Abstract
Introduction
Adenosine is an endogenous nucleoside that accumulates in the extracellular space in response to metabolic stress and cell damage. Extracellular adenosine is a signaling molecule and it signals by activating four G-protein coupled receptors: the A1, A2A, A2B and A3 receptors. Since the discovery of A3 adenosine receptors accumulating evidence has identified these receptors as potential targets for therapeutic intervention.
Areas covered
A3 adenosine receptors are expressed on the surface of most immune cell types, including neutrophils, macrophages, dendritic cells, lymphocytes and mast cells. A3 adenosine receptor activation on immune cells governs a broad array of immune cell functions, which include cytokine production, degranulation, chemotaxis, cytotoxicity, apoptosis, and proliferation. In accordance with their multitudinous immunoregulatory actions, targeting A3 adenosine receptors has been shown to impact the course of a wide spectrum of immune-related diseases, such as asthma, rheumatoid arthritis, cancer, ischaemia, and inflammatory disorders.
Expert opinion
Given the existence of both pre-clinical and early clinical data supporting the utility of A3 adenosine receptor ligands in treating immune-related diseases, further development of A3 adenosine receptor ligands is anticipated.
1. Introduction
1.1. Adenosine and its receptors
The endogenous purine nucleoside, adenosine is a signaling molecule, which is present in the extracellular space at low concentrations in undisturbed tissue. The levels of adenosine are increased in stressful conditions, such as inflammation, ischaemia, hypoxia or trauma [1, 2]. Adenosine exerts organ protective actions through binding to one or more of the 4 transmembrane adenosine receptors, A1, A2A, A2B and A3 [3-8] Adenosine receptors are members of the family of G protein coupled receptors (GPCR) and their expression levels vary in different organs and cell types [9]. In this review, we focus on the A3 adenosine receptor (A3AR) and discuss its regulatory roles in the different cell types of the innate and adaptive immune systems, its role in regulating the course of various immune-related diseases, and the therapeutic potential of its activation or inactivation.
1.2. A3ARs
The human A3AR consists of 318 amino acids. The A3AR shows large interspecies differences in its sequence; the homology between the rat and human A3AR is only 74%. Interestingly, two splice variants of the A3AR receptor can be found in rats but the significance of this phenomenon is unknown [10]. The A3AR is expressed at the mRNA level in most organs of both rodents and human, including testis, lung, kidneys, placenta, heart, brain, spleen, and liver [11]. In addition, the A3AR protein has been detected using radioligand binding or functional tests in all these organs in different species. Finally, most of the cell types of the immune system express functional A3ARs on their surface [2].
1.3. A3AR agonists and antagonists
The prototypical and most widely used A3AR agonists are IB-MECA (figure 1a) and Cl-IB-MECA (figure 1b). Both IB-MECA and Cl-IB-MECA are adenosine derivatives bearing a lipophilic substituent (3-iodobenzyl) at the 6-amino group and ribose modification in the 5′ position [12]. There is an additional 2-chloro substituent in Cl-IB-MECA, which makes it more selective than IB-MECA [13]. Another highly selective agonist is CP532903 (figure 1c). While the methylxanthines caffeine and theophylline are classical antagonists for the A1AR, A2AAR, and A2BAR, their affinity for the A3AR is low. Therefore, antagonists for this subtype have been developed by modifying different molecules with heterocyclic structures. One family of selective A3AR antagonists comprises the derivatives of 1.4-dihydropiridines, which are known as inhibitors of L-type Ca2+ channels. After various modifications, including the introduction of a 6-phenyl group, these molecules bind with high affinity and selectivity for the human A3AR [14]. Since there are significant differences between the sequences of the human and rat A3ARs, most of the antagonists developed for the human receptor bind with much lower affinity to rat and other rodent A3AR. Prominent members of this family are MRS1191, MRS1334 and MRS1523 (figure 2a). The pyridylquinazoline derivative VUF5574 (figure 2b) and the triazoloquinazoline MRS1220 are also used as selective A3AR antagonists [13], both with selectivity only in humans. There are also highly selective antagonists of the human A3AR among the flavonoids, which are naturally occurring phenolic derivatives. MRS1067 is the most important member of this family [15].
Figure 1.
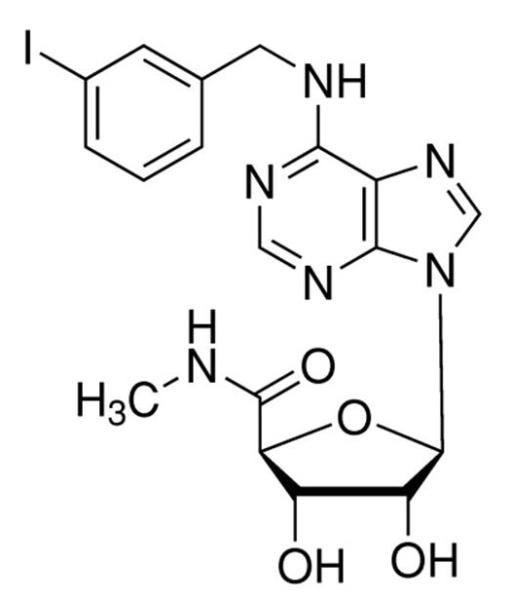
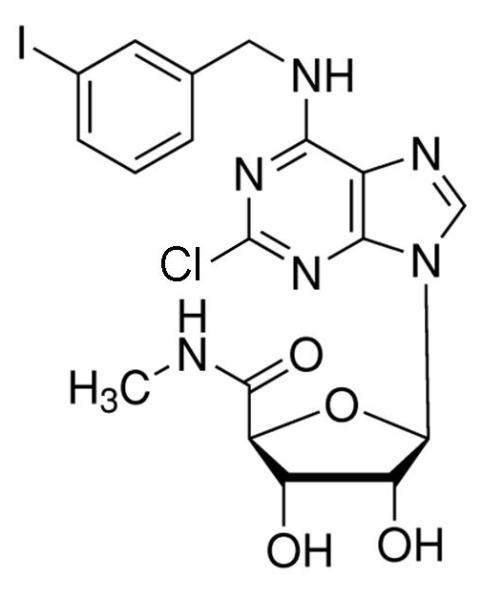
Chemical structures of A3AR agonists: a. IB-MECA, b. Cl-IB-MECA, c. CP532903.
Figure 2.
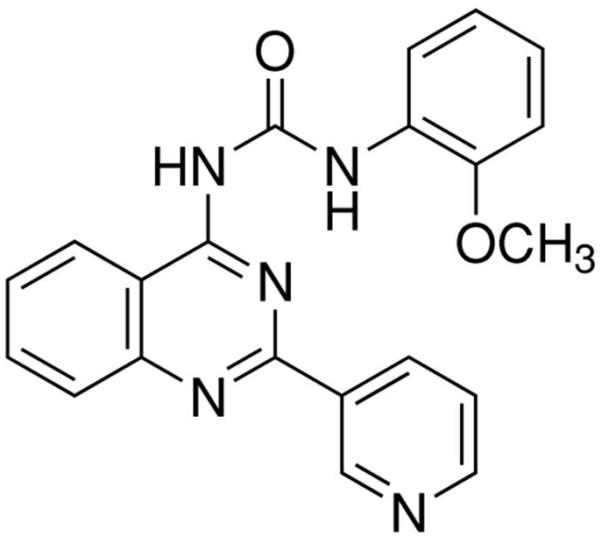
Chemical structures of A3AR antagonists: a. MRS1523, b. VUF5574.
1.4. A3AR signaling
A3AR receptor stimulation inhibits adenylyl cyclase activation via Gi protein, which in turn results in a decrease of cAMP levels [1, 2]. A3AR activation can also stimulate the phospholipase C (PLC) pathway, which results in the elevation of intracellular inositol 1,4,5-trisphosphate and calcium (Ca2+) levels [11]. The A3AR also can stimulate mitogen-activated protein kinases (MAPK), such as extracellular-signal regulated kinase 1/2 (ERK1/2) and p38 through the upstream activation of phosphatidylinositol-3-kinase (PI3K) [16]. The A3AR associated intracellular signaling pathways are summarized on Figure 3.
Figure 3.
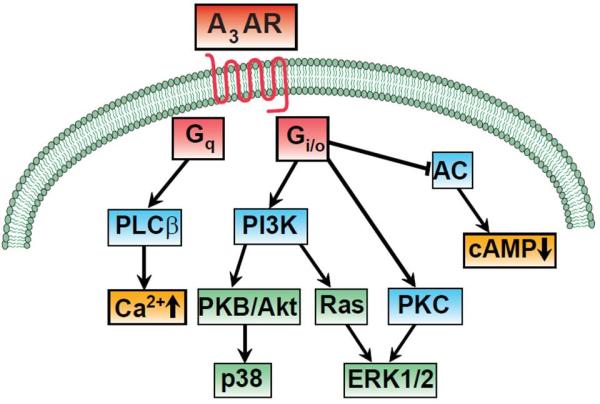
Signaling pathways activated by A3AR.
