Abstract
Endurance training induces the transcriptional coactivator PGC-1α in skeletal muscle, promoting mitochondrial biogenesis and skeletal muscle remodeling. In a recent issue of Cell, Ruas et al. (2012) show that resistance training regulates the splicing of a novel isoform of PGC-1α (PGC-1α4), which is sufficient to stimulate skeletal muscle hypertrophy.
Skeletal muscle is central to the health benefits conferred by exercise. Indeed, engaging in either of the two general forms of exercise, aerobic (e.g., long-distance running and endurance training) and anaerobic (e.g., weightlifting and resistance training), promotes remodeling of skeletal muscle and resistance to cardiovascular disease, obesity, and type 2 diabetes (Golbidi et al., 2012). Thus, intense effort is ongoing to decipher the molecular pathways that alter skeletal muscle properties in response to exercise (Bassel-Duby and Olson, 2006). Skeletal muscle responds to endurance training by altering contractile proteins, generating more mitochondria, and increasing angiogenesis, whereas resistance training primarily stimulates growth of muscle fibers (LeBrasseur et al., 2011; Yan et al., 2011). Peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α (PGC-1α) was identified a decade ago as a major transcriptional activator that regulates the response of skeletal muscle to endurance exercise (Lin et al., 2002; Puigserver et al., 2001). PGC-1α is induced by endurance exercise in both mice and humans and acts as a transcriptional coregulator to coordinate the genetic program for fiber-type switching, mitochondrial biogenesis, and angiogenesis (Figure 1). PGC-1α is clearly a central factor in endurance-dependent muscle remodeling, and until now it has not been linked to resistance training adaptations. In a recent issue of Cell, Ruas and colleagues provide evidence that a novel isoform of PGC-1α governs hypertrophy of skeletal muscle in response to resistance training (Ruas et al., 2012).
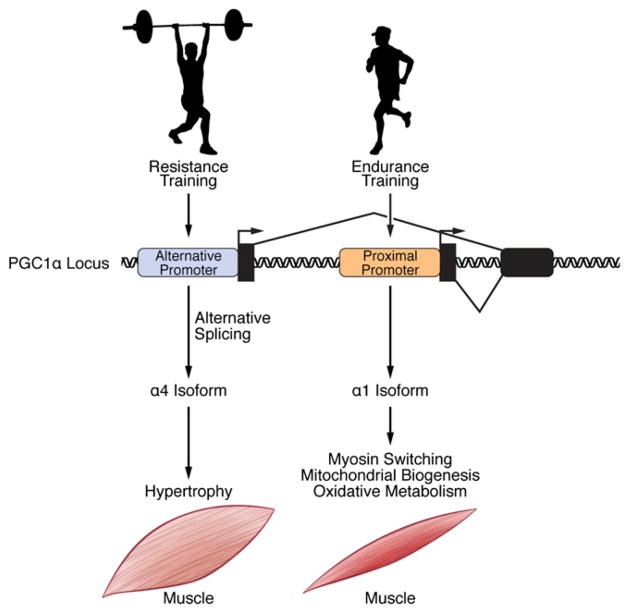
Figure 1. Regulation and Function of PGC-1α Variants by Exercise in Skeletal Muscle.
Endurance training activates the proximal promoter of PGC-1α, whereas resistance exercise stimulates the alternative promoter. Proximal promoter usage and generation of the 1α1 isoform to regulate endurance exercise-dependent skeletal muscle remodeling has been well established. Ruas and colleagues (Ruas et al., 2012) have now proposed a function for a PGC-1α isoform (1α4) during resistance training. Use of the alternative promoter, along with splicing of a premature stop codon between exons 6 and 7, results in 1α4 isoform expression and induction of skeletal muscle hypertrophy.
Previous work identified two independent promoters responsible for PGC-1α transcription (Miura et al., 2008; Yoshioka et al., 2009). Based on this knowledge, the authors cloned four isoforms using a PCR strategy specific for the two promoters and named these species PGC-1α1– PGC-1α4, where PGC-1α1 is the previously described PGC-1α and PGC-1α4 is the isoform whose function was explored. They then asked whether the different splice variants regulate similar or different gene sets, using microarray analysis after infection of cultured primary skeletal myotubes. While there was some overlap between the PGC-1α1, PGC-1α2, and PGC-1α3 regulated genes, PGC-1α4 induced the most divergent set of genes. The first clue to understanding the physiological function of PGC-1α4 was the observation that it did not regulate the same genes as PGC-1α1, which, as expected, increased mitochondrial oxidative and angiogenic genes. Instead, PGC-1α4 regulated genes in the insulin-like growth factor 1 (IGF-1) and myostatin pathways, which are well known to regulate hypertrophy of skeletal muscle. Of note, the α4 isoform contains the activation domain required for PGC-1α1 coactivator activity yet regulates a completely different set of genes from the other isoforms. It will be interesting to understand how domains in each of these variants confer specificity for regulation of their particular gene sets.
