Oculopalatal tremor (myoclonus) is a rare movement disorder that develops after brainstem or cerebellar stroke, tumor, or following radiation. We describe a patient with transient OPT from relapsing-remitting multiple sclerosis (MS) and review the imaging changes over the course of his OPT.
Case report
A 16-year-old boy with mild cognitive impairment presented with vision loss in his left eye of 6 weeks duration. At age 5 years he had undergone 2 surgical resections and radiation for a posterior fossa ependymoma. Postoperatively, he developed a left temporal lobe stroke and subsequently a seizure disorder requiring lamotrigine and levetiracetam. In the last 2 years he had become seizure-free. Serial MRI showed no tumor recurrence in the last 10 years.
On examination, his visual acuity was 20/25 in the right eye and counting fingers in the left eye with a left afferent pupillary defect and normal optic discs in both eyes. His right eye had superotemporal quadrantanopia from his previous stroke. He had abnormal tandem gait and mild dysmetria on finger-nose–finger testing bilaterally.
MRI of the brain and orbit showed abnormal enhancement of the left prechiasmatic optic nerve consistent with optic neuritis and abnormal fluid-attenuated inversion recovery (FLAIR) signal in the left corona radiata and right medial temporal lobe with enhancing lesions consistent with a clinically isolated syndrome. Encephalomalacia of the left inferior temporal lobe from his previous infarct and postoperative changes in the posterior fossa were seen. Spine MRI and CSF examination were unremarkable. He received IV methyl prednisone for 3 days followed by a tapering dose of oral prednisone.
Over the next 6 months his left eye visual acuity improved to 20/30. The left optic disc became pale. A follow-up MRI 6 months after the previous MRI showed multiple new T2 and FLAIR signal abnormalities consistent with demyelination.
Seven months after the optic neuritis, he presented with a 1-week history of oscillopsia lasting for 2–3 hours on waking in the morning. On examination in the afternoon he had palatal myoclonus without nystagmus.
One week later, he was examined in the morning for persistent oscillopsia and new blurred vision on gazing to either side. Extraocular movements showed bilateral internuclear ophthalmoplegia.
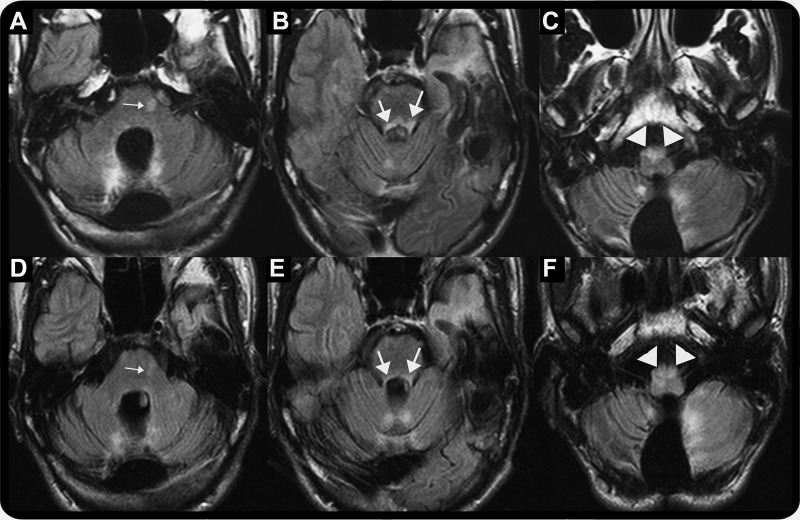
In primary gaze, he had a slow intermittent vertical pendular nystagmus that increased in frequency and amplitude on upgaze. He continued to have palatal myoclonus. Repeat brain MRI (figure, A–C) showed a new contrast-enhancing lesion in the pons and bilateral superior cerebellar peduncles together with hyperintense signal within bilaterally enlarged inferior olivary nuclei (right greater than left), consistent with hypertrophic degeneration. Repeat spinal tap showed 1 oligoclonal band. He received IV methylprednisolone for 3 days. He was then placed on β-interferon (Avonex) 30 mcg weekly.
