Abstract
Restoring p53 activity by inhibiting the interaction between p53 and MDM2 represents an attractive approach for cancer therapy. To this end, a number of small-molecule p53-MDM2 binding inhibitors have been developed during the past several years. Nutlin-3 is a potent and selective small-molecule MDM2 antagonist that has shown considerable promise in pre-clinical studies. This review will highlight recent advances in the development of small-molecule MDM2 antagonists as potential cancer therapeutics, with special emphasis on Nutlin-3.
Keywords: Nutlin-3, p53, MDM2, therapy
1. The function and regulation of p53
P53 is a tumor suppressor that is induced and activated by a variety of potentially tumorigenic stresses, including inappropriate oncogene signaling and DNA damage. The induction of p53 occurs through post-transcriptional and post-translational mechanisms. Upon activation, p53 primarily functions as a transcription factor, recruited to binding sites in chromatin and regulating the expression of genes that control a diverse group of biological activities, including apoptosis, cell cycle regulation, senescence, DNA metabolism, energy metabolism, angiogenesis, immune responses, cell differentiation, motility and migration, and cell–cell communication [1, 2].
Due to its potent tumor suppressor role, the function of p53 is almost always compromised in tumor cells. Inactivating mutations in p53 are found in approximately 50% of all human cancers. The frequencies of reported p53 mutations vary among cancer types, ranging from ∼10% in haematopoietic malignancies, 50–70% in colorectal and head and neck cancers, and nearly 100% in ovarian cancer [3, 4]. The vast majority of tumor-associated p53 mutations are missense mutations within p53's DNA binding domain and inhibit the ability of p53 to bind DNA and activate transcription [5]. In the remaining cancers in which the p53 gene is not mutated, the function of the p53 pathway is often inhibited through other mechanisms, including increased expression of MDM2 or MDMX (both negative regulators of p53) or inactivation of the tumor suppressor protein p14/ARF [6]. The fact that the p53 signaling pathway is inactivated in virtually all cancers has drawn great attention from the world-wide cancer research community to target the p53 pathway for development of improved cancer therapies. Recent studies demonstrated that restoring wild-type p53 activity is sufficient to induce tumor regression in mouse xenograft and endogenous tumor models [7, 8]. The transformed phenotype of tumor cells is especially responsive to restoration of p53 activity, which leads to either apoptotic death or senescence growth arrest followed by immune system-assisted tumor clearance. Thus, p53 could well be the best target for cancer specific treatment development.
Wild-type p53 is a short-lived protein. It remains at low or undetectable levels in most tissues. In normal cells, the activity and level of p53 is tightly controlled by MDM2, an E3 ubiquitin-ligase enzyme responsible for p53 ubiquitination [9, 10]. In humans, the MDM2 gene encodes a 491 amino acid protein with multiple domains, including an N-terminal domain that contains the binding site for p53 and several other proteins, an acidic domain capable of interacting with p300 and the tumor suppressor p14/ARF, and a RING finger domain that harbors the E3 ligase activity responsible for p53 ubiquitination [11]. P53 and MDM2 form an auto-regulatory feedback loop. P53 binds to the P2 promoter of the MDM2 gene and promotes MDM2 gene expression. In turn, MDM2 protein binds to the N-terminal transactivation domain of p53 and inhibits p53, primarily by promoting its ubiquitination and subsequent degradation by the proteasome [12]. Several reports have shown that MDM2 can also inhibit p53 by promoting its ubiquitin-dependent exclusion from the nucleus [13-15]. The increase in p53 levels following stress results, in large part, from stabilization of the p53 protein. P53 stabilization following DNA damaging stress is believed to result from stress-induced modifications in p53 or MDM2 that disrupt p53-MDM2 binding. In contrast, p53 stabilization in response to other stresses (e.g. oncogenic or ribosomal stress) can result from an inhibition of the E3-ligase activity of MDM2 that results from stress-induced interactions of MDM2 with other proteins. For instance, Honda et al. demonstrated that p14/ARF, whose expression increases in response to oncogenic stress, can bind and inhibit the ubiquitin ligase activity of MDM2 and promote p53 stabilization [16]. Furthermore, ribosomal proteins L5, L11 and L23 were also reported to activate the p53 pathway by interacting with and inhibiting the E3 function of MDM2 [17-19]. The importance of MDM2 in regulating p53 has been best demonstrated in mice. MDM2 gene knockout leads to early embryonic lethality in mice, a phenotype that could be rescued by simultaneous deletion of the p53 gene [20, 21].
MDMX is an MDM2-related protein that has also emerged as a key p53 regulator. MDMX has high homology with MDM2, particularly in the N-terminal binding domain for p53 [22]. Like MDM2, MDMX binds with high affinity to the N-terminal transactivation domain of p53 and inhibits the ability of p53 to activate gene transcription. Importantly, however, MDMX does not target p53 for degradation [23]. Most current models suggest that MDMX inhibits the transcriptional activity of p53, whereas MDM2 is responsible for promoting p53 degradation and keeping p53 protein levels in check [24]. It was originally believed that the MDMX gene is not regulated by p53. However, Chen and colleagues [25] recently identified a functional p53-binding site in MDMX intron 1, and showed that p53 activation induced a two-fold increase in MDMX mRNA and protein in testicular germ cell tumors (TGCTs). Given that MDMX can inhibit p53 transcriptional activity, the results suggested MDMX can function in an autoregulatory feedback loop with p53, similar to MDM2. More recently, Jochemsen and colleagues [26] discovered that, like the MDM2 gene, the MDMX gene contains a promoter (P2) within its first intron that is regulated by p53. Activated p53 was shown to induce expression of an MDMX transcript from the P2 promoter that encodes a novel “long-form” of the protein. Importantly, this long-form of MDMX appeared to function with MDM2 in promoting p53 ubiquitination during recovery from stress. Thus, it appears that both MDM2 and MDMX can function in an auto-regulatory feedback loop that keeps p53 levels and activity in check.
2. Nutlin-3, a non-genotoxic activator of p53
Given that MDM2 is a crucial negative regulator of p53 and a major suppressor of p53 function in most p53 wild-type tumors, blocking the interaction between p53 and MDM2 is expected to stabilize p53 and activate the p53 pathway, leading to growth arrest and/or apoptosis in cancer. This notion has led to the development of a number of small-molecule p53-MDM2 binding inhibitors during the past several years. The first potent and selective small-molecule MDM2 antagonists, the Nutlins, were identified from a class of cis-imidazoline compounds using a conventional in vitro biochemical screening method [27]. Nutlins occupy the p53-binding pocket of MDM2 in a way that remarkably mimics the molecular interactions of the crucial amino acid residues from p53. The Nutlins could displace p53 from MDM2 in vitro with nanomolar potency (IC50 = 90 nM for Nutlin-3a, the active enantiomer of Nutlin-3) [27]. (For convenience, we will use Nutlin-3 to refer to all studies including those in which only the active enantiomer, Nutlin-3a, was used). Among Nutlins, Nutlin-3 is most commonly used in anti-cancer studies. With multiple types of cultured cells, Nutlin-3 has been shown to inhibit the p53–MDM2 interaction in the cellular context with a high degree of specificity, leading to p53 stabilization and activation of the p53 pathway [28].
P53 is subject to various post-translational modifications, including phosphorylation, acetylation, methylation, and ubiqitination on different amino acids [29]. Stress-induced phosphorylations have been shown to be important not only in the dissociation of p53 from MDM2 but also in the activation of p53 as a transcription factor. Thompson et al. [30] monitored p53 phosphorylation at six key serine residues (Ser (6), Ser (15), Ser (20), Ser (37), Ser (46), and Ser (392)) in cells in which p53 was induced by either genotoxic stresses (doxorubicin or etoposide) or induced by Nutlin-3. P53 phosphorylations induced by genotoxic stress were not observed in cells in which p53 was induced by Nutlin-3. This led to the conclusion, subsequently supported by other studies [31, 32], that Nutlin-3 stabilizes p53 in a non-genotoxic fashion, as would be expected from simply blocking the binding between p53 and MDM2. Somewhat at odds with this conclusion is a study from Verma et al. [33]. In their study, Nutlin-3 triggered a DNA damage response in azoxymethane-induced mouse AJ02-NM(0) colon cancer cells, characterized by the phosphorylation p53 at Ser 15 and the phosphorylation of H2AX at Ser-139, an accepted marker of DNA double strand breaks. One potential explanation is that the DNA damage response observed in this study was a secondary consequence of DNA fragmentation associated with apoptosis, and not the result of Nutlin-3 itself inducing DNA damage. The notion that Nutlin-3 can activate the p53 pathway in a non-genotoxic fashion is attractive from a therapeutic standpoint. Most cancer therapeutics cause DNA damage, drawbacks being the potential for collateral damage to normal surrounding tissue and the potential for secondary malignancies. By activating p53 through a non-genotoxic fashion, the usage of Nutlin-3 as a therapeutic would presumably be without these potential drawbacks.
