Abstract
Significance: The cell envelope of aerobic bacteria is an oxidizing environment in which most cysteine residues are involved in disulfide bonds. However, reducing redox pathways are also present in this cellular compartment where they provide electrons to a variety of cellular processes. The membrane protein DsbD plays a central role in these pathways by functioning as an electron hub that dispatches electrons received from the cytoplasmic thioredoxin system to periplasmic oxidoreductases. Recent Advances: Recent data have revealed that DsbD provides reducing equivalents to a large array of periplasmic redox proteins. Those proteins use the reducing power received from DsbD to correct non-native disulfides, mature c-type cytochromes, protect cysteines on secreted proteins from irreversible oxidation, reduce methionine sulfoxides, and scavenge reactive oxygen species such as hydrogen peroxide. Critical Issues: Despite the prominent role played by DsbD, we have a poor understanding of how this protein transfers electrons across the inner membrane. Another critical issue will be to grasp the full physiological significance of the new reducing pathways that have been identified in the cell envelope such as the peroxide reduction pathway. Future Directions: A detailed understanding of DsbD's mechanism will require solving the structure of this intriguing protein. Moreover, bioinformatic, biochemical, and genetic approaches need to be combined for a better comprehension of the broad spectrum of periplasmic reducing systems present in bacteria, which will likely lead to the discovery of novel pathways. Antioxid. Redox Signal. 18, 1690–1698.
Introduction
The bacterial periplasm is an oxidizing environment in which most cysteine residues are oxidized to disulfide bonds. In Escherichia coli, the system that introduces disulfide bonds into secreted proteins involves the soluble oxidoreductase DsbA and the membrane protein DsbB (1, 2, 20) (Fig. 1). DsbA, a protein from the thioredoxin superfamily, possesses a catalytic CXXC motif, which is maintained oxidized in vivo. The disulfide bond present in the catalytic site of DsbA is unstable, which makes DsbA a powerful oxidant: upon interacting with a substrate protein, the disulfide bond is transferred from DsbA to the substrate. DsbA is recycled to the oxidized state by DsbB, which generates disulfides de novo from quinone reduction (Fig. 1). As such, DsbB connects the oxidative folding pathway to the electron transport chain. The DsbA/DsbB system has been reviewed in several recent articles (14, 24, 27) and will not be further discussed here. Instead, this review will focus on the reducing pathways that are present in the bacterial cell envelope, where they play important roles in oxidative protein folding and in the protection of envelope proteins against oxidative stress. In particular, we will focus on the membrane protein DsbD, which plays a central role in those reducing pathways by importing electrons from the cytoplasm to the periplasm and distributing them to a variety of periplasmic redox enzymes. After summarizing the current knowledge on DsbD, we will discuss the significant diversity observed within the DsbD proteins family. Then, we will review the different redox processes whose activity depends on electrons delivered by DsbD. Throughout this article, we will pay a particular attention to the new data that have been published in the field, since the last review specifically covering this topic was published by Porat et al. (48).
FIG. 1.
Disulfide bond formation in the Escherichia coli periplasm. Disulfide bonds are introduced by DsbA, which is then recycled by the inner membrane (IM) protein DsbB. DsbB generates disulfides de novo from quinone reduction. The electrons are then transferred to the respiratory chain. Non-native disulfides introduced by DsbA in proteins with multiple cysteine residues are corrected by the protein disulfide isomerase DsbC. DsbC is maintained reduced in the periplasm by the IM protein DsbD. DsbD transfers electrons from the cytoplasmic thioredoxin system to the periplasm. Black arrows indicate the flow of electrons (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
The Discovery of DsbD
Although it is now clear that the bacterial cell envelope harbors a large array of redox enzymes, the presence of redox reactions in the bacterial periplasm was thought to be rather limited. It is the discovery of DsbA in 1991 that opened the way to the study of the redox processes taking place in the envelope (2). The early studies in the field focused on DsbA and DsbB, the two proteins that function in the disulfide formation pathway. Then, in 1994, two different groups independently reported the identification of an additional periplasmic oxidoreductase, which they called DsbC (41, 59). When DsbC was discovered, the protein was first proposed to catalyze disulfide bond formation in the periplasm. However, it is now clear that DsbC rather functions as a protein disulfide isomerase (Fig. 1) correcting the non-native disulfides that can be introduced by DsbA in proteins with multiple cysteine residues (see below).
DsbC is a V-shaped soluble homodimeric protein in which each subunit presents both a catalytic domain and a dimerization domain linked together by an α-helix (39, 67). The catalytic domain adopts a thioredoxin fold and possesses a CXXC motif that is kept reduced in the periplasm (25, 52). This enables DsbC to catalyze the isomerization of incorrect disulfides: the reaction starts by a nucleophilic attack of the first cysteine of the CXXC motif on the non-native disulfide, which results in the formation of a mixed-disulfide between DsbC and the substrate protein. Then, the mixed disulfide undergoes a nucleophilic attack either by another reduced cysteine in the substrate or by the second cysteine of the CXXC motif. In the first scenario, DsbC is released in the reduced state and acts as a true isomerase. In the second scenario, DsbC is released in the oxidized state and acts as a reductase, while the non-native disulfide of the substrate is reduced. The substrate then needs one or more rounds of oxidation by DsbA to acquire its native disulfides. This second scenario is supported by a study showing that expression of a thioredoxin-like protein that only exhibits a reductase activity is able to complement an E. coli dsbC mutant (60).
To allow DsbC to react with non-native disulfides, it is important to maintain the CXXC motif of this protein in the reduced state. A search for mutations that restore disulfide bond formation in a dsbA-null strain led to the identification of DsbD, the protein that provides reducing equivalents to DsbC (42, 51).
