Abstract
Caspases are central to the execution of programmed cell death and their activation constitutes the biochemical hallmark of apoptosis. In this article, we report the successful adaptation of a high content assay method utilizing the DEVD-NucView488™ fluorogenic substrate, and for the first time, we show caspase activation in live cells induced either by drugs or siRNA. The fluorogenic substrate was found to be non-toxic over an exposure period of several days; during which we demonstrate automated imaging and quantification of caspase activation of the same cell population as a function of time. Overexpression of the anti-apoptotic protein Bcl-XL, alone or in combination with the inhibitor Z-VAD-FMK, attenuated caspase activation in HeLa cells exposed to Doxorubicin, Etoposide or cell death siRNA. Our method was further validated against two well characterized NSCLC cell lines reported to be sensitive (H3255) or refractory (H2030) to Erlotinib; where we show a differential time dependent activation was observed for H3255 and no significant changes in H2030, consistent with their respective chemosensitivity profile. In summary, our results demonstrate the feasibility of using this newly adapted and validated high content assay to screen chemical or RNAi libraries for the identification of previously uncovered enhancers and suppressors of the apoptotic machinery in live cells.
Keywords: High content assay, RNAi HT screening, Chemical HT screening, caspase, apoptosis, cancer, live cells
INTRODUCTION
One of the hallmarks of cancer genetics is that cancer cells accumulate mutations to escape apoptotic events leading to their malignant growth1. These events are the processes of programmed cell death (PCD) that may occur in multi-cellular organisms. Once triggered, PCD involves a series of biochemical events leading to a characteristic cell morphology and death; in more specific terms, a series of biochemical events that lead to a variety of morphological changes, including changes to the cell membrane such as the loss of membrane asymmetry and attachment, cell shrinkage, nuclear fragmentation, and chromosomal DNA fragmentation1, 2. PCD is instrumental in maintaining tissue homeostasis by actively eliminating unwanted and mutated cells. It is a highly controlled process triggered by intrinsic or extrinsic stimuli such as DNA damage or cytotoxic agents. Both pathways converge by activating the effector caspases belonging to the Group II class of caspases, namely Caspase 2, 3 and 7 (Figure 1). Given their central role as death effector mediators, activation of Group II caspases ideally reflects progression into apoptosis regardless of the nature of the stimulus and as such provides a good opportunity to screen for and discover the next generation of apoptosis-inducing drugs needed to overcome current drug resistance and to improve prognosis in cancer therapy.
Figure 1. Assay principle.
The caspase-activated DNV fluorogenic substrate allows to continuously monitor caspase activation in live, individual cells over time. Cleavage of the DEVD peptide releases a functional NucView488™ dye able to bind to DNA and to fluoresce when excited at 488 nm.
In addition to target-based assays that could potentially be adapted to being performed with cells – such as the homogeneous β-Galactosidase fragment complementation method for Caspase activity3 – current cell-based assays monitoring apoptosis in microtiter plates that are potentially amenable to high-throughput screening of chemical and RNAi libraries rely on four main biochemical events induced during programmed cell death: mitochondrial membrane depolarization, caspase activation, chromatin condensation and cytosolic release of DNA fragments (Table 1). DNA-specific dyes such as Hoechst 333424 and Acridine orange5 are toxic to cells, prohibiting their use for studying apoptosis in real time. Similarly, MitoTracker probes6 covalently label mitochondria and potentially interfere with the apoptotic process, precluding their use for real time studies. ELISA-based methods to quantify the cytosolic release of DNA fragments7 or caspase activation8 suffer from necessary washing steps, incompatible with real time kinetics. Washing steps are also required for assays relying on the PhiPhiLux9 and FLICA10 fluorogenic substrates. Similarly, cell lysis is necessary when employing the Caspase-Glo assay11 or fluorogenic substrates such as DEVD-AMC12. Finally, several published caspase activation assays rely on the transfection of the cell line of interest with a recombinant caspase substrate13, 14. Severe drawbacks of this approach include lack of versatility since the cells of interest need to be transfected prior to performing the assay, and potentially lack of physiological relevance due to the transformation of the original cell line. To date, no method amenable to microtiter plates provides access to real time kinetics of induction of apoptosis without requiring prior transfection of the cells of interest with a recombinant caspase substrate (Table 1).
Table 1. Current available technologies to monitor apoptosis in microtiter plates.
| Assay | Biochemical Event | Readout | Commercially available |
Used for HTS |
Transfection required |
Real Time |
|---|---|---|---|---|---|---|
| β-Gal. fragment complementation3 | Caspase activation | Mod. fluorogenic substrate | No | No | N/A | N/A |
| Hoechst 333424 | Chromatin condensation | Fluorescent dye | Yes | No | No | No |
| Acridine Orange5 | Chromatin condensation | Fluorescent dye | Yes | No | No | No |
| Cellular DNA Fragmentation ELISA7 | Cytosolic release of DNA fragments | ELISA | Yes | Yes | No | No |
| MitoTracker6 | Mito. membrane depolarization | Fluorescent dye | Yes | No | No | No |
| M30-ELISA8 | Caspase activation | ELISA | Yes | Yes | No | No |
| ANLucBCLuc14 | Caspase activation | Luminescence | No | No | Yes | No |
| Caspase-Glo11 | Caspase activation | Luminescence | Yes | No | No | No |
| PhiPhiLux9 | Caspase activation | Fluorescent dye | Yes | No | No | No |
| FLICA10 | Caspase activation | Fluorescent dye | Yes | No | No | No |
| DEVD-AMC12 | Caspase activation | Fluorogenic substrate | Yes | No | No | No |
| GFP-FRET13 | Caspase activation | Fluorogenic substrate | No | No | Yes | Yes |
Mito.: mitochondrial, β-Gal.: β-Galactosidase, Mod.: Modified.