In addition to Nutlin-3, a number of other compounds that target the p53-MDM2 interaction have been described, most notably MI-219 and RITA (Reactivation of p53 and Induction of Tumor cell Apoptosis). MI-219 was designed using a crystal structure guided technique [34]. Based on the crystal structure of the MDM2–p53 complex, a group of spiro-oxindole molecules were developed as a new class of inhibitors of the MDM2–p53 interaction. Among them, MI-219 was developed with extensive modifications. Similar to Nutlin-3, MI-219 binds to MDM2 and interrupts the p53-MDM2 interaction, stabilizing p53. MI-219 displays a high binding affinity to MDM2 with Ki value of 5 nM (Nutlin-3 has a Ki value of 36 nM under the same assay setting) [34], and is 10,000-fold selective for MDM2 over MDMX. Treatment with MI-219 was reported to cause cell cycle arrest or apoptosis in cells with wild-type p53 [34]. Another small-molecule compound, called RITA, was identified using a cell-based screen [35]. A pair of isogenic cell lines (HCT116 colon carcinoma), which differ only in their p53 status, were treated with the National Cancer Institute library compounds. RITA was identified as it suppressed the growth of HCT116 p53 +/+ cells in a dose-dependent manner but only slightly inhibited the growth of HCT116 p53-/- cells. In contrast to Nutlin-3 and MI-219, RITA binds to p53 but not to MDM2. The interaction of RITA with wild-type p53 prevented its interaction with MDM2 and resulted in accumulation of p53. Consequently, RITA induced p53 target gene expression and triggered massive apoptosis in various tumor cells expressing wild-type p53 [35]. Notably, while all three compounds can block p53-MDM2 binding and thus activate p53, the response of cells to each compound can vary. For example, Rinaldo et al. [36] compared the responses of multiple p53 wild-type cell lines with either Nutlin-3 or RITA. While the primary response to Nutlin-3 in these cell lines was growth arrest, RITA induced abundant apoptosis in these cells. The basis for these different responses resided, at least in part, on levels of the pro-apoptotic protein HIPK2 (Homeodomain-Interacting Protein Kinase 2). Specifically, HIPK2 levels decreased in Nutlin-3-treated cells due to MDM2-mediated degradation, whereas HIPK2 levels were modestly increased in cells exposed to RITA [36].
3. Cell cycle arrest, cell death, senescence, quiescence and other phenotypes induced by Nutlin-3 in normal cells and cancer cells
In wild-type p53 tumor cells, activation of p53 by Nutlin-3 induces p53- and p21-dependent cell cycle arrest and p53 dependent death. Tovar et al. [28] tested the effect of Nutlin-3 on ten wild-type p53 cancer cell lines from different tumor types, including colon, breast, lung, prostate, melanoma, osteosarcoma, and renal cancer. They showed that Nutlin-3 induced cell cycle arrest at the G1/S and G2/M boundaries and the depletion of S-phase cells in all ten cell lines. This indicated that the p53 dependent cell cycle arrest pathway is preserved in these cancer cells. More recently it was demonstrated cells that arrested in a 4N state in response to Nutlin-3 treatment are in a tetraploid G1-state [37, 38]. This is based on the fact that in multiple cell lines Nutlin-3 caused depletion of key G2/M regulators (Cyclin B1, Cyclin A, cdc2 and geminin) and up-regulation of G1-arrest markers (p53, p21, hypophosphorylated pRb) in 4N cells. These effects were dependent on both p53 and p21. The down-regulation of another G2/M phase regulator protein PLK1 (polo-like kinase1) by Nutlin-3 has also been reported [39]. Notably, in some but not all cell lines, 4N-arrested cells underwent endoreduplication after Nutlin-3 removal [37, 38]. It was further demonstrated that this endoreduplication could, in certain cell lines, give rise to stable tetraploid clones that now displayed increased resistance to irradiation and cisplatin-induced apoptosis [37, 38]. The results suggested a potentially adverse side effect of Nutlin-3-based therapies is endoreduplication and the generation of therapy-resistant tetraploid cells.
Ultimately, the outcome of p53 activation (apoptosis vs. growth arrest) by Nutlin-3 treatment may be dependent on cell type and the activation or inactivation of survival signaling pathways. Thus, most hematologic cancer cell lines with wild-type p53 undergo apoptosis as their primary response to Nutlin-3 treatment, whereas most but not all non-hematologic cancer cell lines (sarcomas, carcinomas) undergo a growth arrest. Tovar et al. [28] reported that SJSA-1 and MHM, two osteosarcoma cell lines with amplification of the MDM2 gene, were highly sensitive to Nutlin-3 induced apoptosis, whereas HCT-116 (colon cancer), A549 (lung cancer) and H460 (lung cancer), which contained only one MDM2 gene, were the least sensitive [28]. This suggested amplification of the MDM2 gene in cancer cells may predispose to p53-dependent apoptosis and such cancer cells may therefore be highly sensitive to MDM2 inhibitors. In contrast, in the study by Kitagawa et al. [40], it was found that Nutlin-3 treatment did not cause significant apoptosis in the choriocarcinoma cell line JAR, which is known to have MDM2 gene amplification. This would suggest MDM2 gene amplification is not a perfect predictor of sensitivity to Nutlin-3 induced apoptosis. Nonetheless, being able to target Nutlin-3 treated cells down the more desirable apoptotic pathway could presumably increase its therapeutic potential. Indeed, several factors have been identified that affect the response to Nutlin-3 treatment. As mentioned earlier, MDMX is an MDM2-related protein that can bind to p53 and inhibit its transcriptional activity without promoting p53 degradation. While the binding between p53 and MDM2 is effectively blocked by Nutlin-3, the binding between p53 and MDMX is not [41]. Thus, cancer cells with elevated MDMX levels are reportedly resistant to Nutlin-3 induced apoptosis, due to the ability of MDMX to bind and inhibit p53 that would otherwise be activated by Nutlin-3 [41, 42]. This has led to the realization that targeting the p53-MDMX interaction may be equally important as targeting the interaction between p53 and MDM2, at least in certain cancers. A number of small molecule inhibitors of p53-MDMX binding have been described that in some cases can induce p53-dependent apoptosis and in some cases have been reported to function in an additive or synergistic fashion with Nutlin-3 [41, 43, 44]. In another study, Kitagawa et al. [40] reported cancer cells with relatively high E2F1 activity were more susceptible to apoptosis following Nutlin-3 treatment than cancer cells with low E2F1 activity. This was linked with the ability of E2F1 to increase expression of pro-apoptotic p73, a p53-related protein. Finally, Zhu et al. [45] reported acute lymphoblastic leukemia cells with high PTEN (Phosphatase and TENsin homolog) expression were more susceptible to Nutlin-3 induced apoptosis than low PTEN expressing cells. This effect was linked with the ability of PTEN to inhibit survival signaling by the PI3K/Akt signal transduction pathway.
P53 can induce apoptosis through both transcriptional and non-transcriptional mechanisms, and both mechanisms have been described in response to Nutlin-3. First, p53 that is stabilized in response to stress or Nutlin-3 treatment accumulates in the nucleus where it functions as a transcription factor, activating expression of genes that encode pro-apoptotic proteins such as PUMA, bax, and Noxa, that drive apoptosis by disrupting the outer mitochondrial membrane [28, 42]. In contrast, a smaller though significant portion of p53 can also accumulate directly in mitochondria, where it interacts with pro- and anti-apoptotic members of the Bcl-2 family, resulting in release of factors from the mitochondria that drive apoptosis [46]. Vaseva et al. [47] reported that the transcription-independent mitochondrial p53 program is a major contributor to Nutlin-3 induced apoptosis in RKO cells (colon carcinoma) and ML1 cells (leukemia). They showed that blocking the transcription arm of p53 via α-amanitin treatment, for example, increased apoptosis induced by Nutlin-3. Furthermore, blocking p53 movement to mitochondria inhibited Nutlin-3 induced apoptosis in both cell lines. In contrast, in postnatal neurons it was shown that Nutlin-3-induced apoptosis without p53 accumulation in mitochondria and, further, that inhibition of transcription blocked apoptosis by Nutlin-3 [48]. Thus, Nutlin-3 induced apoptosis in postnatal neurons appears to occur through a p53 transcription-dependent mechanism. Finally, Saha and Chang [49] provided evidence that Nutlin-3 induced apoptosis in multiple myeloma cells involves both p53 transcription-dependent and transcription-independent pathways. It would appear that p53-dependent apoptosis following Nutlin-3 treatment can occur through different mechanisms in different cell types.