Properties of E. coli DsbD
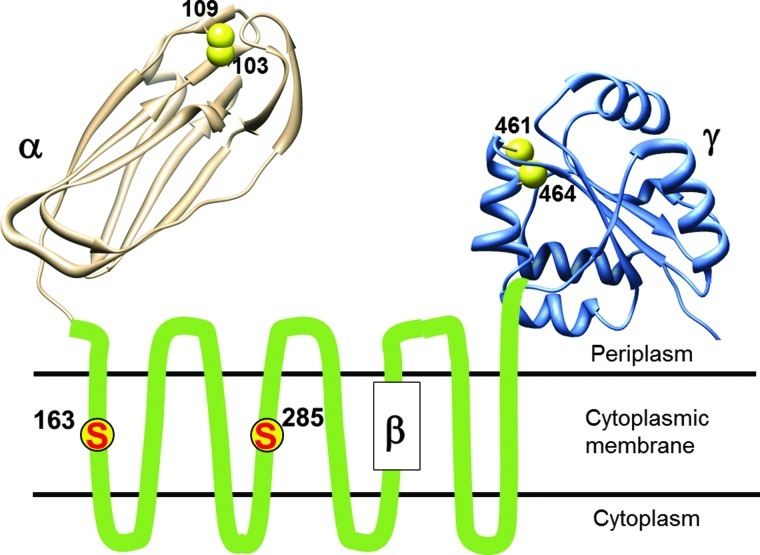
DsbD is a 59-kDa monomeric protein, which is located in the cytoplasmic membrane. DsbD has three distinct domains: an N-terminal periplasmic domain (DsbDα) with an immunoglobulin fold, followed by a membrane-embedded domain (DsbDß) with eight transmembrane segments (TM), and finally, a second periplasmic domain (DsbDγ), with a thioredoxin fold. Each domain of DsbD possesses a pair of redox-active cysteines that are essential for the activity of the protein (Fig. 2) (10, 62). These cysteines form a relay that shuttle electrons from cytoplasmic thioredoxin to periplasmic oxidoreductases via a cascade of thiol–disulfide exchange reactions: electrons are first transferred from the reduced Cys33 and Cys36 residues of the catalytic motif of thioredoxin 1 (Trx1) to the cysteine residues of DsbDß and then successively to the cysteines of DsbDγ and DsbDα. DsbDα then reduces substrate proteins, such as DsbC (28). After donating its electrons to DsbD, Trx1 is released in the oxidized state. It is then converted back to its reduced state by thioredoxin reductase at the expense of nicotinamide adenine dinucleotide phosphate (NADPH). The reaction catalyzed by DsbD is thermodynamically driven as electrons flow from the more reducing protein, Trx1 (E°′=−270 mV), to DsbDß (−246 mV), then to DsbDγ (−241 mV) and DsbDα (−239 mV), and finally to oxidoreductases with significantly more oxidizing redox potentials (DsbC has a redox potential of −130 mV) (11, 55, 67).
FIG. 2.
DsbD is composed of three domains. The structure of DsbDα (in light gray, protein database [PDB] 1JPE) and of DsbDγ (in light blue, PDB 2FWF) were generated using UCSF Chimera version 1.6 (46). The essential cysteines of DsbDß are shown in yellow circles and numbered. “S” represents the sulfur atom of a thiol group. The essential cysteines in the other domains (α and γ) are shown as yellow spheres (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
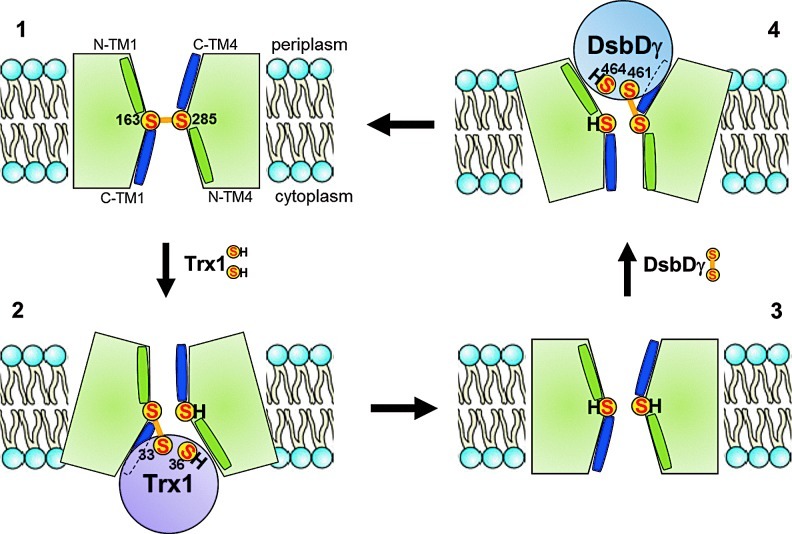
The reaction between DsbDγ and DsbDα has been thoroughly characterized using a combination of biochemical and biophysical techniques (36–38, 54, 56). Moreover, the structures of the two periplasmic domains, either as independent entities or in complex, have been solved (19, 22, 31, 56). In contrast with the abundance of details regarding the interaction between DsbDγ and DsbDα, we only have a partial understanding of the mechanism used by DsbDß to transfer electrons across the membrane. The current working model for the structure of DsbDβ was built by Beckwith and his coworkers (7, 9, 29). The model was constructed by probing the accessibility of selected membrane-embedded residues to membrane-impermeable alkylating reagents. These experiments revealed that the catalytic cysteine residues of DsbDβ (Cys163 and Cys285), which are located in TM1 and TM4, respectively, are exposed to both sides of the membrane. The residues located at the C-terminus of TM1 and TM4 are exposed to the aqueous environment, whereas the residues located at the N-terminus are not (Fig. 3). Moreover, analysis of the amino acid sequence of DsbDß suggests that TM1–3 and TM4–6 present an antiparallel architecture (32). These results, together with the fact that Cys163 and Cys285 can form a mixed disulfide complex with Trx1 (28) and DsbDγ (9), respectively, provide support for a model in which DsbDβ adopts an hourglass structure (9). In this structure, the catalytic cysteine residues are located at the juncture of the two cavities where they are exposed to both thioredoxin in the cytoplasm and DsbDγ in the periplasm (Fig. 3). Interestingly, the water accessibility of the TM residues was shown to be independent of the redox state of the protein, which suggests that the conformation of DsbDß is largely the same in its oxidized and reduced forms (7).
FIG. 3.
Model of the structural changes of DsbDβ during electron transfer. The model shows that DsbDß adopts an hourglass-like structure whose both sides are open while the central pore is maintained narrow. The C-terminal parts of TM1 and TM4 are water-exposed (blue; C-TM1 and C-TM4), while the N-terminal segments are not (green; N-TM1 and N-TM4). The conformation of DsbDβ does not depend on the redox state of Cys163 and Cys285 (conformation 1 is similar to conformation 3). Upon interaction with either Trx1 or DsbDγ, the conformation of DsbDβ changes and opens more widely toward the interacting side of the membrane (conformation 2,4). “S” represents the sulfur atom of a thiol group (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.) TM, transmembrane segments; Trx1, thioredoxin 1.
The properties exhibited by DsbDß are reminiscent of those of channel-like transporters, such as aquaporins and the protein-conducting channel SecYEG complex (43, 64). Channels usually adopt an hourglass-like structure whose both sides remain steadily open while the central pore is maintained small enough to allow the passage of specific ligands (note that in the case of SecY, there is a removable plug in the central pore). In contrast, many pump-like membrane transporters alternate between an open and a closed conformation depending on the binding of ligands (18).
A particularly intriguing question regarding DsbD is how DsbDß alternatively interacts with Trx1 and DsbDγ. An attractive hypothesis is that Trx1 may interact first with some membrane-embedded residues of DsbDß, which would trigger major conformational changes within DsbD and allow the subsequent formation of the mixed-disulfide complex between these two proteins. As shown in Figure 3, DsbDγ and DsbDβ would interact in the same way. The crystal structure of the SecA–SecYEG complex provides support to this hypothesis (68): the binding of SecA, a soluble cytoplasmic protein, to the SecYEG complex leads indeed to conformational changes within the membrane domain of SecY. This subsequently allows specific helices from SecA to displace structural elements within SecY, allowing protein translocation.
