The evaluation of a patient with cognitive impairment or dementia is part of many neurologists' daily practice. Once it has been established that a patient has mild cognitive impairment (MCI) or dementia, our job is to determine the etiology. Why is this important? Although specific pharmacologic treatment options are still limited at present for most dementias, there are a growing number of clinical trials in which patients may participate. Furthermore, it is imperative for the neurologist to guide a comprehensive approach to the treatment of cognitive impairment and dementia including management of behavioral symptoms, programs and strategies to optimize functional independence, caregiver education and support, and assistance with prognostication, planning, and connection with specific resources to assist with these issues.
In 2011, new diagnostic criteria were published for Alzheimer disease (AD),1 behavioral variant frontotemporal dementia (FTD),2 and primary progressive aphasia,3 and a new consensus statement was published on vascular cognitive impairment.4 All of these criteria emphasize the value of structural and functional/molecular neuroimaging (including fluorodeoxyglucose PET [FDG-PET]), as well as CSF markers, in increasing diagnostic confidence or specificity. Although these criteria were in large part aimed at the clinical research communities studying these diseases, they also serve as guidance for practicing clinicians. These reports emphasize elements that can be summarized as the practitioner's 2 major goals: 1) start by establishing a diagnostic hypothesis based on a careful clinical evaluation emphasizing history and examination (including office-based cognitive testing that may be supplemented with formal neuropsychological testing); 2) perform diagnostic testing judiciously to test this hypothesis.
In 2004, the US Centers for Medicare and Medicaid Services approved reimbursement of FDG-PET for the purposes of differential diagnosis of AD vs FTD.
For most clinicians, the first diagnostic test in a patient with cognitive impairment is a brain MRI. In many patients with a presentation that is prototypical for a specific neurodegenerative disease, this test and a few other tests (e.g., vitamin B12, thyroid testing) may be all that is required to establish a confident diagnosis. For example, multiple studies have shown that the original 1984 diagnostic criteria for AD, which advocate essentially this approach, demonstrate a sensitivity and specificity of approximately 81% and 70%, respectively.5
Yet there are many patients in whom the diagnosis is still uncertain after this information has been obtained. The clinician faced with this situation should strongly consider using additional relatively new diagnostic testing to further evaluate the patient: molecular neuroimaging with FDG-PET or a spinal fluid examination for amyloid-β and tau proteins. For example, a patient presenting in her 50s or 60s with a syndrome of executive or language impairment and a relatively unrevealing MRI scan can be challenging to confidently diagnose (figure). In such patients, I find FDG-PET to be an extremely valuable next step in the diagnostic evaluation, since it is minimally invasive and may provide a clear indication of whether the patient has a hypometabolic pattern consistent with atypical AD as opposed to a pattern supportive of FTD or another neurodegenerative disease. Although quantitative MRI and spinal fluid studies are worth considering as well, I will focus the remainder of this brief discussion on FDG-PET.
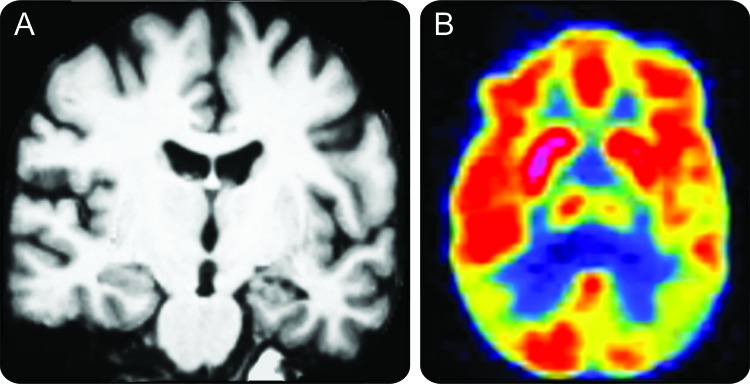
Figure.
A 52-year-old patient presented with insidiously progressive very mild dementia (Clinical Dementia Rating [CDR] 0.5) characterized mainly by a dysexecutive syndrome
The differential diagnosis primarily included Alzheimer disease (AD) or frontotemporal dementia. MRI (A) was relatively unrevealing. FDG-PET (B) showed temporoparietal hypometabolism in a pattern consistent with AD. Spinal fluid analysis supported the diagnosis of AD. She has since progressed to mild dementia (CDR 1) with clinical characteristics consistent with AD and is in a clinical drug trial.
