of exposure to TRA-8 by measuring cellular ATP levels (ATPLite, Perkin Elmer Biosciences, Meriden, CT). For combination treatments, cells were pretreated with chemotherapy drugs for 24 h before adding TRA-8 antibody, then ATP levels were determined 24 h later. ATP values are the mean ± SE from at least three independent experiments with a minimum of four replicates each.
Co-immunoprecipitation and western blot detection of DR5/DDX3/cIAP1 complex
Cell lysates (2 mg total protein in 2 ml 0.5% NP40 lysis buffer) were incubated overnight with 50 μl humanized TRA-8 (Tigatuzumab)-conjugated Sepharose 4B at 4°C. Beads were washed five times with lysis buffer and resuspended in SDS–PAGE loading buffer. Co-immunoprecipitated proteins were separated using SDS–PAGE to detect DDX3, cIAP1, and DR5 (40, 40, and 20% of immunoprecipitate, respectively), then blotted onto nitrocellulose membranes, and probed overnight at 4°C with murine monoclonal anti-DDX3 (3E4) or anti-cIAP1 (4H6) antibodies or rabbit polyclonal anti-DR5 antibody. HRP-conjugated goat anti-murine or anti-rabbit IgG secondary antibodies and chemiluminescent substrate (Thermo Scientific, Cincinnati, OH) were used to reveal proteins.
Quantitative measurement of DR5/DDX3/cIAP1 complex with chemiluminescent ELISA
Chemiluminescent ELISA plates (NUNC, Naperville, IL) were coated with 10 μg/ml TRA-8 or murine IgG1 (Southern Biotech, Birmingham, AL) in PBS for 1 h at 37°C, blocked with SuperBlock Blocking Buffer (Thermo Scientific), and incubated with 50 μg total cell protein in lysis buffer/5% BSA for 1 h at room temperature. Plates were washed five times with 0.1% Tween 20 in PBS, incubated with HRP-conjugated anti-DDX3 (3E4), anti-cIAP1 (4H6) or anti-DR5 (2B9) for 1 h at room temperature, and washed five times. Chemiluminescent substrate (KPL) was added, and plates were counted in a TopCount plate reader (Packard, Hartford, CT). Specific binding was determined by subtracting counts per second bound to control IgG-coated wells from binding to anti-DR5-coated wells.
In vivo therapy studies using orthotopic xenograft models of TNBC
Female athymic nude mice were obtained at 4–6 weeks of age from Harlan Laboratories (Indianapolis, IN). Orthotopic breast cancer xenografts were established by implanting 4 × 106 2LMP or SUM159 cells in a 1:1 mixture with Matrigel (BD Biosciences, San Jose, CA) into the mammary fat pad. Mice were randomized into six treatment groups of 10 mice. Therapy was initiated 14 days after implantation when tumors were 5–7 mm in diameter. Mice were treated with 200 μg TRA-8 given by i.p. injection on days 14, 17, 21, 24, 28, and 31; 6 mg/kg doxorubicin given by i.v. injection on days 15, 19, and 23; 20 mg/kg Abraxane given by i.v. injection on days 15, 19, 23, 27, and 31; TRA-8 plus doxorubicin or Abraxane; or were left untreated. Tumor growth was monitored twice weekly by measuring tumor diameter in the two largest dimensions with calipers. Mean tumor size was calculated from the product of individual tumor diameters and reported relative to tumor size at the start of treatments. Tumor growth, tumor doubling time (TDT), and tumor regression rates were determined. All studies were conducted in accordance with University of Alabama at Birmingham Institutional Animal Care and Use Committee regulations. Mice were examined daily for physical and behavioral changes and weighed twice weekly to assess toxicity of treatments. Tumor growth was monitored until the mean tumor size for each group at least doubled in size or until the study was terminated.
Imaging studies
Female nude mice (4–6 weeks old) were used for in vivo magnetic resonance imaging (MRI) study. 1 × 106 2LMP (groups 1–4) or 4 × 106 SUM159 (groups 5–8) cells were implanted subcutaneously into the left flank (n = 5 for groups 2–4, 6, 7; n = 4 for group 1; n = 6 for groups 5 and 8). Therapy started when tumors were about 5–10 mm in diameter. Mice were treated with 200 μg TRA-8 given by i.p. injection on days 0 and 3 (groups 3 and 7); 20 mg/kg Abraxane given by i.v. injection on days 1 and 5 after therapy initiation (groups 2 and 6); TRA-8 plus Abraxane (groups 4 and 8); or were left untreated (groups 1 and 5). Anatomical MRI and diffusion-weighted imaging (DWI) were performed at days 0 (prior to dosing), 3, and 7 (details presented in Supplementary materials). A total of 3–7 slices (1 mm thick) were used to cover tumor regions of interest. Apparent diffusion coefficient (ADC) of tumor regions and tumor volume measurements were made among groups 1–8 over 7 days and analyzed using two-way repeated measures analysis of variance (RM ANOVA).