The DsbD Family Is Diverse
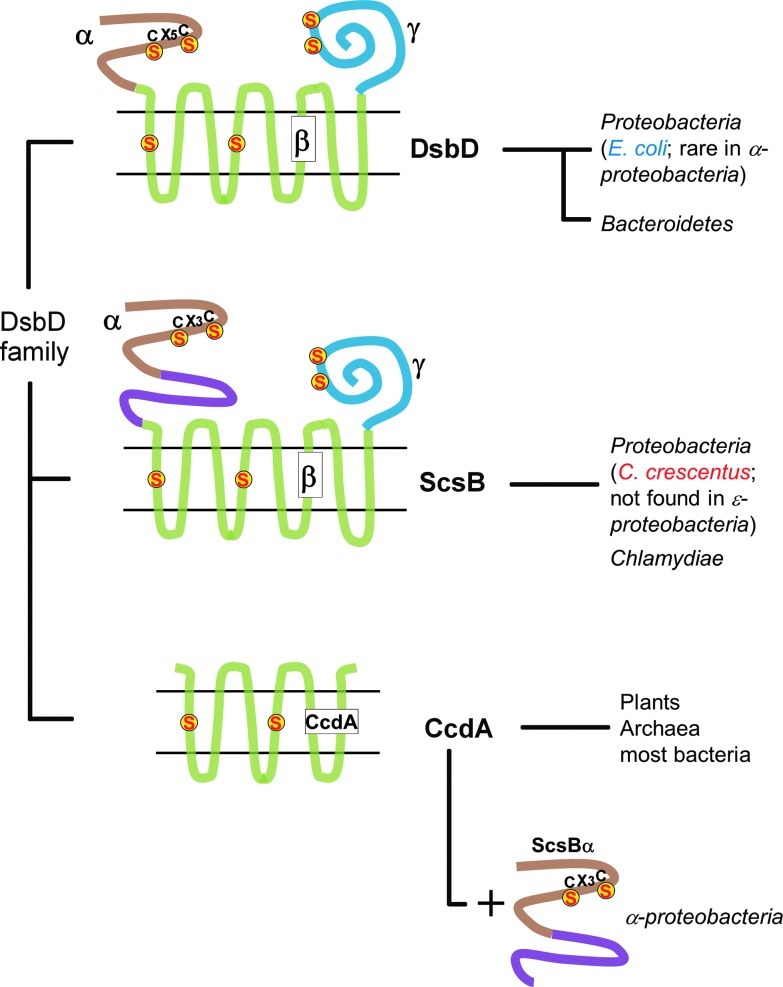
A recent bioinformatic analysis of bacterial genomes has shown that homologs of E. coli DsbD are found in many bacteria, and that they can be divided into three classes: the DsbD-like proteins, the CcdA-like proteins, and the ScsB-like proteins (Fig. 4) (8). As E. coli DsbD is the prototype of the first class, this subgroup will not be further discussed here.
FIG. 4.
Diversity of the DsbD family. The DsbD family is classified into three groups–DsbD, ScsB, and CcdA. DsbD is conserved in most Proteobacteria except α-proteobacteria. ScsB is found in Chlamydiae and many Proteobacteria, except ɛ-proteobacteria. The N-terminal domain of ScsB (ScsBα) is quite different from DsbDα. In particular, ScsBα is much bigger than DsbDα: it appears to consist of two subdomains (shown in brown and purple) of which the N-terminal subdomain contains the two redox-active cysteines, like DsbDα (note the difference in the separation of the two cysteines: CX3C in DsbDα versus CX5C in ScsBα). DsbD homologs from Bacteroidetes have similar sizes with E. coli DsbD, but belong to a different branch of the phylogenetic tree. CcdA, which does not possess the N- and C-terminal periplasmic domains and only comprises six TMs instead of the eight found in E. coli DsbD, is widely distributed among living organisms. Moreover, many α-Proteobacteria that have ScsBα as a separately encoded protein usually express a CcdA-like protein instead of a ScsB-like protein (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
Some bacteria, such as Rhodobacter capsulatus, contain a homolog of DsbD, which is called CcdA (15). CcdA is also found in plants and archaea, and appears to have more broadly evolved compared to the other DsbD family members (8, 30, 45). CcdA is a stripped-down version of DsbD, which does not possess the two periplasmic domains (30) and only comprises six TMs instead of the eight found in E. coli DsbD (Fig. 4). It has been shown that R. capsulatus CcdA can provide electrons to separately encoded thioredoxin-like proteins such as CcmG (details in DsbD provide electrons to a variety of redox periplasmic pathways section). In contrast, the E. coli homolog of CcmG receives electrons from the periplasmic soluble domain of DsbD (DsbDα). Therefore, it can be postulated that DsbD evolved from the fusion of a primitive CcdA-like protein with two soluble periplasmic proteins to facilitate electron transfer (30).
In a recent study, we identified a third distinct class of DsbD-like homologs (8) (Fig. 4) found in proteobacteria and Chlamydiae. The prototype of this new class is Salmonella typhimurium ScsB (suppression of copper sensitivity), a protein that has been shown to confer copper tolerance to E. coli copper-sensitive mutants (21). Like DsbD, the ScsB class has a three-domain structure, each domain, including a pair of cysteines, active in the transfer of electrons. The transmembrane domain (ScsBβ) has eight TMs, and the C-terminal domain (ScsBγ) has a thioredoxin fold. However, the N-terminal domain (ScsBα), which is presumably the final electron donor for substrate proteins, differs significantly from DsbDα (Fig. 4) and acts on a different array of substrate proteins from those already known for DsbD (see below). One interesting feature of ScsBβ is that its N-terminal part (N-subdomain), which harbors the two catalytic cysteines, is relatively well conserved, in contrast to the C-terminal part of ScsBβ (C-subdomain), which is not (Fig. 4). Some organisms do not even have the C-subdomain of ScsBα, which suggests that the acquisition of this subdomain is related to a different substrate specificity. Interestingly, many α-proteobacteria do not express a full-length ScsB, but rather two independent proteins corresponding to ScsBα and either CcdA or ScsBβγ. The co-occurrence of these proteins suggests that they cooperate in electron transfer and supports the gene fusion hypothesis advanced above (Fig. 4).