Because of their central role as death effector mediators, activation of Group II caspases constitutes an attractive biochemical event to follow for the monitoring of apoptosis. However, caspase activation is a transient event in a cell, and cells within any given population are heterogeneous and undergo apoptosis at different rates. It is therefore essential for an high content screening assay monitoring apoptosis to allow multiple measurements in the same well over time. For this reason, we sought to employ a live and homogeneous assay, compatible with the assessment of real time kinetics of apoptosis in high density format. The caspase-activated DEVD-NucView488™ (DNV) fluorogenic substrate appears to be compatible with such requirements15; this cell-permeable substrate consists of a derivative of the DNA intercalating dye thiazole orange attached to the highly negatively charged DEVD peptide15. Presumably, the negative charges provided by the DEVD peptide prevent binding of the NucView488™ dye moiety to DNA in healthy cells where caspase activity is low. In contrast, in the cytoplasm of cells undergoing apoptosis, the DEVD sequence is thought to be cleaved by Caspase-3 - and potentially by other members of Group II caspases. Cleavage of the DEVD peptide releases a functional dye able to bind to DNA and to fluoresce when excited at 488 nm (Figure 1). The dye is not fluorescent until it binds to nucleic acids such as DNA in cell nuclei15; its fluorescence signal remains associated with DNA and is therefore retained within the cell. In addition, the DNV substrate does not appear to cause any toxicity or to interference with the progression of apoptosis15. Consequently, the DNV substrate appears especially useful for live cell monitoring of apoptosis. However, to date, reported uses of the DNV substrate are limited to single time point measurements using FACS analysis16 or fluorescence microscopy17, 18. Based on the characteristics of the DNV substrate, we speculated that we could adapt its use to high-density microtiter plates and to live imaging of apoptosis in high content screens.
In this article, we report the adaptation, optimization and validation of the use of the DNV fluorogenic substrate as a homogeneous, live assay for monitoring real-time kinetics of apoptosis in high density format. We demonstrate that our optimized method enables real time screening of chemical and RNAi libraries for the rapid identification of both early and late modulators of apoptosis.
MATERIALS AND METHODS
Reagents
The DNV substrate was purchased from Biotium Inc. (Hayward, CA). Dulbecco’s modified Eagle’s medium (DMEM), RPMI1640, Glutamine, Penicillin, Streptomycin, OptiMEM, Phosphate Buffered Saline without Mg2+, Ca2+ (PBS), Lipofectamine RNAiMAX, Lipofectamine 2000, Hoechst 33342, Rhodamine phalloidin, Alexa Fluor 633 phalloidin and goat anti-rabbit IgG antibody conjugated with Alexa Fluor 488 were purchased from Invitrogen Life Science (Carlsbad, CA). Rabbit anti-Bcl-XL polyclonal antibody was purchased from Pharmingen (San Diego, CA). Fetal bovine serum (FBS) was obtained from Omega Scientific (Tarzana, CA). Paraformaldehyde was purchased from Electron Microscopy Science (Hatfield, PA). Erlotinib (Tarceva) was purchased from LC Laboratories (Woburn, MA). Doxorubicin, Etoposide, Triton X-100 and GFP siRNA were purchased from Sigma-Aldrich Co. (Saint Louis, MO). Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone) was purchased from Biomol International (Butler Pike, PA). AllStars Hs Cell Death Control siRNA pool was purchased from Qiagen Inc. (Germatown, MD). DRAQ5 is manufactured by Biostatus Limited (Leicestershire, UK) and distributed in the US by Axxora LLC (San Diego, CA).
HeLa-Bcl-XL cells overexpressing the anti-apoptotic protein Bcl-XL were generated in the laboratory of Dr. Xiaodong Wang (University of Texas Southwestern Medical Center at Dallas, Dallas, TX) by stable transfection as previously described, as well as Control HeLa-Empty cells transfected with an empty control vector19. HeLa-Empty and HeLa-Bcl-XL cells were cultured in DMEM supplemented with 10 % FBS, 1 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. Non-small cell lung cancer cell lines H3255 and H2030 were obtained from Dr. Romel Somwar (Memorial Sloan-Kettering Cancer Center, New York, NY) and were cultured in RMPI1640 supplemented with 10 % FBS, 1 mM glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin. All cells were grown under a humidifed atmosphere of 5 % CO2-95 % air at 37 °C.
Instrumentation for cell imaging
The caspase activation assay was performed on a fully automated linear track robotic platform (CRS F3 Robot System, Thermo Fisher Scientific, Waltham, MA) with integrated peripherals for plate handling, liquid dispensing and microscopy imaging. Images were acquired with an IN Cell Analyzer 1000 epifluorescence automated microscope (INCA1000) (GE Healthcare, Piscataway, NJ) equipped with a 10X objective. To image the DNV probe signal, S475/20x excitation filter (475 nm excitation peak; +/− 20 nm bandpass), HQ535/50 emission filter (535 nm emission peak; +/− 50 nm bandpass) and Q505LP dichroic were used, exposing fields for 200 milliseconds for the green channel and 60 milliseconds for brightfield image. Imaging for Hoechst staining of nuclei count was performed on the same platform, using D360/40 excitation filter (360 nm excitation peak; +/− 40 nm bandpass), HQ535/50 emission filter (535 nm emission peak; +/− 50 nm bandpass) and Q505LP dichroic, and exposing fields for 200 milliseconds in the blue channel. Imaging for DRAQ5 staining of nuclei count was performed on the same platform, using HQ622/36 excitation filter (622 nm excitation peak; +/− 36 nm bandpass), HQ700/75 emission filter (700 nm emission peak; +/− 75 nm bandpass) and 51008bs dichroic, and exposing fields for 200 milliseconds in the blue channel. In all cases, four image fields were collected per well, and image analysis was conducted with the IN Cell Developer 1.7 software (GE Healthcare) using custom-developed analysis modules. The analysis modules were developed as follows. The analysis module for the NucView488™ signal conducts object segmentation in the green channel, quantifies the number of objects and the intensity per object, and data is reported as the sum of the fluorescence intensity for all imaged objects in the four fields as relative fluorescence units. The analysis module used for nuclei count performs object segmentation in the blue channel to automatically define and count the number of nuclei. Data is reported as the sum of imaged nuclei.
Confocal microscopy imaging was performed using an IN Cell Analyzer 3000 automated confocal microscope (INCA3000). This laser scanning confocal imager comprises two laser light sources, three excitation lines and three highly sensitive 12-bit CCD cameras allowing simultaneous imaging of three fluorophores with continuous laser-based autofocus. Image acquisition was performed at the following excitation/emission wavelengths (λexcitation, nm / λemission, nm): 364/450, 488/535, 633/695. Images were captured with an exposure time of 1.5 millisecond, collecting four images per well using a 40X objective. Data was acquired using the Raven 1.0 software (GE Healthcare). Image processing was performed using the IN Cell Developer Toolbox 1.7 software.