Further suggesting a role in muscle growth, the authors showed PGC-1α4 mRNA was down- and upregulated during hindlimb suspension and reloading, respectively, in mice. PGC-1α4 was sufficient to induce hypertrophy in vivo by intramuscular injection of either naked plasmids or adenovirus encoding PGC-1α4 and by generation of a muscle-specific PGC-1α4 transgenic mouse (myo-PGC-1α4). When assessing the functionality of a potential hypertrophic molecule in skeletal muscle, it is essential to test it in a setting of muscle atrophy. Importantly, the authors investigated the response of the myo-PGC-1α4 transgenic mice to two atrophic stimuli, hindlimb suspension and cancer cachexia, via inoculation with Lewis Lung Carcinoma (LLC) cells. The myo-PGC-1α4 transgenic mice were resistant to muscle loss in both settings, but most strikingly, gastrocnemius weight was maintained and muscular strength partially preserved in transgenic mice after LLC tumor initiation. It is enticing to speculate that PGC-1α4 may regulate the size of other tissues in which it is expressed, such as the heart and brown adipose tissue. Future work should also focus on developing antibodies that recognize the different PGC-1α isoforms, allowing for definitive analysis of expression and relative abundance of each variant at the protein level.
Are there potential clinical implications related to these findings? That PGC-1α4 is increased modestly (1.5-fold) in humans after weightlifting may suggest the PGC-1α4-hypertrophy signaling axis is functional in human skeletal muscle. Interestingly, PGC-1α4, along with PGC-1α, was most dramatically induced in human skeletal muscle after a combined exercise protocol where the subjects participated in both cycling and weight-lifting. This suggests the most efficient exercise program to maximize beneficial muscle remodeling is a combination of endurance and resistance training. Furthermore, it indicates some overlap in the exercise-induced regulation of PGC-1α1 and PGC-1α4. Thus, one significant avenue of future investigation will be to understand how each of the PGC-1α species is generated in response to various exercise stimuli, whether it is via a different cocktail of transcription factors and/or mRNA splicing mechanisms. Possibly more attractive as a potential therapy are the mechanistic insights of PGC-1α4 function provided by Ruas et al. (2012). They suggest this isoform modulates hypertrophy through histone modifications near the promoters of the genes encoding two potent hypertrophic regulators, IGF-1 and myostatin. Specifically, they propose a model where PGC-1α4 increases the transcription of IGF-1, an inducer of muscle growth, and decreases transcription of myostatin, a negative regulator of muscle growth. If one could design an ideal molecule as a therapy to promote muscle growth, it would likely be a potent inducer of IGF-1 while possessing repressive activity on myostatin. In principle, PGC-1α4 may be that molecule, but much more information must be obtained before moving forward in this regard. For instance, evaluation of IGF-1 and myostatin protein levels, in both serum and tissue, induced by PGC-1α4 are essential, as the associated gene changes are less than robust.
In summary, Ruas et al. (2012) identified a novel isoform of PGC-1α that exerts a prohypertrophic function (Figure 1). More work is needed to delineate whether modulation of PGC-1α4 is an attractive therapeutic candidate for treatment of muscle loss in chronic diseases and aging. These therapies are especially necessary for those patients who are not able to exercise due to their physical condition. However, for relatively healthy individuals, it seems that a simple, old-fashioned exercise program is still the best approach to promote muscle growth.
References
- Bassel-Duby R, Olson EN. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Golbidi S, Mesdaghinia A, Laher I. Oxid Med Cell Longev. 2012;2012:349710. doi: 10.1155/2012/349710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBrasseur NK, Walsh K, Arany Z. Am J Physiol Endocrinol Metab. 2011;300:E3–E10. doi: 10.1152/ajpendo.00512.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, Michael LF, Puigserver P, Isotani E, Olson EN, et al. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Miura S, Kai Y, Kamei Y, Ezaki O. Endocrinology. 2008;149:4527–4533. doi: 10.1210/en.2008-0466. [DOI] [PubMed] [Google Scholar]
- Puigserver P, Rhee J, Lin J, Wu Z, Yoon JC, Zhang CY, Krauss S, Mootha VK, Lowell BB, Spiegelman BM. Mol Cell. 2001;8:971–982. doi: 10.1016/s1097-2765(01)00390-2. [DOI] [PubMed] [Google Scholar]
- Ruas JL, White JP, Rao RR, Kleiner S, Brannan KT, Harrison BC, Greene NP, Wu J, Estall JL, Irving BA, et al. Cell. 2012;151:1319–1331. doi: 10.1016/j.cell.2012.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Z, Okutsu M, Akhtar YN, Lira VA. J Appl Physiol. 2011;110:264–274. doi: 10.1152/japplphysiol.00993.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka T, Inagaki K, Noguchi T, Sakai M, Ogawa W, Hosooka T, Iguchi H, Watanabe E, Matsuki Y, Hiramatsu R, Kasuga M. Biochem Biophys Res Commun. 2009;381:537–543. doi: 10.1016/j.bbrc.2009.02.077. [DOI] [PubMed] [Google Scholar]