Abstract
Background
Menopausal status and use of hormonal contraception or menopausal hormone therapy (HT) may affect treatment response to selective serotonin reuptake inhibitors (SSRIs). This report evaluates whether menopausal status and use of hormonal contraceptives or menopausal HT affect outcome in women treated with citalopram.
Methods
In the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, 896 premenopausal and 544 postmenopausal women were treated with citalopram for 12–14 weeks. Baseline demographic and clinical characteristics were used in adjusted analysis of the effect of menopausal status and use of hormonal contraceptives or menopausal HT on outcomes. Remission was defined as final Hamilton Rating Scale for Depression-17 (HRSD17) ≤7 or Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR16) score ≤5 and response as ≥50% decrease from the baseline QIDS-SR16 score.
Results
Premenopausal and postmenopausal women differed in multiple clinical and demographic baseline variables but did not differ in response or remission rates. Premenopausal women taking hormonal contraceptives had significantly greater unadjusted remission rates on the HRSD17 and the QIDS-SR16 than women not taking contraception. Response and remission rates were not different between postmenopausal women taking vs. not taking HT. Adjusted results showed no significant difference in any outcome measure across menopause status in women who were not taking contraception/HT. There were no significant differences in adjusted results across HT status in premenopausal or postmenopausal women.
Conclusions
In this study, citalopram treatment outcome was not affected by menopausal status. Hormonal contraceptives and HT also did not affect probability of good outcome.
Introduction
Major depressive disorder (MDD) is approximately twice as common in women as in men and is ranked as the second leading cause of health-related disability in women.1,2 Among other factors, the presentation of MDD has been shown to be influenced by gender,3–5 menopausal status,6 and the presence of hormonal contraceptives7 or menopausal hormone therapy (HT).6
Research suggests that antidepressant response to some medications may differ by sex, age, or menopausal status.8–13 In particular, several studies have shown that selective serotonin reputable inhibitors (SSRIs) may be less effective for depression in older women compared to younger women or in postmenopausal women compared to premenopausal or perimenopausal women.10,11 In addition, some studies have reported that response to SSRIs in postmenopausal depressed women is enhanced when the women are also taking HT.10,14,15 There are no published data on the effect of hormonal contraceptives on antidepressant outcome in depressed women, although a study of women with premenstrual dysphoric disorder found no effect of concomitant use of oral contraceptives on sertraline response.16
The purpose of this report is to determine if treatment outcomes with the SSRI citalopram in women differ according to menopausal status or the presence of hormonal contraceptives or menopausal HT. Using data from the National Institute of Mental health (NIMH)-supported Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study, we compared response and remission rates between premenopausal and postmenopausal women with MDD who were not taking hormonal contraceptives or menopausal HT. We also evaluated differences between response and remission rates in premenopausal women who were and were not taking hormonal contraceptives and in postmenopausal women who were and were not taking HT.
Materials and Methods
Study overview and organization
The rationale, design, and methods of STAR*D have been detailed elsewhere.17,18 Briefly, STAR*D aimed to prospectively determine which of several treatments are most effective for outpatients with nonpsychotic MDD who have an unsatisfactory clinical outcome to an initial and, if necessary, subsequent treatment(s). Treatment was provided at 18 primary and 23 psychiatric care, public, or private sector settings.
Study population
To enhance generalizability, STAR*D enrolled only self-declared treatment-seeking outpatients 18–75 years of age, identified by their clinicians as having nonpsychotic MDD requiring treatment. Advertising for symptomatic volunteers was proscribed. Broadly inclusive entry criteria were used.17,18 Patients were eligible if they met DSM-IV criteria for single or recurrent nonpsychotic MDD (established by treating clinician and confirmed by a DSM-IV checklist), scored ≥14 (moderate severity) on the clinical research coordinator (CRC)-rated 17-item Hamilton Rating Scale for Depression (HRSD17),19 and were not treatment resistant to an adequate antidepressant treatment trial during the current MDD episode.20 Exclusion criteria are detailed elsewhere.17,18
All risks, benefits, and adverse events associated with STAR*D participation were explained to participants, who provided written informed consent before study entry. The STAR*D protocol was developed in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Boards at the national and data coordinating centers and the respective regional centers and clinical sites.
Baseline measures
At baseline, trained CRCs based at each site collected standard demographic information, self-reported psychiatric history, and current general medical comorbidites as evaluated by the Cumulative Illness Rating Scale (CIRS),21 which was completed using a manual to guide scoring.22 CRCs administered the initial HRSD17 and assessed depressive symptoms using the 16-item Quick Inventory of Depressive Symptomatology-Clinician-Rated (QIDS-C16).23,24 The participant completed the 16-item Quick Inventory of Depressive Symptomatology-Self-Report (QIDS-SR16)23,24 for assessment of depressive symptoms. Participants also completed the Psychiatric Diagnostic Screening Questionnaire (PDSQ)25 to estimate the presence of 11 potential concurrent DSM-IV psychiatric disorders. Based on prior reports,26 we defined the presence of concomitant axis I disorders using thresholds with a 90% specificity in relation to the gold standard diagnosis rendered by a structured interview.
Research outcomes assessors (ROAs) masked to treatment and not located at any clinical site collected the HRSD17 and the 30-item Inventory of Depressive Symptomatology-Clinician-Rated (IDS-C30)24,27,28 by telephone interview within 72 hours of study entry for assessment of depressive symptoms. Responses to items on these measures were used to estimate the presence of atypical,29 melancholic,30 and anxious31 symptom features.
A telephone-based Interactive voice response system32,33 collected health perceptions via the 12-item Short Form health survey (SF-12),34 and quality of life via the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q)35 and the Work and Social Adjustment Scale (WSAS).36
Course of treatment measures
An integral part of our measurement-based care intervention20,37 was the collection of clinically relevant information at each clinic visit to inform treatment decision making. Depressive symptom severity was obtained at each clinic visit with the QIDS-SR16 and the QIDS-C16. Side effects were assessed using the Frequency, Intensity, and Burden of Side Effects Rating Scale (FIBSER),38 which uses three 7-point subscales to evaluate frequency, intensity, and global burden measures, respectively.
Intervention
Treatment consisted of up to 14 weeks of citalopram in the first step of STAR*D, with the aim of reaching symptom remission, defined as a QIDS-C16 score ≤5 or an HRSD17 score <7. The protocol18 required a fully adequate dose of citalopram for a sufficient time to maximize the likelihood of achieving remission and ensure that participants who did not remit were truly resistant to the medication. Dose adjustments were guided by recommendations in a treatment manual (<www.star-d.org>). Individualized starting doses and dose adjustments were used to minimize side effects, maximize safety, and optimize the chances of therapeutic benefit for each participant. Citalopram was to begin at 20 mg/day and be raised to 40 mg/day by weeks 2–4 and to 60 mg/day (final dose) by weeks 4–6. Dose adjustments were guided by symptom changes based on the QIDS-C16, and side effect burden was based on the FIBSER and on how long a participant had received a particular dose.