DsbD Provides Electrons to a Variety of Redox Periplasmic Pathways
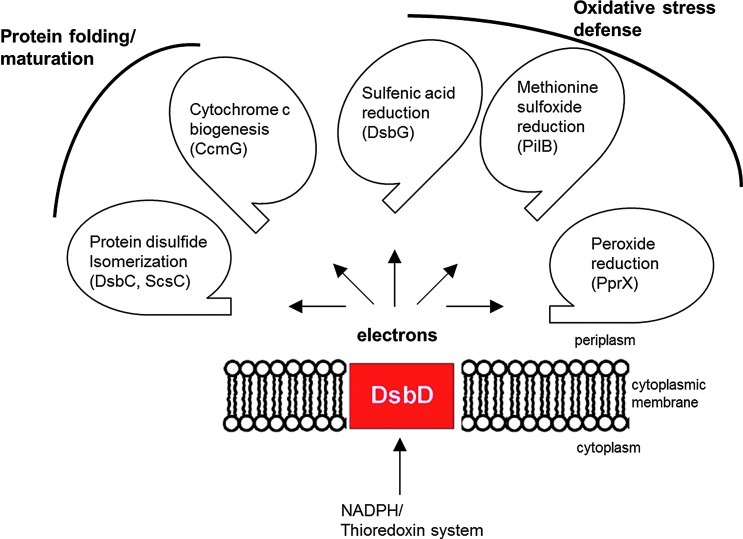
In the previous sections, we focused on the main properties of DsbD and on the diversity of the DsbD family. We will now review recent data that highlight the function of DsbD as an electron hub dispatching reducing equivalents to various redox pathways present in the cell envelope.
DsbD provides electrons for protein folding and maturation
The best-documented function of DsbD proteins is to provide electrons to periplasmic protein disulfide isomerases, such as E. coli DsbC. Envelope proteins are synthesized in the cytoplasm as unfolded polypeptides and are then transported in an unfolded state across the inner membrane. It has been shown that DsbA preferentially introduces disulfides in a vectorial manner in polypeptides entering the periplasm (26). Thus, if disulfides need to be formed between cysteine residues that are not consecutive in the sequence, for example, between Cys1 and Cys3 and between Cys2 and Cys4, DsbA will likely introduce non-native disulfides (Cys1–Cys2 and Cys3–Cys4) in the secreted protein. The function of DsbC and of other periplasmic protein disulfide isomerases, such as the recently described Caulobacter crescentus ScsC (8), is to correct these non-native disulfides using the catalytic mechanism described in The Discovery of DsbD section.
While about half of the E. coli envelope proteins are known or predicted to be DsbA substrates, the number of DsbC substrates is more limited. The list of DsbC substrates includes a few soluble periplasmic enzymes with multiple cysteine residues, such as ribonuclease I (RNase I; 8 cysteines, 1 nonconsecutive disulfide), endonuclease 1 (End1; 8 cysteines, 1 nonconsecutive disulfide), a murein endopeptidase (MepA; 6 cysteines, 3 nonconsecutive disulfides), and a phytase (AppA; 8 cysteines, 1 nonconsecutive disulfide) (3, 23, 35, 40, 65). Noteworthy, a new artificial DsbC substrate has been developed, which confers a semiquantification genetic selection of DsbC or DsbD mutants simply by correlating bacteria ampicillin resistance to DsbC and DsbD activity (50).
Recently, we showed that DsbC is also involved in the oxidative folding of two proteins that localize in the outer membrane (OM), the ß-barrel protein LptD and the lipoprotein RcsF (12, 34). These two OM substrates of DsbC play an important role in the cell: LptD is an essential protein that inserts lipopolysaccharides in the OM, while RcsF is a sensor that upon detection of damages occurring in the cell envelope activates a signaling cascade. The altered oxidative folding of these proteins in strains lacking dsbC leads to specific phenotypes that can be attributed to the lack of disulfide bond formation. This is particularly striking in the case of RcsF: the protein has two nonconsecutive disulfide bonds (Cys1–Cys3 and Cys2–Cys4) that are essential for folding and activity. Failure to form these two disulfides leads to the degradation of RcsF by periplasmic proteases, which renders the bacteria unable to detect and respond to certain envelope perturbations. For instance, deletion of mdoG, a gene coding for periplasmic glycans that play an osmoprotectant role, leads to the constitutive activation of the Rcs phosphorelay. This constitutive activation depends on RcsF and causes the production of colanic acid, an exopolysaccharide that accumulates at the cell surface, yielding a dramatic mucoid phenotype (Fig. 5). However, the decreased levels of properly folded RcsF in mdoG dsbC double-mutants do not allow activation of the Rcs phosphorelay, as illustrated by the nonmucoid phenotype of the double mutant (Fig. 5). Noteworthy, one of the two RcsF disulfide bonds bridges two cysteine residues that are present on two adjacent β-strands (Fig. 5), forming a so-called cross-strand disulfide (CSD). These CSD are described as notoriously unstable (66), which suggests that RcsF may be redox regulated.
FIG. 5.
The absence of DsbC impairs the oxidative folding of RcsF, which prevents the activation of the Rcs phosphorelay. (A) Deletion of mdoG constitutively activates the Rcs phosphorelay, which leads to a mucoid phenotype. This activation depends on RcsF. The mdoG dsbC mutant does not exhibit a mucoid phenotype due to the decreased levels of properly folded RcsF in the outer membrane of that strain. (B) Two nonconsecutive disulfides (in yellow) are present in the three-dimensional structure of RcsF, which is drawn in ribbon form (PDB entry code 2Y1B). The formation of these two disulfide bonds requires DsbC. The first disulfide bond involves Cys74 (Cys1) and Cys118 (Cys3) and links an amino acid in the β2-strand to a residue in the β3–β4 loop. The second disulfide between Cys109 (Cys2) and Cys124 (Cys4) is a cross-strand disulfide (CSD) that connects the β3 and β4 strands. The figures were generated using MacPyMol (Delano Scientific LLC, 2006) (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
DsbD plays an additional role in protein assembly by providing electrons to thioredoxin-like proteins, such as E. coli CcmG, which are involved in cytochrome c maturation in the periplasm (Fig. 6). Cytochromes c are covalently attached to heme groups via thioether bonds between the tetrapyrrole ring of these groups and two cysteine residues of a CXXCH motif of the apoprotein. During the folding of the apocytochromes c in the periplasm, the cysteine residues of the CXXCH motif are thought to be oxidized to a disulfide by DsbA, probably to protect the apocytochromes from degradation (57). Reduction of this disulfide is then required to allow the covalent attachment of heme to the protein. This reaction is carried out by CcmG, which is maintained reduced in the periplasm by DsbD (16, 44, 63).
FIG. 6.
DsbD is an electron hub that transfers reducing equivalents to several oxidoreductases present in the cell envelope. DsbD-like proteins provide reducing equivalents originating from the cytoplasmic thioredoxin system to periplasmic protein disulfide isomerases, such as DsbC in E. coli and ScsC in Caulobacter crescentus, and to oxidoreductases involved in the maturation of cytochrome c, such as E. coli CcmG. Other substrates of DsbD proteins are involved in the defense mechanisms against oxidative stress, such as E. coli DsbG, which controls the global sulfenic acid content of the periplasm, Neisseria gonorrheae PilB, a multidomain protein that exhibits methionine sulfoxide reductase (Msr) activity and C. crescentus PprX, a periplasmic peroxiredoxin (To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars.)