2. A3ARs on immune cells
2.1. Neutrophils
Neutrophils are part of the first line of defense of the immune system; their function is to kill pathogens, remove debris of injured tissue, and secrete factors that regulate inflammation [17]. It was shown recently that the A3AR, along with the P2Y2 receptor, has a major role in the regulation of neutrophil migration to sites of inflammation. Chen and coworkers [18] showed that human neutrophils release ATP at the leading edge of the cell surface, and the released ATP is rapidly hydrolyzed to adenosine by the ecto-enzymes nucleoside triposphate dephosphorylase (CD39) and 5′-ectonuleotidase (CD73). Adenosine potentiates neutrophil migration by activating the A3AR, which is recruited to the leading edge of the migrating neutrophils. This autocrine positive feedback mechanism provides signal amplification and regulates the chemotactic gradient sensing of the cells. Subsequently, the same group of investigators confirmed the potentiating role of A3ARs in neutrophil migration using a mouse sepsis model [19]. In contrast, data published by Van der Hoeven and coworkers suggests that A3AR activation inhibits neutrophil migration and superoxide production by suppressing the monomeric GTP-ase Rac, which small G protein is a central regulator of neutrophil functions [20, 21]. These conflicting results may be explained by the differences between the species and chemoattractants used. Chen et al. used both fMLP-stimulated human neutrophils and W peptide-stimulated neutrophils from mouse bone marrow. In contrast, Van der Hoeven and coworkers used neutrophils from mouse bone marrow that were stimulated with fMLP, C5a, IL-8, and PAF. The A3AR agonists used were also different by the two groups, IB-MECA and CP-532,903 respectively. Although these differences provide a possible explanation for the opposite effects of A3AR activation described by the two groups, further investigation is necessary to reveal more completely the regulatory role of A3AR stimulation on neutrophil functions.
2.2. Macrophages
Macrophages are mononuclear phagocytes that produce high amounts of inflammatory mediators early during inflammation, and later participate in the resolution of inflammation by secreting anti-inflammatory cytokines, and by removal of pathogens, cell debris and apoptotic cells [4]. Tumor necrosis factor-α (TNF-α) is an important inflammatory cytokine produced by macrophages in response to different stimuli including toll-like receptor (TLR) activation. A3AR agonists have been shown to inhibit TNF-α production in various macrophage populations [22-24]. Lee and coworkers studied the effect of A3AR activation on LPS induced TNF-α production by microglia, the resident macrophage of the central nervous system, and found that A3AR stimulation decreased TNF-α production by inhibiting the PI3K/Akt and NF-κB pathways [25]. Martin and coworkers confirmed the inhibitory effect of A3AR activation on TNF-α production by the RAW264.7 mouse macrophage cell line [26]. They also found that the A3AR-mediated inhibition of TNF-α production coincided with reductions in the calcium dependent activation of NF-κB and ERK 1/2. It is noteworthy that the conclusions of all these studies regarding a primary role of A3ARs in decreasing TNF-α production are based on pharmacological A3AR ligands. In contrast, the results of other studies employing adenosine receptor knockout mice suggest that the A2AAR and A2BAR rather than the A3AR mediate the inhibitory effect of adenosine on macrophage TNF-α production [27-29].
In addition to decreasing TNF-α release, A3AR ligands have been shown to inhibit other macrophage functions, as well. We showed using a pharmacological approach that IB-MECA reduced production of the chemokine macrophage inflammatory protein (MIP) 1α in mouse macrophages [28, 30]. Barnholt and coworkers found that A3AR receptor stimulation inhibited IFN-γ–induced gene expression (including IRF1, iNOS and CD36 expression) in RAW264.7 murine and THP-1 human macrophages by decreasing STAT-1 phosphorylation [31]. In addition, IB-MECA inhibited the respiratory burst of human monocytes by blocking NADPH oxidase activity [32, 33]. Besides the regulation of the release of inflammatory mediators, A3AR activation on human macrophages promotes tissue remodeling by enhancing the secretion of matrix metalloproteinases (MMP) [34].
In conclusion, A3AR activation has mainly anti-inflammatory and tissue remodeling effects on macrophages.
2.3. Dendritic cells
Dendritic cells (DC) are specialized antigen-presenting cells that activate naive T cells thereby initiating adaptive immune responses. The expression level of adenosine receptors varies based on the maturation state of dendritic cells. In immature human dendritic cells, A1ARs and A3AR are expressed predominantly and stimulating these receptors induces Ca2+ mobilization from intracellular stores, actin polymerization, and chemotaxis [35]. Mature dendritic cells, however, down-regulate their A3ARs and express mainly A2AARs. In contrast, Dickenson et al found that A2AARs and A3ARs are expressed on the mouse dendritic cell line XS-106, and that stimulating these receptors inhibits TNF-α release [36]. Moreover, recent studies have uncovered an important anti-inflammatory role for the A2BAR in dendritic cells [6, 37-39].
In conclusion, stimulating the A3AR enhances the chemotaxis of human immature dendritic cells; however, the role of A3ARs in regulating mature dendritic cell function is less well established.
2.4. Mast cells
Mast cells are important regulators of not only allergic responses but also many tissue functions like blood flow and coagulation, smooth muscle contraction, wound healing, and various innate and adaptive immune responses [40]. Ramkumar and coworkers showed for the first time that A3AR activation augmented degranulation of the mast cell line RBL-2H3 [41]. Reeves and coworkers confirmed this result by reporting that IB-MECA increased the degranulation of rat mast cells in vitro [42]. Subsequently, Zhong and coworkers demonstrated that the A3AR is highly expressed in murine primary lung mast cells, and that A3AR stimulation in these cells induces degranulation and histamine release, which is mediated through the Gi and PI3K pathways and through an increase of intracellular Ca2+ levels [43].
In vivo studies also addressed the effect of A3AR activation on mast cell degranulation. For example, IB-MECA injection into mice was found to augment plasma protein extravasation and since this effect was abolished in animals treated with the histamine-depleting compound 48/80, these results corroborated the stimulatory effect of IB-MECA on mast cell degranulation [42]. A similar study by Smith and coworkers [44] documented that Cl-IB-MECA treatment of mice augmented serum histamine levels and that this effect was absent in animals pre-treated with compound 48/80. Genetic studies confirmed the role of A3ARs in activating mast cells, as Cl-IB-MECA potentiated antigen-dependent degranulation of murine bone marrow-derived mast cells, and this potentiating effect was not detectable in cells obtained from A3AR deficient mice [45]. However, there is no evidence that the A3AR plays a similar role in human mast cells and the A2BAR appears to assume the role of A3AR in increasing the release of inflammatory mediators in human mast cells [46, 47].
Jin and coworkers reported that inosine stimulated degranulation in RBL-2H3 cells, and caused mast cell-dependent constriction of arterioles in hamsters. These effects were attenuated by A3AR blockade indicating a role for the A3AR in mediating the mast cell-activating effect of inosine [48]. These observations were confirmed by Tilley and coworkers [49], who found that adenosine and inosine increased cutaneous vasopermeability in mice, effects that were abolished in A3AR or mast cell deficient animals.
In addition to degranulation, there is evidence that A3ARs can also control other mast cell functions. For example, Gao and coworkers demonstrated that IB-MECA protected rat RBL-2H3 cells from apoptosis caused by exposure to UV light [50]. Furthermore, Feoktistov and coworkers showed that A3AR activation stimulated angiopoietin-2 expression in HMC-1 human mast cells [51]. In conclusion, A3AR activation influences numerous mast cell functions in rodents and humans including degranulation, apoptosis and regulation of vasopermeability.
2.5. Lymphocytes
Studies using pharmacological approaches or knockout animals have shown that A3ARs do not directly affect CD4+ lymphocyte function [2]. Nevertheless, it appears that A3AR expression is up-regulated in human CD4+ lymphocytes after activation with phytohemagglutinin [52] and in murine macrophages stimulated with anti-CD3 antibody and antigen-presenting cells [53]. In contrast, there is evidence that A3ARs can dictate CD8+ lymphocyte activation. For example, MacKenzie and coworkers reported that adenosine inhibited the adhesion of anti-CD3 antibody-activated cytotoxic T cells to adenocarcinoma cells through A3ARs [54]. Hoskin et al. found that the A3AR inhibited the tumoricidal activity of anti-CD3-activated cytotoxic T cells, and that this inhibition was achieved by reducing the mRNA levels of granzyme, perforin and FAS ligand, and by inhibiting interferon (IFN)-γ and interleukin (IL)-2 release [55]. In addition, Harish and coworkers showed that Cl-IB-MECA treatment potentiated natural killer (NK) cell activity in melanoma bearing mice [56] and Jeffe and coworkers found that IB-MECA increased IFN-α release by IFN-γ stimulated human NK cells [57].
Taken together, the A3AR exerts inhibitory effects on cytotoxic T cell functions and stimulatory effects on NK cell functions.
3. A3AR in disease states
3.1 Rheumatoid arthritis (RA)
RA is a chronic inflammatory disease, which affects the joints. During the course of RA, immune cells infiltrate the synovium, a delicate membrane on the joint surface, and release inflammatory mediators including TNF-α. This chronic inflammation ultimately leads to cartilage destruction, bone erosions, and joint deformities [58, 59]. Methotrexate is an anti-inflammatory drug that is widely used in the therapy of RA. An increasing body of evidence is available that methotrexate promotes adenosine release and that the anti-inflammatory effects of methotrexate are mediated by the released adenosine [60]. This observation coupled with the notion that adenosine receptor activation on innate immune cells inhibits TNF-α release made adenosine receptors promising targets in RA treatment. This idea was further underlined by data showing that the A3AR was up-regulated in peripheral blood mononuclear cells of RA patients and cells of synovial tissue of rats with adjuvant induced arthritis [61-63].