Statistical analysis
IC50 values were estimated using the Hill Equation [17, 18]. The combination index (CI) for dose–effect relationships of TRA-8 and drugs in combination was calculated based on the multiple drug-effect equation of Chou-Talalay [19]. CI = DA/ICX,A + DB/ICX,B where ICX,A and ICX,B are concentrations of drugs producing X% inhibition for each respective drug alone, and DA and DB are concentrations of each drug in the mixture that yield X% inhibition. The CI curve or modified isobologram is generated by plotting CI vs. X, ranging from 0 to 100%. Drug interactions are readily identified at any level of inhibition. The resulting CI theorem offers quantitative definition for additive effect (CI = 1), synergism (CI < 1), and antagonism (CI > 1) in drug combinations [19]. The quantitative diagnostic plot was generated with Statistical Analysis Software (SAS) version 9.1. The synergism effect was further confirmed with concentration–effect curve with nonlinear regression method [20] and isobologram methods (data not shown). Experimental animal treatment groups were composed of 10 animals each to provide evidence of substantial tumor sensitivity to TRA-8 therapy with or without chemotherapy. For xenograft models, TDT was estimated for each animal using empirical distribution, and median TDT between treatment groups was compared using Kruskal–Wallis nonparametric statistical test.
Results
Breast cancer cell line sensitivity to monoclonal anti-DR5-mediated cytotoxicity
Table 1 lists the origin and genotype of 26 breast cancer cell lines examined in this study, including 12/15 (80%) basal-like TNBC cell lines that were highly sensitive to anti-DR5-mediated cytotoxicity (IC50 values < 50 ng/ml). The basal B subtype were all highly sensitive (9/9) with IC50 values ranging from 1.0 to 18.2 ng/ml while 3/6 of basal A cell lines were sensitive with IC50 values of 12.8–48.8 ng/ml. In contrast, all luminal and HER2-over-expressing cell lines (8/11) were totally resistant (IC50 > 1,000 ng/ml) or moderately sensitive (IC50 = 304–388 ng/ml). Sensitivity or resistance did not correlate with tumor origin of cell lines.
Table 1.
TRA-8 cytotoxicity against breast cancer cell lines
| Phenotype | Cell line | IC50 TRA-8 (ng/ml) | Tumor origin |
|---|---|---|---|
| Luminal | MCF-7 | >1,000 | Met AC, PE |
| T-47D | >1,000 | IDC, PE | |
| ZR-75-1 | 388 | IDC, AF | |
| MDA-MB-134 | >1,000 | IDC, PE | |
| HER2-amplified luminal (ER+) | BT-474 | >1,000 | IDC, 1° |
| DY36T2 (subclone MDA-MB-361) | >1,000 | Met AC, BR | |
| ZR-75-30 | >1,000 | IDC, AF | |
| HER2-amplified luminal (ER−) | MDA-MB-453 | >1,000 | MC, PE |
| SK-BR-3 | >1,000 | AC, PE | |
| HER2-amplified basal (ER−) | HCC1569 | 304 | Met C, 1° |
| HCC1954 | 325 | DC, 1° | |
| TNBC (basal A) | MDA-MB-468 | 12.8 | Met AC, PE |
| HCC1187 | 24.1 | DC, 1° | |
| BT-20 | 48.8 | AC, 1° | |
| HCC1937 | >1,000 | DC, 1° | |
| HCC1143 | >1,000 | DC, 1° | |
| HCC1599 | >1,000 | DC, 1° | |
| TNBC (basal B) | SUM149 | 1.0 | Inf DC, 1° |
| HCC38 | 1.0 | DC, 1° | |
| 2LMP (subclone MDA-MB-231) | 2.1 | LM | |
| SUM159 | 1.9 | AnCa, 1° | |
| MDA-MB-436 | 3.3 | AC, PE | |
| SUM102 | 6.5 | Int DC, 1° | |
| MDA-MB-157 | 11.8 | Med, PE | |
| BT-549 | 13.4 | IDCp, 1° | |
| MDA-MB-231 | 18.2 | Met AC, PE |
Tumor type: AC adenocarcinoma, AnCa anaplastic carcinoma, DC ductal carcinoma, IDC invasive ductal carcinoma, IDCp invasive ductal carcinoma, papillary, Inf DC inflammatory ductal carcinoma, Int DC intraductal carcinoma, Med medullary carcinoma, Met AC metastatic adenocarcinoma, Met C metaplastic carcinoma, MC metastatic carcinoma
Source: 1° primary tumor, AF ascitic fluid, BR brain metastasis, LM lung metastasis, PE pleural effusion, PF pericardial effusion
Effect of chemotherapy on anti-DR5-mediated cytotoxicity to breast cancer cell lines
We previously reported [14, 16, 21–25] that chemotherapy agents can enhance anti-DR5-mediated cytotoxicity to various tumor types. Treatment with TRA-8 and doxorubicin or paclitaxel produced synergistic cytotoxicity against 12/14 or 10/14 basal-like cell lines, respectively (Fig. 1; Table 2). In general, stronger synergistic effects occurred in combination with TRA-8 in cell lines resistant to TRA-8 cytotoxicity compared to TRA-8 sensitive cell lines, as dramatic synergy occurred with TRA-8 and doxorubicin in T-47D and HCC1937 but only additive effects occurred with 2LMP.