Immunostaining of Bcl-XL anti-apoptotic protein in HeLa-Empty and HeLa-Bcl-XL cells
HeLa-Empty and HeLa-Bcl-XL cell suspensions were dispensed into a 384-well assay plate (Corning 3985, Corning Inc., Corning, NY) at a cell seeding density of 1,000 cells per well in 45 μl medium using a Multidrop 384 dispenser (Thermo Electron Corporation, Waltham, MA). At 24h post-cell seeding, cells were pre-treated with 40 μM Z-VAD-FMK in PBS or with control PBS. One hour later, cells were treated with 25 μM Doxorubicin. At 48h post-treatment, cells were fixed for 20 minutes using 4% (v/v) paraformaldehyde, washed with PBS and permeabilized with 0.1% (v/v) Triton X-100 in PBS for 15 minutes. After a wash in PBS, cells were incubated for 30 minutes with 5% (v/v) FBS in PBS. Bcl-XL immunostaining was performed using a rabbit anti-Bcl-XL polyclonal antibody and an anti-rabbit secondary antibody conjugated with Alexa Fluor 488. Actin staining was performed with rhodamine phalloidin at a final dilution of 1/40 for 20 minutes. After three washes in PBS, the cells nuclei were stained with 40 μg/mL Hoechst 33342 for 15 minutes and 50 μL PBS was added to the wells after a final wash in PBS. Imaging was performed using the INCA1000 as described above.
Evaluation and colocalization of the NucView488™ dye
HeLa-Empty and HeLa-Bcl-XL cell suspensions were seeded as previously described. At 24h post seeding, 12-point doubling dilutions of Etoposide in 10% (v/v) DMSO ranging from 0.5 μM to 1 mM were prepared in a polypropylene 384-well microplate (ABgene, Rockford, IL), and 5 μL of each dilution were transferred to the assay plate using a PP-384-M Personal Pipettor (Apricot Designs, Monrovia, CA) to reach a final concentration of Etoposide ranging from 0.05 to 100 μM in 1% DMSO (v/v), after which 5 μL of 5 μM DNV substrate solution in PBS were dispensed to the assay plates using a FlexDrop IV (Perkin Elmer, Waltham, MA). The assay plate was incubated for 72h in an automated Steri-Cult incubator (Thermo Scientific Inc., Waltham, MA). Cells were then fixed and stained with Alexa Fluor 633 phalloidin and Hoechst 33342 according to the protocol previously descibed. Images were acquired on the INCA3000 as described above.
Automated imaging and quantification of caspase activation using the DNV fluorogenic substrate
Cells (1,000 per well for HeLa-Empty and HeLa-Bcl-XL; 2,000 per well for H3255 and H2030) were dispensed in 45 μL medium in 384-well microplates (Corning 3985, Corning Inc., Corning, NY) using the Multidrop 384 dispenser and incubated in the Steri-Cult incubator. If pre-treatment with Z-VAD-FMK pan-caspase inhibitor was tested, it was performed 1h prior to treatment at a final concentration of 40 μM in 1% DMSO (v/v). Transfection with siRNAs or treatment with small molecule was performed 24h post-cell seeding by transferring siRNA complexes or drug dilutions from a polypropylene 384-well source plate to the 384-well assay plates using the PP-384-M Personal Pipettor, after which 5 μL of 5 μM DNV solution in PBS were dispensed to the assay plates using the FlexDrop IV. Images were acquired on the INCA1000 as described above, over a time course of up to 96h post-DNV substrate addition. Each assay condition was performed in duplicate and reported data corresponds to the average of two wells, except for RNA knockdowns experiments which were performed in quadruplicate; reported data in that case correspond to the average of four wells and error bars represent the standard deviation of the data obtained in quadruplicate. For comparison of the NucView488™ signal with nuclei count, DRAQ5 live staining of nuclei was performed after the last timepoint by adding DRAQ5 to the cells diluted in PBS to reach a final concentration of 2.5 μM. Images were acquired on the INCA1000 as described above.
siRNA transfection
Cells were seeded in 384-well microplates as descrived above and transfection with GFP or cell death siRNA pool was conducted 24h post-cell seeding. Transfection of cell death siRNA pool in HeLa-Empty cells described in Figure 3 was performed using 0.025 μL Lipofectamine RNAi Max per well; siRNA transfection in HeLa-Empty and HeLa-Bcl-XL cells described in Figure 6B was performed using 0.075 μL Lipofectamine 2000 per well. siRNAs were preincubated with the transfection reagent for 20 minutes at room temperature in OptiMEM, and 10 μL of the complex were transferred to the assay plates. The siRNA final concentration was 100 nM for all transfections. Following transfection, 5 μL DNV substrate solution in PBS was added to each well using the FlexDrop IV, and automated imaging and quantification of caspase activation was performed as described above 48h post-transfection.
Figure 3. Confocal microscopy imaging of caspase activation using the DNV fluorogenic substrate.
Hoechst, DNV, Alexa Fluor 633 phalloidin triple staining of HeLa-Empty cells treated with 10 μM Doxorubicin for 72h and imaged on the INCA3000. A. Blue channel: Hoechst staining of nuclei. B. Green channel: NucView488™ staining. C. Overlay of blue and green channel image. D. Overlay of red channel: Alexa Fluor 633 phalloidin staining of actin, with blue and green channels.
Figure 6. Assessment of the stability of the DNV fluorogenic substrate signal.
A. HeLa-Empty cells or B. HeLa-Bcl-XL cells were treated with DMSO control or concentrations of Etoposide (Etop.) ranging from 0.005 μM to 10 μM for up to 72h and imaged every 24h. DNV addition was performed right after preparation of the DNV solution or after 3, 6, 12 or 24h storage on our automated platform in the conditions of screening.
Assessment of the effect of the DNV substrate on the proliferation of HeLa cells
HeLa-Empty and HeLa-Bcl-XL cell suspensions were seeded as described above; at 24h post seeding, 12-point doubling dilutions of the DNV substrate in 10 % (v/v) DMSO ranging from 0.5 μM to 1 mM were prepared in a polypropylene 384-well microplate, and 5 μL of each dilution were transferred to the assay plates to reach a final concentration of DNV substrate ranging from 0.05 to 100 μM in 1% DMSO (v/v). The assay plates were incubated for 24, 48, 72 and 96h in the Steri-Cult incubator. At each time point, cells were fixed for 20 minutes using 4% (v/v) paraformaldehyde, washed with PBS, and the cells nuclei were stained with 40 μg/mL Hoechst 33342 for 15 min. Images were acquired on the INCA1000 as described above. Each assay condition was performed in duplicate and reported data corresponds to the average of two wells.