The roles of DsbD in the defense mechanisms against oxidative stress
Oxidative stress occurs when cells are exposed to elevated levels of reactive oxygen species (ROS) such as superoxide (O2−), hydrogen peroxide (H2O2), and alkyl hydroperoxides (ROOH) (53). Oxidative stress can cause DNA damage and mutations, disassembly of iron–sulfur clusters, and lipid peroxidation. It also affects proteins by oxidizing cysteine and methionine residues, which can lead to the inactivation of cellular proteins and cellular death. The presence of protection mechanisms against oxidative stress is therefore essential for cell survival. When DsbD was discovered, the function of the protein was thought to be restricted to providing electrons for disulfide isomerization and cytochrome c maturation. However, recent data obtained on E. coli, Neisseria meningitides, and C. crescentus, three gram-negative bacteria, have revealed that DsbD also plays an important role in the defense mechanisms against oxidative stress by providing electrons to envelope pathways that either reduce proteins that have been oxidatively damaged, or directly reduce harmful ROS in the cell envelope (6, 8, 13).
DsbD provides electrons for sulfenic acid reduction in the envelope
E. coli DsbG is a periplasmic protein that shares 26% sequence identity with DsbC. Like DsbC, it is also a V-shaped dimeric protein whose CXXC catalytic motif is kept reduced by DsbD (4). Because of the similarities between DsbC and DsbG, DsbG was proposed to function as a second protein disulfide isomerase in the E. coli periplasm. However, a search for DsbG substrates led us to find out that this is not the case. We found that the function of DsbG is to protect periplasmic proteins that contain single cysteine residues from oxidation (Fig. 6) (13). We identified three l,d-transpeptidases as DsbG substrates; these proteins employ a conserved cysteine residue to catalyze the cross-linking of peptidoglycan for cell wall synthesis. Their catalytic cysteine residue is sensitive to oxidation by ROS, which leads to the formation of sulfenic acid derivatives (–SOH). Sulfenic acids are highly unstable intermediates that, unless they are stabilized within a protein microenvironment, are further oxidized to sulfinic (–SO2H) and sulfonic (–SO3H) acids, two irreversible modifications (49). Thus, the function of DsbG is to reduce the sulfenic acid derivatives that form on these proteins to prevent their irreversible inactivation. Other proteins likely depend on DsbG for protection against ROS, as suggested by the observation that many envelope proteins were labeled by a sulfenic acid-specific probe (13). Moreover, recent data from our laboratory suggest that DsbC also protects the single cysteine residue of certain proteins from irreversible oxidation (unpublished data).
DsbD provides electrons for methionine sulfoxide reduction in the envelope
The examples of reductive pathways we have talked about so far are largely the ones that are specific to the bacterial cell envelope. However, it has recently become clear that certain antioxidant enzymes that have previously been detected in the cytoplasm of cells have their counterparts in the bacterial periplasm. For example, methionine residues, like cysteine residues, are also sensitive to ROS and can be oxidized to methionine sulfoxides (MetO). Two diastereoisomers of MetO are generated, referred to as R and S, owing to the asymmetric position of the sulfur atom in the lateral chain. The oxidation of methionine residues in proteins leads, if unrepaired, to changes in hydrophobicity, alterations in protein conformation, and loss of biological activity. For this reason, most cells contain methionine sulfoxide reductases (Msr) that catalyze the reduction of MetO back to methionine [see the following review: (5)]. The bacterial cytoplasm contains MsrA and MsrB proteins that reduce the S and R form of MetO, respectively (5), the electrons being provided to them by the thioredoxin system.
The discovery of a periplasmic protein PilB involved in MetO reduction in Neisseria gonorrhoeae revealed that such antioxidant enzymes can be found in both the cytoplasm and the cell envelope (Fig. 6) (61). PilB is composed of three domains. The N-terminal domain has a thioredoxin fold and functions as a reductase, while the second and third domains are homologous to MsrA and MsrB, respectively (6). DsbD plays a role in periplasmic MetO reduction by providing electrons to the N-terminal domain of PilB. The latter then transfers the electrons to the MsrA and MsrB domains of the protein. Interestingly, deletion of dsbD was shown to increase the oxidative stress sensitivity of N. meningitidis mutants lacking the genes coding for two DsbA (dsbA1 and dsbA2). It has been proposed that the increased sensitivity may result from the lack of reducing equivalents delivered to the N. meningitidis PilB homolog (33).
Although the PilB protein is only found in a limited number of bacterial species, other bacteria contain gene clusters involving a CcdA-like protein and periplasmic homologs of thioredoxin, MsrA and MsrB. It seems likely that these proteins cooperate in periplasmic MetO reduction, illustrating the importance of this process in the cell envelope.
DsbD provides electrons for peroxide reduction in the envelope
In the cytoplasm, in addition to enzymes that reduce oxidatively damaged proteins, bacterial cells also express several catalases and thiol-dependent peroxidases, such as peroxiredoxins (Prxs), to directly scavenge harmful peroxides. Catalases deal with high concentrations of peroxides (mM), while Prxs are the major scavengers for peroxides generated at physiological concentrations (μM) (58).
Prx enzymes utilize a reactive cysteine residue to attack the O–O bond of the substrate, which leads to the formation of sulfenic acid. The latter is then attacked by a second cysteine residue, generating an intra- or intermolecular disulfide bond, depending on whether the resolving cysteine belongs to the same or to another Prx molecule. The Prx activity is then regenerated by reduction of the disulfide bond, a reaction that is usually catalyzed by proteins from the thioredoxin family [recently reviewed in (17, 47)]. While cytoplasmic peroxides scavengers are widespread in bacteria, none had been identified in the cell envelope. A recent search for substrates of C. crescentus ScsB (see above) leads to the identification of the first peroxide reduction pathway active in the bacterial periplasm (Fig. 6) (8). In this bacterium, ScsB was shown to deliver electrons to TlpA, a thioredoxin-like protein present in the periplasm. TlpA then uses the electrons to reduce a periplasmic peroxiredoxin, PprX (8). Characterization of PprX revealed that this enzyme is active against H2O2 and cumene hydroperoxide. Although the physiological importance of PprX remains to be determined, these results highlight the importance to directly scavenge peroxides in the cell envelope before they reach the cytoplasm.
