Summary
Mechanical thrombectomy devices comprise a wide array of endovascular tools cleared for removing thrombi from the neurovasculature in acute ischemic stroke patients. In the United States, 3 classes of mechanical thrombectomy devices have been cleared by the Food and Drug Administration: coil retrievers in 2004, aspiration devices in 2008, and stent retrievers in 2012. Available evidence and fundamental physiologic principles suggest that mechanical thrombectomy is appropriate for patients with large, proximal intracranial artery occlusions due to emboli of cardiac or arterial origin and is most effective when performed as soon as feasible after onset in patients known to still be harboring salvageable penumbral tissue. This review summarizes the mechanism of action of these devices, clinical trial results for efficacy and safety, and clinical use.
Mechanism of action
Mechanical thrombectomy devices seek to salvage ischemic, but not yet fully infarcted, brain by restoring perfusion through the initially occluded artery. In randomized trials and large series, recanalization has been shown to increase the odds of good final functional outcome more than 4-fold.1
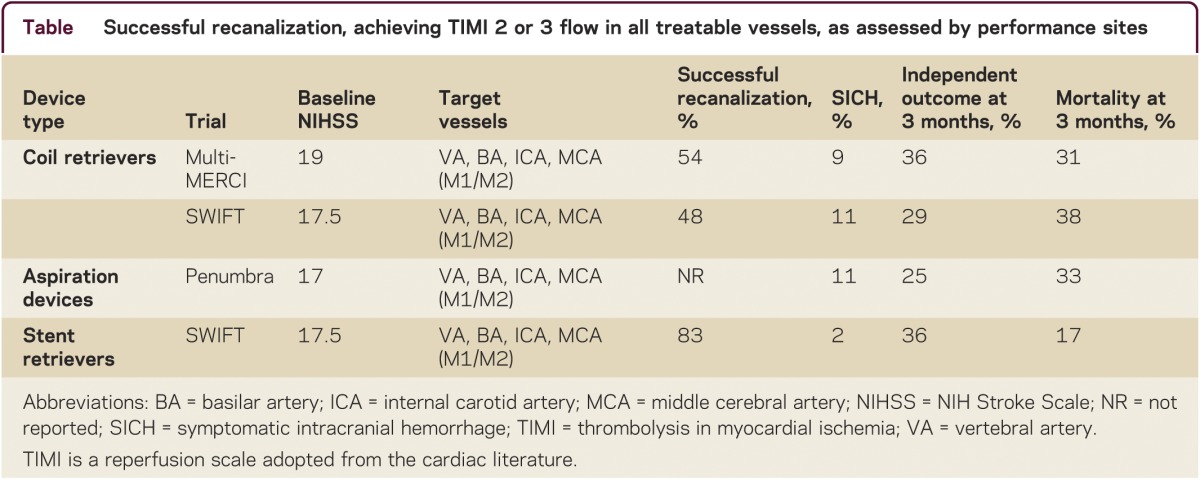
Each class of mechanical thrombectomy devices achieves recanalization through somewhat different biomechanical mechanisms (table).
Table.
Successful recanalization, achieving TIMI 2 or 3 flow in all treatable vessels, as assessed by performance sites
Coil retrievers
The coil retrievers are composed of Nitinol shape-memory wire and delivered through a microcatheter across the target clot. As the device is extruded from delivery catheter, it immediately reassumes its native coil form. The neurointerventionalist deploys the loops of the coil through the clot to engage the thrombus, and then pulls both coil and clot back into the catheter, like pulling a cork from a wine bottle. Currently 10 coil retriever devices are available, all under the trade name Merci (Stryker), differing in helix length, diameter, curvature, and presence of additional cascading filaments, to enable use in a variety of target arteries and clot types.
Aspiration devices
Suction thrombectomy devices employ vacuum aspiration to remove occlusive clot in acute ischemic stroke. While manual aspiration of target thrombi can be performed through any microcatheter, progress in developing suction thrombectomy devices required a technical solution to the problem of clogging of aspiration tips, a common occurrence when applying suction through a bore small enough to fit within intracranial arteries. The Penumbra System overcomes this obstacle by adding an in bore separator wire with a bulbous tip that the operator continually advances and retracts, disrupting attached clot and pulling in thrombus ahead of the catheter.