Nutlin-3 was reported to cause permanent, irreversible arrest (senescence) in fibroblasts and fibrosarcoma cells that was dependent in some cases on the length of Nutlin-3 exposure [31, 50]. In contrast, recent studies have reported that cell cycle arrest induced by prolonged Nutlin-3 treatment in p53 wild-type cancer cells is largely if not entirely reversible [51, 52]. Specifically, exposure to Nutlin-3 for up to 6 days induced a senescent-like arrest that included expression of senescence-associated beta-galactosidase in multiple p53 wild-type cancer cell lines. Despite this arrest, nearly 100% of Nutlin-3 treated cells resumed cycling after Nutlin-3 removal, as determined by BrdU incorporation. This suggested Nutlin-3 mediated cell cycle arrest is not permanent. These findings revealed a potential complication of Nutlin-3 based therapies. Namely, if Nutlin-3 induced cell cycle arrest is not permanent in some p53 wild-type cancer cells, these cells could recover after treatment and resume cycling. Nonetheless, the findings also provided a rationale for the potential use of Nutlin-3 against p53-null or mutant cancers. In this rationale, pre-treatment of p53-null or mutant tumors is predicted to arrest normal tissues and cells surrounding the tumor but allow the tumor cells to continue proliferation. Subsequent exposure to drugs that target proliferating cells would then selectively kill the tumor cells while leaving the normal surrounding cells unaffected [53-55].
More recent studies have implicated mTOR (mammalian target of rapamycin) signaling as a determinant of whether growth arrest induced by Nutlin-3 is permanent (senescence) or transient (quiescence) [56-58]. mTOR is a cytoplasmic kinase whose activity is often elevated in cancer [59]. mTOR converts signals from activated growth factor receptors into downstream events that promote cell proliferation and survival. Blagosklonny and colleagues noted that in some cases p53 induction did not induce senescence while ectopic expression of p21 did [52]. This led them to question whether p53 could unexpectedly suppress senescence. To address this, they used a cell line in which p21 was expressed from an inducible (IPTG-driven) promoter [60]. In this cell line, transient p21 expression induced by IPTG caused the cells to undergo a senescent arrest characterized by flat-cell phenotype, expression of senescence-associated beta galactosidase, and a complete loss of proliferative potential after IPTG removal [58]. To test the effect of p53 on this senescent arrest, the authors first induced p21 by IPTG, and then induced p53 expression in the same cells by addition of Nutlin-3. Remarkably, cells in which p53 was induced by Nutlin-3 maintained their proliferative potential and were able to resume cycling and fully recover after IPTG removal. These results indicated that p53 expression converted the normal senescence response in these cells to quiescence. The suppression of senescence they observed appears to be associated with a p53-dependent inhibition of mTOR activity [58]. This is based on the finding that shRNA-mediated knockdown of TSC2, a negative regulator of mTOR and p53 target gene [61], imposed senescence in these Nutlin-3 treated cells. The results supported a model in which p53 can suppress senescence through up-regulation of TSC2 and inhibition of mTOR. These findings, if correct, have obvious clinical implications. For example, if p53 activation by Nutlin-3 can suppress senescence in cancer cells and cause them to arrest in a quiescent state, then these cells could recover after treatment and resume cycling. This would conceivably limit the effectiveness of Nutlin-3-based therapies. The findings of Blagosklonny and colleagues suggest the status of the mTOR pathway can determine, at least in part, the choice between senescence and quiescence in Nutlin-3 and p53-arrested cells [57]. In fact, Nutlin-3 failed to inhibit mTOR in melanoma-derived cell lines and mouse embryo fibroblasts that undergo senescence as their primary response to p53 activation [57]. The findings imply that Nutlin-3 could be effective as a single treatment agent, but only against cancers in which p53 fails to inhibit or only partially inhibits mTOR.
Finally, effects of Nutlin-3 on the invasive and migratory capacity of cells have also been described. For example, Sechierro et al. reported an inhibitory effect of Nutlin-3 on angiogenesis, which was related at least partially to decreased migratory capacity of treated endothelial cells [62]. In other studies it was shown that Nutlin-3 could inhibit cancer cell migration and invasion indirectly through repression of stromal-derived factor-1/CXCL12 expression in stromal fibroblasts [63]. In studies from our lab it was found that Nutlin-3 treatment caused a cytoskeletal rearrangement in p53 wild-type human cancer cells from multiple etiologies [64]. Specifically, Nutlin-3 decreased actin stress fibers and reduced the size and number of focal adhesions in treated cells. This process was dependent on p53 expression but was independent of p21 expression and growth arrest. Consistent with this, Nutlin-3 treated cells failed to form filamentous actin–based motility structures (lamellipodia) and displayed significantly decreased directional persistence in response to migratory cues. Finally, chemotactic assays showed a p53-dependent/p21-independent decrease in migratory and invasive capacity of Nutlin-3-treated cells. Taken together, these findings reveal that Nutlin-3 treatment can inhibit the migration and invasion capacity of p53 wild-type cells, adding to the potential therapeutic benefit of Nutlin-3 and other small molecule MDM2 inhibitors.
4. Other targets of Nutlin-3
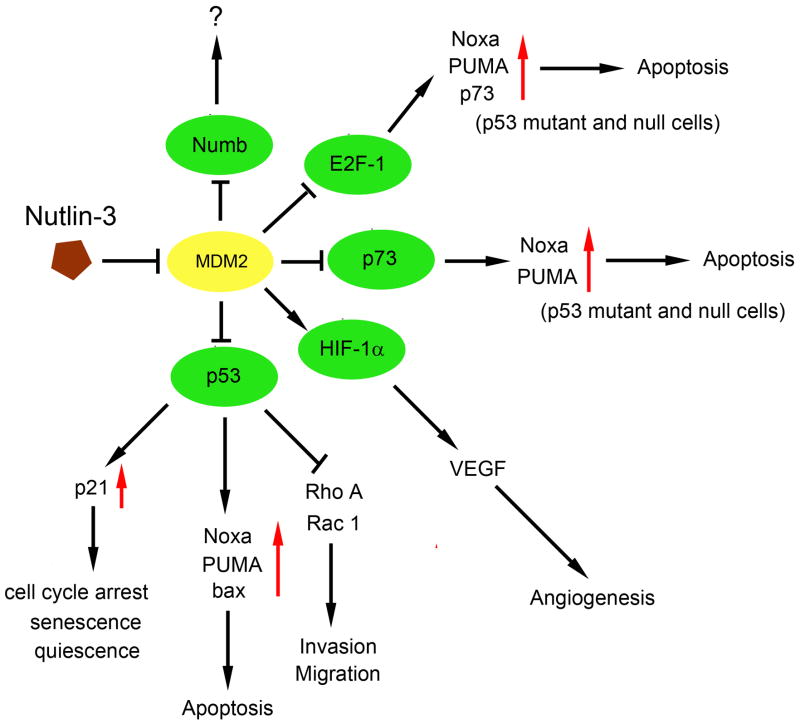
Since Nutlin-3 occupies the N-terminal p53-binding pocket of MDM2, it would likely also interfere with proteins that bind MDM2 at or near this same pocket. In addition to p53, the N-terminal domain of MDM2 has been shown to functionally interact with other proteins, including E2F1 [65], HIF-1α [66], p73 [67] and Numb [68]. In some cases, Nutlin-3 has been shown to modulate the levels/activity of these other MDM2-interacting proteins (Figure 1).
Figure 1. Molecular Mechanisms of the actions of Nutlin-3.
Nutlin-3 binds to the p53 binding pocket in MDM2 and inhibits the p53/MDM2 interaction, leading to stabilization of p53 and induction of p53-dependent cell cycle arrest or apoptosis. Nutlin-3 can also inhibit cancer cell migration and invasion in a p53-dependent manner, most likely through inhibition of RhoA and Rac1 activity. In addition to p53, Nutlin-3 also blocks the interaction of MDM2 with p73, E2F1, HIF-1α, resulting in increased apoptosis (p73, E2F1) and decreased VEGF production and decreased angiogenesis (HIF-1α). Nutlin-3 is predicted to block the interaction of MDM2 and Numb.
P73 is a p53 homolog that, like p53, can activate proapoptotic genes and induce cell death. P73 binds the MDM2 N-terminal hydrophobic pocket, which is the region targeted by Nutlin-3. Lau et al. [69] were the first to report that Nutlin-3 could disrupt the interaction between endogenous p73 and MDM2 and, as a result, enhance p73 function. In p53-null and mutant cells it was demonstrated that dissociation of p73 and MDM2 by Nutlin-3 increased p73 transcriptional activity, with up-regulation of p73 target genes PUMA and Noxa. The increase in p73 levels was due in part to stabilization of the p73 protein. Finally siRNA knockdown of p73 prevented Nutlin-3 induced apoptosis, indicating the apoptosis was at least in part p73-dependent [69]. These findings provide a rationale for the use of Nutlin-3 against p53-null or mutant tumors, due to its ability to stabilize the p73 protein and promote p73-dependent death.