We tested for the first time the potential of IB-MECA, the prototypical A3AR agonist at the time, in alleviating the course of collagen-induced arthritis in mice. We found that IB-MECA reduced the severity of joint inflammation by inhibiting the formation of MIP-1α, the cytokine IL-12, and the infiltration of neutrophils [30]. These results were later confirmed by Baharav and coworkers, who found that both IB-MECA and Cl-IB-MECA ameliorated clinical and histological features of arthritis in various mouse and rat arthritis models [64]. Further experiments revealed that IB-MECA decreased the expression of PI3K, IKK, and NF-κB, implicating these signaling pathways as mediating the alleviating effect of IB-MECA on the course of arthritis. In addition to IB-MECA, another A3AR agonist, CF502 also inhibited the clinical and pathological manifestations of adjuvant induced arthritis in rats [65, 66].
Ochaion et al. found that combined methotrexate and IB-MECA treatment was more effective than the two drugs alone. In addition, methotrexate augmented the expression of A3AR on immune cells rendering them more sensitive to the effect of IB-MECA [67]. IB-MECA was found to be protective also in a rat osteoarthritis model, and the protective effect was associated with a down-regulation of the NF-κB pathway [68].
Finally, in agreement with animal model data, clinical trials using IB-MECA as a therapeutic agent for RA patients showed that it was safe and well-tolerated and that IB-MECA treatment resulted in improvements of symptoms of the disease [69].
3.2. Asthma
There is substantial evidence that adenosine can induce bronchoconstriction in patients with asthma or chronic obstructive pulmonary disorder (COPD) but not in healthy individuals, and that adenosine levels are elevated in the bronchoalveolar lavage fluid and exhaled breath condensate of patients with asthma [70-72]. In addition, A3AR expression was found to be elevated in the airways of asthma patients, and in vitro studies suggest that A3ARs on human eosinophils mediates inhibition of chemotaxis, degranulation and superoxide anion release, which are important pathophysiological events during airway inflammation [73-75]. Tilley and coworkers exposed mice to aerosolized adenosine and found that it caused bronchoconstriction, degranulation of airway mast cells and neutrophil infiltration in wild-type mice, but not in A3AR deficient mice [76]. Also, 5′ N-ethylcarboxamide (NECA), a non-selective adenosine receptor agonist, caused airway hyperresponsiveness in wild-type mice, and this effect was abolished in A3AR or mast cell deficient mice [77].
Mucin hypersecretion, besides airway hyperresponsiveness, is also an important feature of asthma and other chronic lung diseases. Young and coworkers investigated the role of A3AR in regulating mucin hypersecretion in mice in which chronic inflammation in the lung is induced by deficiency in adenosine deaminase (ADA), which converts adenosine to inosine. Genetic or pharmacological inactivation of A3AR in ADA deficient mice prevented mucin production and also decreased the migration of eosinophils into the airways [78]. In another model in which pulmonary inflammation was induced by ovalbumin, IB-MECA treatment increased mucin secretion in wild-type mice and this effect was absent in A3AR deficient mice [79]. In conclusion, A3ARs contribute to mucin hypersecretion in the setting of pulmonary inflammation.
From a translational point of view, it is noteworthy that there are marked differences in the role of A3ARs in regulating certain aspects of airway inflammatory diseases between different species. In murine models, A3AR activation causes eosinophil infiltration into the airways, which suggests the therapeutic potential of A3AR antagonists in the treatment of asthma. On the other hand, A3AR activation appears to inhibit the functions of human eosinophils, indicating that A3AR agonists might be used in asthma therapy in humans. Further investigation is necessary to evaluate which approach is more promising in the treatment of human asthma.
3.3 Cancer
There are several lines of evidence indicating that the expression of A3AR is higher in tumors, such as breast and colorectal carcinoma, as compared to relevant adjacent normal tissue [80, 81]. A3AR expression is also elevated in the peripheral blood cells of patients with colorectal cancer compared to healthy inviduals [81]. These observations raise the possibility of using the A3AR as a diagnostic marker and therapeutic target for the treatment of cancer [80, 81]. This idea is also supported by extensive data showing that A3AR ligands can induce apoptosis in tumor cells. For example, Fishman and coworkers [82] injected mice with colon carcinoma cells, and then studied the effect of oral treatment with IB-MECA on tumor growth. They found that tumor growth was suppressed in the group treated with IB-MECA in comparison with the group treated with vehicle [82]. The beneficial effect of IB-MECA was associated with up-regulation of GSK-3β and down-regulation of NF-κB and the oncogenes cyclin D1 and c-Myc in extract prepared from tumor lesions. Similar to these results, Cl-IB-MECA induced apoptosis and inhibited tumor growth in rats with hepatocellular carcinoma [83]. Cl-IB-MECA treatment also inhibited tumor growth in mice injected with Hep-3B hepatoma cells and induced apoptosis in tumor cells in vitro [84]. Furthermore, A3AR agonists inhibited cancer cell proliferation also in lung and thyroid cancer, and leukaemia [85-87]. In addition to the tumor growth inhibitory effect, the A3AR is also able to suppress the formation of metastases in a model of rat prostate cancer and in the liver of mice inoculated with colon carcinoma cells [88, 89]. Finally, it is noteworthy that in some of these studies the agonists exerted effects only at high concentrations [85] or independently of the A3AR [86].
In contrast, there is also evidence to suggest a pro-tumoral role for A3AR activation. For example, Gessi et al. reported that A3AR activation augmented the invasion of U87MG glioblastoma cells by increasing MMP-9 levels [90]. Also, Gessi and coworkers found that A3AR activation stimulated the proliferation of colon cancer cell lines via a mechanism involving ERK1/2 activation [91]. It was recently shown that A3 receptor stimulation elevates hypoxia inducible factor 1 (HIF-1) expression in A375 human melanoma and in human glioblastoma cells under hypoxic conditions [92-94]. In addition, A3AR activation increases the production of vascular endothelial growth factor (VEGF) and angiopoietin-2 [92-94]. Since HIF-1, VEGF, and angiopoietin-2 are critical factors that support tumor angiogenesis, A3AR activation may have undesired pro-tumor effects that warrant further investigation.
In summary, data about the role of the A3AR in regulating tumor growth are somewhat controversial as there is evidence for both pro-tumoral and also anti-tumoral activity.
3.4. Ischaemia
Although A3AR expression in myocardial tissue is low, A3AR agonists have cardioprotective effects in myocardial ischaemia-reperfusion models [11]. Wan et al. found that the A3AR agonist CP-532,903 reduced infarct size in an in vivo mouse model and was also protective in an isolated heart model of global ischaemia/reperfusion injury, and the protective mechanism involved activating sarcolemmal KATP channels [95]. Ge and coworkers investigated the effects of Cl-IB-MECA in an in vivo mouse model of infarction induced by 30 minutes of coronary occlusion and 24 hours of reperfusion. Treatment of wild type mice with Cl-IB-MECA during reperfusion reduced myocardial infarct size, but failed to reduce the infarct size in A3AR knockout mice and in chimeric mice lacking the expression of A3AR on bone marrow derived cells [96]. These results suggest a role of neutrophil A3AR in the protective effect of Cl-IB-MECA and correlate with the earlier results of Jordan and coworkers who reported that IB-MECA inhibits neutrophil associated reperfusion injury [97].
Doxorubicin (DOX) is a drug used in the treatment of leukaemias and solid tumors but its clinical use is limited by its cardiotoxicity [98-103]. A3AR activation has been shown to protect against DOX-induced cardiotoxicity by restoring Ca2+ homeostasis [104], which suggests that the combination of DOX with A3AR agonists may prevent the side effects of DOX and allow wider use of this drug in anti-cancer therapy.
A3AR activation protects not only against cardiac ischaemia but also against stroke. Chen et al. reported that Cl-IB-MECA pre-treatment was protective against hypoxia in primary cortical cultures and decreased the infarct size in an in vivo model of cerebral ischeamia induced by transient middle cerebral artery (MCA) ligation in wild-type mice but not in A3AR knockout mice. In addition the infarct size induced by MCA ligation was increased in A3AR knockout mice compared to wild-type controls [105]. Shen et al. found that inosine pre-treatment reduced MCA occlusion-induced cerebral infarction in rats and that this effect was inhibited by administering the A3AR antagonist, MRS1191, which suggests that the effect of inosine is A3AR dependent [106]. Finally, A3AR activation was also found to be protective in a model of subarrachnoid hemorrhage [107].
In contrast, there are also data that support the neurotoxic effects of A3AR activation. Sei and coworkers treated cultured rat cerebellar neurons with AR agonists and found that Cl-IB-MECA, but not A1AR, A2AAR or A2BAR agonists, induced cell death in cultured neurons [108]. Cl-IB-MECA induced apoptosis also in primary cultures of rat astrocytes and in C6 glial cells by reducing the expression of Bcl-2 and enhancing caspase-3 activation [109]. Von Lubitz and coworkers studied the effect of IB-MECA on forebrain ischaemia in gerbils and found that although chronic IB-MECA treatment improved postischemic cerebral blood circulation, survival, and neuronal preservation, acute preischemic administration of IB-MECA impaired blood flow, enhanced mortality and neuronal damage [110]. The opposite effects of chronic and acute treatment may be related to the desensitization of A3AR by chronic activation, which is the consequence of the down-regulation of Gi protein [111]. Taken together, both neuroprotective and neurotoxic effects of A3AR activation have been described.