Figure. Serial brain MRI at oculopalatal tremor (OPT) onset (A–C) and following resolution of OPT (D–F).
(A, B) Axial fluid-attenuated inversion recovery (FLAIR) study shows new hyperintense signal in the pons (small arrow) and bilateral superior cerebellar peduncles (large arrows). (C) Axial FLAIR study shows hyperintense signal in enlarged inferior olivary nuclei (right greater than left) (arrowhead) consistent with hypertrophic degeneration. (D, E) Axial FLAIR study shows resolving hyperintense signal in the pons (small arrow) and bilateral superior cerebellar peduncles (large arrows). (F) Axial FLAIR study shows persistent hyperintense signal in enlarged inferior olivary nuclei (right greater than left) (arrowhead).
Four months later, his oscillopsia improved. The bilateral internuclear ophthalmoplegia, vertical pendular nystagmus, and palatal myoclonus resolved. Serial MRI (figure, D–F) showed improving signal changes in both superior cerebellar peduncles and pons. However, T2 signal changes in both inferior olivary nuclei persisted for 3 years though enlargement had subsided.
DISCUSSION
We have described a patient with relapsing-remitting MS who developed oculopalatal tremor, which then resolved. We wondered if his radiation 11 years earlier caused the OPT. However, he had clinical and radiologic evidence of relapsing-remitting MS at the time of his OPT. We believe that the new signal changes in both superior cerebellar peduncles and the pons close to the central tegmental tract (in the Guillain-Mollaret triangle), which changed with serial imaging, were demyelinating in origin and lead to the OPT. Recent experimental data show that inferior olivary synchronization and modulation by the cerebellum play a role in OPT.1
A literature search showed few reports of OPT from MS. An article that reviewed 171 publications on palatal myoclonus found 9 cases of MS among 287 cases.2-6 However, on review many of these cases do not meet the current clinical criteria for diagnosis of MS. Neuroimaging or pathology was not available in any. There is one report of bilateral OPT with abnormal T2 signal in the pons in a case of clinically definite MS.7
We believe ours is the first case where resolution of OPT was seen in MS. It is possible that patients with MS and pendular nystagmus have not had the palate examined and actually had OPT. Subsequent imaging documented the improvement of signal changes in both the superior cerebellar peduncles and pons, possibly explaining why the OPT resolved, similar to resolution of other brainstem findings in patients with MS. The inferior olivary nuclei, however, continued to show increased T2 signal on serial MRI.
Examination of the palate and careful imaging review should be done in patients with MS and pendular nystagmus.
DISCLOSURES
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/cp for full disclosures.
REFERENCES
- 1.Shaikh AG, Hong S, Liao K, et al. Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity. Brain 2010;133:923–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deuschl G, Mischke G, Schenck E, Schulte-Monting J, Lucking CH. Symptomatic and essential rhythmic palatal myoclonus. Brain 1990;113:1645–1672 [DOI] [PubMed] [Google Scholar]
- 3.Herrmann C, Brown JM. Palatal myoclonus: a reappraisal. J Neurol Sci 1967;5:473–492 [DOI] [PubMed] [Google Scholar]
- 4.Yap CB, Mayo C, Barron K. ‘Ocular bobbing’ in palatal myoclonus. Arch Neurol 1968;18:304–310 [DOI] [PubMed] [Google Scholar]
- 5.Chokroverty S, Barron KD. Palatal myoclonus and rhythmic ocular movements: a polygraphic study. Neurology 1969;19:975–982 [DOI] [PubMed] [Google Scholar]
- 6.Gresty MA, Ell JJ, Findley LJ. Acquired pendular nystagmus: its characteristics, localizing value and pathophysiology. J Neurol Neurosurg Psychiatry 1982;45:431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Revol A, Vighetto A, Confavreux C, Trillet M, Aimard G. Myoclonisoculo-vtlo-palatines et sclerose en plaques. Rev Neurol 1990;146:518–521 [PubMed] [Google Scholar]