E2F1 is a transcription factor that interacts with pRb and regulates the G1/S phase transition. While E2F1 can promote cell cycle progression, it is also believed to have a tumor suppressor function, based on its ability to induce apoptosis when highly expressed through induction of pro-apoptotic target genes, such as p73, PUMA, and Noxa, and also on the fact that E2F1 knockout mice develop a broad spectrum of tumors [70, 71]. E2Fs function as heterodimers with members of the DP family of transcription factors. Martin et al. [65] reported that MDM2 can bind directly to E2F1 through a region in MDM2 that includes the p53-binding pocket. They reported this binding can stimulate the activation capacity of E2F1/DP1. Blattner et al. [72] later showed that E2F1 protein was stabilized in cells transfected with MDM2 antisense oligonucleotides and in cells microinjected with antibodies that target the p53-binding pocket in MDM2, suggesting MDM2 might target E2F1 for degradation. This notion was later supported by studies from Loughran and La Thangue [73] showing MDM2 could decrease E2F1 and DP1 protein levels when all three proteins were co-expressed in p53-null cells. In their study, MDM2 blocked apoptosis in E2F1 overexpressing cells, but increased S-phase entry (measured by BrdU incorporation) and clonogenicity. Based on this it was proposed MDM2 decreases E2F1 to a level at which it can no longer promote apoptosis, but can promote cell cycle progression. The effect of Nutlin-3 on E2F1 levels appears to depend on p53 status. In p53 wild-type cells, Nutlin-3 treatment is reported to cause a drastic decrease in E2F1 levels through mechanisms that are unclear but may involve transcriptional repression of E2F1 mediated by p53-p21, or enhanced degradation [51, 71, 74]. In contrast, E2F1 levels remain unchanged or are increased in p53-null or mutant cells treated with Nutlin-3 [71, 75, 76]. In studies by Ambrosini et al. [71] it was shown that Nutlin-3 blocked endogenous interactions between E2F1 and MDM2, and E2F1 and pRb, in p53-null/mutant cells. This was associated with a decrease in E2F1 ubiquitination, consistent with MDM2 promoting E2F1 ubiquitination. Finally, in at least two separate studies Nutlin-3 enhanced apoptosis in p53-null/mutant cells when combined with chemotherapeutic drugs (cisplatin, carboplatin, and doxorubicin), and this apoptosis was E2F1 and p73-dependent apoptosis [71, 76]. At least one emerging model based on these findings is that in p53-null/mutant cells Nutlin-3 can stabilize and increase the levels of “free” E2F1, in part, by blocking MDM2-E2F1 binding. Free E2F1 may then be subject to therapy-induced modifications that direct it to pro-apoptotic promoters.
HIF-1α (Hypoxia-inducible factor 1α) is a heterodimeric transcription factor that responds to hypoxic conditions and activates expression of genes, such as vascular endothelial growth factor gene (VEGF) that promote angiogenesis [77]. HIF-1α has been shown to form a complex with MDM2 and, in one report, increased association of HIF-1α and MDM2 protected p53 from MDM2-mediated degradation [66]. Mayo and colleagues used mapping and competition studies to show that HIF-1α binds the p53-binding domain of MDM2 [78]. Further, they demonstrated that Nutlin-3 blocked the interaction between MDM2 and HIF-1α. Under normoxic or hypoxic conditions, MDM2 binding increased the ability of HIF-1α to induce VEGF production [78, 79]. Blocking the association of MDM2 and HIF-1α by Nutlin-3 diminished the level of VEGF under normoxic or hypoxic conditions [78]. The findings suggest Nutlin-3 can diminish VEGF production and thus may inhibit tumor angiogenesis by blocking the interaction between HIF-1α and MDM2.
Numb is a cell fate regulatory protein involved in a variety of developmental processes, most notably development of the nervous system [80]. Oren and colleagues reported MDM2 can bind Numb and promote its degradation [68]. Analysis of MDM2 mutant proteins demonstrated that Numb binding occurs through the N-terminal p53-binding region of MDM2 [68]. Moreover, point mutations within the p53-binding domain that are known to disrupt p53-MDM2 binding also disrupted the binding between MDM2 and Numb. This suggests that Numb binds the same region of MDM2 as does p53 and, therefore, that the interaction between MDM2 and Numb may be disrupted by Nutlin-3. To our knowledge, the effect of Nutlin-3 on Numb levels, activity, and interaction with MDM2 has not been explored.
5. Nutlin-3 used as a combination agent for cancer cell killing
Most currently available genotoxic therapies, such as irradiation (IR) and chemotherapeutic drugs, activate p53 pathway by inducing DNA damage. However a potential drawback of such therapies is that they can impose a high genotoxic burden to normal tissues that may lead to development of secondary malignancies later in life. As discussed earlier, Nutlin-3 increases p53 through a non-genotoxic mechanism. Therefore, combining Nutlin-3 with lower doses of conventional genotoxic agents may achieve the desired level/activity of p53 while reducing the genotoxic burden on normal tissues. In vivo experiments with nude mice demonstrated that Nutlin-3 has little toxicity to animals at therapeutically efficacious dose schedules [27]. These data suggest that the non-genotoxic activation can be less toxic and better tolerated than genotoxic p53 activation in vivo.
Perhaps the most promising therapy potential for Nutlin-3 and related compounds is in the treatment of blood malignancy. In vitro experiments demonstrated that acute myeloid leukemia (AML), B-chronic lymphocytic leukemia (B-CLL), and multiple myeloma patient specimens mainly underwent apoptosis in response to Nutlin-3 treatment [81-83]. Since only 10% of AML, B-chronic lymphocytic leukemia and multiple myeloma have p53 mutation or deletion [3], they are potentially attractive tumor types for Nutlin-3 based therapy or other therapies that target p53-MDM2 binding. Nutlin-3 was also reported to display additive and/or synergistic effects when used in combination with genotoxic therapy commonly used for the treatment of haematological malignancies (Table 1). For example, Nutlin-3 demonstrated synergetic effect with the genotoxic drugs doxorubicin, chlorambucil, and fludarabine in B-cell chronic lymphocytic leukemia cells [81]; Also, Nutlin-3 has been combined with CpG-oligodeoxynucleotide to induces strong immune stimulation coupled to cytotoxicity in B-cell chronic lymphocytic leukemia cells [84]. These studies suggested MDM2 antagonists such as Nutlin-3 may enhance the activity of established chemotherapeutics in vitro.
Table 1. Antitumor activity of Nutlin-3 in combination with other drugs or therapies.
| Cancer Type | Combination Therapy (Nutlin-3 + Treatment) | P53 status | Effect of Nutlin-3 | Reference |
|---|---|---|---|---|
| Acute Lymphoblastic Leukemia | Ly294002 (PI3K inhibitor) | WT | Synergistic apoptosis induction | [45] |
| Acute Myeloid Leukemia | 1,25-dihydroxyvitamin D(3) | WT | Enhanced apoptosis | [90] |
| Acute Myeloid Leukemia | AZD6244 (inhibitor of MEK signaling) | WT | Synergistic apoptosis induction | [91] |
| Acute Myeloid Leukemia | inhibition of X-linked inhibitor of apoptosis (XIAP) by antisense oligonucleotide | WT | Synergistic apoptosis induction | [92] |
| Acute Myeloid Leukemia | MK-0457 (Pan-Aurora kinase inhibitor) | WT | Synergistic apoptosis induction | [93] |
| Acute Myeloid Leukemia | PI-103 (dual PI3 kinase/mTOR inhibitor) | WT | Synergistic apoptosis induction | [94] |
| Acute Myeloid Leukemia | TRAIL (death ligand) | WT | Synergistic apoptosis induction | [89] |
| Acute Myeloid Leukemia | PD98059 (inhibitor of mitogen- activated protein kinase kinase) | WT | Synergistic apoptosis induction | [95] |
| Acute Myeloid Leukemia | ABT-737 (inhibitor of Bcl-2) | WT | Synergistic apoptosis induction | [96] |
| B Lymphoid & Myeloid Leukemia | Perifosine (alkylphospholipids) | WT | Synergistic Cytoxicity | [97] |
| B-cell chronic lymphocytic leukemia cells | Dasatinib (Multi-kinase inhibitor) | WT & Mutant | Synergistic Cytoxicity | [88] |
| B-cell chronic lymphocytic leukemia cells | Doxorubicin, Chlorambucil & fludarabin (Chemotherapy Drugs) | WT | Synergistic apoptosis induction | [81] |
| Endometrial and Ovarian cancer cells | Low-dose Mithramycin (inhibitor of Sp1 DNA binding) | WT | Synergistic apoptosis induction | [98] |
| Hepatocellular carcinoma | Doxorubicin (Chemotherapy Drug) | WT & Mutant | Enhanced Chemosensitivity | [99] |
| Laryngeal Carcinoma | Radiation | WT | Enhanced Radiosensitivity | [85] |
| Lung Cancer | Radiation | WT | Enhanced Radiosensitivity | [100] |
| Malignant Peripheral Nerve Sheath & Colon Cancer cells | Cisplatin, Carboplatin & Doxorubicin (Chemotherapy Drugs) | Mutant & Null | Synergistic apoptosis induction | [71] |
| Multiple Myeloma | RITA (inhibitor of p53-MDM2 interaction) | WT | Synergistic Cytoxicity | [101] |
| Melanoma, Colon & Breast Cancer cells | Roscovitin & DRB (CDK inhibitors) | WT | Synergistic apoptosis induction | [87] |
| Mantle Cell Lymphoma | Bortezomib (Proteasome inhibitor) | Mutant | Synergistic apoptosis induction | [102] |
| Multiple Myeloma | Velcade (Proteasome inhibitor) | WT | Synergistic Cytoxicity | [103] |
| Neuroblastoma | Doxorubicin (Chemotherapy Drug) | Null | Enhanced Chemosensitivity | [76] |
| Neuroblastoma | Cisplatin & Etoposide (Chemotherapy Drugs) | WT | Synergistic apoptosis induction | [104] |
| Prostate Cancer Cells | Radiation | WT & Mutant | Radio-sensitized under hypoxic conditions | [86] |
The effects of combining Nutlin-3 and irradiation have also been examined in several studies (Table 1). Arya et al reported that wild-type p53 laryngeal carcinoma cells were significantly radiosensitized by Nutlin-3 and displayed enhanced cell cycle arrest in the absence of increased apoptosis [85]. In another study, Nutlin-3 has been shown to radiosensitize hypoxic prostate cancer cells via p53 independent mechanism under low O2 conditions [86].