Assessment of the stability of the DNV substrate signal
12-point doubling dilutions of Etoposide in 10 % (v/v) DMSO ranging from 0.05 μM to 100 μM were prepared in a polypropylene 384-well microplate and 5 μL of each dilution were transferred to 384-well assay plates to reach a final concentration of Etoposide ranging from 0.005 to 10 μM in 1% DMSO (v/v). HeLa-Empty and HeLa-Bcl-XL cell suspensions were dispensed into the assay plates at a cell seeding density of 1,000 cells per well in 45 μl medium using the Multidrop 384 dispenser. For each cell line, after the initital cell seeding, cells were dispensed 3, 6, 12, and 24h later into four plates corresponding to the 3, 6, 12, and 24h time points post-preparation of the DNV substrate solution (5 μM in PBS, protected from the light). The assay plates were incubated in the automated Steri-Cult incubator and 24h post-cell seeding, 5 μL of the DNV substrate were dispensed into each plate using the FlexDrop IV. Automated imaging and quantification of caspase activation for each plate 24, 48, 72 and 96h post-substrate addition was performed as described above. Each assay condition was performed in duplicate and reported data corresponds to the average of two wells.
RESULTS
Evaluation of apoptosis resistant HeLa-Bcl-XL cells
For the purpose of evaluating, optimizing and validating the use of the DNV substrate for real time monitoring of apoptosis, we took advantage of a well-described HeLa cell line stably transfected with the anti-apoptotic protein Bcl-XL (HeLa-Bcl-XL), which is resistant to apoptosis. Control HeLa cells were transfected with the vector only (HeLa-Empty)19. We confirmed by immunostaining that HeLa-BCl-XL cells overexpress Bcl-XL compared to HeLa-Empty cells (Figure 2A and B). As expected, the signal corresponding to the immunostaining of Bcl-XL in the green channel is practically non-existant for HeLa-Empty cells (Figure 2A), whereas green staining is intense for almost all imaged HeLa-Bcl-XL cells (Figure 2B). After exposure for 48h to Doxorubicin, most HeLa-Empty apoptosis-sensitive cells have been decimated; interestingly, surviving HeLa-Empty cells were found to overexpress Bcl-XL as observed in the green channel (Figure 2C), highlighting the heterogeneity of Bcl-XL expression in the cell population. In contrast, most of the HeLa-BcL-XL cells were resistant to exposure to 25 μM Doxorubicin for 48h (Figure 2D). Altogether, these results validate Hela-Bcl-XL cells as a model of apoptosis-resistant cells. As a control, we employed the broad spectrum caspase inhibitor Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethylketone). Z-VAD-FMK is a cell-permeant pan-caspase inhibitor that irreversibly binds to the catalytic site of caspase proteases and can inhibit induction of apoptosis20. When pre-treated with the pan-caspase inhibitor Z-VAD-FMK, HeLa-Bcl-XL cells were in part protected from apoptosis, as attested by the presence of a greater number of healthy cells compared to no pre-treatment (Figure 2E and C). Pre-treatment with the caspase inhibitor had little effect on the apoptosis-resistant cells HeLa-Bcl-XL, as expected (Figure 2F). The HeLa-Empty/Hela-Bcl-XL isogenic pair is used in this study as a main tool for validating our live caspase activation monitoring method.
Figure 2. A to F. Immunostaining of Bcl-XL anti-apoptotic protein in HeLa-Empty and HeLa-Bcl-XL cells.
Triple staining of A, C, E. HeLa-Empty and B, D, F. HeLa-Bcl-XL cells. Blue channel: Hoechst staining of nuclei. Green channel: Alexa Fluor 488 immunostaining of Bcl-XL. Red channel: Phalloidin-rhodamine staining of actin. A, B. No pre-treatment, no treatment control. C, D. No pre-treatment, treatment with 25 μM Doxorubicin for 48h. E, F. Pre-treatment with 40 μM Z-VAD-FMK pan-caspase inhibitor for one hour, treatment with 25 μM Doxorubicin for 48h. G, H. Assessment of the specificity of the DNV caspase activation signal. Bar graph of caspase activation signal induced by Doxorubicin in G. HeLa-Empty apoptosis-sensitive cells and H. HeLa-Bcl-XL apoptosis-resistant cells. Cells were pre-treated with 40 μM Z-VAD-FMK pan-caspase inhibitor or control and treated with DMSO control or 10 μM Doxorubicin (Dox.) for up to 72h.
Imaging of caspase activation using the DNV substrate
The DNV substrate is reported to stain the nucleus of apoptotic cells after cleavage by activated Caspase-3 in the cytoplasm15. To confirm this hypothesis, we performed a triple staining with Hoechst, DNV, and phalloidin rhodamine of HeLa-Empty cells pre-treated with 10 μM Doxorubicin in a 384-well microplate. Imaging on an automated confocal microscope reveals that the NucView488™ signal visualized in the green channel is co-localized with Hoechst staining of DNA visualized in the blue channel (Figure 3). The overlay of the red channel corresponding to rhodamine staining of actin filaments with the blue and green channel shows that NucView488™ positive cells have a condensed nucleus and a collapsed cytoskeleton. In addition, the intensely bright and condensed hoechst staining of NucView488™ positive cells is indicative of chromatin condensation (Figure 3). These observations are in agreement with the morphological features of apoptotic cells: chromatin condensation, nuclear and cell shrinkage, nuclear fragmentation, membrane blebbing and formation of apoptotic bodies. Altogether, our results seem to suggest that the DNV substrate specifically stains the nucleus of apoptotic cells after treatment with Doxorubicin.
We then investigated the feasibility of an automated caspase activation assay relying on the DNV substrate. HeLa-Empty cells transfected in 384-well microplate format with a cell death siRNA pool targeting human genes essential for survival were imaged on an automated epifluorescence microscope. Intense staining in the green channel can be observed for a majority of the cells 72h post transfection (Figure 4D). This result is in sharp contrast with control HeLa-Empty cells treated with the cell death siRNA pool in absence of transfection reagent, for which almost no signal can be detected (Figure 4A). Brightfield imaging of the same field reveals a sparse population of cells with a shrunken cytoplasm for the transfected cells (Figure 4E), whereas the control cells are present in large number and have a healthy morphology (Figure 4B). Our results indicate that the DNV substrate specifically stains apoptotic cells after transfection with siRNAs targeting genes essential for survival. For the purpose of automatically quantifying the NucView488™ signal, we developed an image analysis algorithm based on object segmentation. Our custom-developed analysis module can accurately recognize the stained objects in the green channel, as shown in Figure 4F. The software then quantifies the number of objects and the intensity per object for each of the fields imaged. The data can also be summed and averaged per well. All following quantifications described in this article rely on this image analysis module.
Figure 4. Automated imaging and quantification of caspase activation using the DNV fluorogenic substrate.
HeLa-Empty cells imaged with the INCA1000 after transfection for 72h with: A, B, C. Control cell death siRNA pool transfection in absence of transfection reagent; or D, E, F. Cell death siRNA pool transfection with RNAiMAX transfection reagent. A, D. Green channel. B,E. Overlay of green channel and brightfield image. C, F. Overlay of image analysis mask and green channel image.