Fig. 1.

In vitro cytotoxicity of TRA-8 in combination with doxorubicin. Breast cancer cells were pretreated with drug for 24 h followed by treatment with drug alone, TRA-8 alone, or drug plus TRA-8 for an additional 24 h, and then cell viability was evaluated by measuring ATP levels. Values are mean and SE determined from at least three independent experiments with a minimum of four replicates in each
Table 2.
In vitro cytotoxicity of TRA-8 and chemotherapy drugs against breast cancer cell lines
| Phenotype | Cell line | Doxorubicin |
Paclitaxel |
||||
|---|---|---|---|---|---|---|---|
| Synergy | Dose (nM) | CIa | Synergy | Dose (nM) | CI | ||
| Luminal | T-47D | Yes | 100–1,000 | 0.033–0.006 | Yes | 2.5–200 | 0.570–0.053 |
| MDA-MB-134 | Yes | 250–1,000 | 0.020–0.005 | No | 2.5–200 | 2.5–64.3 | |
| HER2-amplified luminal (ER+) | BT-474 | Yes | 250–5,000 | 0.007–0.001 | Yes | 2.5–200 | 0.009–0.048 |
| ZR-75-30 | Yes | 500–1,000 | 0.011–0.007 | No | 5–200 | 12.6–29.4 | |
| HER2-amplified basal (ER−) | HCC1569 | Yes | 100–1,000 | 0.254–0.002 | Yes | 2.5–200 | 0.377–0.050 |
| HCC1954 | Yes | 250–1,000 | 0.709–0.002 | Yes | 5–200 | 0.300–0.101 | |
| Basal A | MDA-MB-468 | Yes | 50–1,000 | 0.790–0.065 | Yes | 2.5–5, 20 | 0.874–0.656 |
| HCC1187 | Yes | 100–1,000 | 0.452–0.020 | Yes | 20–200 | 0.662–0.640 | |
| BT-20 | Yes | 100–500 | 0.450–0.004 | Yes | 5–200 | 0.882–0.254 | |
| HCC1937 | Yes | 250–1,000 | 0.046–0.001 | Yes | 2.5–200 | 0.960–0.022 | |
| HCC1143 | Yes | 100–1,000 | 0.230–0.002 | Yes | 2.5–200 | 0.743–0.009 | |
| Basal B | SUM149 | Yes | 375–1,000 | 0.675–0.067 | No | 2.5–200 | 2.1–17.0 |
| HCC38 | No | 100–375 | 10.3–90.0 | No | 2.5–200 | 3.6–23.1 | |
| 2LMP | No | 25–250 | 3.2–19.5 | Yes | 5–200 | 0.989–0.152 | |
| SUM159 | Yes | 250–500 | 0.752–0.496 | Yes | 20–200 | 0.591–0.348 | |
| MDA-MB-436 | Yes | 250–1,000 | 0.730–0.264 | Yes | 10–200 | 0.898–0.607 | |
| SUM102 | Yes | 375–500 | 0.097–0.037 | No | 2.5–200 | 3.9–118.7 | |
| MDA-MB-157 | Yes | 50–1,000 | 0.740–0.070 | No | 2.5–10, 200 | 1.2–1.6, 188.0 | |
| BT-549 | Yes | 100–1,000 | 0.815–0.036 | Yes | 2.5–200 | 0.760–0.087 | |
| MDA-MB-231 | Yes | 25–500 | 0.651–0.088 | Yes | 5–200 | 0.497–0.104 | |
Cl combination index as determined at 50% effect level
Correlation of DR5-associated DDX3/cIAP1 and sensitivity to anti-DR5-mediated cytotoxicity
Our previous studies identified a novel regulatory mechanism of DR5-mediated apoptosis [26, 27]. In this model, DDX3, a Helicard protein, associates with DR5 and recruits cIAP1 to form a protein complex at the DR5 death domain, which may inhibit death domain signal transduction initiated by caspase 8/10. To determine whether sensitivity of basal-like cell lines to DR5-mediated apoptosis correlates with DR5/DDX3/cIAP1 protein complex formation, we examined DR5/DDX3/cIAP1 complexes in 22 human breast cancer cell lines with defined TRA-8 sensitivity by co-immunoprecipitation and chemiluminescent ELISA. The majority of non-TNBC cell lines were resistant to TRA-8-induced apoptosis (IC50 > 1,000 ng/ml), except HCC1569 and HCC1954 (Table 1). All cell lines showed positive DR5 immunoprecipitation (Fig. 2a, b, lower). All TRA-8-resistant non-TNBC cell lines except T-47D expressed higher levels of DDX3 and cIAP1 (Fig. 2a) co-immunoprecipitated with DR5, while two moderately sensitive HER2-amplified basal (ER−) cell lines (HCC1569 and HCC1954) had lower levels of DDX3 and cIAP1. TRA-8-resistant HCC1937 and HCC1143 basal A cells expressed high levels of DR5-associated DDX3 and cIAP1. In contrast, the majority of TRA-8-sensitive TNBC cell lines, particularly the basal B type, expressed nondetectable or lower levels of DR5-associated DDX3 and cIAP1 (Fig. 2b). Levels of DR5, DDX3 and cIAP1 in the complex were quantitatively measured using chemiluminescent ELISA. The ratio of DDX3 or cIAP1 to DR5 in the majority of DR5-resistant non-TNBC cell lines was greater than 1, except two moderately sensitive cell lines (HCC1569 and HCC1954) (Fig. 2c), but far less than 1 in the majority of DR5-sensitive basal-like cell lines (Fig. 2d). These results suggest that levels of DR5-associated DDX3 and cIAP1 may account for the exceptional sensitivity of basal-like cell lines to DR5-mediated apoptosis.