In addition to genotoxic therapy, Nutlin-3 has also been shown to synergize the cytoxicity of other drugs (Table 1). Cheok et al. reported that the combination of CDK inhibitors (Roscovitine and DRB) and Nutlin-3 showed a clear synergism in the activation of p53 and the promotion of p53 dependent apoptosis in a range of p53 wild-type tumor cells, including melanoma, colon carcinoma, breast adenocarcinoma, and hepatocarcinoma cells [87]. The molecular basis of this combined therapeutic activity is that the use of CDK inhibitors strongly enhanced Nutlin-3 dependent induction of p53 activity. Nutlin-3 has also shown synergistic anti-leukemic activity with a small molecule multikinase inhibitor Dasatinib in both p53 wild-type and p53 mutated B chronic lymphocytic leukemia by inhibiting the Akt pathway [88]. In p53 wild-type AML cell lines Nutlin-3 has been demonstrated to synergize with recombinant TRAIL in inducing apoptosis [89]. One possible underlying mechanism is that Nutlin-3 was reported to up-regulate TRAIL-R2 both at the mRNA level and at the cell surface level.
5. Final Remarks
Wild-type p53 is a tumor suppressor activated by DNA damage and other stresses. There has been considerable interest in restoring wild-type p53 function as a therapeutic strategy. This goal has led to the development of Nutlin-3, a small molecule that activates p53 by blocking its interaction with MDM2, the primary negative regulator of p53 activity in cells. Notably, Nutlin-3 activates p53 through a non-genotoxic mechanism, and thus its use as a therapeutic agent may spare tissues of deleterious side-effects associated with common DNA damaging drugs. Nutlin-3 has shown considerable promise in preclinical studies against p53 wild-type cancers [27, 28, 83]. Thus, Nutlin-3 treatment induced robust growth arrest or apoptosis in cultured cell lines that express wild-type p53, and inhibited the growth of p53 wild-type human tumors grown as mouse xenografts. Most currently available genotoxic therapies activate the p53 pathway by inducing DNA damage. When combined with radiation or chemotherapeutic drugs, Nutlin-3 can function in an additive or synergistic fashion to enhance tumor cell killing (see Table 1). Thus, in a clinical setting, combining Nutlin-3 with lower doses of conventional genotoxic agents may achieve equivalent tumor killing while reducing the genotoxic burden to normal tissues.
There is also interest/support for the use of Nutlin-3 against p53-null or mutant tumors. In one strategy, Nutlin-3 would promote cell cycle arrest in normal tissues that surround a p53-null/mutant tumor, while the tumor cells themselves would continue to proliferate. Subsequent treatment with drugs that target proliferating cells would then selectively kill the tumor cells while having no effect on the arrested normal cells. Support for this strategy comes from studies in which p53 wild-type cells arrested in G1 and G2-phase by Nutlin-3 pretreatment were resistant to killing by the S-phase drug gemcitabine or the microtubule poison taxol [54, 55]. Nutlin-3 has also been shown to have some direct growth inhibitory effects in p53-null/mutant cells. For example, Nutlin-3 triggered p73-dependent apoptosis in p53-null cells by disrupting the interaction between MDM2 and p73 [69]. In other studies, Nutlin-3 enhanced therapy-induced apoptosis that was p73- and E2F1-dependent in p53-null/mutant cells by disrupting the interactions between MDM2 and p73, and MDM2 and E2F1 [71, 76]. In sum, there is support for the use of Nutlin-3 against both p53 wild-type and p53-null/mutant cancers.
The therapeutic potential of Nutlin-3 is not limited to its ability to induce growth arrest or apoptosis. For example, Mayo and colleagues reported that Nutlin-3 can block VEGF production by disrupting the interaction between MDM2 and HIF-1α [78]. These results suggest Nutlin-3 may have anti-angiogenic properties. Nutlin-3 treatment also decreased expression in stromal fibroblasts of stromal-derived factor 1 (SDF1) and, as a result, blocked SDF-1 mediated cancer cell migration [63]. In our lab, Nutlin-3 decreased the migration and invasive capacity of p53 wild-type cancer cells, most likely by decreasing the activities of RhoA and Rac1 [64].
Finally, it is worth pointing out that Nutlin-3 is not only a potential therapeutic agent, but also a useful tool for studying the p53 pathway. In the past, the p53 pathway was often studied in cells exposed to DNA damaging stress. However, the multitude of signaling events activated by DNA damage made it a challenge to identify those events/outcomes that were solely p53-dependent. Given its high specificity, Nutlin-3 can be used to identify those pathways that are modulated specifically and solely by non-genotoxic p53 activation.
Acknowledgments
This study is supported by NCI grant 1RO1CA137598-01A1 to CGM.
List of Abbreviations
- MDM2
Murine Double Minute 2
- PI3K
Phosphatidylinositol 3-Kinases
- PUMA
P53 Upregulated Modulator of Apoptosis
- TSC2
Tuberous sclerosis protein 2
- MEK
Mitogen-activated protein kinase
- DRB
5,6-dichlorobenzimidazole riboside
- CDK
Cyclin-dependent kinase
References
- 1.Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer. 2009;9:749–58. doi: 10.1038/nrc2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 3.Olivier M, Hollstein M, Hainaut P. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb Perspect Biol. 2010;2:a001008. doi: 10.1101/cshperspect.a001008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed AA, Etemadmoghadam D, Temple J, Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio A, Bowtell D, Brenton JD. Driver mutations in TP53 are ubiquitous in high grade serous carcinoma of the ovary. J Pathol. 2010;221:49–56. doi: 10.1002/path.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bullock AN, Fersht AR. Rescuing the function of mutant p53. Nat Rev Cancer. 2001;1:68–76. doi: 10.1038/35094077. [DOI] [PubMed] [Google Scholar]
- 6.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–73. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 7.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R, Jacks T. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 8.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature. 2007;445:656–60. doi: 10.1038/nature05529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature. 1997;387:299–303. doi: 10.1038/387299a0. [DOI] [PubMed] [Google Scholar]
- 10.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature. 1997;387:296–9. doi: 10.1038/387296a0. [DOI] [PubMed] [Google Scholar]
- 11.Iwakuma T, Lozano G. MDM2, an introduction. Mol Cancer Res. 2003;1:993–1000. [PubMed] [Google Scholar]
- 12.Michael D, Oren M. The p53-Mdm2 module and the ubiquitin system. Semin Cancer Biol. 2003;13:49–58. doi: 10.1016/s1044-579x(02)00099-8. [DOI] [PubMed] [Google Scholar]
- 13.Geyer RK, Yu ZK, Maki CG. The MDM2 RING-finger domain is required to promote p53 nuclear export. Nat Cell Biol. 2000;2:569–73. doi: 10.1038/35023507. [DOI] [PubMed] [Google Scholar]
- 14.O'Keefe K, Li H, Zhang Y. Nucleocytoplasmic shuttling of p53 is essential for MDM2-mediated cytoplasmic degradation but not ubiquitination. Mol Cell Biol. 2003;23:6396–405. doi: 10.1128/MCB.23.18.6396-6405.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boyd SD, Tsai KY, Jacks T. An intact HDM2 RING-finger domain is required for nuclear exclusion of p53. Nat Cell Biol. 2000;2:563–8. doi: 10.1038/35023500. [DOI] [PubMed] [Google Scholar]