Unlike in the case of cardiac and cerebral ischeamia, A3AR activation seems to be consistently harmful in renal ischaemic injury. Lee and coworkers studied the role of A3ARs in ischaemia-induced renal failure in rats by inducing ischaemia with micro aneurysm clips after pre-treatment with IB-MECA or the A3AR antagonist, MRS1191. The data showed that MRS1191 pre-treatment decreased blood urea nitrogen and creatinin concentrations and morphological damage in the kidney, and IB-MECA was harmful [112]. In further experiments, they confirmed these results in murine models of renal ischaemia-reperfusion and myoglobinuric injury: renal failure was attenuated in A3AR deficient mice and WT mice pre-treated with an A3AR antagonist [113].
3.5. Other inflammatory diseases
A3AR activation has been found to be protective in a variety of inflammatory diseases including intestinal, pulmonary and systemic inflammation. IB-MECA attenuated colitis-induced inflammatory cell infiltration and colon inflammatory cytokine and chemokine levels in dextran sodium sulphate-induced colitis and also in the spontaneous colitis of IL-10 deficient mice [114]. A3AR stimulation with IB-MECA has been found to be beneficial also in 2,4,6-trinitrobenzene sulfonic acid-induced colitis in rats [115]. In addition, Antonioli and coworkers found that the A3AR inhibits colonic motility in both normal and inflamed colon [116]
A3AR activation has been found to be anti-inflammatory in the lung. For example, Cl-IB-MECA inhibited the migration of neutrophils and attenuated microvascular permeability in the lung following LPS inhalation of mice [117]. The results of Morschl and coworkers suggest that along with neutrophil migration, A3ARs also inhibit the invasion of eosinophils into the lung, as there was an increase in the number of eosinophils and the levels of related cytokines and chemokines in the lung of A3AR deficient vs. wild-type mice exposed to bleomycin [118].
Dry eye syndrome is a disease caused by decreased tear production or increased tear film evaporation and it is associated with inflammation in the eye [119]. Data from a Phase 2 clinical trial show that orally administered IB-MECA improved various parameters of the disease, including corneal staining, tear break-up time and tear meniscus height and decreased intraocular pressure, in comparison to the group treated with placebo [120].
We studied the effects of A3AR in a sepsis model induced by intraperitoneal administration of LPS and found that pre-treatment with IB-MECA decreased LPS induced plasma levels of IL-12, IFN-γ and nitrite, and enhanced the level of IL-10. In addition IB-MECA pre-treatment protected the mice from LPS-induced lethality [24]. Lee et al also investigated the effect of A3AR on sepsis using a different model, cecal ligation and puncture. They found that the mortality, as well as the expression of inflammatory factors in plasma and renal cortices of A3AR deficient mice was higher than that of wild-type littermates. IB-MECA treatment of WT animals subjected to cecal ligation and puncture improved survival and renal and hepatic function, and these protective effects were absent in A3AR knockout animals [121].
4. Conclusion
In conclusion, a considerable amount of data from in vitro and in vivo experiments suggests that the A3AR is expressed in various cell types of the innate and adaptive immune systems and that the A3AR regulates the functions of both systems (Table 1). In addition the A3AR regulates the course of numerous diseases, which include RA, cancer and asthma (Table 2).
Table 1.
In vitro effects of A3AR activation
| Cell type | Effect |
|---|---|
| neutrophil | chemotaxis ↑ or ↓, superoxide ↓ |
| macrophage | TNF-α ↓, MIP-1α ↓, IRF1 ↓, iNOS ↓, CD36 ↓, NADPH ↓, MMP-9 ↑ |
| dendritic cells | chemotaxis ↑, TNF-α ↓ |
| mas cells | degranulation ↑, histamine ↑, apoptosis ↓, Ang-2 ↑ |
| citotoxic T cells | tumoricidal activity ↓, granzyme ↓, perforin ↓, FAS ligand ↓, IFN-γ ↓, IL-2 ↓ |
| NK cells | IFN-α↑ |
| eosinophils | chemotaxis ↓, degranulation ↓, superoxide release ↓ |
| Hep-3B hepatoma cells | apoptosis ↑ |
| U87MG glioblastoma cells |
MMP-9 ↑ |
| A375 melanoma cells | HIF-1 ↑, VEGF ↑, Ang-2 ↑ |
| neurons | hypoxia induced cell death ↓, apoptosis ↑ |
Table 2.
In vivo effects of A3AR activation
| Disease | Effect |
|---|---|
| Rheumatoid arthritis | Joint: MIP-1α ↓, IL-12 ↓, neutrophil infiltration ↓, deformation ↓ |
| asthma | bronchoconstriction ↑, degranulation of airway mast cells ↑, neutrophil infiltration ↑, hyperresponsiveness ↑, mucin ↑ |
| cancer | Tumor growth ↓, metastasis ↓ |
| cardiac ischaemia | infarct size ↓ |
| cerebral ischaemia | infarct size ↓ |
| renal ischaemia | kidney damage ↑, blood urea ↑ |
| colitis | neutrophil infiltration ↓, IL-1 ↓, IL-6 ↓, IL-12 ↓, MIP-1α ↓, MIP-2 ↓ |
| sepsis | plasma IL-12 ↓, IFN-γ ↓, NO ↓, IL-10 ↑; lethality ↓ |
5. Expert opinion
Since the recent discovery of the A3AR, it has become clear that this receptor represents a viable target for the treatment of various immune-related diseases. There are substantial preclinical and clinical data supporting the efficacy of A3AR agonists in ameliorating the symptoms of RA. A3AR ligands are also promising therapeutic agents in the treatment of COPD, inflammatory bowel diseases, and ischemia. In addition, a considerable amount of evidence has accumulated that A3AR agonists are effective in inhibiting tumor growth and the formation of metastases in animal models of cancer.
However, there are numerous uncertainties with regard to the potential use of A3AR ligands. The A3AR displays the largest inter-species structural and pharmacological variation of all the adenosine receptors. The variation in the affinity of A3AR agonists and antagonists, as well as the differential expression and function of the A3AR among different species makes extrapolation from the preclinical to clinical scenario strenuous. This problem is further compounded by the broad expression of A3ARs on the various immune cell types and the myriad roles of A3ARs in regulating physiological functions. Side effects, such as a systemic suppression of the immune system causing augmented vulnerability to infections may limit the usefulness of A3AR agonists in treating systemic diseases. Developing tissue or cell specific A3AR ligands or targeted delivery of these ligands may assist in circumventing systemic side effects. Also problematic are the observations that in some of the preclinical studies, A3AR agonists were effective only at high concentrations raising the possibility of effects mediated via other adenosine receptors. Overcoming these issues will require the development of more selective A3AR-targeting drugs. These topics may be areas of significant progress in the coming years.
Optimism is, however, fueled by clinical trials, in which IB-MECA was tested in various conditions, which included RA, dry eye syndrome, and psoriasis. Oral administration of IB-MECA was safe and well-tolerated and resulted in improvements in some of the symptoms of diseases in the various trials. Further clinical trials are in progress with IB-MECA in RA and with Cl-IB-MECA in hepatocellular carcinoma and chronic hepatitis C.
Therefore we can be optimistic that new A3AR ligands with greater specificity and affinity will offer a promising approach to treating inflammatory and immune-related diseases.
Article highlights box.
A3AR activation has mainly anti-inflammatory effects in various cell types of the immune system
A3AR agonist treatment is beneficial in multiple in vivo models of rheumatoid arthritis.
In vitro data support the anti-tumoral and also the pro-tumoral effects of A3AR activation, but in vivo experiments suggest the anti-tumoral role of A3AR signaling.
A3AR activation can be both protective and harmful in different animal models of ischaemia.
There are various completed and ongoing clinical trials for the use of A3AR agonists in the treatment of numerous diseases like rheumatoid arthritis, cancer and hepatitis.
Acknowledgments
This work was supported by National Institutes of Health Grant R01GM66189, USAMRMC grant 09065004, Hungarian Scientific Research Fund (OTKA) grant CK 78275, and Intramural Research Program of NIH/NIAAA.