Fig. 2.
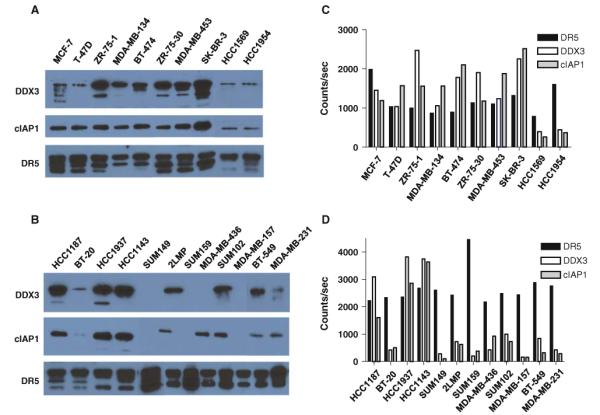
Analysis of DR5/DDX3/cIAP1 protein complex in breast cancer cell lines. Co-immunoprecipitation and western blot analysis of DR5/DDX3/cIAP1 complex in a panel of non-TNBC (a) and TNBC (b) cell lines. Total cell lysates were prepared and immunoprecipitated with Tigatuzumab-conjugated Sepharose 4B. The immunoprecipitated proteins were separated in SDS–PAGE and western blotted. The blots were probed with anti-DDX3, anti-cIAP1, and anti-DR5 antibodies. The representative results of three experiments are shown. c, d Quantitative measurement of DR5-associated DDX3 and cIAP1 by chemiluminescent ELISA. Total cell lysates were incubated in the anti-DR5 antibody and isotype control antibody-coated ELISA plates. The DR5-associated DDX3 and cIAP1 were detected with HRP-conjugated anti-DDX3 and anti-cIAP1 antibody, and the DR5 levels were detected with rabbit polyclonal antibody. The results are shown as the average counts per second of duplicates. The specific binding was calculated by subtracting the isotype control background counts
Anti-tumor efficacy of anti-DR5 alone or combined with Abraxane or doxorubicin in basal B xenografts
Single-agent TRA-8, Abraxane, and doxorubicin all produced significant inhibition of 2LMP tumor growth compared with untreated tumors (Fig. 3a) with TDT of 58, 46, and 51 days, respectively, compared with control of 9 days (P < 0.0001 each). The combination of TRA-8/Abraxane or TRA-8/doxorubicin produced substantial tumor regression to <50% of original tumor size and tumor growth inhibition with TDTs of 87 and 78 days, respectively. TRA-8/Abraxane was more effective than single-agent TRA-8 (P < 0.0029) or Abraxane (P < 0.0001), while TRA-8/doxorubicin was more effective than TRA-8 (P < 0.032) or doxorubicin (P < 0.005). Two complete tumor regressions without recurrence over 100 days occurred with TRA-8 alone and TRA-8/Abraxane as well as one complete regression with single-agent Abraxane.
Fig. 3.

In vivo efficacy of TRA-8 alone or in combination with Abraxane or doxorubicin against orthotopic basal B xenografts in athymic nude mice. 2LMP (a) or SUM159 (b) cells were implanted in the mammary fat pad, and treatments began 14 days later when tumors were well established. The bars indicate the interval during which treatment was administered, and the numbers in parentheses, the fraction of animals in which tumors completely regressed (n = 9–10 mice/group)
A similar study was carried out in the slower-growing SUM159 orthotopic model (Fig. 3b) where single-agent TRA-8 and both combination regimens produced comparable efficacy (TDT = 81, 75, and 85 days) that was significantly better than control TDT of 18 days (P < 0.0001) or Abraxane (TDT = 49 days; P < 0.012) or doxorubicin (TDT = 42 days; P < 0.0001). In this model, the striking efficacy of TRA-8 prevented observation of any benefit from combination therapy.
MRI assessment of anti-tumor efficacy
We have previously shown that increases in the ADC at early time points post-therapy in mouse xenograft models correlate with tumor cell apoptosis and anti-tumor efficacy [28]. Figure 4a illustrates the percent change in ADC of 2LMP tumors at day 3 and 7 post-therapy. At day 3, the three treatment groups have a rising ADC as compared to the falling control group ADC, which reaches statistical significance by day 7 for the TRA-8/Abraxane (P < 0.001) and Abraxane (P = 0.047) groups. Figure 4b provides ADC data for SUM159 tumors, illustrating a similar pattern that did not reach statistical significance. In both models, MRI assessment of tumor volumes was unchanged over 7 days of observations. Thus, alterations in ADC occurred prior to any demonstrable anti-tumor efficacy as assessed by tumor size.