- 16.Honda R, Yasuda H. Association of p19(ARF) with Mdm2 inhibits ubiquitin ligase activity of Mdm2 for tumor suppressor p53. EMBO J. 1999;18:22–7. doi: 10.1093/emboj/18.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lohrum MA, Ludwig RL, Kubbutat MH, Hanlon M, Vousden KH. Regulation of HDM2 activity by the ribosomal protein L11. Cancer Cell. 2003;3:577–87. doi: 10.1016/s1535-6108(03)00134-x. [DOI] [PubMed] [Google Scholar]
- 18.Dai MS, Lu H. Inhibition of MDM2-mediated p53 ubiquitination and degradation by ribosomal protein L5. J Biol Chem. 2004;279:44475–82. doi: 10.1074/jbc.M403722200. [DOI] [PubMed] [Google Scholar]
- 19.Dai MS, Zeng SX, Jin Y, Sun XX, David L, Lu H. Ribosomal protein L23 activates p53 by inhibiting MDM2 function in response to ribosomal perturbation but not to translation inhibition. Mol Cell Biol. 2004;24:7654–68. doi: 10.1128/MCB.24.17.7654-7668.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montes de Oca Luna R, Wagner DS, Lozano G. Rescue of early embryonic lethality in mdm2-deficient mice by deletion of p53. Nature. 1995;378:203–6. doi: 10.1038/378203a0. [DOI] [PubMed] [Google Scholar]
- 21.Jones SN, Roe AE, Donehower LA, Bradley A. Rescue of embryonic lethality in Mdm2-deficient mice by absence of p53. Nature. 1995;378:206–8. doi: 10.1038/378206a0. [DOI] [PubMed] [Google Scholar]
- 22.Shvarts A, Steegenga WT, Riteco N, van Laar T, Dekker P, Bazuine M, van Ham RC, van der Houven van Oordt W, Hateboer G, van der Eb AJ, Jochemsen AG. MDMX: a novel p53-binding protein with some functional properties of MDM2. EMBO J. 1996;15:5349–57. [PMC free article] [PubMed] [Google Scholar]
- 23.Stad R, Little NA, Xirodimas DP, Frenk R, van der Eb AJ, Lane DP, Saville MK, Jochemsen AG. Mdmx stabilizes p53 and Mdm2 via two distinct mechanisms. EMBO Rep. 2001;2:1029–34. doi: 10.1093/embo-reports/kve227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wade M, Wang YV, Wahl GM. The p53 orchestra: Mdm2 and Mdmx set the tone. Trends Cell Biol. 2010;20:299–309. doi: 10.1016/j.tcb.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li B, Cheng Q, Li Z, Chen J. p53 inactivation by MDM2 and MDMX negative feedback loops in testicular germ cell tumors. Cell Cycle. 2010;9:1411–20. doi: 10.4161/cc.9.7.11255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips A, Teunisse A, Lam S, Lodder K, Darley M, Emaduddin M, Wolf A, Richter J, de Lange J, Verlaan-de Vries M, Lenos K, Bohnke A, Bartel F, Blaydes JP, Jochemsen AG. HDMX-L is expressed from a functional p53-responsive promoter in the first intron of the HDMX gene and participates in an autoregulatory feedback loop to control p53 activity. J Biol Chem. 2010;285:29111–27. doi: 10.1074/jbc.M110.129726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 28.Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, Myklebost O, Heimbrook DC, Vassilev LT. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proc Natl Acad Sci U S A. 2006;103:1888–93. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meek DW, Anderson CW. Posttranslational modification of p53: cooperative integrators of function. Cold Spring Harb Perspect Biol. 2009;1:a000950. doi: 10.1101/cshperspect.a000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thompson T, Tovar C, Yang H, Carvajal D, Vu BT, Xu Q, Wahl GM, Heimbrook DC, Vassilev LT. Phosphorylation of p53 on key serines is dispensable for transcriptional activation and apoptosis. J Biol Chem. 2004;279:53015–22. doi: 10.1074/jbc.M410233200. [DOI] [PubMed] [Google Scholar]
- 31.Kumamoto K, Spillare EA, Fujita K, Horikawa I, Yamashita T, Appella E, Nagashima M, Takenoshita S, Yokota J, Harris CC. Nutlin-3a activates p53 to both down-regulate inhibitor of growth 2 and up-regulate mir-34a, mir-34b, and mir-34c expression, and induce senescence. Cancer Res. 2008;68:3193–203. doi: 10.1158/0008-5472.CAN-07-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drakos E, Thomaides A, Medeiros LJ, Li J, Leventaki V, Konopleva M, Andreeff M, Rassidakis GZ. Inhibition of p53-murine double minute 2 interaction by nutlin-3A stabilizes p53 and induces cell cycle arrest and apoptosis in Hodgkin lymphoma. Clin Cancer Res. 2007;13:3380–7. doi: 10.1158/1078-0432.CCR-06-2581. [DOI] [PubMed] [Google Scholar]
- 33.Verma R, Rigatti MJ, Belinsky GS, Godman CA, Giardina C. DNA damage response to the Mdm2 inhibitor nutlin-3. Biochem Pharmacol. 2010;79:565–74. doi: 10.1016/j.bcp.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shangary S, Qin D, McEachern D, Liu M, Miller RS, Qiu S, Nikolovska-Coleska Z, Ding K, Wang G, Chen J, Bernard D, Zhang J, Lu Y, Gu Q, Shah RB, Pienta KJ, Ling X, Kang S, Guo M, Sun Y, Yang D, Wang S. Temporal activation of p53 by a specific MDM2 inhibitor is selectively toxic to tumors and leads to complete tumor growth inhibition. Proc Natl Acad Sci U S A. 2008;105:3933–8. doi: 10.1073/pnas.0708917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Issaeva N, Bozko P, Enge M, Protopopova M, Verhoef LG, Masucci M, Pramanik A, Selivanova G. Small molecule RITA binds to p53, blocks p53-HDM-2 interaction and activates p53 function in tumors. Nat Med. 2004;10:1321–8. doi: 10.1038/nm1146. [DOI] [PubMed] [Google Scholar]
- 36.Rinaldo C, Prodosmo A, Siepi F, Moncada A, Sacchi A, Selivanova G, Soddu S. HIPK2 regulation by MDM2 determines tumor cell response to the p53-reactivating drugs nutlin-3 and RITA. Cancer Res. 2009;69:6241–8. doi: 10.1158/0008-5472.CAN-09-0337. [DOI] [PubMed] [Google Scholar]
- 37.Shen H, Maki CG. Persistent p21 expression after Nutlin-3a removal is associated with senescence-like arrest in 4N cells. J Biol Chem. 2010;285:23105–14. doi: 10.1074/jbc.M110.124990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen H, Moran DM, Maki CG. Transient nutlin-3a treatment promotes endoreduplication and the generation of therapy-resistant tetraploid cells. Cancer Res. 2008;68:8260–8. doi: 10.1158/0008-5472.CAN-08-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKenzie L, King S, Marcar L, Nicol S, Dias SS, Schumm K, Robertson P, Bourdon JC, Perkins N, Fuller-Pace F, Meek DW. p53-dependent repression of polo-like kinase-1 (PLK1) Cell Cycle. 2010;9:4200–12. doi: 10.4161/cc.9.20.13532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitagawa M, Aonuma M, Lee SH, Fukutake S, McCormick F. E2F-1 transcriptional activity is a critical determinant of Mdm2 antagonist-induced apoptosis in human tumor cell lines. Oncogene. 2008;27:5303–14. doi: 10.1038/onc.2008.164. [DOI] [PubMed] [Google Scholar]
- 41.Hu B, Gilkes DM, Farooqi B, Sebti SM, Chen J. MDMX overexpression prevents p53 activation by the MDM2 inhibitor Nutlin. J Biol Chem. 2006;281:33030–5. doi: 10.1074/jbc.C600147200. [DOI] [PubMed] [Google Scholar]
- 42.Xia M, Knezevic D, Tovar C, Huang B, Heimbrook DC, Vassilev LT. Elevated MDM2 boosts the apoptotic activity of p53-MDM2 binding inhibitors by facilitating MDMX degradation. Cell Cycle. 2008;7:1604–12. doi: 10.4161/cc.7.11.5929. [DOI] [PubMed] [Google Scholar]
- 43.Wang H, Ma X, Ren S, Buolamwini JK, Yan C. A Small-molecule Inhibitor of MDMX Activates p53 and Induces Apoptosis in Cancer Cells. Mol Cancer Ther. 2010 doi: 10.1158/1535-7163.MCT-10-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernal F, Wade M, Godes M, Davis TN, Whitehead DG, Kung AL, Wahl GM, Walensky LD. A stapled p53 helix overcomes HDMX-mediated suppression of p53. Cancer Cell. 2010;18:411–22. doi: 10.1016/j.ccr.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhu N, Gu L, Li F, Zhou M. Inhibition of the Akt/survivin pathway synergizes the antileukemia effect of nutlin-3 in acute lymphoblastic leukemia cells. Mol Cancer Ther. 2008;7:1101–9. doi: 10.1158/1535-7163.MCT-08-0179. [DOI] [PubMed] [Google Scholar]