Optimization and Validation of a high content assay for monitoring live caspase activation based on the DNV substrate
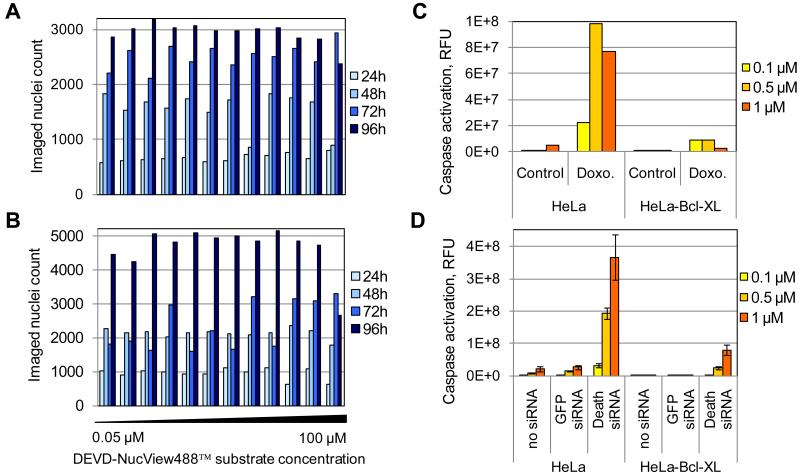
In order to assess the cytotoxic effects of the DNV substrate over the course of a typical cell-based screen, we treated HeLa-Empty and HeLa-Bcl-XL cells with 12 doubling dilutions of the DNV substrate ranging from 0.05 μM to 100 μM for 96h, and performed automated nuclei count of the treated cells at 24, 48, 72 and 96h post-treatment. No significant effect on the proliferation of HeLa-Empty or HeLa-Bcl-XL cells was observed with up to 100 μM substrate for as long as 96h treatment, confirming that the DNV substrate is not toxic (Figure 5A and B).
Figure 5. A, B. Assessment of the effect of the DNV fluorogenic substrate on the proliferation of HeLa cells.
Bar graph of the number of imaged nuclei count as a function of substrate concentration at four time points: 24; 48; 72; 96h post-cell seeding. A. HeLa-Empty cells. B. HeLa-Bcl-XL cells. C, D. Optimization of DNV fluorogenic substrate concentration. Bar graph of caspase activation signal induced by Doxorubicin at 72h on HeLa-Empty or HeLa-Bcl-XL cells using three substrate concentrations: 0.1; 0.5; 1 μM. C. Optimization of substrate concentration for chemical screens. Cells were treated with DMSO control or 10 μM Doxorubicin. D. Optimization of substrate concentration for siRNA screens. Cells were treated with no siRNA control, GFP siRNA control, or cell death siRNA pool for 48h.
In order to identify the optimal concentration of DNV substrate to use in high content screens, we performed titration experiments in 384-well format in the context of both a small molecule and a siRNA screen. We tested three concentrations of DNV substrate: 0.1; 0.5 and 1 μM based on the recommendations from the supplier (1 μM). As expected, the signal obtained with HeLa-Empty and HeLa-Bcl-XL cells treated with Doxorubicin was higher than for cells treated with the DMSO control (Figure 5C). Not surprisingly, the signal obtained with HeLa-Bcl-XL cells resistant to apoptosis was consistently lower compared to HeLa-Empty cells. Using 0.5 μM substrate provided an 80 to 1 signal to noise ratio between Doxorubicin and DMSO-treated HeLa-Empty cells. Interestingly, we observed a 10 to 1 signal to noise ratio between Doxorubicin-treated HeLa-Empty cells and Doxorubicin-treated HeLa-Bcl-XL cells, in agreement with the level of overexpression of Bcl-XL protein in HeLa-Bcl-XL cells compared to HeLa-Empty cells. Our results strongly suggest that the DNV substrate accurately reflects the level of apoptosis for the imaged cells, and that our custom image analysis module correctly quantitates the NucView488™ signal for the imaged well. To test the automated quantification of apoptosis induced by siRNA knockdown in the context of an RNA interference screen, we performed a pilot experiment using a cell death siRNA pool targeting human genes essential to survival. Controls consisted of cells treated with the cell death siRNA pool in absence of transfection reagent and of cells transfected with untargeted control siRNA. Significant caspase activation was observed and quantified for both HeLa-Empty and HeLa-Bcl-XL cells transfected with the cell death siRNA pool compared to the untargeted and mock transfection controls (Figure 5D). As expected, the NucView488™ signal induced by the cell death siRNA pool was significantly lower for the HeLa-Bcl-XL apoptosis resistant cells compared to HeLa-Empty cells. Using 0.5 μM substrate provided an optimal signal to noise ratio of 8 to 1 between the two cell lines. Altogether, our observations suggest that the optimal concentration of DNV substrate to use with HeLa-Empty and HeLa-Bcl-XL cells is 0.5 μM.
To test the specificity of the caspase activation signal obtained with the DNV substrate, we employed the pan-caspase inhibitor Z-VAD-FMK. HeLa-Empty cells treated with Doxorubicin and monitored using the DNV substrate demonstrated time-dependent caspase activation over a 72h period, with a peak at at 66h (Figure 2G). In contrast, the NucView488™ signal was close to non-existant for cells treated with control DMSO. Importantly, HeLa-Empty cells pre-treated with the pan-caspase inhibitor Z-VAD-FMK had their caspase activation signal reduced by five fold (Figure 2G, demonstrating that the NucView488™ signal that we imaged and quantified specifically reflects the status of caspase activation in the imaged cells. In Hela-Bcl-XL apoptosis resistant cells, the caspase activation signal induced by Doxorucin was nine-fold lower compared to HeLa-Empty cells (Figure 2H), consistant with our previous observation (Figure 5C). As expected, Z-VAD-FMK also reduced the intensity of caspase activation in these cells (Figure 2H).