The protocol recommended treatment visits at weeks 0 (baseline), 2, 4, 6, 9, and 12 (with an optional week 14 visit if needed). After an optimal trial (based on dose and duration), participants who reached remission (QIDS-C16 <5) or response (response defined as an improvement ≥50% over the baseline QIDS-C16 score without remission) could enter a 12-month naturalistic follow-up, but all who did not reach remission were encouraged to enter the subsequent randomized trial (Level 2 of STAR*D). Participants could discontinue citalopram before 12 weeks if (1) intolerable side effects required a medication change, (2) an optimal dose increase was not possible because of side effects or participant choice, or (3) significant symptoms (QIDS-C16 score ≥9) were present after 9 weeks at maximally tolerated doses. Participants could opt to move to the next treatment level if they had intolerable side effects or if their QIDS-C16 score was >5 after an adequate trial in terms of dose and duration. Intensive efforts were made to provide consistent high-quality care, including the use of a treatment manual, initial didactic instruction, ongoing support and guidance by the CRC, the use of the QIDS-C16 and the FIBSER at each visit, and a centralized treatment monitoring and feedback system (<www.star-d.org>).20,39
Safety assessments
Side effects were monitored clinically. Serious adverse events (SAEs) were monitored using a multitiered approach39 that involved the CRCs, study clinicians, the interactive voice response system, the clinical manager, safety officers, regional center directors, and the NIMH data safety and monitoring board.
Concomitant medications
Concomitant treatments for current medical illnesses, associated symptoms of depression (e.g., sleep and agitation), and citalopram side effects were permitted at study entry and during the treatments, based on clinical judgment.
Main outcome measures
The primary outcome measure was the HRSD17 collected by ROAs using telephone-based structured interviews at entry and exit from citalopram treatment. The secondary outcomes included the QIDS-SR16 and FIBSER collected at baseline and at each treatment visit.
Group assignment/definition of menopausal status
Participants were divided into three groups, premenopausal, perimenopausal, and postmenopausal, according to (1) age and (2) self-report. Menopausal status was determined based on the participant's response to the following question: Is the patient postmenopausal or posthysterectomy or male? A woman was classified as premenopausal if she was <40 years of age. Of those classified as premenopausal, 49 (4.74 %) answered yes to the above question (indicating that they were postmenopausal or posthysterectomy). These women were still included in the premenopausal group because of the very low prevalence (1%–2%) of naturally occurring menopause in women <40 years old. Those in this age group who were posthysterectomy but still had ovaries were likely to still be premenopausal, and those who also had both ovaries removed were likely to be taking menopausal HT and would thus be excluded from the analyses. Women who were at least 40 years old and who reported not being postmenopausal or posthysterectomy were classified as perimenopausal. Finally, women who were at least 40 years old and reported being postmenopausal or posthysterectomy were operationally defined as postmenopausal. A small number of these women may have had a hysterectomy without ovariectomy and were still cycling. Women who either had experienced natural menopause or had undergone a hysterectomy were included in the study, with the exception of those women on HT. Concomitant medications were assessed at baseline and at every study visit. We reviewed the list of medications reported by each participant in order to determine which participants were taking either hormonal contraceptives of any kind, including parenteral formulations, or menopausal HT.
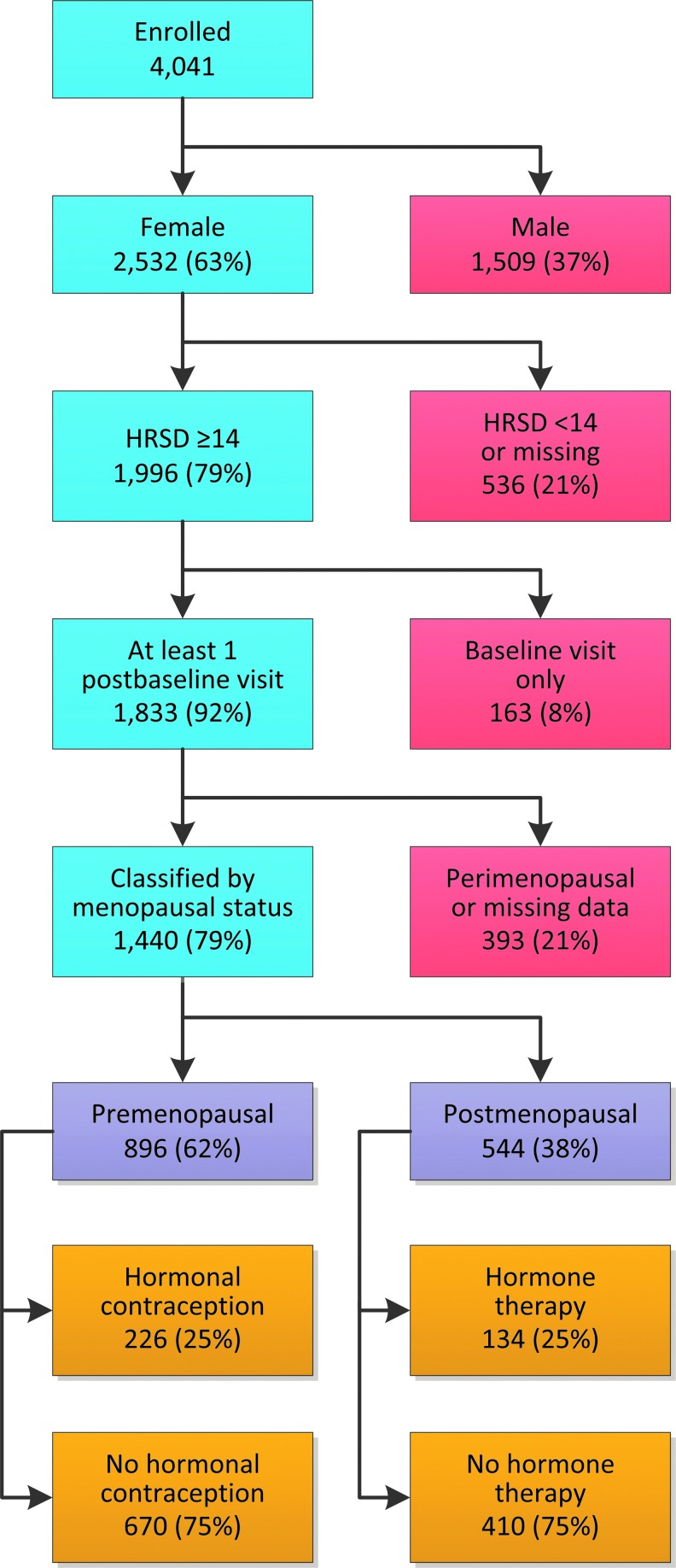
Of the 4041 participants enrolled in the STAR*D study, 62.7% were women (n=2532). The study sample consists of all women who enrolled in STAR*D, completed one return visit, and had a baseline ROA call to obtain the primary outcome measure, HRSD17.20 Of the 2532 women enrolled, 1996 (79%) met the HRSD17 inclusion criteria, and 1833 (92%) completed at least one return visit. Of the analyzable sample (n=1883), 393 (21%) were classified as perimenopausal or were not classified and were excluded from the analysis because of missing data. Of the remaining 1440 (76%) women, 896 (62%) were classified as premenopausal, and 544 (38%) were classified as postmenopausal. Of the 896 premenopausal women, 226 (25%) were taking hormonal contraceptives. Of the 544 postmenopausal women, 134 (25%) were taking menopausal HT. Figure 1 is a graphic representation of study flow.
FIG. 1.
Consort chart. Distribution of menopausal status and hormone therapy. Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. HRSD, Hamilton Rating Scale for Depression.
Administration of hormonal medications, such as hormonal contraceptives and menopausal HT, could affect the presentation of depressive and menopausal symptoms as well as treatment response. Because of the variation in hormonal medications taken and their unknown effects, we excluded women taking these medications (n=360) from the analyses comparing premenopausal and postmenopausal women. Thus, the analyses regarding effect of menopausal status on treatment response were conducted with a sample of 1080 women. Of these, 670 (62%) were classified as premenopausal, and 410 (38%) were classified as postmenopausal.