Abbreviations
- A1AR
A1 adenosine receptor
- A2AAR
A2A adenosine receptor
- A2BAR
A2B adenosine receptor
- A3AR
A3 adenosine receptor
- ADA
adenosine deaminase
- cAMP
cyclic adenosinemonophosphate
- CD39
ectonucleoside triphosphate diphosphohydrolase 1
- CD73
ecto-5′-nucleotidase
- Cl-IB-MECA
2-chloro-N6-(3-iodobenzyl)-adenosine-5′-methylcarboxamide
- COPD
chronic obstructive pulmonary disorder
- DC
dendritic cell
- DOX
doxorubicin
- ERK
extracellular signal-regulated kinase
- GPCR
G protein coupled receptors
- HIF-1
hypoxia-inducible factor-1
- IB-MECA
N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide
- IKK
IκB kinase
- IL-10
interleukin-10
- IL-12
interleukin-12
- IP3
Inositol 1,4,5-trisphosphate
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MIP-1α
macrophage inflammatory protein-1α
- MMP
matrix metalloproteinase
- MRS1067
3,6-dichloro-2′-isopropyloxy-4′-methyl-flavone
- MRS1191
3-Ethyl-5-benzyl-2-methyl-4-phenylethynyl-6-phenyl-1,4-(±)-dihydropyridine-3,5-dicarboxylate
- MRS1334
1,4-Dihydro-2-methyl-6-phenyl-4-(phenylethynyl)-3,5-pyridinedicarboxylic acid 3-ethyl-5-[(3-nitrophenyl)methyl] ester
- MRS1523
3-Propyl-6-ethyl-5-[(ethylthio)carbonyl]-2 phenyl-4-propyl-3-pyridine carboxylate
- MRS 3558
(1′S,2′R,3′S,4′R,5′S)-4-(2-chloro-6-(3-chlorobenzylamino)-9H-purin-9-yl)-2,3-dihydroxy-N-methylbicyclo [3.1.0] hexane-1-carboxamide
- NF-κB
nuclear factor κB
- PI3K
Phosphatidylinositol 3-kinases
- PLC
phospholipase C
- RA
rheumatoid arthritis
- TLR
toll-like receptor
- TNF-α
tumor necrosis factor-α
- VEGF
vascular endothelial growth factor
- VUF5574
N-(2-Methoxyphenyl)-N’-[2-(3-pyrindinyl)-4-quinazolinyl]-urea
Footnotes
Declaration of interest: The authors declare no conflict of interests.
References
- 1.Hasko G, Cronstein BN. Adenosine: an endogenous regulator of innate immunity. Trends Immunol. 2004 Jan;25(1):33–9. doi: 10.1016/j.it.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 2.Hasko G, Linden J, Cronstein B, Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat Rev Drug Discov. 2008 Sep;7(9):759–70. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasko G, Pacher P, Vizi ES, Illes P. Adenosine receptor signaling in the brain immune system. Trends Pharmacol Sci. 2005 Oct;26(10):511–6. doi: 10.1016/j.tips.2005.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hasko G, Pacher P, Deitch EA, Vizi ES. Shaping of monocyte and macrophage function by adenosine receptors. Pharmacol Ther. 2007 Feb;113(2):264–75. doi: 10.1016/j.pharmthera.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasko G, Pacher P. A2A receptors in inflammation and injury: lessons learned from transgenic animals. J Leukoc Biol. 2008 Mar;83(3):447–55. doi: 10.1189/jlb.0607359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasko G, Csoka B, Nemeth ZH, Vizi ES, Pacher P. A(2B) adenosine receptors in immunity and inflammation. Trends Immunol. 2009 Jun;30(6):263–70. doi: 10.1016/j.it.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Csoka B, Nemeth ZH, Rosenberger P, Eltzschig HK, Spolarics Z, Pacher P, et al. A2B adenosine receptors protect against sepsis-induced mortality by dampening excessive inflammation. J Immunol. Jul 1;185(1):542–50. doi: 10.4049/jimmunol.0901295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001 Dec;53(4):527–52. [PMC free article] [PubMed] [Google Scholar]
- 9.Bours MJL, Swennen ELR, Di Virgilio F, Cronstein BN, Dagnelie PC. Adenosine 5′-triphosphate and adenosine as endogenous signaling molecules in immunity and inflammation. Pharmacology & Therapeutics. 2006;112(2):358–404. doi: 10.1016/j.pharmthera.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 10.Sajjadi FG, Boyle DL, Domingo RC, Firestein GS. cDNA cloning and characterization of A3i, an alternatively spliced rat A3 adenosine receptor variant. FEBS Lett. 1996 Mar 11;382(1-2):125–9. doi: 10.1016/0014-5793(96)00150-0. [DOI] [PubMed] [Google Scholar]
- 11.Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA. The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther. 2008 Jan;117(1):123–40. doi: 10.1016/j.pharmthera.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 12.Muller CE. Medicinal chemistry of adenosine A(3) receptor ligands. Current Topics in Medicinal Chemistry. 2003;3(4):445–62. doi: 10.2174/1568026033392174. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006 Mar;5(3):247–64. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobson KA. Adenosine A3 receptors: novel ligands and paradoxical effects. Trends Pharmacol Sci. 1998 May;19(5):184–91. doi: 10.1016/s0165-6147(98)01203-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jacobson KA, Park KS, Jiang JL, Kim YC, Olah ME, Stiles GL, et al. Pharmacological characterization of novel A3 adenosine receptor-selective antagonists. Neuropharmacology. 1997 Sep;36(9):1157–65. doi: 10.1016/s0028-3908(97)00104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schulte G, Fredholm BB. Signalling from adenosine receptors to mitogen-activated protein kinases. Cellular Signalling. 2003;15(9):813–27. doi: 10.1016/s0898-6568(03)00058-5. [DOI] [PubMed] [Google Scholar]
- 17.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6(3):173–82. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science. 2006 Dec 15;314(5806):1792–5. doi: 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 19.Inoue Y, Chen Y, Hirsh MI, Yip L, Junger WG. A3 and P2Y2 receptors control the recruitment of neutrophils to the lungs in a mouse model of sepsis. Shock. 2008 Aug;30(2):173–7. doi: 10.1097/shk.0b013e318160dad4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van der Hoeven D, Wan TC, Auchampach JA. Activation of the A3 Adenosine Receptor Suppresses Superoxide Production and Chemotaxis of Mouse Bone Marrow Neutrophils. Molecular Pharmacology. 2008 Sep 1;74(3):685–96. doi: 10.1124/mol.108.048066. 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Hoeven D, Gizewski ET, Auchampach JA. Activation of the A3 adenosine receptor inhibits fMLP-induced Rac activation in mouse bone marrow neutrophils. Biochemical Pharmacology. 79(11):1667–73. doi: 10.1016/j.bcp.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sajjadi FG, Takabayashi K, Foster AC, Domingo RC, Firestein GS. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J Immunol. 1996 May 1;156(9):3435–42. [PubMed] [Google Scholar]
- 23.McWhinney CD, Dudley MW, Bowlin TL, Peet NP, Schook L, Bradshaw M, et al. Activation of adenosine A3 receptors on macrophages inhibits tumor necrosis factor-alpha. Eur J Pharmacol. 1996 Aug 29;310(2-3):209–16. doi: 10.1016/0014-2999(96)00272-5. [DOI] [PubMed] [Google Scholar]
- 24.Hasko G, Nemeth ZH, Vizi ES, Salzman AL, Szabo C. An agonist of adenosine A3 receptors decreases interleukin-12 and interferon-gamma production and prevents lethality in endotoxemic mice. Eur J Pharmacol. 1998 Oct 9;358(3):261–8. doi: 10.1016/s0014-2999(98)00619-0. [DOI] [PubMed] [Google Scholar]
- 25.Lee JY, Jhun BS, Oh YT, Lee JH, Choe W, Baik HH, et al. Activation of adenosine A3 receptor suppresses lipopolysaccharide-induced TNF-[alpha] production through inhibition of PI 3-kinase/Akt and NF-[kappa]B activation in murine BV2 microglial cells. Neuroscience Letters. 2006;396(1):1–6. doi: 10.1016/j.neulet.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Martin L, Pingle SC, Hallam DM, Rybak LP, Ramkumar V. Activation of the Adenosine A3 Receptor in RAW 264.7 Cells Inhibits Lipopolysaccharide-Stimulated Tumor Necrosis Factor-α Release by Reducing Calcium-Dependent Activation of Nuclear Factor-κB and Extracellular Signal-Regulated Kinase 1/2. Journal of Pharmacology and Experimental Therapeutics. 2006 Jan 1;316(1):71–8. doi: 10.1124/jpet.105.091868. 2006. [DOI] [PubMed] [Google Scholar]
- 27.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. Faseb J. 2000 Oct;14(13):2065–74. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 28.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996 Nov 15;157(10):4634–40. [PubMed] [Google Scholar]
- 29.Kreckler LM, Wan TC, Ge ZD, Auchampach JA. Adenosine inhibits tumor necrosis factor-alpha release from mouse peritoneal macrophages via A2A and A2B but not the A3 adenosine receptor. J Pharmacol Exp Ther. 2006 Apr;317(1):172–80. doi: 10.1124/jpet.105.096016. [DOI] [PubMed] [Google Scholar]