Fig. 4.

Analysis of 2LMP and SUM159 subcutaneous tumor response to treatment. a, b Intratumoral apparent diffusion coefficient (ADC) change of a 2LMP and b SUM159 tumors, measured at 0 (baseline), 3, and 7 days after initiation of therapy, when mice were untreated or treated with Abraxane (20 mg/kg at days 1 and 5, i.v.), TRA-8 (200 μg at days 0 and 3, i.p.), and combined therapy. Statistical difference between control and each treated group is indicated by asterisk (two asterisks indicate that P < 0.001)
Discussion
Identification of specific tumor subtypes that are sensitive to TRAIL or death receptor antibodies would be very beneficial to the clinical development of these agents. Buchsbaum et al. identified breast cancer cell lines of low, intermediate, and high sensitivity to TRA-8 anti-DR5 antibody in vitro and reported anti-tumor efficacy of TRA-8 against 2LMP (metastatic subclone of MDA-MB-231) basal xenografts, an effect that was enhanced by chemotherapy, radiation, or combination treatment with anti-DR4 antibody [14, 29]. However, the observation that the basal subtype might be particularly sensitive to strategies targeting death receptors was not appreciated until Rahman et al. reported that TRAIL produced significant in vitro cytotoxicity against a subset of breast cancer cell lines, particularly TN basal-like lines exhibiting a mesenchymal phenotype, whereas luminal breast cancer cell lines were resistant to TRAIL [13].
The present studies examined 26 breast cancer cell lines of TN basal-like, HER2-amplified basal and luminal subtypes, and luminal cell lines with normal HER2 levels to evaluate sensitivity to TRA-8-mediated cytotoxicity alone or in combination with chemotherapy. TRA-8 preferentially induced killing of TN basal-like cell lines with IC50 values of 1.0–4.8 ng/ml obtained for 12/15 cell lines, as compared to IC50 values of 304–325 ng/ml for 2 HER2 expressing basal and >1,000 ng/ml for 8/9 luminal cell lines; 25 ng/ml TRA-8 reduced viability by ~50% in some resistant basal, HER2-positive, and luminal cell lines (HCC1143, HCC1937, T-47D, and SK-BR-3), but higher doses of TRA-8 had little additional effect.
Rahman et al. reported that TNBC cell lines of mesenchymal or basal B subtype were sensitive to TRAIL (IC50 < 250 ng/ml) [13], while we found that basal B cell lines were very sensitive to TRA-8 (IC50 < 18 ng/ml). Rahman et al. also found that 3 of 3 cell lines that express a TN epithelial or basal A phenotype were resistant to TRAIL (IC50 > 1,000 ng/ml) [13], whereas we found that 3 of 6 basal A cell lines were sensitive to both TRA-8 and TRAIL (IC50 < 50 ng/ml). This apparent discrepancy may be related to differences in TRAIL preparations used in the two studies, as we used SuperKiller™ TRAIL instead of the GST-TRAIL fusion protein employed by them.
The TRA-8-sensitive TN basal-like cell lines included inflammatory (SUM149) and medullary (MDA-MB-157) subtypes. BRCA1 function did not directly correlate with TRA-8 sensitivity in three basal-like cell lines with BRCA1 mutations [30, 31]. TRA-8-sensitive TN basal-like cell lines included 7/12 derived from primary tumors and 5/12 from metastatic tumors. Three HER2-expressing cell lines were derived from primary tumors, and 8/9 luminal cell lines were isolated from metastatic tumors, including pleural effusions, ascitic fluids, and one brain metastasis. It is encouraging that all five TN basal-like cell lines isolated from metastatic tumors were sensitive to TRA-8, suggesting that TRA-8 may affect both primary and metastatic tumors.
Doxorubicin and taxanes are frequently used in first-line therapy of TNBC (adjuvant or metastatic). Therefore, we examined the interaction of these drugs with TRA-8 in combination therapy of TN basal, HER2-positive and luminal cell lines. The majority of TN basal-like cell lines (11/14) were sensitive to doxorubicin (IC50 ≤ 500 nM). Combination treatment with TRA-8 and doxorubicin produced synergistic killing of 85% of TN basal-like cell lines, including BRCA-1 mutant cell lines (Table 2). Combination treatment with TRA-8 and doxorubicin also produced synergistic killing of HER2-positive and luminal cell lines (Table 2 and Ref. [14]). Although paclitaxel produced mainly additive cytotoxicity with TRA-8 against TNBC cell lines, this combination may be more effective in a therapeutic setting in which tumors are exposed to both agents for a longer period.