Statistical analysis
Remission was defined as an exit HRSD17 score ≤7 (or last observed QIDS-SR16 score ≤5). As defined by the original proposal, participants for whom the exit HRSD17 score was missing were designated as not reaching remission. Response was defined as a reduction of ≥50% from the baseline QIDS-SR16 at the last assessment. Intolerance was defined a priori as either leaving treatment before 4 weeks or leaving at or after 4 weeks, with intolerance as the identified reason. The alpha level was set at 0.05 (two-sided). No adjustments were made for multiple comparisons, so results must be interpreted accordingly.
Summary statistics are presented as means and standard deviations (SD) for continuous variables and percentages for discrete variables. Student's t tests and Mann-Whitney U tests were used to compare continuous baseline clinical and demographic features, treatment features, and side effect and SAE rates between premenopausal women (not taking hormonal contraceptives) and postmenopausal women (not taking menopausal HT), as well as between premenopausal women taking vs. not taking hormonal contraceptives and between postmenopausal women taking vs. not taking menopausal HT. Chi-square tests compared discrete characteristics.
Logistic regression models were used to compare remission and response rates after adjusting for factors shown to differ between the groups at baseline. Kaplan-Meier curves were used to present the cumulative probability of first remission and cumulative probability of first response, both measured using the QIDS-SR16. Log-rank statistics were used to test if there was a statistically significant difference in the cumulative proportions.
Results
Baseline characteristics
Table 1 shows the sociodemographic characteristics of premenopausal women (not taking hormonal contraceptives) vs. postmenopausal women (not taking menopausal HT), as well as for premenopausal women by hormonal contraceptive status and for postmenopausal women by HT status. Postmenopausal women were older, more likely to be divorced or widowed, less educated, and less likely to be employed and had a lower household income than premenopausal women. Among all premenopausal women, those taking hormonal contraceptives were younger, more educated, more likely to be single, and more likely to have private insurance. Among all postmenopausal women, those taking menopausal HT were more likely to be white and less likely to be black or Hispanic and more likely to be married and were more educated and had a higher household income.
Table 1.
Demographic Measures by Menopausal and Hormone Status
| |
Premenopausal |
Postmenopausal |
p |
||||
|---|---|---|---|---|---|---|---|
| Measure | +HC (n=226) | −HC (n=670) | +HT (n=134) | −HT (n=410) | +HC vs. −HC within pre | +HT vs. −HT within post | Pre vs. Post No HC/HT |
| Age | 27.2±5.8 | 29.8±5.7 | 54.7±8.1 | 54.3±7.6 | <0.01 | 0.61 | <0.01 |
| Race | 0.09 | 0.02 | 0.09 | ||||
| White | 174 (77.0) | 464 (69.4) | 111 (82.8) | 292 (71.4) | |||
| Black | 35 (15.5) | 141 (21.1) | 21 (15.7) | 93 (22.7) | |||
| Other | 17 (7.5) | 64 (9.6) | 2 (1.5) | 24 (5.9) | |||
| Hispanic | 26 (11.5) | 112 (16.7) | 14 (10.4) | 86 (21.0) | 0.06 | <0.01 | 0.08 |
| Education, years | 14.0±3.1 | 13.4±2.9 | 13.2±3.7 | 12.3±3.9 | 0.01 | 0.02 | <.001 |
| Employment status | 0.24 | 0.14 | <0.01 | ||||
| Employed | 153 (67.7) | 415 (62.0) | 64 (47.8) | 175 (42.8) | |||
| Unemployed | 73 (32.3) | 252 (37.7) | 49 (36.6) | 187 (45.7) | |||
| Retired | 0 (0.0) | 2 (0.3) | 21 (15.7) | 47 (11.5) | |||
| Monthly household income | 2684±3410 | 2209±2597 | 2166±2072 | 1928±2688 | 0.09 | 0.04 | <0.01 |
| Medical insurance | 0.05 | 0.07 | 0.04 | ||||
| Any private | 134 (60.6) | 327 (51.0) | 78 (59.1) | 189 (47.8) | |||
| Public only | 27 (12.2) | 102 (15.9) | 21 (15.9) | 88 (22.3) | |||
| None | 60 (27.1) | 212 (33.1) | 33 (25.0) | 118 (29.9) | |||
| Marital status | <0.01 | 0.02 | <0.01 | ||||
| Never married | 113 (50.0) | 265 (39.6) | 7 (5.2) | 49 (12.0) | |||
| Married/cohabiting | 88 (38.9) | 268 (40.0) | 60 (44.8) | 139 (34.0) | |||
| Divorced/separated | 25 (11.1) | 133 (19.9) | 57 (42.5) | 169 (41.3) | |||
| Widowed | 0 (0.0) | 4 (0.6) | 10 (7.5) | 52 (12.7) | |||
HC, hormonal contraceptive; HT, hormone therapy; Post, postmenopausal; Pre, premenopausal.
Table 2 shows clinical measures at baseline. Excluding those taking hormonal contraceptives or menopausal HT, postmenopausal women had an older age at first episode, longer duration of illness, fewer episodes, a longer duration of index episode, and greater likelihood of chronicity than premenopausal women. They also had lower QIDS-SR16 scores, more anxious features, and higher mental but lower physical subscale scores on the SF-12. They were less likely to have a family history of depression and less likely to have attempted suicide and had less psychiatric but more general medical comorbidities. Among all premenopausal women, those taking hormonal contraceptives had a lower age at first episode, a lower HRSD17 score, lower QIDS-SR16 scores, higher Q-LES-Q scores, higher SF-12 physical subscale scores, and lower WSAS scores. Among all postmenopausal women, those taking HT had a greater number of episodes, less anxious features, and higher physical functioning on the SF-12.
Table 2.