An automated screen campaign involves pre-dispensing and storing reagents on deck over the entire course of the screen; therefore, the stability of the DNV substrate in the conditions of screening is an important factor to assess. For this reason, we performed an experiment where we performed live monitoring of caspase activation in HeLa-Empty and HeLa-Bcl-XL cells treated with Etoposide. The DNV substrate was stored on our automated platform for 0, 3, 6, 12 or 24h in the conditions of screening before being dispensed into the wells. After 48 and 72h incubation with Etoposide or DMSO control we performed imaging and quantification of the NucView488 signal on an automated epifluorescence microscope. Importantly, we found that the high signal induced by Etoposide on HeLa-Empty cells after 72h incubation remained practically constant for up to 12h storage (Figure 6A). Furthermore, the low signal induced by control DMSO remained consistantly low for up to 24h storage (Figure 6A), as well as the low signal observed with HeLa-Bcl-XL apoptosis-resistant cells (Figure 6B), as expected. This important results demonstrates that storage of the diluted substrate in the conditions of screening did not alter its specificity for apoptic cells and did not induce any increase in background noise. We conclude that a batch of DNV reagent can be used for dispensing in the conditions of screening for up to 12h continuously.
Validation of the newly-developed method for live monitoring of real time kinetics of caspase activation in high content screens
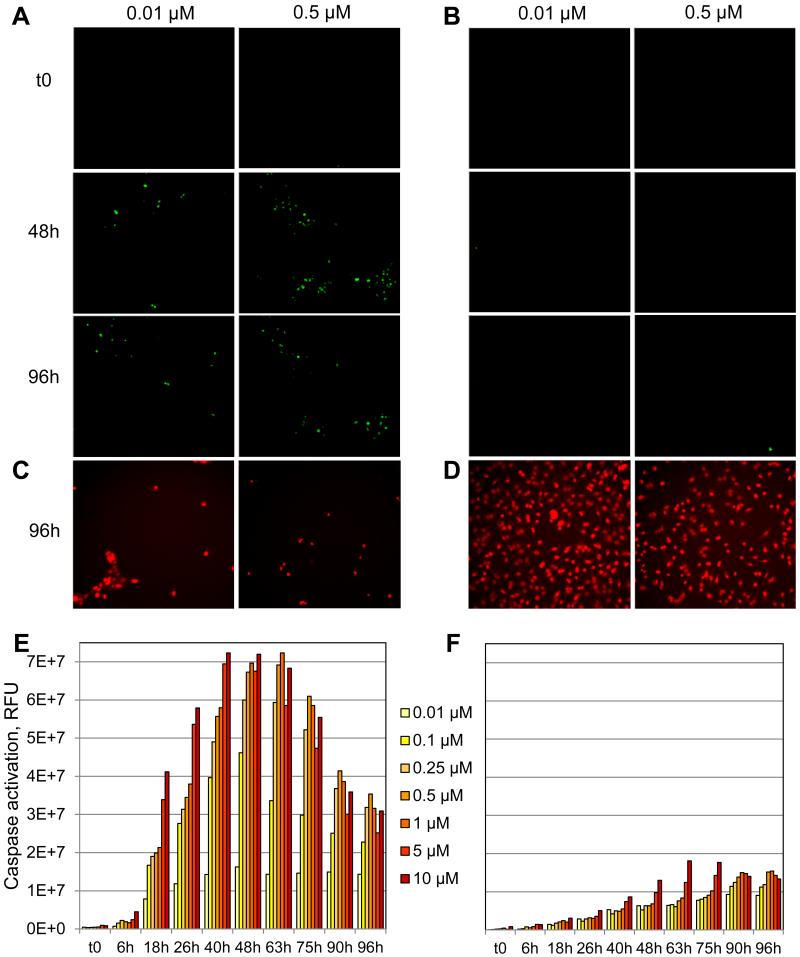
We further validated our newly-developed method for monitoring real time kinetics of caspase activation using the well-characterized pair of Non Small Cell Lung Cancer (NSCLC) cell lines: H3255 and H2030 cell lines21. Both lines were derived from patients with NSCLC arising from oncogenic EGFR or KRAS. H3255 cells harbor the L858R mutation in the EGFR gene and are sensitive to the EGFR tyrosine kinase inhibitor Erlotinib (Tarceva). In contrast, H2030 cells express wildtype EGFR and mutated KRAS and are refractory to Erlotinib. This pair of NSCLC cell lines therefore provides a good platform for validating our newly-developed method to quantify apoptosis in high content screens. We monitored the real time kinetics of caspase activation induced by concentrations of Erlotinib ranging from 0.01 μM to 10 μM in both cell lines, imaging the cells at regular time intervals after treatment over a course of 96h (Figure 7). We found that we could visualize (Figure 7A) and quantify (Figure 7E) Erlotinib-induced caspase activation in H3255 Erlotinib-sensitive cells as early as 18h post-treatment, gradually increasing over time to reach a plateau at 48 and 63h, and decreasing from 63h to 96h post-treatment. In addition, Erlotinib-induced caspase activation in these cells was dose-dependent at any of the imaged time points (Figure 7E). In contrast, monitoring of NucView488™ signal induced by Erlotinib in the Erlotinib-refractory H2030 cells revealed low caspase activation at any time point and for any of the tested concentrations, in agreement with their chemosensitivity profile (Figure 7B and F). These results were supported by imaging of the nuclei after 96h treatment: few nuclei could be visualized for H3255 cells treated with 0.01 and 0.5 μM Erlotinib, in sharp contrast with H2030 cells (Figure 7C and D).
Figure 7. Live monitoring of real time kinetics of caspase activation induced by Erlotinib in NSCLC cell lines.
A, C. E. Erlotinib-sensitive H3255 cells and B, D, F. Erlotinib-refractory H2030 cells were treated with concentrations of Erlotinib ranging from 0.01 μM to 10 μM. A, B. Automated imaging and quantification of caspase activation using the DNV fluorogenic substrate at the time of treatment or 48 and 96h post-treatment. C, D. DRAQ5 nuclei staining at 96h post-treatment. E, F. Real time automated quantification of caspase activation using the DNV fluorogenic substrate.
DISCUSSION
Apoptosis is central to a number of pathological proliferative disorders, including cancer. Therefore, the ability to monitor apoptosis in high content screens is highly sought for the discovery of drugs in a wide range of therapeutic areas. Current methods to follow apoptosis rely on quantifying caspase activation, given the central role of this class of enzymes as death effector molecules. However, direct monitoring of caspase activation in live cells in the context of a high content screen is a difficult task for two reasons. First, cell death signaling in response to pro-apoptotic stimuli is limited in time and cultured cells are typically not synchronized. Therefore, caspase activation in cultured cells is a transient and heterogeneous event. Second, technical hurdles have so far limited the monitoring of caspase activation to single time point measurements. For these combined reasons, to our knowledge no method currently exists that allows continuous, live monitoring of caspase activation in high content screens. The requirements for such an assay are: 1. Amenable to high density format, 2. Live and continuous, 3. Non-toxic and not interfering with apoptosis. 4. Versatile. A previous report suggests that the DNV substrate meets the requirements for such an assay15, but reported uses of the DNV substrate are limited so far to single time point measurements using FACS analysis16 or fluorescence microscopy17, 18. For this reason, we sought to evaluate and optimize the use of the DNV substrate as a novel method to monitor the real time kinetics of caspase activation in high content screens.