Concluding Remarks
Since the discovery of DsbA in 1991, much of the attention given to the bacterial redox pathways was focused on the disulfide bond forming system involving DsbA and DsbB. The role of the DsbC/DsbG reducing pathway was thought to be mostly limited to the correction of non-native disulfides in a small number of periplasmic proteins. However, an abundance of data has recently revealed the unsuspected diversity of reducing pathways present in the bacterial cell envelope. As discussed above, envelope-reducing pathways provide electrons that are used not only proof read the oxidative folding process but also to protect envelope proteins from the harmful action of ROS. Many questions remain to be addressed. For instance, the physiological importance of the newly discovered redox pathways such as those involved in sulfenic acid reduction and peroxide scavenging needs to be established. Moreover, how the expression of DsbD and of its substrates is regulated depending on the conditions of the environment should also be investigated, as it is possible that in certain bacteria, these proteins are upregulated in response to oxidative stress. Thorough bioinformatic, biochemical, and genetic analyses, such as those that have led to the identification of the ScsB class of DsbD-like proteins and to the discovery of PprX, also need to be undertaken to fully understand the broad spectrum of the periplasmic reducing systems present in the hundreds of sequenced bacterial genomes, and also to discover novel reducing pathways. Finally, the electron transporter protein DsbD clearly plays a central role in these pathways by transferring electrons from the cytoplasm to the periplasm. Yet, the mechanism used by this protein to catalyze this reaction is still unknown. A detailed understanding of DsbD's mechanism will undoubtedly require solving the structure of the transmembrane domain of DsbD (DsbDß) or of its homologous protein, CcdA.
Abbreviations Used
- CSD
cross-strand disulfide
- Dsb
disulfide bond
- e−
electron
- IM
inner membrane
- MetO
methionine sulfoxide
- Msr
methionine sulfoxide reductase
- NADPH
nicotinamide adenine dinucleotide phosphate
- OM
outer membrane
- PDB
protein database
- Prx
peroxiredoxin
- ROS
reactive oxygen species
- TM
transmembrane segments
- Trx1
thioredoxin 1
Acknowledgments
We thank Pauline Leverrier, Katleen Denoncin, and Alexandra Gennaris for critical reading of the manuscript and helpful comments. JFC is Chercheur Qualifié of the FRS-FNRS. This work was supported by the European Research Council (FP7/2007–2013) ERC independent researcher starting grant 282335–Sulfenic. We apologize to authors whose work was not cited directly due to reference limitations.
References
- 1.Bardwell JC. Lee JO. Jander G. Martin N. Belin D. Beckwith J. A pathway for disulfide bond formation in vivo. Proc Natl Acad Sci U S A. 1993;90:1038–1042. doi: 10.1073/pnas.90.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bardwell JC. McGovern K. Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 3.Berkmen M. Boyd D. Beckwith J. The nonconsecutive disulfide bond of Escherichia coli phytase (AppA) renders it dependent on the protein-disulfide isomerase, DsbC. J Biol Chem. 2005;280:11387–11394. doi: 10.1074/jbc.M411774200. [DOI] [PubMed] [Google Scholar]
- 4.Bessette PH. Cotto JJ. Gilbert HF. Georgiou G. In vivo and in vitro function of the Escherichia coli periplasmic cysteine oxidoreductase DsbG. J Biol Chem. 1999;274:7784–7792. doi: 10.1074/jbc.274.12.7784. [DOI] [PubMed] [Google Scholar]
- 5.Boschi-Muller S. Gand A. Branlant G. The methionine sulfoxide reductases: catalysis and substrate specificities. Arch Biochem Biophys. 2008;474:266–273. doi: 10.1016/j.abb.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 6.Brot N. Collet JF. Johnson LC. Jonsson TJ. Weissbach H. Lowther WT. The thioredoxin domain of Neisseria gonorrhoeae PilB can use electrons from DsbD to reduce downstream methionine sulfoxide reductases. J Biol Chem. 2006;281:32668–32675. doi: 10.1074/jbc.M604971200. [DOI] [PubMed] [Google Scholar]
- 7.Cho SH. Beckwith J. Two snapshots of electron transport across the membrane: insights into the structure and function of DsbD. J Biol Chem. 2009;284:11416–11424. doi: 10.1074/jbc.M900651200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho SH. Parsonage D. Thurston C. Dutton RJ. Poole LB. Collet JF. Beckwith J. A new family of membrane electron transporters and its substrates, including a new cell envelope peroxiredoxin, reveal a broadened reductive capacity of the oxidative bacterial cell envelope. MBio. 2012;3:pii. doi: 10.1128/mBio.00291-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho SH. Porat A. Ye J. Beckwith J. Redox-active cysteines of a membrane electron transporter DsbD show dual compartment accessibility. EMBO J. 2007;26:3509–3520. doi: 10.1038/sj.emboj.7601799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung J. Chen T. Missiakas D. Transfer of electrons across the cytoplasmic membrane by DsbD, a membrane protein involved in thiol-disulphide exchange and protein folding in the bacterial periplasm. Mol Microbiol. 2000;35:1099–1109. doi: 10.1046/j.1365-2958.2000.01778.x. [DOI] [PubMed] [Google Scholar]
- 11.Collet JF. Riemer J. Bader MW. Bardwell JC. Reconstitution of a disulfide isomerization system. J Biol Chem. 2002;277:26886–26892. doi: 10.1074/jbc.M203028200. [DOI] [PubMed] [Google Scholar]
- 12.Denoncin K. Vertommen D. Paek E. Collet JF. The protein-disulfide isomerase DsbC cooperates with SurA and DsbA in the assembly of the essential beta-barrel protein LptD. J Biol Chem. 2010;285:29425–29433. doi: 10.1074/jbc.M110.119321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Depuydt M. Leonard SE. Vertommen D. Denoncin K. Morsomme P. Wahni K. Messens J. Carroll KS. Collet JF. A periplasmic reducing system protects single cysteine residues from oxidation. Science. 2009;326:1109–1111. doi: 10.1126/science.1179557. [DOI] [PubMed] [Google Scholar]
- 14.Depuydt M. Messens J. Collet JF. How proteins form disulfide bonds. Antioxid Redox Signal. 2011;15:49–66. doi: 10.1089/ars.2010.3575. [DOI] [PubMed] [Google Scholar]
- 15.Deshmukh M. Brasseur G. Daldal F. Novel Rhodobacter capsulatus genes required for the biogenesis of various c-type cytochromes. Mol Microbiol. 2000;35:123–138. doi: 10.1046/j.1365-2958.2000.01683.x. [DOI] [PubMed] [Google Scholar]