Clinical Measures by Menopausal and Hormone Status
| |
Premenopausal |
Postmenopausal |
p |
||||
|---|---|---|---|---|---|---|---|
| Measure | +HC (n=226) | −HC (n=670) | +HT (n=134) | −HT (n=410) | +HC vs. −HC within Pre | +HT vs. −HT within Post | Pre vs. Post No HC/HT |
| Age at first episode | 17.0±7.0 | 18.7±7.9 | 32.2±16.7 | 34.5±17.6 | <0.01 | 0.19 | <0.01 |
| Age at first episode <18 | 137 (60.6) | 338 (51.3) | 34 (25.8) | 93 (22.8) | 0.02 | 0.49 | <0.01 |
| Duration of illness, years | 10.3±7.8 | 11.1±8.0 | 22.7±16.6 | 19.9±16.6 | 0.16 | 0.06 | <0.01 |
| Number of episodes | 4.2±5.6 | 4.6±7.7 | 6.4±11.4 | 4.2±8.7 | 0.93 | <0.01 | <0.01 |
| At least 1 prior episode | 165 (78.9) | 483 (77.7) | 104 (81.9) | 257 (69.6) | 0.70 | <0.01 | <0.01 |
| Family history of depression | 148 (65.8) | 392 (59.0) | 76 (56.7) | 209 (51.2) | 0.07 | 0.27 | 0.01 |
| Ever attempted suicide | 53 (23.5) | 169 (25.3) | 21 (15.7) | 57 (13.9) | 0.59 | 0.61 | <0.01 |
| No. of psychiatric comorbiditiesa | 0.06 | 0.47 | <0.01 | ||||
| 0 | 80 (35.6) | 184 (28.0) | 58 (44.3) | 143 (36.2) | |||
| 1 | 59 (26.2) | 151 (23.0) | 37 (28.2) | 118 (29.9) | |||
| 2 | 41 (18.2) | 132 (20.1) | 12 (9.2) | 52 (13.2) | |||
| 3 | 19 (8.4) | 78 (11.9) | 11 (8.4) | 33 (8.4) | |||
| 4+ | 26 (11.6) | 112 (17.0) | 13 (9.9) | 49 (12.4) | |||
| No. of general medical comorbiditiesb | 0.26 | 0.18 | <0.01 | ||||
| 0 | 151 (66.8) | 409 (61.0) | 24 (17.9) | 115 (28.0) | |||
| 1 | 44 (19.5) | 135 (20.1) | 39 (29.1) | 95 (23.2) | |||
| 2 | 17 (7.5) | 82 (12.2) | 25 (18.7) | 78 (19.0) | |||
| 3 | 10 (4.4) | 25 (3.7) | 20 (14.9) | 56 (13.7) | |||
| 4+ | 4 (1.8) | 19 (2.8) | 26 (19.4) | 66 (16.1) | |||
| Duration of index episode, months | 16.8±30.8 | 20.4±43.2 | 31.9±58.4 | 31.7±59.9 | 0.30 | 0.80 | <0.01 |
| Duration of index episode ≥2 years | 45 (20.0) | 130 (19.6) | 47 (35.1) | 146 (35.9) | 0.90 | 0.87 | <0.01 |
| HRSD17 | 22.8±4.5 | 24.1±5.2 | 23.6±5.1 | 24.1±5.2 | <0.01 | 0.27 | 0.87 |
| QIDS-C16 | 12.4±4.3 | 12.6±4.5 | 12.9±4.6 | 13.1±4.5 | 0.53 | 0.59 | 0.05 |
| QIDS-SR16 | 16.2±3.7 | 17.0±3.9 | 15.7±4.3 | 16.4±4.1 | <0.01 | 0.10 | 0.01 |
| Anxious features (HRSD) | 113 (50.0) | 358 (53.4) | 68 (50.7) | 261 (63.7) | 0.37 | <0.01 | <0.01 |
| Atypical features (IDS-C) | 51 (22.6) | 155 (23.1) | 22 (16.4) | 78 (19.0) | 0.86 | 0.50 | 0.11 |
| Melancholic features (IDS-C) | 46 (20.4) | 170 (25.4) | 21 (15.7) | 91 (22.2) | 0.13 | 0.10 | 0.24 |
| Q-LES-Q | 42.7±13.1 | 38.2±14.2 | 40.9±15.6 | 38.2±15.3 | <0.01 | 0.07 | 0.94 |
| SF-12 Mental | 23.6±7.7 | 23.8±7.7 | 27.6±8.6 | 27.7±8.8 | 0.84 | 0.87 | <0.01 |
| SF-12 Physical | 54.5±9.3 | 51.6±10.5 | 45.5±12.2 | 42.5±11.8 | <0.01 | 0.01 | <0.01 |
| WSAS | 23.3±8.2 | 25.5±8.6 | 24.6±9.1 | 25.1±9.8 | <0.01 | 0.55 | <0.01 |
From the Psychiatric Diagnostic Screening Questionnaire (PDSQ).
From the Cumulative Illness Rating Scale (CIRS).
HRSD, Hamilton Rating Scale For depression; IDS-C, clinician-rated Inventory of Depressive Symptomatology; QIDS-C, -SR Clinician, Self-Rated, Quick Inventory of Depressive Symptomatology; Q-LES-Q, Quality of Life Enjoyment and Satisfaction Questionnaire; SF, Short-form health survey; WSAS, Work and Social Adjustment Scale.
Treatment characteristics and outcome
Treatment characteristics are shown in Table 3. Excluding those taking hormonal contraceptives or menopausal HT, postmenopausal women were less likely than premenopausal women to be seen in primary care settings. Postmenopausal women were also less likely to drop out of treatment before week 8, achieved a higher citalopram dose, and were less likely to develop a psychiatric SAE. Among all premenopausal women, those taking hormonal contraceptives were less likely to drop out of treatment before week 8 and reached a higher citalopram dose than those not taking contraceptives. Among all postmenopausal women, there were no differences in treatment characteristics by HT status.
Table 3.
Treatment Measures by Menopausal and Hormone Status
| |
Premenopausal |
Postmenopausal |
p |
||||
|---|---|---|---|---|---|---|---|
| Measure | +HC (n=226) | −HC (n=670) | +HT (n=134) | −HT (n=410) | +HC vs. −HC within Pre | +HT vs. −HT within Post | Pre vs. Post No HC/HT |
| Psychiatric care | 153 (67.7) | 450 (67.2) | 60 (44.8) | 178 (43.4) | 0.88 | 0.78 | <0.01 |
| Weeks in treatment | 10.6±4.1 | 9.7±4.5 | 9.9±4.3 | 10.3±4.2 | 0.02 | 0.24 | 0.02 |
| <4 | 22 (9.7) | 97 (14.5) | 18 (13.4) | 46 (11.2) | 0.07 | 0.49 | 0.13 |
| <8 | 49 (21.7) | 228 (34.0) | 38 (28.4) | 106 (25.9) | <0.01 | 0.57 | <0.01 |
| No. of postbaseline visits | 4.1±1.5 | 3.6±1.6 | 3.9±1.5 | 3.9±1.6 | <0.01 | 0.73 | <0.01 |
| Weeks to first postbaseline visit | 2.3±1.0 | 2.4±1.1 | 2.4±1.1 | 2.4±1.2 | 0.15 | 0.79 | 0.42 |
| Maximum citalopram dose | 43.5±16.0 | 39.7±15.9 | 40.6±17.2 | 42.0±16.9 | <0.01 | 0.46 | 0.03 |
| Exit citalopram dose | 43.5±16.0 | 39.7±15.9 | 40.6±17.2 | 42.0±16.9 | <0.01 | 0.46 | 0.03 |
| Weeks on exit citalopram dose | 2.8±1.9 | 2.6±2.2 | 2.6±1.5 | 2.7±2.0 | 0.13 | 0.84 | 0.51 |
| Maximum SE frequencya | 0.62 | 0.41 | 0.25 | ||||
| No side effects | 26 (13.1) | 93 (16.6) | 16 (15.2) | 72 (20.3) | |||
| 10%–25% of the time | 59 (29.6) | 159 (28.4) | 28 (26.7) | 108 (30.5) | |||
| 50%–75% of the time | 64 (32.2) | 182 (32.6) | 32 (30.5) | 96 (27.1) | |||
| 90%–100% of the time | 50 (25.1) | 125 (22.4) | 29 (27.6) | 78 (22.0) | |||
| Maximum SE intensitya | 0.68 | 0.72 | 0.17 | ||||
| No side effects | 26 (13.1) | 93 (16.6) | 16 (15.2) | 70 (19.8) | |||
| Minimal to mild | 57 (28.6) | 157 (28.1) | 30 (28.6) | 103 (29.1) | |||
| Moderate to marked | 86 (43.2) | 233 (41.7) | 41 (39.0) | 123 (34.7) | |||
| Severe to intolerable | 30 (15.1) | 76 (13.6) | 18 (17.1) | 58 (16.4) | |||
| Maximum SE burdena | 0.21 | 0.15 | 0.08 | ||||
| No side effects | 35 (17.6) | 130 (23.3) | 18 (17.1) | 98 (27.7) | |||
| Minimal to mild | 95 (47.7) | 223 (39.9) | 43 (41.0) | 126 (35.6) | |||
| Moderate to marked | 58 (29.1) | 171 (30.6) | 35 (33.3) | 96 (27.1) | |||
| Severe to intolerable | 11 (5.5) | 35 (6.3) | 9 (8.6) | 34 (9.6) | |||
| Exited level due to intoleranceb | 32 (14.2) | 119 (17.8) | 25 (18.7) | 69 (16.8) | 0.21 | 0.63 | 0.70 |
| At least 1 SAE | 8 (3.5) | 24 (3.6) | 4 (3.0) | 13 (3.2) | 0.98 | 1.00 | 0.72 |
| At least 1 psychiatric SAE | 5 (2.2) | 16 (2.4) | 1 (0.7) | 3 (0.7) | 0.88 | 1.00 | 0.04 |
From the Frequency, Intensity, and Burden of Side Effects Rating Scale (FIBSER).