Our confocal imaging experiments confirm that following DNV addition, the NucView488™ signal is confined to the nucleus or the perinuclear region of cells undergoing apoptosis (Figure 3). Together with data reported by others15, these results suggest that the DNV substrate is non-fluorescent until it is cleaved by activated effector caspases, thereby allowing the NucView488 substrate to stain the nuclei of apoptotic cells. Since the DEVD peptide corresponds to the optimal substrate sequence for both Caspase-3 and Caspase-7, it is therefore probably cleaved by both enzymes22. This sequence could also potentially be cleaved at a slower rate by other members of the Group II family of caspases with slightly different specificities22. The assay requires a unique addition of DNV substrate, in absence of any washing step. In addition, we show that the DNV substrate is not toxic to HeLa cells (Figure 5A and B). Altogether, these observations confirm that a method based on the use of the DNV substrate could allow continuous monitoring of caspase activation. After optimizing the substrate concentration with HeLa cells (Figure 5C and D), we sought to validate the use of the DNV substrate for live monitoring of apoptosis in high content screens. We demonstrated that the NucView488™ signal observed in the green channel can be imaged in high density format on a robotic platform equipped with an automated epifluorescence microscope. Imaging of the same well can be performed as many times as needed over the course of a screen, and the obtained images can easily be processed by automated analysis software and quantified (Figure 4 and 7). Data is collected at the single cell level, allowing to study heterogeneous populations of cells (Figure 4). We show that a high signal is observed and quantified when HeLa-Empty cells are treated with Doxorubicin or Etoposide, both drugs known to induce apoptosis in cancer cell lines (Figure 2, 3, 5 and 6). However, pre-treatment with a pan-caspase inhibitor can antagonize this high signal, demonstrating the specificity of the signal imaged using the DNV substrate (Figure 2). Of note, we noticed that control cells treated with DMSO exhibit a strong nuclear staining using Hoechst 33342 (Figure 2B), while the nuclear staining for cells pre-treated with Doxorubicin and stained with Hoechst in the same conditions is very weak (Figure 2D and F). It is likely that we are observing the quenching of Hoechst fluorescence by energy transfer to Doxorubicin, as the maximum emission wavelength of Hoechst bound to DNA is 458 nm, which is near the maximum excitation wavelength of Doxorubicin bound to DNA (473 nm)23. This probably leads to energy transfer between the two dyes, which results in the quenching of Hoechst fluorescence as previously observed24, 25. Nuclei staining with an alternative dye such as DRAQ5 is therefore recommended when performing nuclei count after doxorubicin treatment. In addition, in HeLa-Bcl-XL overexpressing the anti-apoptotic protein Bcl-XL, a much lower NucView488™ signal was observed when those cells were treated with Doxorubicin (Figure 2 and 5C). Similarly, transfection of HeLa-Empty cells with an siRNA pool targeting genes essential for cell survival resulted in a significant increase in NucView488™ signal compared to a no siRNA control or transfection with an untargeted siRNA (Figure 5D); in HeLa-Bcl-XL cells, the quantified NucView488™ signal was dramatically reduced compared to HeLa-Empty cells. Altogether, our results validate the new method we have developed, in that we could reliably track and quantify apoptosis induced by small molecules or RNA interference over time, and its inhibition by caspase inhibition or by overexpression of the anti-apoptotic Bcl-XL protein. In contrast to previous uses of the DNV substrate, we demonstrate that our method allows monitoring of apoptosis for the same cell population at multiple time points. Of note, the fact that our method could be employed to either track apoptosis induced by a small molecule or by knockdown of gene expression demonstrates its great versatility. For further validation, we sought to test our method in a different cell system; we used for this purpose the well-described NSCLC cell lines H3255 and H203021. We could precisely monitor the real time kinetics of caspase activation induced by Erlotinib in the Erlotinib-sensitive cell line H3255. As expected, the strong caspase activation induced by Erlotinib in this cell line was time- and dose-dependent (Figure 7A and E). In contrast, only low levels of caspase activation could be detected in the Erlotinib-refractory H2030 cell line at any time point or tested concentration (Figure 7B and F). These results strongly validate our method and demonstrate its flexibility, in that we could easily employ our newly-developed assay with different cell lines without any prior cell engineering or any dedicated optimization for the new lines. In addition, our live, real-time method allowed us to take multiple snapshots of the cells during the same experiment. This result is important because it demonstrates that our method can detect early as well as late inducers of apoptosis in the same screen. Furthermore, our method allows for a high throughput screen to be performed without compromising plate to plate variability. Based on our experience and according to simulations using the POLARA® scheduling software, we estimate that a throughput of 100 384-well plates a week can be achieved by staggering plates. This estimation is based on a fully automated screen with three readouts every 24h over a course of 72h, where all plates are read at exact same intervals of 24h. This throughput allows for the screening of roughly 35,000 compounds a week. We have performed such a screen with a total of 28 plates and we will be submitting the results in a manuscript shortly.
In summary, our method meets all the requirements for a live assay aimed at quantifying apoptosis in high density format. We conclude that our method allows for the first time the monitoring of real-time kinetics of apoptosis in high content screens and could be used in combination with other readouts as a multiplexed assay for cell death. We expect that the flexibility of our method will allow to dissect apoptosis signaling pathways using both chemical and functional genomics, thereby enabling the rapid identification of novel modulators of apoptosis.
ACKNOWLEDGEMENTS
The authors wish to thank the members of the High Throughput Screening Core Facility for their help during the course of this study, Dr. Xuejun Jiang for providing HeLa-Empty and HeLa-Bcl-XL cells, Dr. Romel Somwar (Varmus lab) for providing H3255 and H2030 cells, and Tony J. Riley (Medical Graphics, MKCC) for his contribution to the artwork in this paper. The HTS Core Facility is partially supported by Mr. William H. Goodwin and Mrs. Alice Goodwin and the Commonwealth Foundation for Cancer Research, the Experimental Therapeutics Center of MSKCC, the William Randolph Hearst Fund in Experimental Therapeutics, the Lilian S Wells Foundation and by an NIH/NCI Cancer Center Support Grant 5 P30 CA008748-44.