DDX3 was identified as a novel DR5-associated adaptor protein that mediates apoptosis resistance at the DR5 death domain by recruiting inhibitor of apoptosis proteins (IAPs) to form DR5/DDX3/cIAP1 complexes [26, 27]. We hypothesize that crucial post-translational modifications of components in the complex, such as phosphorylation and/or cleavage, lead to differential function of DR5 in sensitive and resistant cells. Expression levels of each component in the complex may influence signal transduction or, alternatively, the presence of DDX3 with absent CARD region would function as a naturally occurring dominant negative molecule and impair cIAP1 binding, resulting in high sensitivity to TRA-8-mediated apoptosis. This model predicts that the DDX3/cIAP1 complex is dominant over the death domain complex, producing resistance to DR5-mediated apoptosis. Li et al. [26] described DDX3 binding to DR5, which correlated with reduced caspase-8 activation after TRA-8 treatment in MDA-MB-231 cells with induced resistance to TRA-8. Doxorubicin treatment reduced DDX3 binding to DR5 in these cells, producing caspase activation and TRA-8 sensitization, suggesting that DR5/DDX3/cIAP1 complex modulation by doxorubicin overcomes DDX3-mediated inhibition of TRA-8-induced apoptosis [26]. Thus, DR5-associated DDX3 and cIAP1 may serve as a biomarker for predicting response to anti-DR5 (TRA-8)-mediated apoptosis, and these observations represent an initial investigation of this potential biomarker to help select patients in a clinical trial.
The anti-tumor efficacy of TRA-8 and doxorubicin or Abraxane was evaluated in two orthotopic xenograft models of basal breast cancer in athymic nude mice. SUM159 tumor growth was significantly inhibited by TRA-8, but no complete tumor regressions occurred. The mean tumor growth inhibition was less pronounced in 2LMP xenografts treated with TRA-8 alone, but 2/10 complete tumor regressions occurred. Combination treatment with TRA-8 and Abraxane or doxorubicin produced tumor growth inhibition and significant delays in TDT of 2LMP and SUM159 orthotopic tumors (Fig. 2). Combination treatment with TRA-8 and doxorubicin produced 1/10 complete tumor regressions in mice bearing SUM159 tumors, whereas 2/9 complete tumor regressions occurred in 2LMP tumors treated with TRA-8 and Abraxane. DWI measured effective therapy early with combined TRA-8 and Abraxane for TNBC xenografts at 7 days post-treatment. Thus, combination treatment with either Abraxane or doxorubicin enhanced the anti-tumor efficacy of TRA-8 against basal-like orthotopic xenografts.
The observation that basal-like breast cancers generally exhibit high sensitivity to TRA-8 or TRAIL [13] suggests that selecting such patients for DR5 therapy is likely to improve the odds of obtaining clinical benefit from these agents, similar to selecting patients with HER2-over-expressing tumors for Herceptin therapy [32]. Tigatuzumab, the humanized version of TRA-8, has completed Phase I studies with no dose-limiting toxicity detected [33]. A randomized Phase II trial of Abraxane ± Tigatuzumab treatment for patients with metastatic TNBC is being performed by the Translational Breast Cancer Research Consortium (ClinicalTrials.gov NCT01307891). Tissue samples will be analyzed for DR5-associated DDX3 and cIAP1, and the results correlated with efficacy.
The availability of an effective targeted therapy for aggressive basal-like cancer subtypes would be important, as the relapse rate from chemotherapy is significant and other therapeutic options are limited [8]. The finding that combination treatment with TRA-8 and doxorubicin or paclitaxel produced enhanced cytotoxicity against the majority of TRA-8-sensitive and TRA-8-resistant breast cancer cell lines suggests that concurrently targeting more than one cytotoxic pathway may increase TRA-8 efficacy and reduce the probability of developing resistance. The lack of overlapping toxicities between TRA-8 or TRAIL and chemotherapy drugs is another important factor in designing clinical trials with death receptor agonists. The generally high initial chemosensitivity of primary basal-like breast tumors suggests that neoadjuvant treatment with death receptor agonists and combination chemotherapy might inhibit both primary and metastatic tumor growth to decrease relapse rates and produce the greatest clinical response.
In summary, the current studies suggest that TN basal-like breast tumors may be susceptible to the therapeutic effects of an agonistic antibody to DR5 and justify an approach targeting TRAIL death receptors in combination with chemotherapy in clinical trials with the basal-like breast cancer subtype.
Supplementary Material
Acknowledgments
We thank Sally Lagan for help in preparing the manuscript. Supported in part by Komen for the Cure Promise Grant KG090969 and the UAB SPORE in Breast Cancer 5P50 CA089019-08.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s10549-011-1755-0) contains supplementary material, which is available to authorized users.