- 46.Vaseva AV, Moll UM. The mitochondrial p53 pathway. Biochim Biophys Acta. 2009;1787:414–20. doi: 10.1016/j.bbabio.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vaseva AV, Marchenko ND, Moll UM. The transcription-independent mitochondrial p53 program is a major contributor to nutlin-induced apoptosis in tumor cells. Cell Cycle. 2009;8:1711–9. doi: 10.4161/cc.8.11.8596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Uo T, Kinoshita Y, Morrison RS. Apoptotic actions of p53 require transcriptional activation of PUMA and do not involve a direct mitochondrial/cytoplasmic site of action in postnatal cortical neurons. J Neurosci. 2007;27:12198–210. doi: 10.1523/JNEUROSCI.3222-05.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saha MN, Jiang H, Chang H. Molecular mechanisms of nutlin-induced apoptosis in multiple myeloma: evidence for p53-transcription-dependent and -independent pathways. Cancer Biol Ther. 2010;10:567–78. doi: 10.4161/cbt.10.6.12535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Efeyan A, Ortega-Molina A, Velasco-Miguel S, Herranz D, Vassilev LT, Serrano M. Induction of p53-dependent senescence by the MDM2 antagonist nutlin-3a in mouse cells of fibroblast origin. Cancer Res. 2007;67:7350–7. doi: 10.1158/0008-5472.CAN-07-0200. [DOI] [PubMed] [Google Scholar]
- 51.Huang B, Deo D, Xia M, Vassilev LT. Pharmacologic p53 activation blocks cell cycle progression but fails to induce senescence in epithelial cancer cells. Mol Cancer Res. 2009;7:1497–509. doi: 10.1158/1541-7786.MCR-09-0144. [DOI] [PubMed] [Google Scholar]
- 52.Korotchkina LG, Demidenko ZN, Gudkov AV, Blagosklonny MV. Cellular quiescence caused by the Mdm2 inhibitor nutlin-3A. Cell Cycle. 2009;8:3777–81. doi: 10.4161/cc.8.22.10121. [DOI] [PubMed] [Google Scholar]
- 53.Tokalov SV, Abolmaali ND. Protection of p53 wild type cells from taxol by nutlin-3 in the combined lung cancer treatment. BMC Cancer. 2010;10:57. doi: 10.1186/1471-2407-10-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kranz D, Dobbelstein M. Nongenotoxic p53 activation protects cells against S-phase-specific chemotherapy. Cancer Res. 2006;66:10274–80. doi: 10.1158/0008-5472.CAN-06-1527. [DOI] [PubMed] [Google Scholar]
- 55.Carvajal D, Tovar C, Yang H, Vu BT, Heimbrook DC, Vassilev LT. Activation of p53 by MDM2 antagonists can protect proliferating cells from mitotic inhibitors. Cancer Res. 2005;65:1918–24. doi: 10.1158/0008-5472.CAN-04-3576. [DOI] [PubMed] [Google Scholar]
- 56.Leontieva OV, Gudkov AV, Blagosklonny MV. Weak p53 permits senescence during cell cycle arrest. Cell Cycle. 2010;9:4323–7. doi: 10.4161/cc.9.21.13584. [DOI] [PubMed] [Google Scholar]
- 57.Korotchkina LG, Leontieva OV, Bukreeva EI, Demidenko ZN, Gudkov AV, Blagosklonny MV. The choice between p53-induced senescence and quiescence is determined in part by the mTOR pathway. Aging (Albany NY) 2010;2:344–52. doi: 10.18632/aging.100160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci U S A. 2010;107:9660–4. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27(2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang BD, Broude EV, Fang J, Kalinichenko TV, Abdryashitov R, Poole JC, Roninson IB. p21Waf1/Cip1/Sdi1-induced growth arrest is associated with depletion of mitosis-control proteins and leads to abnormal mitosis and endoreduplication in recovering cells. Oncogene. 2000;19:2165–70. doi: 10.1038/sj.onc.1203573. [DOI] [PubMed] [Google Scholar]
- 61.Feng Z, Hu W, de Stanchina E, Teresky AK, Jin S, Lowe S, Levine AJ. The regulation of AMPK beta1, TSC2, and PTEN expression by p53: stress, cell and tissue specificity, and the role of these gene products in modulating the IGF-1-AKT-mTOR pathways. Cancer Res. 2007;67:3043–53. doi: 10.1158/0008-5472.CAN-06-4149. [DOI] [PubMed] [Google Scholar]
- 62.Secchiero P, Corallini F, Gonelli A, Dell'Eva R, Vitale M, Capitani S, Albini A, Zauli G. Antiangiogenic activity of the MDM2 antagonist nutlin-3. Circ Res. 2007;100:61–9. doi: 10.1161/01.RES.0000253975.76198.ff. [DOI] [PubMed] [Google Scholar]
- 63.Moskovits N, Kalinkovich A, Bar J, Lapidot T, Oren M. p53 Attenuates cancer cell migration and invasion through repression of SDF-1/CXCL12 expression in stromal fibroblasts. Cancer Res. 2006;66:10671–6. doi: 10.1158/0008-5472.CAN-06-2323. [DOI] [PubMed] [Google Scholar]
- 64.Moran DM, Maki CG. Nutlin-3a induces cytoskeletal rearrangement and inhibits the migration and invasion capacity of p53 wild-type cancer cells. Mol Cancer Ther. 2010;9:895–905. doi: 10.1158/1535-7163.MCT-09-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martin K, Trouche D, Hagemeier C, Sorensen TS, La Thangue NB, Kouzarides T. Stimulation of E2F1/DP1 transcriptional activity by MDM2 oncoprotein. Nature. 1995;375:691–4. doi: 10.1038/375691a0. [DOI] [PubMed] [Google Scholar]
- 66.Chen D, Li M, Luo J, Gu W. Direct interactions between HIF-1 alpha and Mdm2 modulate p53 function. J Biol Chem. 2003;278:13595–8. doi: 10.1074/jbc.C200694200. [DOI] [PubMed] [Google Scholar]
- 67.Balint E, Bates S, Vousden KH. Mdm2 binds p73 alpha without targeting degradation. Oncogene. 1999;18:3923–9. doi: 10.1038/sj.onc.1202781. [DOI] [PubMed] [Google Scholar]
- 68.Juven-Gershon T, Shifman O, Unger T, Elkeles A, Haupt Y, Oren M. The Mdm2 oncoprotein interacts with the cell fate regulator Numb. Mol Cell Biol. 1998;18:3974–82. doi: 10.1128/mcb.18.7.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lau LM, Nugent JK, Zhao X, Irwin MS. HDM2 antagonist Nutlin-3 disrupts p73-HDM2 binding and enhances p73 function. Oncogene. 2008;27:997–1003. doi: 10.1038/sj.onc.1210707. [DOI] [PubMed] [Google Scholar]
- 70.Yamasaki L, Jacks T, Bronson R, Goillot E, Harlow E, Dyson NJ. Tumor induction and tissue atrophy in mice lacking E2F-1. Cell. 1996;85:537–48. doi: 10.1016/s0092-8674(00)81254-4. [DOI] [PubMed] [Google Scholar]
- 71.Ambrosini G, Sambol EB, Carvajal D, Vassilev LT, Singer S, Schwartz GK. Mouse double minute antagonist Nutlin-3a enhances chemotherapy-induced apoptosis in cancer cells with mutant p53 by activating E2F1. Oncogene. 2007;26:3473–81. doi: 10.1038/sj.onc.1210136. [DOI] [PubMed] [Google Scholar]
- 72.Blattner C, Sparks A, Lane D. Transcription factor E2F-1 is upregulated in response to DNA damage in a manner analogous to that of p53. Mol Cell Biol. 1999;19:3704–13. doi: 10.1128/mcb.19.5.3704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Loughran O, La Thangue NB. Apoptotic and growth-promoting activity of E2F modulated by MDM2. Mol Cell Biol. 2000;20:2186–97. doi: 10.1128/mcb.20.6.2186-2197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wunderlich M, Berberich SJ. Mdm2 inhibition of p53 induces E2F1 transactivation via p21. Oncogene. 2002;21:4414–21. doi: 10.1038/sj.onc.1205541. [DOI] [PubMed] [Google Scholar]
- 75.Ray RM, Bhattacharya S, Johnson LR. Mdm2 inhibition induces apoptosis in p53 deficient human colon cancer cells by activating p73- and E2F1-mediated expression of PUMA and Siva-1. Apoptosis. 2010 doi: 10.1007/s10495-010-0538-0. [DOI] [PubMed] [Google Scholar]
- 76.Peirce SK, Findley HW. The MDM2 antagonist nutlin-3 sensitizes p53-null neuroblastoma cells to doxorubicin via E2F1 and TAp73. Int J Oncol. 2009;34:1395–402. [PubMed] [Google Scholar]
- 77.Otrock ZK, Hatoum HA, Awada AH, Ishak RS, Shamseddine AI. Hypoxia-inducible factor in cancer angiogenesis: structure, regulation and clinical perspectives. Crit Rev Oncol Hematol. 2009;70:93–102. doi: 10.1016/j.critrevonc.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 78.LaRusch GA, Jackson MW, Dunbar JD, Warren RS, Donner DB, Mayo LD. Nutlin3 blocks vascular endothelial growth factor induction by preventing the interaction between hypoxia inducible factor 1alpha and Hdm2. Cancer Res. 2007;67:450–4. doi: 10.1158/0008-5472.CAN-06-2710. [DOI] [PubMed] [Google Scholar]