REFERENCES
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naqvi T, Lim A, Rouhani R, Singh R, Eglen RM. Beta galactosidase enzyme fragment complementation as a high-throughput screening protease technology. J Biomol Screen. 2004;9:398–408. doi: 10.1177/1087057104264040. [DOI] [PubMed] [Google Scholar]
- 4.Eymin B, Leduc C, Coll JL, Brambilla E, Gazzeri S. p14ARF induces G2 arrest and apoptosis independently of p53 leading to regression of tumours established in nude mice. Oncogene. 2003;22:1822–1835. doi: 10.1038/sj.onc.1206303. [DOI] [PubMed] [Google Scholar]
- 5.Tucker B, Lardelli M. A rapid apoptosis assay measuring relative acridine orange fluorescence in zebrafish embryos. Zebrafish. 2007;4:113–116. doi: 10.1089/zeb.2007.0508. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore K, Wilson M. The use of chloromethyl-X-rosamine (Mitotracker red) to measure loss of mitochondrial membrane potential in apoptotic cells is incompatible with cell fixation. Cytometry. 1999;36:355–358. doi: 10.1002/(sici)1097-0320(19990801)36:4<355::aid-cyto11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 7.MacKeigan JP, Murphy LO, Blenis J. Sensitized RNAi screen of human kinases and phosphatases identifies new regulators of apoptosis and chemoresistance. Nat Cell Biol. 2005;7:591–600. doi: 10.1038/ncb1258. [DOI] [PubMed] [Google Scholar]
- 8.Hagg M, Biven K, Ueno T, Rydlander L, Bjorklund P, Wiman KG, Shoshan M, Linder S. A novel high-through-put assay for screening of pro-apoptotic drugs. Invest New Drugs. 2002;20:253–259. doi: 10.1023/a:1016249728664. [DOI] [PubMed] [Google Scholar]
- 9.Fennell M, Chan H, Wood A. Multiparameter measurement of caspase 3 activation and apoptotic cell death in NT2 neuronal precursor cells using high-content analysis. J Biomol Screen. 2006;11:296–302. doi: 10.1177/1087057105284618. [DOI] [PubMed] [Google Scholar]
- 10.Bedner E, Smolewski P, Amstad P, Darzynkiewicz Z. Activation of caspases measured in situ by binding of fluorochrome-labeled inhibitors of caspases (FLICA): correlation with DNA fragmentation. Exp Cell Res. 2000;259:308–313. doi: 10.1006/excr.2000.4955. [DOI] [PubMed] [Google Scholar]
- 11.Fan F, Wood KV. Bioluminescent assays for high-throughput screening. Assay Drug Dev Technol. 2007;5:127–136. doi: 10.1089/adt.2006.053. [DOI] [PubMed] [Google Scholar]
- 12.Yu K, Kennedy CA, O’Neill MM, Barton RW, Tatake RJ. Disparate cleavage of poly-(ADP-ribose)-polymerase (PARP) and a synthetic tetrapeptide, DEVD, by apoptotic cells. Apoptosis. 2001;6:151–160. doi: 10.1023/a:1011375024832. [DOI] [PubMed] [Google Scholar]
- 13.Tawa P, Tam J, Cassady R, Nicholson DW, Xanthoudakis S. Quantitative analysis of fluorescent caspase substrate cleavage in intact cells and identification of novel inhibitors of apoptosis. Cell Death Differ. 2001;8:30–37. doi: 10.1038/sj.cdd.4400769. [DOI] [PubMed] [Google Scholar]
- 14.Coppola JM, Ross BD, Rehemtulla A. Noninvasive imaging of apoptosis and its application in cancer therapeutics. Clin Cancer Res. 2008;14:2492–2501. doi: 10.1158/1078-0432.CCR-07-0782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cen H, Mao F, Aronchik I, Fuentes RJ, Firestone GL. DEVD-NucView488: a novel class of enzyme substrates for real-time detection of caspase-3 activity in live cells. FASEB J. 2008;22:2243–2252. doi: 10.1096/fj.07-099234. [DOI] [PubMed] [Google Scholar]
- 16.Shan P, Pu J, Yuan A, Shen L, Chai D, He B. RXR agonists inhibit oxidative stress-induced apoptosis in H9c2 rat ventricular cells. Biochem Biophys Res Commun. 2008;375:628–633. doi: 10.1016/j.bbrc.2008.08.074. [DOI] [PubMed] [Google Scholar]
- 17.Jones S, Martel C, Belzacq-Casagrande AS, Brenner C, Howl J. Mitoparan and target-selective chimeric analogues: membrane translocation and intracellular redistribution induces mitochondrial apoptosis. Biochim Biophys Acta. 2008;1783:849–863. doi: 10.1016/j.bbamcr.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Zhu QS, Ren W, Korchin B, Lahat G, Dicker A, Lu Y, Mills G, Pollock RE, Lev D. Soft tissue sarcoma cells are highly sensitive to AKT blockade: a role for p53-independent up-regulation of GADD45 alpha. Cancer Res. 2008;68:2895–2903. doi: 10.1158/0008-5472.CAN-07-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nijhawan D, Fang M, Traer E, Zhong Q, Gao W, Du F, Wang X. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 2003;17:1475–1486. doi: 10.1101/gad.1093903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao RG, Cao CX, Pommier Y. Activation of PKCalpha downstream from caspases during apoptosis induced by 7-hydroxystaurosporine or the topoisomerase inhibitors, camptothecin and etoposide, in human myeloid leukemia HL60 cells. J Biol Chem. 1997;272:31321–31325. doi: 10.1074/jbc.272.50.31321. [DOI] [PubMed] [Google Scholar]
- 21.Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thornberry NA, Rano TA, Peterson EP, Rasper DM, Timkey T, Garcia-Calvo M, Houtzager VM, Nordstrom PA, Roy S, Vaillancourt JP, Chapman KT, Nicholson DW. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J Biol Chem. 1997;272:17907–17911. doi: 10.1074/jbc.272.29.17907. [DOI] [PubMed] [Google Scholar]
- 23.Gabelica V, Rosu F, De Pauw E, Antoine R, Tabarin T, Broyer M, Dugourd P. Electron photodetachment dissociation of DNA anions with covalently or noncovalently bound chromophores. J Am Soc Mass Spectrom. 2007;18:1990–2000. doi: 10.1016/j.jasms.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Krishan A. Flow Cytometric Studies on Intracellular Drug Fluorescence. In: Darzynkiewicz JWGaZ., editor. Techniques in Cell Cycle Analysis. Humana Press; 1987. pp. 337–366. [Google Scholar]
- 25.Preisler HD. Alteration of binding of the supravital dye Hoechst 33342 to human leukemic cells by adriamycin. Cancer Treat Rep. 1978;62:1393–1396. [PubMed] [Google Scholar]