- 30.Szabo C, Scott GS, Virag L, Egnaczyk G, Salzman AL, Shanley TP, et al. Suppression of macrophage inflammatory protein (MIP)-1alpha production and collagen-induced arthritis by adenosine receptor agonists. Br J Pharmacol. 1998 Sep;125(2):379–87. doi: 10.1038/sj.bjp.0702040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnholt KE, Kota RS, Aung HH, Rutledge JC. Adenosine Blocks IFN-γ-Induced Phosphorylation of STAT1 on Serine 727 to Reduce Macrophage Activation. The Journal of Immunology. 2009 Nov 15;183(10):6767–77. doi: 10.4049/jimmunol.0900331. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Broussas M, Cornillet-Lefebvre P, Potron G, Nguyen P. Inhibition of fMLP-triggered respiratory burst of human monocytes by adenosine: involvement of A3 adenosine receptor. J Leukoc Biol. 1999 Sep;66(3):495–501. [PubMed] [Google Scholar]
- 33.Thiele A, Kronstein R, Wetzel A, Gerth A, Nieber K, Hauschildt S. Regulation of adenosine receptor subtypes during cultivation of human monocytes: role of receptors in preventing lipopolysaccharide-triggered respiratory burst. Infect Immun. 2004 Mar;72(3):1349–57. doi: 10.1128/IAI.72.3.1349-1357.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Velot E, Haas B, Léonard Fdr, Ernens I, Rolland-Turner M, Schwartz C, et al. Activation of the adenosine-A3 receptor stimulates matrix metalloproteinase-9 secretion by macrophages. Cardiovascular Research. 2008 Nov 1;80(2):246–54. doi: 10.1093/cvr/cvn201. 2008. [DOI] [PubMed] [Google Scholar]
- 35.Panther E, Idzko M, Herouy Y, Rheinen H, Gebicke-Haerter PJ, Mrowietz U, et al. Expression and function of adenosine receptors in human dendritic cells. FASEB J. 2001 Sep;15(11):1963–70. doi: 10.1096/fj.01-0169com. [DOI] [PubMed] [Google Scholar]
- 36.Dickenson JM, Reeder S, Rees B, Alexander S, Kendall D. Functional expression of adenosine A2A and A3 receptors in the mouse dendritic cell line XS-106. European Journal of Pharmacology. 2003;474(1):43–51. doi: 10.1016/s0014-2999(03)02041-7. [DOI] [PubMed] [Google Scholar]
- 37.Addi A Ben, Lefort A, Hua X, Libert F, Communi D, Ledent C, et al. Modulation of murine dendritic cell function by adenine nucleotides and adenosine: involvement of the A(2B) receptor. Eur J Immunol. 2008 Jun;38(6):1610–20. doi: 10.1002/eji.200737781. [DOI] [PubMed] [Google Scholar]
- 38.Novitskiy SV, Ryzhov S, Zaynagetdinov R, Goldstein AE, Huang Y, Tikhomirov OY, et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood. 2008 Sep 1;112(5):1822–31. doi: 10.1182/blood-2008-02-136325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson JM, Ross WG, Agbai ON, Frazier R, Figler RA, Rieger J, et al. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J Immunol. 2009 Apr 15;182(8):4616–23. doi: 10.4049/jimmunol.0801279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bischoff SC. Role of mast cells in allergic and non-allergic immune responses: comparison of human and murine data. Nature Reviews Immunology. 2007 Feb;7(2):93–104. doi: 10.1038/nri2018. [DOI] [PubMed] [Google Scholar]
- 41.Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993 Aug 15;268(23):16887–90. [PubMed] [Google Scholar]
- 42.Reeves JJ, Jones CA, Sheehan MJ, Vardey CJ, Whelan CJ. Adenosine A3 receptors promote degranulation of rat mast cells both in vitro and in vivo. Inflamm Res. 1997 May;46(5):180–4. doi: 10.1007/s000110050169. [DOI] [PubMed] [Google Scholar]
- 43.Zhong H, Shlykov SG, Molina JG, Sanborn BM, Jacobson MA, Tilley SL, et al. Activation of murine lung mast cells by the adenosine A3 receptor. J Immunol. 2003 Jul 1;171(1):338–45. doi: 10.4049/jimmunol.171.1.338. [DOI] [PubMed] [Google Scholar]
- 44.Smith SR, Denhardt G, Terminelli C. A role for histamine in cytokine modulation by the adenosine A(3) receptor agonist, 2-Cl-IB-MECA. Eur J Pharmacol. 2002 Dec 13;457(1):57–69. doi: 10.1016/s0014-2999(02)02645-6. [DOI] [PubMed] [Google Scholar]
- 45.Salvatore CA, Tilley SL, Latour AM, Fletcher DS, Koller BH, Jacobson MA. Disruption of the A(3) adenosine receptor gene in mice and its effect on stimulated inflammatory cells. J Biol Chem. 2000 Feb 11;275(6):4429–34. doi: 10.1074/jbc.275.6.4429. [DOI] [PubMed] [Google Scholar]
- 46.Feoktistov I, Biaggioni I. Adenosine A2b receptors evoke interleukin-8 secretion in human mast cells. An enprofylline-sensitive mechanism with implications for asthma. J Clin Invest. 1995 Oct;96(4):1979–86. doi: 10.1172/JCI118245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryzhov S, Goldstein AE, Matafonov A, Zeng D, Biaggioni I, Feoktistov I. Adenosine-activated mast cells induce IgE synthesis by B lymphocytes: an A2B-mediated process involving Th2 cytokines IL-4 and IL-13 with implications for asthma. J Immunol. 2004 Jun 15;172(12):7726–33. doi: 10.4049/jimmunol.172.12.7726. [DOI] [PubMed] [Google Scholar]
- 48.Jin X, Shepherd RK, Duling BR, Linden J. Inosine binds to A3 adenosine receptors and stimulates mast cell degranulation. J Clin Invest. 1997 Dec 1;100(11):2849–57. doi: 10.1172/JCI119833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tilley SL, Wagoner VA, Salvatore CA, Jacobson MA, Koller BH. Adenosine and inosine increase cutaneous vasopermeability by activating A(3) receptors on mast cells. J Clin Invest. 2000 Feb;105(3):361–7. doi: 10.1172/JCI8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gao Z, Li BS, Day YJ, Linden J. A3 adenosine receptor activation triggers phosphorylation of protein kinase B and protects rat basophilic leukemia 2H3 mast cells from apoptosis. Mol Pharmacol. 2001 Jan;59(1):76–82. doi: 10.1124/mol.59.1.76. [DOI] [PubMed] [Google Scholar]
- 51.Feoktistov I, Ryzhov S, Goldstein AE, Biaggioni I. Mast cell-mediated stimulation of angiogenesis: cooperative interaction between A2B and A3 adenosine receptors. Circ Res. 2003 Mar 21;92(5):485–92. doi: 10.1161/01.RES.0000061572.10929.2D. [DOI] [PubMed] [Google Scholar]
- 52.Gessi S, Varani K, Merighi S, Cattabriga E, Avitabile A, Gavioli R, et al. Expression of A(3) adenosine receptors in human lymphocytes: Up-regulation in T cell activation. Molecular Pharmacology. 2004 Mar 1;65(3):711–9. doi: 10.1124/mol.65.3.711. [DOI] [PubMed] [Google Scholar]
- 53.Csoka B, Himer L, Selmeczy Z, Vizi ES, Pacher P, Ledent C, et al. Adenosine A2A receptor activation inhibits T helper 1 and T helper 2 cell development and effector function. FASEB J. 2008 Oct;22(10):3491–9. doi: 10.1096/fj.08-107458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.MacKenzie WM, Hoskin DW, Blay J. Adenosine inhibits the adhesion of anti-CD3-activated killer lymphocytes to adenocarcinoma cells through an A3 receptor. Cancer Res. 1994 Jul 1;54(13):3521–6. [PubMed] [Google Scholar]
- 55.Hoskin DW, Butler JJ, Drapeau D, Haeryfar SM, Blay J. Adenosine acts through an A3 receptor to prevent the induction of murine anti-CD3-activated killer T cells. Int J Cancer. 2002 May 20;99(3):386–95. doi: 10.1002/ijc.10325. [DOI] [PubMed] [Google Scholar]
- 56.Harish A, Hohana G, Fishman P, Arnon O, Bar-Yehuda S. A3 adenosine receptor agonist potentiates natural killer cell activity. Int J Oncol. 2003 Oct;23(4):1245–9. [PubMed] [Google Scholar]
- 57.Jeffe F, Stegmann KA, Broelsch F, Manns MP, Cornberg M, Wedemeyer H. Adenosine and IFN-{alpha} synergistically increase IFN-gamma production of human NK cells. J Leukoc Biol. 2009 Mar;85(3):452–61. doi: 10.1189/jlb.0108046. [DOI] [PubMed] [Google Scholar]
- 58.McInnes IB, O’Dell JR. State-of-the-art: rheumatoid arthritis. Annals of the Rheumatic Diseases. 2010 Nov;69(11):1898–906. doi: 10.1136/ard.2010.134684. [DOI] [PubMed] [Google Scholar]
- 59.Isaacs JD. The changing face of rheumatoid arthritis: sustained remission for all? Nat Rev Immunol. 10(8):605–11. doi: 10.1038/nri2804. [DOI] [PubMed] [Google Scholar]
- 60.Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN. The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum. 2007 May;56(5):1440–5. doi: 10.1002/art.22643. [DOI] [PubMed] [Google Scholar]
- 61.Madi L, Cohen S, Ochayin A, Bar-Yehuda S, Barer F, Fishman P. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: involvement of nuclear factor-kappaB in mediating receptor level. The Journal of Rheumatology. 2007 Jan 1;34(1):20–6. 2007. [PubMed] [Google Scholar]