- 79.Nieminen AL, Qanungo S, Schneider EA, Jiang BH, Agani FH. Mdm2 and HIF-1alpha interaction in tumor cells during hypoxia. J Cell Physiol. 2005;204:364–9. doi: 10.1002/jcp.20406. [DOI] [PubMed] [Google Scholar]
- 80.Zilian O, Saner C, Hagedorn L, Lee HY, Sauberli E, Suter U, Sommer L, Aguet M. Multiple roles of mouse Numb in tuning developmental cell fates. Curr Biol. 2001;11:494–501. doi: 10.1016/s0960-9822(01)00149-x. [DOI] [PubMed] [Google Scholar]
- 81.Coll-Mulet L, Iglesias-Serret D, Santidrian AF, Cosialls AM, de Frias M, Castano E, Campas C, Barragan M, de Sevilla AF, Domingo A, Vassilev LT, Pons G, Gil J. MDM2 antagonists activate p53 and synergize with genotoxic drugs in B-cell chronic lymphocytic leukemia cells. Blood. 2006;107:4109–14. doi: 10.1182/blood-2005-08-3273. [DOI] [PubMed] [Google Scholar]
- 82.Stuhmer T, Chatterjee M, Hildebrandt M, Herrmann P, Gollasch H, Gerecke C, Theurich S, Cigliano L, Manz RA, Daniel PT, Bommert K, Vassilev LT, Bargou RC. Nongenotoxic activation of the p53 pathway as a therapeutic strategy for multiple myeloma. Blood. 2005;106:3609–17. doi: 10.1182/blood-2005-04-1489. [DOI] [PubMed] [Google Scholar]
- 83.Kojima K, Konopleva M, Samudio IJ, Shikami M, Cabreira-Hansen M, McQueen T, Ruvolo V, Tsao T, Zeng Z, Vassilev LT, Andreeff M. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–9. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Secchiero P, Melloni E, Tiribelli M, Gonelli A, Zauli G. Combined treatment of CpG-oligodeoxynucleotide with Nutlin-3 induces strong immune stimulation coupled to cytotoxicity in B-chronic lymphocytic leukemic (B-CLL) cells. J Leukoc Biol. 2008;83:434–7. doi: 10.1189/jlb.0707459. [DOI] [PubMed] [Google Scholar]
- 85.Arya AK, El-Fert A, Devling T, Eccles RM, Aslam MA, Rubbi CP, Vlatkovic N, Fenwick J, Lloyd BH, Sibson DR, Jones TM, Boyd MT. Nutlin-3, the small-molecule inhibitor of MDM2, promotes senescence and radiosensitises laryngeal carcinoma cells harbouring wild-type p53. Br J Cancer. 2010;103:186–95. doi: 10.1038/sj.bjc.6605739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Supiot S, Hill RP, Bristow RG. Nutlin-3 radiosensitizes hypoxic prostate cancer cells independent of p53. Mol Cancer Ther. 2008;7:993–9. doi: 10.1158/1535-7163.MCT-07-0442. [DOI] [PubMed] [Google Scholar]
- 87.Cheok CF, Dey A, Lane DP. Cyclin-dependent kinase inhibitors sensitize tumor cells to nutlin-induced apoptosis: a potent drug combination. Mol Cancer Res. 2007;5:1133–45. doi: 10.1158/1541-7786.MCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 88.Zauli G, Voltan R, Bosco R, Melloni E, Marmiroli S, Rigolin GM, Cuneo A, Secchiero P. Dasatinib plus Nutlin-3 shows synergistic anti-leukemic activity in both p53wild-type and p53mutated B chronic lymphocytic leukemias by inhibiting the Akt pathway. Clin Cancer Res. 2010 doi: 10.1158/1078-0432.CCR-22-1596. [DOI] [PubMed] [Google Scholar]
- 89.Secchiero P, Zerbinati C, di Iasio MG, Melloni E, Tiribelli M, Grill V, Zauli G. Synergistic cytotoxic activity of recombinant TRAIL plus the non-genotoxic activator of the p53 pathway nutlin-3 in acute myeloid leukemia cells. Curr Drug Metab. 2007;8:395–403. doi: 10.2174/138920007780655432. [DOI] [PubMed] [Google Scholar]
- 90.Thompson T, Andreeff M, Studzinski GP, Vassilev LT. 1,25-dihydroxyvitamin D3 enhances the apoptotic activity of MDM2 antagonist nutlin-3a in acute myeloid leukemia cells expressing wild-type p53. Mol Cancer Ther. 2010;9:1158–68. doi: 10.1158/1535-7163.MCT-09-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang W, Konopleva M, Burks JK, Dywer KC, Schober WD, Yang JY, McQueen TJ, Hung MC, Andreeff M. Blockade of mitogen-activated protein kinase/extracellular signal-regulated kinase kinase and murine double minute synergistically induces Apoptosis in acute myeloid leukemia via BH3-only proteins Puma and Bim. Cancer Res. 2010;70:2424–34. doi: 10.1158/0008-5472.CAN-09-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Carter BZ, Mak DH, Schober WD, Koller E, Pinilla C, Vassilev LT, Reed JC, Andreeff M. Simultaneous activation of p53 and inhibition of XIAP enhance the activation of apoptosis signaling pathways in AML. Blood. 2010;115:306–14. doi: 10.1182/blood-2009-03-212563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kojima K, Konopleva M, Tsao T, Nakakuma H, Andreeff M. Concomitant inhibition of Mdm2-p53 interaction and Aurora kinases activates the p53-dependent postmitotic checkpoints and synergistically induces p53-mediated mitochondrial apoptosis along with reduced endoreduplication in acute myelogenous leukemia. Blood. 2008;112:2886–95. doi: 10.1182/blood-2008-01-128611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kojima K, Shimanuki M, Shikami M, Samudio IJ, Ruvolo V, Corn P, Hanaoka N, Konopleva M, Andreeff M, Nakakuma H. The dual PI3 kinase/mTOR inhibitor PI-103 prevents p53 induction by Mdm2 inhibition but enhances p53-mediated mitochondrial apoptosis in p53 wild-type AML. Leukemia. 2008;22:1728–36. doi: 10.1038/leu.2008.158. [DOI] [PubMed] [Google Scholar]
- 95.Kojima K, Konopleva M, Samudio IJ, Ruvolo V, Andreeff M. Mitogen-activated protein kinase kinase inhibition enhances nuclear proapoptotic function of p53 in acute myelogenous leukemia cells. Cancer Res. 2007;67:3210–9. doi: 10.1158/0008-5472.CAN-06-2712. [DOI] [PubMed] [Google Scholar]
- 96.Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5:2778–86. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- 97.Voltan R, Celeghini C, Melloni E, Secchiero P, Zauli G. Perifosine plus nutlin-3 combination shows a synergistic anti-leukaemic activity. Br J Haematol. 2010;148:957–61. doi: 10.1111/j.1365-2141.2009.08018.x. [DOI] [PubMed] [Google Scholar]
- 98.Ohgami T, Kato K, Kobayashi H, Sonoda K, Inoue T, Yamaguchi S, Yoneda T, Wake N. Low-dose mithramycin exerts its anticancer effect via the p53 signaling pathway and synergizes with nutlin-3 in gynecologic cancers. Cancer Sci. 2010;101:1387–95. doi: 10.1111/j.1349-7006.2010.01543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zheng T, Wang J, Song X, Meng X, Pan S, Jiang H, Liu L. Nutlin-3 cooperates with doxorubicin to induce apoptosis of human hepatocellular carcinoma cells through p53 or p73 signaling pathways. J Cancer Res Clin Oncol. 2010;136:1597–604. doi: 10.1007/s00432-010-0817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao C, Shinohara ET, Subhawong TK, Geng L, Woon Kim K, Albert JM, Hallahan DE, Lu B. Radiosensitization of lung cancer by nutlin, an inhibitor of murine double minute 2. Mol Cancer Ther. 2006;5:411–7. doi: 10.1158/1535-7163.MCT-05-0356. [DOI] [PubMed] [Google Scholar]
- 101.Saha MN, Jiang H, Mukai A, Chang H. RITA inhibits multiple myeloma cell growth through induction of p53-mediated caspase-dependent apoptosis and synergistically enhances nutlin-induced cytotoxic responses. Mol Cancer Ther. 2010;9:3041–51. doi: 10.1158/1535-7163.MCT-10-0471. [DOI] [PubMed] [Google Scholar]
- 102.Jin L, Tabe Y, Kojima K, Zhou Y, Pittaluga S, Konopleva M, Miida T, Raffeld M. MDM2 antagonist Nutlin-3 enhances bortezomib-mediated mitochondrial apoptosis in TP53-mutated mantle cell lymphoma. Cancer Lett. 2010;299:161–70. doi: 10.1016/j.canlet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 103.Saha MN, Jiang H, Jayakar J, Reece D, Branch DR, Chang H. MDM2 antagonist nutlin plus proteasome inhibitor velcade combination displays a synergistic anti-myeloma activity. Cancer Biol Ther. 2010;9:936–44. doi: 10.4161/cbt.9.11.11882. [DOI] [PubMed] [Google Scholar]
- 104.Barbieri E, Mehta P, Chen Z, Zhang L, Slack A, Berg S, Shohet JM. MDM2 inhibition sensitizes neuroblastoma to chemotherapy-induced apoptotic cell death. Mol Cancer Ther. 2006;5:2358–65. doi: 10.1158/1535-7163.MCT-06-0305. [DOI] [PubMed] [Google Scholar]