References
- 1.SEER . Cancer Statistics Review, 1975–2007. National Cancer Institute; 2010. http://seer.cancer.gov/csr/1975_2007/ [Google Scholar]
- 2.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 3.Sorlie T, Tibshirani R, Parker J, Hastie T, Marron JS, Nobel A, Deng S, Johnsen H, Pesich R, Geisler S, Demeter J, Perou CM, Lonning PE, Brown PO, Borresen-Dale AL, Botstein D. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. doi:10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lacroix M, Leclercq G. Relevance of breast cancer cell lines as models for breast tumours: an update. Breast Cancer Res Treat. 2004;83(3):249–289. doi: 10.1023/B:BREA.0000014042.54925.cc. doi:10.1023/B:BREA.0000014042.54925.cc. [DOI] [PubMed] [Google Scholar]
- 5.Charafe-Jauffret E, Ginestier C, Monville F, Finetti P, Adélaïde J, Cervera N, Fekairi S, Xerri L, Jacquemier J, Birnbaum D, Bertucci F. Gene expression profiling of breast cell lines identifies potential new basal markers. Oncogene. 2006;25(15):2273–2284. doi: 10.1038/sj.onc.1209254. doi: 10.1038/sj.onc.1209254. [DOI] [PubMed] [Google Scholar]
- 6.Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10(6):515–527. doi: 10.1016/j.ccr.2006.10.008. doi:10.1016/j.ccr.2006. 10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liedtke C, Gonzalez-Angulo A-M, Pusztai L. Definition of triple-negative breast cancer and relationship to basal-like molecular subtype. PPO Updates Prin Prac Oncol. 2010;24:1–6. [Google Scholar]
- 8.Perez EA, Moreno-Aspitia A, Aubrey Thompson E, Andorfer CA. Adjuvant therapy of triple negative breast cancer. Breast Cancer Res Treat. 2010;120(2):285–291. doi: 10.1007/s10549-010-0736-z. doi:10.1007/s10549-010-0736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gonzalvez F, Ashkenazi A. New insights into apoptosis signaling by Apo2L/TRAIL. Oncogene. 2010;29(34):4752–4765. doi: 10.1038/onc.2010.221. doi: 10.1038/onc.2010.221. [DOI] [PubMed] [Google Scholar]
- 10.Ashkenazi A, Herbst RS. To kill a tumor cell: the potential of proapoptotic receptor agonists. J Clin Invest. 2008;118(6):1979–1990. doi: 10.1172/JCI34359. doi:10.1172/JCI34359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amm HM, Oliver PG, Lee CH, Li Y, Buchsbaum DJ. Combined modality therapy with TRAIL or agonistic death receptor antibodies. Cancer Biol Ther. 2011;11:431–449. doi: 10.4161/cbt.11.5.14671. doi:10.4161/cbt.11. 5.14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiezorek J, Holland P, Graves J. Death receptor agonists as a targeted therapy for cancer. Clin Cancer Res. 2010;16(6):1701–1708. doi: 10.1158/1078-0432.CCR-09-1692. doi:10.1158/1078-0432.CCR-09-1692. [DOI] [PubMed] [Google Scholar]
- 13.Rahman M, Davis SR, Pumphrey JG, Bao J, Nau MM, Meltzer PS, Lipkowitz S. TRAIL induces apoptosis in triple-negative breast cancer cells with a mesenchymal phenotype. Breast Cancer Res Treat. 2009;113:217–230. doi: 10.1007/s10549-008-9924-5. doi:10.1007/s10549-008-9924-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchsbaum DJ, Zhou T, Grizzle WE, Oliver PG, Hammond CJ, Zhang S, Carpenter M, LoBuglio AF. Antitumor efficacy of TRA-8 anti-DR5 monoclonal antibody alone or in combination with chemotherapy and/or radiation therapy in a human breast cancer model. Clin Cancer Res. 2003;9:3731–3741. [PubMed] [Google Scholar]
- 15.Ichikawa K, Liu W, Zhao L, Wang Z, Liu D, Ohtsuka T, Zhang H, Mountz JD, Koopman WJ, Kimberly RP, Zhou T. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. doi:10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 16.Oliver PG, LoBuglio AF, Zinn KR, Kim H, Nan L, Zhou T, Wang W, Buchsbaum DJ. Treatment of human colon cancer xenografts with TRA-8 anti-death receptor 5 antibody alone or in combination with CPT-11. Clin Cancer Res. 2008;14:2180–2189. doi: 10.1158/1078-0432.CCR-07-1392. doi:10.1158/1078-0432.CCR-07-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill AV. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J Physiol. 1910;40(Suppl):4–5. [Google Scholar]
- 18.Sheiner LB, Stanski DR, Vozeh S, Miller RD, Ham J. Simultaneous modeling of pharmacokinetics and pharmacodynamics: application to d-tubocurarine. Clin Pharmacol Ther. 1979;25(3):358–371. doi: 10.1002/cpt1979253358. [DOI] [PubMed] [Google Scholar]
- 19.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 20.Zhao L, Feng SS. Effects of lipid chain length on molecular interactions between paclitaxel and phospholipid within model biomembranes. J Colloid Interface Sci. 2004;274(1):55–68. doi: 10.1016/j.jcis.2003.12.009. doi: 10.1016/j.jcis.2003.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Ohtsuka T, Buchsbaum D, Oliver P, Makhija S, Kimberly R, Zhou T. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene. 2003;22:2034–2044. doi: 10.1038/sj.onc.1206290. doi:10.1038/sj.onc.1206290. [DOI] [PubMed] [Google Scholar]