- 16.Fabianek RA. Hennecke H. Thony-Meyer L. The active-site cysteines of the periplasmic thioredoxin-like protein CcmG of Escherichia coli are important but not essential for cytochrome c maturation in vivo. J Bacteriol. 1998;180:1947–1950. doi: 10.1128/jb.180.7.1947-1950.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Flohe L. Toppo S. Cozza G. Ursini F. A comparison of thiol peroxidase mechanisms. Antioxid Redox Signal. 2011;15:763–780. doi: 10.1089/ars.2010.3397. [DOI] [PubMed] [Google Scholar]
- 18.Gadsby DC. Ion channels versus ion pumps: the principal difference, in principle. Nature Reviews Mol Cell Biol. 2009;10:344–352. doi: 10.1038/nrm2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goulding CW. Sawaya MR. Parseghian A. Lim V. Eisenberg D. Missiakas D. Thiol-disulfide exchange in an immunoglobulin-like fold: structure of the N-terminal domain of DsbD. Biochemistry. 2002;41:6920–6927. doi: 10.1021/bi016038l. [DOI] [PubMed] [Google Scholar]
- 20.Guilhot C. Jander G. Martin NL. Beckwith J. Evidence that the pathway of disulfide bond formation in Escherichia coli involves interactions between the cysteines of DsbB and DsbA. Proc Natl Acad Sci U S A. 1995;92:9895–9899. doi: 10.1073/pnas.92.21.9895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta SD. Wu HC. Rick PD. A Salmonella typhimurium genetic locus which confers copper tolerance on copper-sensitive mutants of Escherichia coli. J Bacteriol. 1997;179:4977–4984. doi: 10.1128/jb.179.16.4977-4984.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haebel PW. Goldstone D. Katzen F. Beckwith J. Metcalf P. The disulfide bond isomerase DsbC is activated by an immunoglobulin-fold thiol oxidoreductase: crystal structure of the DsbC-DsbDalpha complex. EMBO J. 2002;21:4774–4784. doi: 10.1093/emboj/cdf489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiniker A. Bardwell JCA. In vivo substrate specificity of periplasmic disulfide oxidoreductases. J Biol Chem. 2004;279:12967–12973. doi: 10.1074/jbc.M311391200. [DOI] [PubMed] [Google Scholar]
- 24.Ito K. Inaba K. The disulfide bond formation (Dsb) system. Curr Opin Struct Biol. 2008;18:450–458. doi: 10.1016/j.sbi.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Joly JC. Swartz JR. In vitro and in vivo redox states of the Escherichia coli periplasmic oxidoreductases DsbA and DsbC. Biochemistry. 1997;36:10067–10072. doi: 10.1021/bi9707739. [DOI] [PubMed] [Google Scholar]
- 26.Kadokura H. Beckwith J. Detecting folding intermediates of a protein as it passes through the bacterial translocation channel. Cell. 2009;138:1164–1173. doi: 10.1016/j.cell.2009.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kadokura H. Beckwith J. Mechanisms of oxidative protein folding in the bacterial cell envelope. Antioxid Redox Signal. 2010;13:1231–1246. doi: 10.1089/ars.2010.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katzen F. Beckwith J. Transmembrane electron transfer by the membrane protein DsbD occurs via a disulfide bond cascade. Cell. 2000;103:769–779. doi: 10.1016/s0092-8674(00)00180-x. [DOI] [PubMed] [Google Scholar]
- 29.Katzen F. Beckwith J. Role and location of the unusual redox-active cysteines in the hydrophobic domain of the transmembrane electron transporter DsbD. Proc Natl Acad Sci U S A. 2003;100:10471–10476. doi: 10.1073/pnas.1334136100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzen F. Deshmukh M. Daldal F. Beckwith J. Evolutionary domain fusion expanded the substrate specificity of the transmembrane electron transporter DsbD. EMBO J. 2002;21:3960–3969. doi: 10.1093/emboj/cdf405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim JH. Kim SJ. Jeong DG. Son JH. Ryu SE. Crystal structure of DsbDgamma reveals the mechanism of redox potential shift and substrate specificity(1) FEBS Lett. 2003;543:164–169. doi: 10.1016/s0014-5793(03)00434-4. [DOI] [PubMed] [Google Scholar]
- 32.Kimball RA. Martin L. Saier MH., Jr. Reversing transmembrane electron flow: the DsbD and DsbB protein families. J Mol Microbiol Biotechnol. 2003;5:133–149. doi: 10.1159/000070263. [DOI] [PubMed] [Google Scholar]
- 33.Kumar P. Sannigrahi S. Scoullar J. Kahler CM. Tzeng YL. Characterization of DsbD in Neisseria meningitidis. Mol Microbiol. 2011;79:1557–1573. doi: 10.1111/j.1365-2958.2011.07546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leverrier P. Declercq JP. Denoncin K. Vertommen D. Hiniker A. Cho SH. Collet JF. Crystal structure of the outer membrane protein RcsF, a new substrate for the periplasmic protein-disulfide isomerase DsbC. J Biol Chem. 2011;286:16734–16742. doi: 10.1074/jbc.M111.224865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marcyjaniak M. Odintsov SG. Sabala I. Bochtler M. Peptidoglycan amidase MepA is a LAS metallopeptidase. J Biol Chem. 2004;279:43982–43989. doi: 10.1074/jbc.M406735200. [DOI] [PubMed] [Google Scholar]
- 36.Mavridou DA. Saridakis E. Kritsiligkou P. Goddard AD. Stevens JM. Ferguson SJ. Redfield C. Oxidation state-dependent protein-protein interactions in disulfide cascades. J Biol Chem. 2011;286:24943–24956. doi: 10.1074/jbc.M111.236141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mavridou DA. Stevens JM. Ferguson SJ. Redfield C. Active-site properties of the oxidized and reduced C-terminal domain of DsbD obtained by NMR spectroscopy. J Mol Biol. 2007;370:643–658. doi: 10.1016/j.jmb.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 38.Mavridou DA. Stevens JM. Goddard AD. Willis AC. Ferguson SJ. Redfield C. Control of periplasmic interdomain thiol:disulfide exchange in the transmembrane oxidoreductase DsbD. J Biol Chem. 2009;284:3219–3226. doi: 10.1074/jbc.M805963200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCarthy AA. Haebel PW. Torronen A. Rybin V. Baker EN. Metcalf P. Crystal structure of the protein disulfide bond isomerase, DsbC, from Escherichia coli. Nat Struct Biol. 2000;7:196–199. doi: 10.1038/73295. [DOI] [PubMed] [Google Scholar]
- 40.Messens J. Collet JF. Van Belle K. Brosens E. Loris R. Wyns L. The oxidase DsbA folds a protein with a nonconsecutive disulfide. J Biol Chem. 2007;282:31302–31307. doi: 10.1074/jbc.M705236200. [DOI] [PubMed] [Google Scholar]