Stent retrievers
The stent retrievers are self-expanding stents that are deployed in the occluded vessel within the thrombus, pushing it aside and entangling it within the stent struts. The stent and thrombus are then withdrawn back into the delivery catheter. The first retrievable stent approved in the United States is the Solitaire Flow Restoration Device (Covidien), and several others have already been approved in Europe, including Trevo (Stryker), Revive (Codman), MindFrame (MindFrame Inc.), ReStore (Reverse Medical), and Pulse (which combines a stent retriever and an aspiration device, Penumbra).
Efficacy results in clinical trials
To date, the mechanical thrombectomy devices have been cleared by regulatory authorities and are recognized in national treatment guidelines as tools rather than treatments. Their registration trials required them to demonstrate technical efficacy in removing clots from the neurovasculature, not clinical efficacy in improving final patient outcome.
Coil retrievers were approved based on 2 trials: Mechanical Embolus Removal in Cerebral Ischemia (MERCI) and Multi-MERCI. The Multi-MERCI trial is more relevant to current practice, as it tested a later generation of devices and enrolled both patients who were ineligible for and who failed IV tissue plasminogen activator (tPA). Multi-MERCI was a single-arm, multicenter study that enrolled 177 patients.2 Substantial partial or complete recanalization was achieved in 55% of patients with the coil retrievers alone and 69% with rescue use of additional endovascular therapies. This recanalization rate substantially exceeded the historical comparator (the heparin arm of the PROACT 2 trial, with an 18% partial recanalization rate), indicating technical efficacy. Independent neurologic outcome (modified Rankin Disability Scale 0–2) at 90 days was more frequent (49% vs 10%, p < 0.001), and mortality rates lower (25% vs 52%, p < 0.001) with successful compared with unsuccessful recanalization.
The Penumbra suction thrombectomy system was cleared based on results from a prospective single-arm multicenter trial that tested the safety and efficacy of the device in 125 patients.3 Inclusion/exclusion criteria were similar to the ones used in the MERCI and Multi-MERCI trials. Partial or better recanalization was reported in 82% and complete recanalization in 23% of patients, the latter value equivalent to that attained with coil retrievers in MERCI and Multi-MERCI. Independent neurologic outcome tended to be more frequent with successful compared with unsuccessful recanalization (29% vs 9%, p = 0.06).
The first stent retriever to be cleared by the Food and Drug Administration, the SOLITAIRE FR, was authorized based on the SOLITAIRE FR with the Intention for Thrombectomy (SWIFT) study. In this multicenter, randomized trial, Solitaire was compared to the Merci Retriever as the initial device intervention in 113 acute ischemic stroke patients.4 The primary efficacy outcome, successful recanalization (per Core Lab) without SICH, was achieved more often in SOLITAIRE vs MERCI patients, 60.7% vs 24.1% (p = 0.0001). Good neurologic outcome at 90 days was more frequent (58% vs 33%, p = 0.02) and mortality rates lower (17% vs 38%, p = 0.02) when the stent rather than the coil retriever was employed.
Definitive data regarding the efficacy of mechanical thrombectomy devices in improving final clinical outcome over medical therapy alone awaits the conclusion of ongoing trials. One trial, the Interventional Management of Stroke 3 (IMS 3) Trial, has been stopped for futility. However, the implications for IMS 3 for mechanical thrombectomy may be limited, as many patients enrolled in the endovascular arm of IMS 3 were treated with intra-arterial fibrinolytics drugs rather than mechanical thrombectomy, essentially none of the patients were treated with the most technically efficacious device class, the stent retrievers, and the trial included large number of patients with no occlusions or small, distal occlusions, which are less likely to benefit from mechanical retrieval.
Adverse events
The most clinically salient adverse effects of mechanical thrombectomy are 1) intracerebral hemorrhage, as with all reperfusion therapies, 2) subarachnoid hemorrhage, related to device injury to the vessel wall, 3) fragmentation of the target thrombus leading to embolization to a new, more distal territory. Symptomatic intracranial (intracerebral or subarachnoid) hemorrhage (SICH) occurs in ∼10% of patients with first generation devices (Merci and Penumbra), but is substantially lower with the Solitaire stent retriever (2%–4%).