Exited prior to week 4 for any reason or after week 4 citing intolerable side effects.
SAE, serious adverse event; SE, side effect.
Remission rates for each treatment group are shown in Table 4. Excluding those who were taking hormonal contraceptives or menopausal HT, 28.4% (190 of 670) of premenopausal women and 24.6% (101 of 410) of postmenopausal women remitted based on HDRS17 scores. Using the QIDS-SR16, the remission rates were 32.8% (220 of 670) for premenopausal and 28.6% (117 of 409) for postmenopausal women. Examining premenopausal women by hormonal contraceptive status using the HDRS17, we found that 36.3% (82 of 226) of women who were taking contraceptives remitted compared to 28.4% (190 of 670) of women not taking contraceptives. Based on QIDS-SR16 criteria, 41.2% (93 of 226) of premenopausal women taking hormonal contraceptives and 32.8% (220 of 670) of premenopausal women not taking contraceptives remitted. Among postmenopausal women examined by HT status, 29.1% (39 of 134)) of women taking HT remitted on the HDRS17 compared to 24.6% (101 of 410) of women not taking HT. Applying QIDS-SR16 criteria, remission rates were 35.8% (48 of 134) for women taking HT and 28.6% (117 of 409) for those not taking HT.
Table 4.
Remission Rates by Menopause and Hormone Therapy Status
| |
Within premenopausal |
Within postmenopausal |
||||||
|---|---|---|---|---|---|---|---|---|
| |
+HC (n=226) |
−HC (n=670) |
+HT (n=134) |
−HT (n=410) |
||||
| Remission | n | % | n | % | n | % | n | % |
| HRSD17 | 82 | 39.3 | 190 | 28.4 | 39 | 29.1 | 101 | 24.6 |
| QIDS-SR16 | 93 | 41.2 | 220 | 32.8 | 48 | 35.8 | 117 | 28.6 |
Q-LES-Q, Quality of Life Enjoyment and Satisfaction Questionnaire.
Treatment outcome measures from the unadjusted and adjusted models are shown in Table 5. There were no outcome differences between premenopausal and postmenopausal women, with the exception that premenopausal women showed an increase in percent change on the QIDS-SR16. Among premenopausal women, those taking hormonal contraceptives showed significantly greater remission rates, as measured by both the HRSD17 (primary outcome) and the QIDS-SR16, and lower exit QIDS-SR16 scores. Postmenopausal women taking HT showed similar response and remission rates on both the HRSD17 and the QIDS-SR16 compared to those not taking HT. Because the groups differed on a number of baseline variables, adjusted analyses were performed for remission and response rates. Adjustments included baseline demographic and clinical features that differed significantly between groups at baseline (e.g., race, marital status, family history, suicide attempts, number of episodes, and baseline QIDS-SR16), with the exception of age. Table 4 shows specific adjustment factors for each analysis. After adjustment, there was no significant difference in HRDS17 remission or QIDS-SR16 response or remission rate between premenopausal and postmenopausal women. There was also no significant difference in QIDS-SR16 exit score or percent change. When compared by hormonal contraceptive status, premenopausal women showed no differences in remission rates on the HRDS17 and QIDS-SR16 or in response rates, exit scores, or percent change on the QIDS-SR16. Similarly, postmenopausal women did not show significant differences in remission rates on the HRDS17 and QIDS-SR16 or in response rates, exit scores, or percent change on the QIDS-SR16 when compared by HT status.
Table 5.
Unadjusted and Adjusted Outcomes by Menopausal and Hormone Therapy Status
| |
+HT vs. −HT |
Post vs. Pre |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Within Pre |
Within Post |
Within −HT |
|||||||||||||||
| |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
||||||||||||
| Outcome | OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p | OR | (95% CI) | p |
| HRSD17 remission | 1.439 | (1.046-1.979) | 0.03 | 1.194a | (0.908-1.831) | 0.31 | 1.256 | (0.813-1.941) | 0.30 | 1.004e | (0.629-1.604) | 0.99 | 0.826 | (0.624-1.093) | 0.18 | 1.145i | (0.818-1.602) | 0.43 |
| QIDS-SR16 remission | 1.430 | (1.049-1.950) | 0.02 | 1.168b | (0.903-1.874) | 0.37 | 1.393 | (0.922-2.106) | 0.12 | 1.148f | (0.738-1.787) | 0.54 | 0.820 | (0.627-1.072) | 0.15 | 1.226j | (0.823-1.825) | 0.32 |
| QIDS-SR16 response | 1.346 | (0.993-1.823) | 0.06 | 1.164c | (0.845-1.603) | 0.35 | 1.021 | (0.690-1.512) | 0.92 | 0.855f | (0.563-1.297) | 0.46 | 0.818 | (0.639-1.047) | 0.11 | 1.245k | (0.901-1.720) | 0.18 |
| |
+HT vs. −HT |
Post vs. Pre |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Within Pre |
Within Post |
Within −HT |
|||||||||||||||
| |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
Unadjusted |
Adjusted |
||||||||||||
| Outcome | β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p | β | (95% CI) | p |
| QIDS-SR16 exit score | −1.146 | (−2.059-−0.234) | 0.01 | −0.525d | (−1.370-0.320) | 0.62 | −0.576 | (−1.705-0.554) | 0.32 | 1.070g | (−0.032-2.172) | 0.06 | 0.392 | (−0.342-1.126) | 0.29 | −0.632l | (−1.599-0.336) | 0.20 |
| QIDS-SR16 % change | −4.162 | (−9.468-1.144) | 0.12 | −1.726d | (−8.243-2.355) | 0.54 | −1.528 | (−8.198-5.143) | 0.65 | 5.801h | (−1.057-12.66) | 0.10 | 4.844 | (0.567-9.121) | 0.03 | −4.541m | (−10.56-1.477) | 0.14 |
Adjusted for
Education, number of psychiatric comorbidities, Q-LES-Q, SF-12 Physical, and QIDS-C16.
Recurrent depression, number of psychiatric comorbidities, Q-LES-Q, SF-12 Physical, and QIDS-C16.
Number of psychiatric comorbidities, Q-LES-Q, and SF-12 Physical.
Education, recurrent depression, number of psychiatric comorbidities, number of general medical comorbidities, Q-LES-Q, SF-12 Physical, WSAS, and QIDS-C16.