- 22.Straughn JM, Jr, Oliver PG, Zhou T, Wang W, Alvarez RD, Grizzle WE, Buchsbaum DJ. Anti-tumor activity of TRA-8 anti-death receptor 5 (DR5) monoclonal antibody in combination with chemotherapy and radiation therapy in a cervical cancer model. Gynecol Oncol. 2006;101:46–54. doi: 10.1016/j.ygyno.2005.09.053. doi:10.1016/j.ygyno.2005.09.053. [DOI] [PubMed] [Google Scholar]
- 23.DeRosier LC, Buchsbaum DJ, Oliver PG, Huang Z-Q, Sellers JC, Grizzle WE, Wang W, Zhou T, Zinn KR, Long JW, Vickers SM. Combination treatment with TRA-8 anti-death receptor-5 antibody and CPT-11 induces tumor regression in an orthotopic model of pancreatic cancer. Clin Cancer Res. 2007;13:5535s–5543s. doi: 10.1158/1078-0432.CCR-07-1075. doi:10.1158/1078-0432.CCR-07-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeRosier LC, Vickers SM, Zinn KR, Huang Z, Wang W, Grizzle WE, Sellers JC, Stockard CR, Jr, Zhou T, Oliver PG, Arnoletti JP, LoBuglio AF, Buchsbaum DJ. TRA-8 anti-DR5 monoclonal antibody and gemcitabine induce apoptosis and inhibit radiologically validated orthotopic pancreatic tumor growth. Mol Cancer Ther. 2007;6:3198–3207. doi: 10.1158/1535-7163.MCT-07-0299. doi:10.1158/1535-7163.MCT-07-0299. [DOI] [PubMed] [Google Scholar]
- 25.Fiveash JB, Gillespie GY, Oliver PG, Zhou T, Belenky ML, Buchsbaum DJ. Enhancement of glioma radiation therapy and chemotherapy response with targeted antibody therapy against death receptor 5. Int J Radiat Oncol Biol Phys. 2008;71:507–516. doi: 10.1016/j.ijrobp.2008.02.005. doi: 10.1016/j.ijrobp.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Wang H, Wang Z, Makhija S, Buchsbaum D, LoBuglio A, Kimberly R, Zhou T. Inducible resistance of tumor cells to tumor necrosis factor-related apoptosis-inducing ligand receptor 2-mediated apoptosis by generation of a blockade at the death domain function. Cancer Res. 2006;66:8520–8528. doi: 10.1158/0008-5472.CAN-05-4364. doi:10.1158/0008-5472.CAN-05-4364. [DOI] [PubMed] [Google Scholar]
- 27.Sun M, Song L, Li Y, Zhou T, Jope RS. Identification of an antiapoptotic protein complex at death receptors. Cell Death Differ. 2008;15(12):1887–1900. doi: 10.1038/cdd.2008.124. doi:10.1038/cdd.2008.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim H, Morgan DE, Buchsbaum DJ, Zeng H, Grizzle WE, Warram JM, Stockard CR, McNally LR, Long JW, Sellers JC, Forero A, Zinn KR. Early therapy evaluation of combined anti-death receptor 5 antibody and gemcitabine in orthotopic pancreatic tumor xenografts by diffusion-weighted magnetic resonance imaging. Cancer Res. 2008;68:8369–8376. doi: 10.1158/0008-5472.CAN-08-1771. doi:10.1158/0008-5472.CAN-08-1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buchsbaum DJ, Zhou T, Oliver PG, Hammond CJ, Carpenter M, LoBuglio AF. Striking antitumor efficacy of monoclonal antibodies to DR4 and DR5 with or without chemotherapy in a human breast cancer model. AACR special conference in cancer research apoptosis and cancer: basic mechanisms and therapeutic opportunities in the post-genomic era meeting; 13–17 Feb 2002; Waikoloa, HI: 2002. [Google Scholar]
- 30.Tomlinson GE, Chen TT, Stastny VA, Virmani AK, Spillman MA, Tonk V, Blum JL, Schneider NR, Wistuba II, Shay JW, Minna JD, Gazdar AF. Characterization of a breast cancer cell line derived from a germ-line BRCA1 mutation carrier. Cancer Res. 1998;58(15):3237–3242. [PubMed] [Google Scholar]
- 31.Elstrodt F, Hollestelle A, Nagel JH, Gorin M, Wasielewski M, van den Ouweland A, Merajver SD, Ethier SP, Schutte M. BRCA1 mutation analysis of 41 human breast cancer cell lines reveals three new deleterious mutants. Cancer Res. 2006;66(1):41–45. doi: 10.1158/0008-5472.CAN-05-2853. doi:10.1158/0008-5472.CAN-05-2853. [DOI] [PubMed] [Google Scholar]
- 32.Abramson V, Arteaga CL. New strategies in HER2-over-expressing breast cancer: many combinations of targeted drugs available. Clin Cancer Res. 2011;17(5):952–958. doi: 10.1158/1078-0432.CCR-09-1947. doi:10.1158/1078-0432. CCR-09-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Forero-Torres A, Shah J, Wood T, Posey J, Carlisle R, Copigneaux C, Luo FR, Wojtowicz-Praga S, Percent I, Saleh M. Phase I trial of weekly Tigatuzumab, an agonistic humanized monoclonal antibody targeting death receptor 5 (DR5) Cancer Biother Radiopharm. 2010;25:13–19. doi: 10.1089/cbr.2009.0673. doi:10.1089/cbr.2009.0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