- 62.Ochaion A, Bar-Yehuda S, Cohen S, Barer F, Patoka R, Amital H, et al. The anti-inflammatory target A(3) adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn’s disease. Cellular Immunology. 2009;258(2):115–22. doi: 10.1016/j.cellimm.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 63.Varani K, Massara A, Vincenzi F, Tosi A, Padovan M, Trotta F, et al. Normalization of A(2A) and A(3) Adenosine Receptor Up-Regulation in Rheumatoid Arthritis Patients by Treatment With Anti-Tumor Necrosis Factor alpha but Not Methotrexate. Arthritis and Rheumatism. 2009 Oct;60(10):2880–91. doi: 10.1002/art.24794. [DOI] [PubMed] [Google Scholar]
- 64.Baharav E, Bar-Yehuda S, Madi L, Silberman D, Rath-Wolfson L, Halpren M, et al. Antiinflammatory effect of A3 adenosine receptor agonists in murine autoimmune arthritis models. J Rheumatol. 2005 Mar;32(3):469–76. [PubMed] [Google Scholar]
- 65.Fishman P, Bar-Yehuda S, Madi L, Rath-Wolfson L, Ochaion A, Cohen S, et al. The PI3K-NF-kappa B signal transduction pathway is involved in mediating the anti-inflammatory effect of IB-MECA in adjuvant-induced arthritis. Arthritis Research & Therapy. 2006;8(1) doi: 10.1186/ar1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ochaion A, Bar-Yehuda S, Cohen S, Amital H, Jacobson KA, Joshi BV, et al. The A(3) adenosine receptor agonist CF502 inhibits the PI3K, PKB/Akt and NF-kappa B signaling pathway in synoviocytes from rheumatoid arthritis patients and in adjuvant-induced arthritis rats. Biochemical Pharmacology. 2008 Aug 15;76(4):482–94. doi: 10.1016/j.bcp.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ochaion A, Bar-Yehuda S, Cohn S, Del Valle L, Perez-Liz G, Madi L, et al. Methotrexate enhances the anti-inflammatory effect of CF101 via up-regulation of the A3 adenosine receptor expression. Arthritis Res Ther. 2006;8(6):R169. doi: 10.1186/ar2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bar-Yehuda S, Rath-Wolfson L, Del Valle L, Ochaion A, Cohen S, Patoka R, et al. Induction of an Antiinflammatory Effect and Prevention of Cartilage Damage in Rat Knee Osteoarthritis by CF101 Treatment. Arthritis and Rheumatism. 2009 Oct;60(10):3061–71. doi: 10.1002/art.24817. [DOI] [PubMed] [Google Scholar]
- 69.Silverman MH, Strand V, Markovits D, Nahir M, Reitblat T, Molad Y, et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: data from a phase II clinical trial. J Rheumatol. 2008 Jan;35(1):41–8. [PubMed] [Google Scholar]
- 70.Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in Bronchoalveolar Lavage Fluid in Asthma. American Review of Respiratory Disease. 1993 Jul;148(1):91–7. doi: 10.1164/ajrccm/148.1.91. [DOI] [PubMed] [Google Scholar]
- 71.Huszár Á , Vass G, Vizi Á , Csoma Z, Barát E, Világos G Molnár, et al. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. European Respiratory Journal. 2002 Dec 1;20(6):1393–8. doi: 10.1183/09031936.02.00005002. 2002. [DOI] [PubMed] [Google Scholar]
- 72.Cushley MJ, Tattersfield AE, Holgate ST. Inhaled adenosine and guanosine on airway resistance in normal and asthmatic subjects. Br J Clin Pharmacol. 1983 Feb;15(2):161–5. doi: 10.1111/j.1365-2125.1983.tb01481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Walker BA, Jacobson MA, Knight DA, Salvatore CA, Weir T, Zhou D, et al. Adenosine A3 receptor expression and function in eosinophils. Am J Respir Cell Mol Biol. 1997 May;16(5):531–7. doi: 10.1165/ajrcmb.16.5.9160835. [DOI] [PubMed] [Google Scholar]
- 74.Knight D, Zheng X, Rocchini C, Jacobson M, Bai T, Walker B. Adenosine A3 receptor stimulation inhibits migration of human eosinophils. J Leukoc Biol. 1997 Oct;62(4):465–8. doi: 10.1002/jlb.62.4.465. [DOI] [PubMed] [Google Scholar]
- 75.Ezeamuzie CI, Philips E. Adenosine A(3) receptors on human eosinophils mediate inhibition of degranulation and superoxide anion release. British Journal of Pharmacology. 1999 May;127(1):188–94. doi: 10.1038/sj.bjp.0702476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tilley SL, Tsai M, Williams CM, Wang ZS, Erikson CJ, Galli SJ, et al. Identification of A3 receptor- and mast cell-dependent and -independent components of adenosine-mediated airway responsiveness in mice. J Immunol. 2003 Jul 1;171(1):331–7. doi: 10.4049/jimmunol.171.1.331. [DOI] [PubMed] [Google Scholar]
- 77.Hua X, Chason KD, Fredholm BB, Deshpande DA, Penn RB, Tilley SL. Adenosine induces airway hyperresponsiveness through activation of A3 receptors on mast cells. J Allergy Clin Immunol. 2008 Jul;122(1):107–13. 13 e1–7. doi: 10.1016/j.jaci.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Young HW, Molina JG, Dimina D, Zhong H, Jacobson M, Chan LN, et al. A3 adenosine receptor signaling contributes to airway inflammation and mucus production in adenosine deaminase-deficient mice. J Immunol. 2004 Jul 15;173(2):1380–9. doi: 10.4049/jimmunol.173.2.1380. [DOI] [PubMed] [Google Scholar]
- 79.Young HW, Sun CX, Evans CM, Dickey BF, Blackburn MR. A3 adenosine receptor signaling contributes to airway mucin secretion after allergen challenge. Am J Respir Cell Mol Biol. 2006 Nov;35(5):549–58. doi: 10.1165/rcmb.2006-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Madi L, Ochaion A, Rath-Wolfson L, Bar-Yehuda S, Erlanger A, Ohana G, et al. The A(3) adenosine receptor is highly expressed in tumor versus normal cells: Potential target for tumor growth inhibition. Clinical Cancer Research. 2004 Jul 1;10(13):4472–9. doi: 10.1158/1078-0432.CCR-03-0651. [DOI] [PubMed] [Google Scholar]
- 81.Gessi S, Cattabriga E, Avitabile A, Gafa R, Lanza G, Cavazzini L, et al. Elevated expression of A(3) adenosine receptors in human colorectal cancer is reflected in peripheral blood cells. Clinical Cancer Research. 2004 Sep 1;10(17):5895–901. doi: 10.1158/1078-0432.CCR-1134-03. [DOI] [PubMed] [Google Scholar]
- 82.Fishman P, Bar-Yehuda S, Ohana G, Barer F, Ochaion A, Erlanger A, et al. An agonist to the A(3) adenosine receptor inhibits colon carcinoma growth in mice via modulation of GSK-3 beta and NF-kappa B. Oncogene. 2004 Apr 1;23(14):2465–71. doi: 10.1038/sj.onc.1207355. [DOI] [PubMed] [Google Scholar]
- 83.Bar-Yehuda S, Stemmer SM, Madi L, Castel D, Ochaion A, Cohen S, et al. The A(3) adenosine receptor agonist CF102 induces apoptosis of hepatocellular carcinoma via de-regulation of the Wnt and NF-kappa B signal transduction pathways. International Journal of Oncology. 2008 Aug;33(2):287–95. [PubMed] [Google Scholar]
- 84.Cohen S, Stemmer S, Zozulya G, Ochaion A, Patoka R, Barer F, et al. CF102 an A(3) adenosine receptor agonist mediates anti-tumor and anti-inflammatory effects in the liver. J Cell Physiol. Dec 14; doi: 10.1002/jcp.22593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim SJ, Min HY, Chung HJ, Park EJ, Hong JY, Kang YJ, et al. Inhibition of cell proliferation through cell cycle arrest and apoptosis by thio-Cl-IB-MECA, a novel A3 adenosine receptor agonist, in human lung cancer cells. Cancer Lett. 2008 Jun 18;264(2):309–15. doi: 10.1016/j.canlet.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 86.Morello S, Petrella A, Festa M, Popolo A, Monaco M, Vuttariello E, et al. Cl-IB-MECA inhibits human thyroid cancer cell proliferation independently of A3 adenosine receptor activation. Cancer Biol Ther. 2008 Feb;7(2):278–84. doi: 10.4161/cbt.7.2.5301. [DOI] [PubMed] [Google Scholar]
- 87.Mlejnek P, Dolezel P. Induction of apoptosis by A3 adenosine receptor agonist N-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide in human leukaemia cells: a possible involvement of intracellular mechanism. Acta Physiol (Oxf) Jun;199(2):171–9. doi: 10.1111/j.1748-1716.2010.02087.x. [DOI] [PubMed] [Google Scholar]
- 88.Jajoo S, Mukherjea D, Watabe K, Ramkumar V. Adenosine A(3) receptor suppresses prostate cancer metastasis by inhibiting NADPH oxidase activity. Neoplasia. 2009 Nov;11(11):1132–45. doi: 10.1593/neo.09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ohana G, Bar-Yehuda S, Arich A, Madi L, Dreznick Z, Rath-Wolfson L, et al. Inhibition of primary colon carcinoma growth and liver metastasis by the A3 adenosine receptor agonist CF101. Br J Cancer. 2003 Oct 20;89(8):1552–8. doi: 10.1038/sj.bjc.6601315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gessi S, Sacchetto V, Fogli E, Merighi S, Varani K, Baraldi PG, et al. Modulation of metalloproteinase-9 in U87MG glioblastoma cells by A3 adenosine receptors. Biochem Pharmacol. May 15;79(10):1483–95. doi: 10.1016/j.bcp.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 91.Gessi S, Merighi S, Varani K, Cattabriga E, Benini A, Mirandola P, et al. Adenosine receptors in colon carcinoma tissues and colon tumoral cell lines: focus on the A(3) adenosine subtype. J Cell Physiol. 2007 Jun;211(3):826–36. doi: 10.1002/jcp.20994. [DOI] [PubMed] [Google Scholar]