In 2004, the US Centers for Medicare and Medicaid Services approved reimbursement of FDG-PET for the purposes of differential diagnosis of AD vs FTD. Several studies have supported the value of this neuroimaging tool in dementia diagnosis.6,7 In a clinicopathologic study, Jagust et al.8 demonstrated that FDG-PET improved upon the sensitivity and specificity of clinical diagnostic evaluation in predicting a pathologic diagnosis of AD vs non-AD. Foster et al.9 have performed a series of studies evaluating the practical value of FDG-PET in the diagnostic assessment of patients with dementia, with a particular emphasis on AD and FTD, and showed that FDG-PET improved upon clinical assessment with particular value in situations in which clinical diagnostic confidence was not high; this concept has recently received further support from another group that performed a similar study in patients with MCI or dementia.10 Thus, while the 2001 American Academy of Neurology practice parameter for the diagnostic evaluation of dementia did not recommend the use of FDG-PET or related techniques, citing the need for “further prospective studies … to establish the value that it brings to diagnosis over and above a competent clinical diagnosis,”5 a number of studies in the decade since then have begun to provide evidence of added value in certain situations (summarized in a recent review11).
Further research in clinical practice settings will be necessary to determine the best place for FDG-PET in relation to structural MRI, other forms of MRI, and spinal fluid markers of diseases causing cognitive impairment and dementia.
Nevertheless, FDG-PET faces several challenges in becoming more routinely used in the diagnosis of AD and related disorders. First, the clinician needs to be familiar with its utility and have access to a facility in which it is performed. Second, reimbursement for FDG-PET in the diagnostic evaluation of dementia or cognitive impairment needs to improve in the private sector; it is often particularly difficult (and sometimes impossible) to obtain authorization from private insurers for FDG-PET in patients younger than Medicare-eligible age, ironically the patients in whom it may be most useful. Finally, improved standardization of interpretation and quantification of FDG-PET scans is an important goal; a number of research groups are working on comparisons of different quantitative techniques and on comparisons of visual interpretation vs quantitative analysis, partly with the goal of incorporating FDG-PET into clinical trials.
Further research in clinical practice settings will be necessary to determine the best place for FDG-PET in relation to structural MRI, other forms of MRI, and spinal fluid markers of diseases causing cognitive impairment and dementia. Investigations of the comparative utility of FDG-PET vs amyloid PET imaging are badly needed. As these new tests become more widely available, practically oriented studies will contribute importantly to dialogue among neurologists aiming to balance diagnostic rigor with cost effectiveness. These discussions will move to center stage if we are fortunate enough to move into an era in which we have effective disease-modifying therapies.
STUDY FUNDING
The writing of this manuscript was supported by NIH grants AG-029411 and AG-005134.
DISCLOSURES
Dr. Dickerson serves on the editorial boards of Hippocampus and Neurodegenerative Disease Management; has served as a consultant for Pfizer and Envivo Pharmaceuticals; and receives research support from the NIH and from the Alzheimer's Association.
REFERENCES
- 1. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging and the Alzheimer's Association workgroup. Alzheimers Dement Epub 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011;134:2456–2477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;42:2672–2713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001;56:1143–1153 [DOI] [PubMed] [Google Scholar]
- 6. Hoffman JM, Welsh-Bohmer KA, Hanson M, et al. FDG PET imaging in patients with pathologically verified dementia. J Nucl Med 2000;41:1920–1928 [PubMed] [Google Scholar]
- 7. Silverman DH, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: Regional brain metabolism and long-term outcome. JAMA 2001;286:2120–2127 [DOI] [PubMed] [Google Scholar]
- 8. Jagust W, Reed B, Mungas D, Ellis W, Decarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology 2007;69:871–877 [DOI] [PubMed] [Google Scholar]
- 9. Foster NL, Heidebrink JL, Clark CM, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer's disease. Brain 2007;130:2616–2635 [DOI] [PubMed] [Google Scholar]
- 10. Laforce R, Jr, Buteau JP, Paquet N, Verret L, Houde M, Bouchard RW. The value of PET in mild cognitive impairment, typical and atypical/unclear dementias: a retrospective memory clinic study. Am J Alzheimers Dis Other Dement 2010;25:324–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bohnen NI, Djang DS, Herholz K, Anzai Y, Minoshima S. Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: a review of the recent literature. J Nucl Med 2012;53:59–71 [DOI] [PubMed] [Google Scholar]