Use in practice
Given the paucity of randomized trial literature to guide therapy, all practice recommendations must be considered provisional. Our current approach is guided by the following 4 fundamental physiologic principles:
Large arteries, not small: Mechanical thrombectomy devices work well in large, proximal arteries, rapidly debulking large clot burdens that are resistant to chemical fibrinolysis. Conversely, mechanical thrombectomy devices are currently not well suited for distal arterial branches (hard to navigate to and device diameters too large) and are not options for penetrator occlusions, while chemical fibrinolysis works well on those targets.
Cardiac or arterial origin emboli, not in situ atherothrombi: Mechanical thrombectomy devices work well when the target intracranial occlusion is an embolus that has arisen from the heart or a proximal aortocervical arterial source and landed in a relatively normal recipient artery. When the occlusive lesion is an in situ intracranial atherosclerotic plaque with supervening thrombosis, retrieval devices may catch on the plaque and aspiration devices are effective only for the thrombus component. In situ atherothrombi are probably better treated with balloon angioplasty and stenting, which accomplish controlled cracking and dissection of the underlying atherosclerotic lesions.
Faster, faster, faster: Speed is of the essence in treating large artery cerebral ischemia, as 2 million more nerve cells are lost every minute in which reperfusion does not occur. All sites performing mechanical thrombectomy should have vigorous continuous quality improvement programs, targeting door to arterial puncture times of less than 90 minutes. Since IV fibrinolysis can be started sooner than endovascular therapy and does not increase the risk of endovascular therapy,2–4 patients who are candidates for IV tPA should receive standard dose (0.9 mg/kg) IV tPA on their way to the neurocath lab.
Imaging selection beyond 3 hours: In the first 3 hours after a large artery vascular occlusion, virtually every patient still harbors at least some salvageable penumbral tissue and may benefit from intervention. Thereafter, a steadily increasing proportion of patients will have fully completed their initial infarct. Multimodal CT or MRI may assist in identifying the patients in the 3- to 14-hour window who may still benefit from reperfusion.5 Most likely to benefit are patients with a small core (<20 cc) of irreversibly infarcted tissue and a large penumbral, salvageable region (>20 cc). Highly unlikely to benefit are patients with large cores (>90 cc) and patients with no penumbra. With intermediate patterns, additional patient-specific factors guide therapy decisions, including age, dominant vs nondominant hemisphere involvement, prestroke function, and patient and family outcome preferences.
DISCLOSURES
R. Raychev reports no disclosures. J. Saver reports the following disclosures: The University of California, Regents receive funding for Dr. Saver's services as a scientific consultant regarding trial design and conduct to BrainsGate, CoAxia, Covidien, Talecris, PhotoThera. Dr. Saver is an investigator in the NIH FAST-MAG, MR RESCUE, ICES, CUFFS, CLEAR-ER, and IMS 3 multicenter clinical trials for which the UC Regents receive payments based clinical trial performance; has served as an unpaid site investigator in a multicenter trials run by Lundbeck and Mitsubishi for which the UC Regents received payments based on the clinical trial contracts for the number of subjects enrolled; is an employee of the University of California, which holds a patent on retriever devices for stroke; and is funded by NIH-National Institute of Neurological Disorders and Stroke Awards P50 NS044378 and U01 NS 44364. Go to Neurology.org/cp for full disclosures.

REFERENCES
- 1.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007;38:967–973 [DOI] [PubMed] [Google Scholar]
- 2.Smith WS, Sung G, Saver J, et al. Mechanical thrombectomy for acute ischemic stroke: final results of the Multi MERCI trial. Stroke 2008;39:1205–1212 [DOI] [PubMed] [Google Scholar]
- 3.The penumbra pivotal stroke trial: safety and effectiveness of a new generation of mechanical devices for clot removal in intracranial large vessel occlusive disease. Stroke 2009;40:2761–2768 [DOI] [PubMed] [Google Scholar]
- 4.Saver J, Jahan R, Levy EI, et al. Primary results of the SOLITAIRE™ With the Intention for Thrombectomy (SWIFT) multicenter, randomized clinical trial [online]. Available at:http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@global/documents/downloadable/ucm_436093.pdf Accessed May 5, 2012.
- 5.Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol 2006;60:508–517 [DOI] [PubMed] [Google Scholar]