Education, number of general medical comorbidities, and SF-12 Physical.
Number of general medical comorbidities and SF-12 Physical.
Education, number of episodes, number of psychiatric comorbidities, number of general medical comorbidities, SF-12 Mental and physical, QIDS-C16, and anxious features.
Education, number of episodes, number of general medical comorbidities, SF-12 Physical, QIDS-C16, and anxious features.
Education, number of psychiatric comorbidities, number of general medical comorbidities, SF-12 Physical, QIDS-C16, and anxious features.
Education, age at first episode, number of episodes, recurrent depression, number of general medical comorbidities, SF-12 Physical, and QIDS-C16.
Education, number of episodes, recurrent depression, number of general medical comorbidities, SF-12 Physical, QIDS-C16, and anxious features.
Education, age at first episode, number of episodes, number of psychiatric comorbidities, number of general medical comorbidities, Q-LES-Q, SF-12 Physical, QIDS-C16, and anxious features.
Education, age at first episode, number of episodes, recurrent depression, number of general medical comorbidities, SF-12 Physical, QIDS-C16, and anxious features.
CI, confidence interval; OR, odds ratio.
Discussion
In this study, clinical outcomes in depressed women taking citalopram were unrelated to either menopausal or prescribed hormone status. Although we did find a trend in the unadjusted models toward higher response and remission rates in premenopausal compared to postmenopausal women, the differences were not statistically significant, with the exception of a greater percent change in premenopausal women on the QIDS-SR16. We did find significantly greater remission rates in the unadjusted models between premenopausal women who were taking hormonal contraceptives compared to those who were not, as measured by both the HRSD17 and the QIDS-SR16, as well as lower exit QIDS-SR16 scores. Among postmenopausal women, we did not find that HT caused any outcome differences in unadjusted or adjusted models. When adjusted for demographic and clinical differences between groups, outcome differences were no longer statistically significant. These results suggest that, overall, neither menopausal status nor hormonal contraception or menopausal HT status impacts outcomes of depression treatment with citalopram.
These findings are in contrast to several previous results.10,11,14,15 Thase et al.10 examined antidepressant outcomes by age, sex, and menopausal HT use in a large pooled dataset. They found poorer SSRI response among older women compared to younger women; however, concomitant HT use appeared to eliminate this difference. In a study by Pinto-Meza et al.11 of women patients taking SSRIs in primary care practices, postmenopausal women showed worse treatment response and poorer self-evaluation of global health status compared to premenopausal women. Schneider et al.14 and Zanardi et al.15 performed post-hoc analyses of older (postmenopausal) women taking SSRIs and found that those taking concomitant HT had higher rates of remission. However, the Zanardi sample included women with bipolar disorder taking mood stabilizers as well as unipolar patients and is, thus, difficult to compare directly to the STAR*D sample. The Schneider et al. analysis included only estrogen therapy—women taking formulations containing progesterone were excluded—raising the possibility that this accounted for the difference in results. None of these analyses were adjusted for sociodemographic and baseline differences that may have contributed to outcomes.
Our findings suggest that previously seen differences in outcomes with SSRIs based on menopausal status or HT may be due to other factors. Premenopausal and postmenopausal women differ not just in hormonal status and age but also in numerous social and biologic ways that shift over the life span. Many specific stresses that impact depression, such as childrearing and widowhood, vary across age. Also, we know that many of the diseases of aging, such as cardiovascular disease, may affect or even cause depression, which appears to be different neurobiologically than depression in younger patients. In addition, our group found within the same sample a range of differences in presentation of depression between premenopausal and postmenopausal women.6 Perhaps these differences may account for the differences found in other studies.
This is the first published report, to our knowledge, on the effect of hormonal contraceptives on antidepressant response in depressed women. Contraceptives differ in formulation, particularly in estrogen/progesterone ratio, from menopausal HT and may have had different effects on mood and antidepressant outcome. Sex hormones are known to exert effects on monoamines as well as the hypothalamic-pituitary-adrenal (HPA) axis, both key factors in mood regulation. Differences in sex hormones are often used to explain the higher overall rates of depression in women and the fact that the gender difference in prevalence of MDD appears at puberty. Our finding that treatment outcome was similar in women taking and not taking hormonal contraceptives, however, suggests that clinicians should view clinical decision making for depression in premenopausal women independent of that for contraception. Similarly, our finding of no significant difference in outcomes in postmenopausal women across HT status in a large sample and controlling for group differences suggests that MDD should not be considered a clinical indication for adding HT in postmenopausal depressed women. Nevertheless, there may be a subgroup of women whose depression is particularly sensitive to hormonal fluctuations (e.g., history of postpartum depression, history of premenstrual dysphoric disorder [PMDD]) for whom HT may improve treatment outcome; this should be the subject of future research.
Several limitations should be noted. The sample consisted of outpatients with nonpsychotic MDD and, therefore, may not be generalizable to all depressed women. Another limitation was the definition of menopausal status used, which was based on a combination of age and a question about menopause status and hysterectomy status. More detailed questions about irregular or skipped menstrual cycles and timing of cessation of menses would have allowed more accurate classification of menopausal status. Because this study was not randomized, we cannot well differentiate between effects caused by medications and those resulting from social or behavioral factors. Finally, there may be differences in the type of hormones prescribed for contraception that could affect outcomes and were not considered here; this is particularly true for contraceptive types that work through means other than ovarian suppression, such as intrauterine devices.
Further research in this area could clarify some of these issues. Although the STAR*D sample is large and reasonably well characterized and these secondary analyses were conducted to minimize confounding differences, future studies should more specifically characterize hormonal status in order to confirm these findings. The STAR*D sample used age and self-reported menopausal status and did not confirm reports with thorough clinical interview or laboratory values, which would have provided better sample characterization. Although there are potential ethical and practical issues in the design of such studies, the effect of hormonal contraceptives and menopausal HT on depression treatment outcomes could be tested prospectively. Specifically, premenopausal depressed women could be randomized to hormonal contraception or placebo as an adjunctive treatment (while using other methods of contraception). Similarly, postmenopausal depressed women could be randomized to HT or placebo as an adjunctive treatment. Such studies would provide high-quality evidence for or against efficacy by removing biases in the sample affecting women's likelihood of use of contraceptives or HT. Alternatively, studies comparing two or more types of antidepressant treatments or different formulations of hormonal contraceptives or menopausal HT could indicate specific medications or combinations to be further pursued or avoided depending on menopausal status. Finally, it is possible that careful collection of historical or biologic data could reveal a subgroup of women whose depression is responsive to adjunctive HT.
Acknowledgments
This project was funded by the National Institute of Mental Health under Contract No. N01MH90003 to UT Southwestern Medical Center at Dallas (Pls: A.J.R. and M.H.T.). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. We appreciate the support of Bristol-Myers Squibb, Forest Laboratories, GlaxoSmithKline, King Pharmaceuticals, Organon, Pfizer, and Wyeth for providing medications at no cost for this trial.
Disclosure Statement
M.T. and J.L. have no conflicts of interest to report. S.G.K. has received grants/research support from the National Institute of Mental Health; Bristol-Myers Squibb; Lilly; Euthymics; Forest Laboratories; Wyeth; Novartis; Boehringer-Ingelheim; Pfizer; Otsuka; Rexahn. She has been on the advisory board or received honoraria from Pfizer; Lilly; Bristol-Myers Squibb; Forest Laboratories; Dey Pharma; Rexahn; PGxHealth; Wyeth; Takeda; Trovis; Lundbeck. She has also received book royalties from Guilford Press.