- 92.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, et al. A3 adenosine receptors modulate hypoxia-inducible factor-1alpha expression in human A375 melanoma cells. Neoplasia. 2005 Oct;7(10):894–903. doi: 10.1593/neo.05334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Leung E, et al. Adenosine modulates vascular endothelial growth factor expression via hypoxia-inducible factor-1 in human glioblastoma cells. Biochem Pharmacol. 2006 Jun 28;72(1):19–31. doi: 10.1016/j.bcp.2006.03.020. [DOI] [PubMed] [Google Scholar]
- 94.Merighi S, Benini A, Mirandola P, Gessi S, Varani K, Simioni C, et al. Caffeine inhibits adenosine-induced accumulation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and interleukin-8 expression in hypoxic human colon cancer cells. Mol Pharmacol. 2007 Aug;72(2):395–406. doi: 10.1124/mol.106.032920. [DOI] [PubMed] [Google Scholar]
- 95.Wan TC, Ge ZD, Tampo A, Mio Y, Bienengraeber MW, Tracey WR, et al. The A3 adenosine receptor agonist CP-532,903 [N6-(2,5-dichlorobenzyl)-3′-aminoadenosine-5′-N-methylcarboxamide] protects against myocardial ischemia/reperfusion injury via the sarcolemmal ATP-sensitive potassium channel. J Pharmacol Exp Ther. 2008 Jan;324(1):234–43. doi: 10.1124/jpet.107.127480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ge Z-D, der Hoeven Dv, Maas JE, Wan TC, Auchampach JA. A3 adenosine receptor activation during reperfusion reduces infarct size through actions on bone marrow-derived cells. Journal of Molecular and Cellular Cardiology. 49(2):280–6. doi: 10.1016/j.yjmcc.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jordan JE, Thourani VH, Auchampach JA, Robinson JA, Wang NP, Vinten-Johansen J. A(3) adenosine receptor activation attenuates neutrophil function and neutrophil-mediated reperfusion injury. Am J Physiol. 1999 Nov;277(5 Pt 2):H1895–905. doi: 10.1152/ajpheart.1999.277.5.H1895. [DOI] [PubMed] [Google Scholar]
- 98.Pacher P, Liaudet L, Bai P, Virag L, Mabley JG, Hasko G, et al. Activation of poly(ADP-ribose) polymerase contributes to development of doxorubicin-induced heart failure. J Pharmacol Exp Ther. 2002 Mar;300(3):862–7. doi: 10.1124/jpet.300.3.862. [DOI] [PubMed] [Google Scholar]
- 99.Pacher P, Liaudet L, Mabley JG, Cziraki A, Hasko G, Szabo C. Beneficial effects of a novel ultrapotent poly(ADP-ribose) polymerase inhibitor in murine models of heart failure. Int J Mol Med. 2006 Feb;17(2):369–75. [PMC free article] [PubMed] [Google Scholar]
- 100.Mukhopadhyay P, Batkai S, Rajesh M, Czifra N, Harvey-White J, Hasko G, et al. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007 Aug 7;50(6):528–36. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mukhopadhyay P, Rajesh M, Batkai S, Kashiwaya Y, Hasko G, Liaudet L, et al. Role of superoxide, nitric oxide, and peroxynitrite in doxorubicin-induced cell death in vivo and in vitro. Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1466–83. doi: 10.1152/ajpheart.00795.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, et al. CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res. 2010 Mar 1;85(4):773–84. doi: 10.1093/cvr/cvp369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mukhopadhyay P, Horvath B, Rajesh M, Matsumoto S, Saito K, Batkai S, et al. Fatty acid amide hydrolase is a key regulator of endocannabinoid-induced myocardial tissue injury. Free Radic Biol Med. 2011 Jan 1;50(1):179–95. doi: 10.1016/j.freeradbiomed.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Emanuelov AK, Shainberg A, Chepurko Y, Kaplan D, Sagie A, Porat E, et al. Adenosine A3 receptor-mediated cardioprotection against doxorubicin-induced mitochondrial damage. Biochem Pharmacol. Jan 15;79(2):180–7. doi: 10.1016/j.bcp.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 105.Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y. Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res. 2006 Dec;84(8):1848–55. doi: 10.1002/jnr.21071. [DOI] [PubMed] [Google Scholar]
- 106.Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2005 Mar;36(3):654–9. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- 107.Luo C, Yi B, Tao G, Li M, Chen Z, Tang W, et al. Adenosine A3 receptor agonist reduces early brain injury in subarachnoid haemorrhage. Neuroreport. Sep 15;21(13):892–6. doi: 10.1097/WNR.0b013e32833dbd13. [DOI] [PubMed] [Google Scholar]
- 108.Sei Y, vonLubitz DKJE, Abbracchio MP, Ji XD, Jacobson KA. Adenosine A(2) receptor agonist-induced neurotoxicity in rat cerebellar granule neurons. Drug Development Research. 1997 Mar;40(3):267–73. [Google Scholar]
- 109.Appel E, Kazimirsky G, Ashkenazi E, Kim SG, Jacobson KA, Brodie C. Roles of BCL-2 and caspase 3 in the adenosine A3 receptor-induced apoptosis. J Mol Neurosci. 2001 Dec;17(3):285–92. doi: 10.1385/JMN:17:3:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vonlubitz DKJE, Lin RCS, Popik P, Carter MF, Jacobson KA. Adenosine a(3) Receptor Stimulation and Cerebral-Ischemia. European Journal of Pharmacology. 1994 Sep 22;263(1-2):59–67. doi: 10.1016/0014-2999(94)90523-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Palmer TM, Harris CA, Coote J, Stiles GL. Induction of multiple effects on adenylyl cyclase regulation by chronic activation of the human A3 adenosine receptor. Mol Pharmacol. 1997 Oct;52(4):632–40. doi: 10.1124/mol.52.4.632. [DOI] [PubMed] [Google Scholar]
- 112.Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A1 and A3receptors. American Journal of Physiology - Renal Physiology. 2000 Mar 1;278(3):F380–F7. doi: 10.1152/ajprenal.2000.278.3.F380. 2000. [DOI] [PubMed] [Google Scholar]
- 113.Lee HT, Ota-Setlik A, Xu H, D’Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003 Feb;284(2):F267–73. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- 114.Mabley J, Soriano F, Pacher P, Hasko G, Marton A, Wallace R, et al. The adenosine A3 receptor agonist, N6-(3-iodobenzyl)-adenosine-5′-N-methyluronamide, is protective in two murine models of colitis. Eur J Pharmacol. 2003 Apr 18;466(3):323–9. doi: 10.1016/s0014-2999(03)01570-x. [DOI] [PubMed] [Google Scholar]
- 115.Guzman J, Yu JG, Suntres Z, Bozarov A, Cooke H, Javed N, et al. ADOA3R as a therapeutic target in experimental colitis: proof by validated high-density oligonucleotide microarray analysis. Inflamm Bowel Dis. 2006 Aug;12(8):766–89. doi: 10.1097/00054725-200608000-00014. [DOI] [PubMed] [Google Scholar]
- 116.Antonioli L, Fornai M, Colucci R, Ghisu N, Tuccori M, Awwad O, et al. Control of enteric neuromuscular functions by purinergic A(3) receptors in normal rat distal colon and experimental bowel inflammation. Br J Pharmacol. 2010 Oct;161(4):856–71. doi: 10.1111/j.1476-5381.2010.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wagner R, Ngamsri KC, Stark S, Vollmer I, Reutershan J. Adenosine receptor A3 is a critical mediator in LPS-induced pulmonary inflammation. Am J Physiol Lung Cell Mol Physiol. Oct;299(4):L502–12. doi: 10.1152/ajplung.00083.2010. [DOI] [PubMed] [Google Scholar]
- 118.Morschl E, Molina LG, Volmer JB, Mohsenin A, Pero RS, Hong JS, et al. A(3) Adenosine Receptor Signaling Influences Pulmonary Inflammation and Fibrosis. American Journal of Respiratory Cell and Molecular Biology. 2008 Dec;39(6):697–705. doi: 10.1165/rcmb.2007-0419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mariette X, Gottenberg JE. Pathogenesis of Sjogren’s syndrome and therapeutic consequences. Current Opinion in Rheumatology. 2010 Sep;22(5):471–7. doi: 10.1097/BOR.0b013e32833c36c5. [DOI] [PubMed] [Google Scholar]
- 120.Avni I, Garzozi HJ, Barequet IS, Segev F, Varssano D, Sartani G, et al. Treatment of Dry Eye Syndrome with Orally Administered CF101 Data from a Phase 2 Clinical Trial. Ophthalmology. 2010 Jul;117(7):1287–93. doi: 10.1016/j.ophtha.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lee HT, Kim M, Joo JD, Gallos G, Chen JF, Emala CW. A3 adenosine receptor activation decreases mortality and renal and hepatic injury in murine septic peritonitis. Am J Physiol Regul Integr Comp Physiol. 2006 Oct;291(4):R959–69. doi: 10.1152/ajpregu.00034.2006. [DOI] [PubMed] [Google Scholar]