- 41.Missiakas D. Georgopoulos C. Raina S. The Escherichia coli dsbC (xprA) gene encodes a periplasmic protein involved in disulfide bond formation. EMBO J. 1994;13:2013–2020. doi: 10.1002/j.1460-2075.1994.tb06471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Missiakas D. Schwager F. Raina S. Identification and characterization of a new disulfide isomerase-like protein (DsbD) in Escherichia coli. EMBO J. 1995;14:3415–3424. doi: 10.1002/j.1460-2075.1995.tb07347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Murata K. Mitsuoka K. Hirai T. Walz T. Agre P. Heymann JB. Engel A. Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407:599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 44.Page MD. Ferguson SJ. Paracoccus denitrificans CcmG is a periplasmic protein-disulphide oxidoreductase required for c- and aa3-type cytochrome biogenesis; evidence for a reductase role in vivo. Mol Microbiol. 1997;24:977–990. doi: 10.1046/j.1365-2958.1997.4061775.x. [DOI] [PubMed] [Google Scholar]
- 45.Page ML. Hamel PP. Gabilly ST. Zegzouti H. Perea JV. Alonso JM. Ecker JR. Theg SM. Christensen SK. Merchant S. A homolog of prokaryotic thiol disulfide transporter CcdA is required for the assembly of the cytochrome b6f complex in Arabidopsis chloroplasts. J Biol Chem. 2004;279:32474–32482. doi: 10.1074/jbc.M404285200. [DOI] [PubMed] [Google Scholar]
- 46.Pettersen EF. Goddard TD. Huang CC. Couch GS. Greenblatt DM. Meng EC. Ferrin TE. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 47.Poole LB. Hall A. Nelson KJ. Overview of peroxiredoxins in oxidant defense and redox regulation. Curr Protoc Toxicol Chapter. 2011;7 doi: 10.1002/0471140856.tx0709s49. Unit7.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Porat A. Cho SH. Beckwith J. The unusual transmembrane electron transporter DsbD and its homologues: a bacterial family of disulfide reductases. Res Microbiol. 2004;155:617–622. doi: 10.1016/j.resmic.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 49.Reddie KG. Carroll KS. Expanding the functional diversity of proteins through cysteine oxidation. Curr Opin Chem Biol. 2008;12:746–754. doi: 10.1016/j.cbpa.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 50.Ren G. Bardwell JC. Engineered pathways for correct disulfide bond oxidation. Antioxid Redox Signal. 2011;14:2399–2412. doi: 10.1089/ars.2010.3782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rietsch A. Belin D. Martin N. Beckwith J. An in vivo pathway for disulfide bond isomerization in Escherichia coli. Proc Natl Acad Sci U S A. 1996;93:13048–13053. doi: 10.1073/pnas.93.23.13048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rietsch A. Bessette P. Georgiou G. Beckwith J. Reduction of the periplasmic disulfide bond isomerase, DsbC, occurs by passage of electrons from cytoplasmic thioredoxin. J Bacteriol. 1997;179:6602–8. doi: 10.1128/jb.179.21.6602-6608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roos G. Messens J. Protein sulfenic acid formation: from cellular damage to redox regulation. Free Radic Biol Med. 2011;51:314–326. doi: 10.1016/j.freeradbiomed.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 54.Rozhkova A. Glockshuber R. Kinetics of the intramolecular disulfide exchange between the periplasmic domains of DsbD. J Mol Biol. 2007;367:1162–1170. doi: 10.1016/j.jmb.2006.12.033. [DOI] [PubMed] [Google Scholar]
- 55.Rozhkova A. Glockshuber R. Thermodynamic aspects of DsbD-mediated electron transport. J Mol Biol. 2008;380:783–788. doi: 10.1016/j.jmb.2008.05.050. [DOI] [PubMed] [Google Scholar]
- 56.Rozhkova A. Stirnimann CU. Frei P. Grauschopf U. Brunisholz R. Grutter MG. Capitani G. Glockshuber R. Structural basis and kinetics of inter- and intramolecular disulfide exchange in the redox catalyst DsbD. EMBO J. 2004;23:1709–1719. doi: 10.1038/sj.emboj.7600178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sanders C. Turkarslan S. Lee DW. Daldal F. Cytochrome c biogenesis: the Ccm system. Trends Microbiol. 2010;18:266–274. doi: 10.1016/j.tim.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seaver LC. Imlay JA. Alkyl hydroperoxide reductase is the primary scavenger of endogenous hydrogen peroxide in Escherichia coli. J Bacteriol. 2001;183:7173–7181. doi: 10.1128/JB.183.24.7173-7181.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shevchik VE. Condemine G. Robert-Baudouy J. Characterization of DsbC, a periplasmic protein of Erwinia chrysanthemi and Escherichia coli with disulfide isomerase activity. EMBO J. 1994;13:2007–2012. doi: 10.1002/j.1460-2075.1994.tb06470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shouldice SR. Cho SH. Boyd D. Heras B. Eser M. Beckwith J. Riggs P. Martin JL. Berkmen M. In vivo oxidative protein folding can be facilitated by oxidation-reduction cycling. Mol Microbiol. 2010;75:13–28. doi: 10.1111/j.1365-2958.2009.06952.x. [DOI] [PubMed] [Google Scholar]
- 61.Skaar EP. Tobiason DM. Quick J. Judd RC. Weissbach H. Etienne F. Brot N. Seifert HS. The outer membrane localization of the Neisseria gonorrhoeae MsrA/B is involved in survival against reactive oxygen species. Proc Natl Acad Sci U S A. 2002;99:10108–10113. doi: 10.1073/pnas.152334799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart EJ. Katzen F. Beckwith J. Six conserved cysteines of the membrane protein DsbD are required for the transfer of electrons from the cytoplasm to the periplasm of Escherichia coli. EMBO J. 1999;18:5963–5971. doi: 10.1093/emboj/18.21.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stirnimann CU. Rozhkova A. Grauschopf U. Grutter MG. Glockshuber R. Capitani G. Structural basis and kinetics of DsbD-dependent cytochrome c maturation. Structure. 2005;13:985–993. doi: 10.1016/j.str.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 64.Van den Berg B. Clemons WM., Jr. Collinson I. Modis Y. Hartmann E. Harrison SC. Rapoport TA. X-ray structure of a protein-conducting channel. Nature. 2004;427:36–44. doi: 10.1038/nature02218. [DOI] [PubMed] [Google Scholar]
- 65.Vertommen D. Depuydt M. Pan J. Leverrier P. Knoops L. Szikora JP. Messens J. Bardwell JC. Collet JF. The disulphide isomerase DsbC cooperates with the oxidase DsbA in a DsbD-independent manner. Mol Microbiol. 2008;67:336–349. doi: 10.1111/j.1365-2958.2007.06030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wouters MA. Fan SW. Haworth NL. Disulfides as redox switches: from molecular mechanisms to functional significance. Antioxid Redox Signal. 2010;12:53–91. doi: 10.1089/ars.2009.2510. [DOI] [PubMed] [Google Scholar]
- 67.Zapun A. Missiakas D. Raina S. Creighton TE. Structural and functional characterization of DsbC, a protein involved in disulfide bond formation in Escherichia coli. Biochemistry. 1995;34:5075–5089. doi: 10.1021/bi00015a019. [DOI] [PubMed] [Google Scholar]
- 68.Zimmer J. Nam Y. Rapoport TA. Structure of a complex of the ATPase SecA and the protein-translocation channel. Nature. 2008;455:936–943. doi: 10.1038/nature07335. [DOI] [PMC free article] [PubMed] [Google Scholar]