A.J.R. has received consultant fees from Advanced Neuromodulation Systems; AstraZeneca; Best Practice Project Management; Brain Resource Inc.; Bristol-Myers Squibb/Otsuka; Cyberonics; Forest Pharmaceuticals; Gerson Lehrman Group; GlaxoSmithKline; Jazz Pharmaceuticals; Magellan Health Services; Merck & Company; Neuronetics; Novartis Pharmaceuticals; Ono Pharmaceuticals; Organon; Otsuka Pharmaceuticals; Pamlab; Pfizer; The University of Michigan; Transcept Pharmaceuticals; Urban Institute; and Wyeth Ayerst; speaking fees from Cyberonics Inc.; Forest Laboratories; GlaxoSmithKline; and Otsuka; royalties from Guilford Publications; Healthcare Technology Systems; and The University of Texas Southwestern Medical Center; and research support from National Institute of Mental Health and the Stanley Medical Research Institute. He has owned shares of stock in Pfizer.
S.R.W. has been a consultant for Cyberonics, Inc.; ImaRx Therapeutics, Inc.; Bristol-Myers Squibb; Organon; Case-Western University; Singapore Clinical Research Institute; Dey Pharmaceuticals; Venebio; and Dey.
M.E.T. has provided scientific consultation to Astra-Zeneca; Bristol-Myers Squibb; Dey Pharma, L.P.; Eli Lilly & Company; Forest Pharmaceuticals, Inc.; Gerson Lehman Group; GlaxoSmithKline; Guidepoint Global; H. Lundbeck A/S; MedAvante, Inc.; Merck and Company; Neuronetics, Inc.; Novartis; Otsuka; Ortho-McNeil Pharmaceuticals; PamLab; Pfizer (formerly Wyeth-Ayerst Laboratories); Schering-Plough (formerly Organon, Inc.); Shire US Inc.; Supernus Pharmaceuticals; Takeda (Lundbeck); and Transcept Pharmaceuticals. He has been a member of the speakers' bureaus for AstraZeneca; Bristol-Myers Squibb; Eli Lilly & Company; Merck and Company; and Pfizer (formerly Wyeth-Ayerst Laboratories). He receives grant funding from Eli Lilly & Company; GlaxoSmithKline; National Institute of Mental Health; and Agency for Healthcare Research and Quality, Sepracor, Inc. He has equity holdings in MedAvante, Inc., and receives royalty income from American Psychiatric Foundation, Inc.; Guilford Publications; Herald House; Oxford University Press; and W.W. Norton & Company. His wife is employed as the Group Scientific Director for Embryon, formerly Advogent, which does business with BMS and Pfizer/Wyeth.
D.W. has owned stock in Pfizer, Inc. and Bristol-Myers Squibb within the last 5 years. She has also received funding from National Alliance for Research on Schizophrenia & Depression (NARSAD).
M.F. has received research support from Abbott Laboratories; Alkermes, Inc.; Aspect Medical Systems; AstraZeneca; BioResearch; BrainCells Inc.; Bristol-Myers Squibb; Cephalon, Inc.; CeNeRx BioPharma; Clinical Trials Solutions, LLC; Clintara, LLC; Covidien; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Icon Clinical Research; i3 Innovus/Ingenix; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; NARSAD; National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmavite LLC; Photothera; Roche; RCT Logic, LLC; Sanofi-Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Synthelabo; Wyeth-Ayerst Laboratories. He has provided advisory/consulting services to Abbott Laboratories; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Bayer AG; Best Practice Project Management, Inc.; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol-Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Clinical Trials Solutions, LLC; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co., Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Company; MSI Methylation Sciences, Inc.; Naurex, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG; Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Otsuka Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; RCT Logic, LLC; Sanofi-Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering-Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc. He has speaking/publishing relationships with Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol-Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth-Ayerst Laboratories. He has equity holdings (excluding mutual funds/blinded trusts) with Compellis. He has received royalty/patent or other income for Sequential Parallel Comparison Design (SPCD) and patent application for a combination of azapirones and bupropion in Major Depressive Disorder; has received copyright royalties for the MGH Cognitive & Physical Functioning Questionnaire (CPFQ), Sexual Functioning Inventory (SFI), Antidepressant Treatment Response Questionnaire (ATRQ), Discontinuation-Emergent Signs & Symptoms (DESS), and SAFER; and has received a patent for research and licensing of SPCD with RCT Logic; Lippincott, Williams & Wilkins; World Scientific Publishing Co. Pte.Ltd.
M.H.T. has been a consultant for Abbott Laboratories, Inc.; Akzo (Organon Pharmaceuticals Inc.); AstraZeneca; Bayer; Bristol-Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Fabre-Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica Products, LP; Johnson & Johnson PRD; Eli Lilly & Company; Meade Johnson; Neuronetics; Parke-Davis Pharmaceuticals, Inc.; Pfizer, Inc.; Pharmacia & Upjohn; Sepracor; Solvay Pharmaceuticals, Inc.; VantagePoint; and Wyeth-Ayerst Laboratories. He has served on speakers bureaus for Abdi Brahim; Akzo (Organon Pharmaceuticals Inc.); Bristol-Myers Squibb Company; Cephalon, Inc.; Cyberonics, Inc.; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica Products, LP; Eli Lilly & Company; Pharmacia & Upjohn; Solvay Pharmaceuticals, Inc.; and Wyeth-Ayerst Laboratories. He has also received grant support from Bristol-Myers Squibb; Cephalon, Inc.; Corcept Therapeutics, Inc.; Cyberonics, Inc.; Eli Lilly & Company; Forest Pharmaceuticals; GlaxoSmithKline; Janssen Pharmaceutica; Merck; National Institute of Mental Health; NARSAD; Novartis; Pfizer Inc.; Pharmacia & Upjohn; Predix Pharmaceuticals; Solvay Pharmaceuticals, Inc.; and Wyeth-Ayerst Laboratories.
References
- 1.Kessler RC. Epidemiology of women and depression. J Affect Disord. 2003;74:5–13. doi: 10.1016/s0165-0327(02)00426-3. [DOI] [PubMed] [Google Scholar]
- 2.Michaud CM. Murray CJL. Bloom BR. Burden of disease—implications for future research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 3.Kornstein SG. Schatzberg AF. Thase ME, et al. Gender differences in chronic major and double depression. J Affect Disord. 2000;60:1–11. doi: 10.1016/s0165-0327(99)00158-5. [DOI] [PubMed] [Google Scholar]
- 4.Marcus SM. Young EA. Kerber KB, et al. Gender differences in depression: Findings from the STAR*D study. J Affect Disord. 2005;87:141–150. doi: 10.1016/j.jad.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Marcus SM. Kerber K. Rush AJ, et al. Gender differences in depression symptoms in treatment seeking adults: STAR*D confirmatory analyses. Compr Psychiatry. 2008;49:238–246. doi: 10.1016/j.comppsych.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kornstein SG. Young EA. Harvey AT, et al. The influence of menopausal status and postmenopausal use of hormone therapy on presentation of major depression in women. Menopause. 2010;17:828–839. doi: 10.1097/gme.0b013e3181d770a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young EA. Kornstein SG. Harvey A, et al. Influences of hormone-based contraception on depressive symptoms in premenopausal women with major depression. Psychoneuroendocrinology. 2007;32:843–853. doi: 10.1016/j.psyneuen.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kornstein SG. Schatzberg AF. Thase ME, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression. Am J Psychiatry. 2000;157:1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 9.Martényi F. Dossenbach M. Mraz K. Metcalfe S. Gender differences in the efficacy of fluoxetine and maprotiline in depressed patients: A double-blind trial of antidepressants with serotonergic or norepinephrinergic reuptake inhibition profile. Eur Neuropsychopharmacol. 2001;11:227–232. doi: 10.1016/s0924-977x(01)00089-x. [DOI] [PubMed] [Google Scholar]
- 10.Thase ME. Entsuah R. Cantillon M. Kornstein SG. Relative antidepressant efficacy of venlafaxine and SSRIs: Sex-age interactions. J Womens Health. 2005;14:609–616. doi: 10.1089/jwh.2005.14.609. [DOI] [PubMed] [Google Scholar]
- 11.Pinto-Meza A. Usall J. Serrano-Blanco A. Suarez D. Haro JM. Gender differences in response to antidepressant treatment prescribed in primary care. Does menopause make a difference? J Affect Disord. 2006;93:53–60. doi: 10.1016/j.jad.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 12.Grigoriadis S. Kennedy SH. Bagby RM. A comparison of antidepressant response in younger and older women. J Clin Psychopharmacol. 2003;23:405–407. doi: 10.1097/01.jcp.0000085415.08426.c6. [DOI] [PubMed] [Google Scholar]
- 13.Raskin A. Age-sex differences in response to antidepressant drugs. J Nerv Ment Dis. 1974;159:120–130. doi: 10.1097/00005053-197408000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Schneider LS. Small GW. Clary CM. Estrogen replacement therapy and antidepressant response to sertraline in older depressed women. Am J Geriatr Psychiatry. 2001;9:393–399. [PubMed] [Google Scholar]
- 15.Zanardi R. Rossini D. Magri L. Malaguti A. Colombo C. Smeraldi E. Response to SSRIs and role of the hormonal therapy in post-menopausal depression. Eur Neuropsychopharmacol. 2007;17:400–405. doi: 10.1016/j.euroneuro.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 16.Freeman EW. Rickels K. Sondheimer SJ. Polansky M. Concurrent use of oral contraceptives with antidepressants for premenstrual syndromes. J Clin Psychopharmacol. 2001;21:450–542. doi: 10.1097/00004714-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 17.Fava M. Rush AJ. Trivedi MH, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003;26:457–494. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 18.Rush AJ. Fava M. Wisniewski SR, et al. STAR*D Investigators Group. Sequenced Treatment Alternatives to Relieve Depression (STAR*D): Rationale and design. Control Clin Trials. 2004;25:119–142. doi: 10.1016/s0197-2456(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 19.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Trivedi MH. Rush AJ. Wisniewski SR, et al. STAR*D Study Team. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: Implications for clinical practice. Am J Psychiatry. 2006;163:28–40. doi: 10.1176/appi.ajp.163.1.28. [DOI] [PubMed] [Google Scholar]
- 21.Linn BS. Linn MW. Gurrel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16:622–626. doi: 10.1111/j.1532-5415.1968.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 22.Miller MD. Towers A. Pittsburgh, PA: University of Pittsburgh; 1991. A manual of guidelines for scoring the Cumulative Rating Scale for Geriatrics (CIRS-G) [Google Scholar]
- 23.Rush AJ. Trivedi MH. Ibrahim HM, et al. The 16-item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 24.Trivedi MH. Rush AJ. Ibrahim HM, et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychol Med. 2004;34:73–82. doi: 10.1017/s0033291703001107. [DOI] [PubMed] [Google Scholar]
- 25.Zimmerman M. Mattia J. A self-report scale to help make psychiatric diagnoses: The Psychiatric Diagnostic Screening Questionnaire. Arch Gen Psychiatry. 2001;58:787–794. doi: 10.1001/archpsyc.58.8.787. [DOI] [PubMed] [Google Scholar]
- 26.Rush AJ. Zimmerman M. Wisniewski SR, et al. Comorbid psychiatric disorders in depressed outpatients: Demographic and clinical features. J Affect Disord. 2005;87:43–55. doi: 10.1016/j.jad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Rush AJ. Giles DE. Schlesser MA. Fulton C. Weissenburger JE. Burns C. The Inventory for Depressive Symptomatology (IDS): Preliminary findings. Psychiatry Res. 1986;18:65–87. doi: 10.1016/0165-1781(86)90060-0. [DOI] [PubMed] [Google Scholar]
- 28.Rush AJ. Gullion CM. Basco MR. Jarrett RB. Trivedi MH. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 29.Novick JS. Stewart JW. Wisniewski SR, et al. Clinical and demographic features of atypical depression in outpatients with major depression: Preliminary findings from STAR*D. J Clin Psychiatry. 2004;66:1002–1011. doi: 10.4088/jcp.v66n0807. [DOI] [PubMed] [Google Scholar]
- 30.Khan AY. Carrithers J. Preskorn SH, et al. Clinical and demographic factors associated with DSM-IV melancholic depression. Ann Clin Psychiatry. 2006;18:91–98. doi: 10.1080/10401230600614496. [DOI] [PubMed] [Google Scholar]
- 31.Fava M. Alpert JE. Carmin CN, et al. Clinical correlates and symptom patterns of anxious depression among patients with major depressive disorder in STAR*D. Psychol Med. 2004;34:1299–1308. doi: 10.1017/s0033291704002612. [DOI] [PubMed] [Google Scholar]
- 32.Mundt JC. Interactive voice response systems in clinical research and treatment. Psychiatr Serv. 1997;48:611–612. doi: 10.1176/ps.48.5.611. [DOI] [PubMed] [Google Scholar]
- 33.Kobak KA. Greist JH. Jefferson JW. Mundt JC. Katzelnick DJ. Computerized assessment of depression and anxiety over the telephone using interactive voice response. MD Comput. 1999;16:64–68. [PubMed] [Google Scholar]
- 34.Ware JE., Jr Sherbourne DC. The MOS 36-item Short Form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 35.Endicott J. Nee J. Harrison W. Blumenthal R. Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q): A new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 36.Mundt JC. Marks IM. Shear MK. Greist JH. The Work and Social Adjustment Scale: A simple measure of impairment in functioning. Br J Psychiatry. 2002;180:461–464. doi: 10.1192/bjp.180.5.461. [DOI] [PubMed] [Google Scholar]
- 37.Trivedi MH. Rush AJ. Gaynes BN, et al. Maximizing the adequacy of medication treatment in controlled trials and clinical practice: STAR*D measurement-based care. Neuropsychopharmacology. 2007;32:2479–2489. doi: 10.1038/sj.npp.1301390. [DOI] [PubMed] [Google Scholar]
- 38.Wisniewski SR. Rush AJ. Balasubramani GK. Trivedi MH. Nierenberg AA for the STAR*D Investigators. Self-rated global measure of the Frequency, Intensity, and Burden of Side Effects. J Psychiatr Pract. 2006;12:71–79. doi: 10.1097/00131746-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 39.Wisniewski SR. Eng H. Meloro L, et al. Web-based communications and management of a multi-center clinical trial: The Sequenced Treatment Alternatives to Relieve Depression (STAR*D) project. Clin Trials. 2004;1:387–398. doi: 10.1191/1740774504cn035oa. [DOI] [PubMed] [Google Scholar]