Abstract
Objective
Most studies suggest that hysterectomies are more common in African Americans than in other ethnic groups. To assess this ethnic surgical disparity in a novel way, our main goal was to determine whether admixture (the proportion of sub-Saharan African or European origin in individuals) is associated with hysterectomy frequency in African American women in the Women’s Health Initiative (WHI).
Study Design
In this retrospective study, we used ancestry informative single nucleotide polymorphisms (SNPs) to estimate admixture proportions in >10,000 African American women from the WHI. Logistic regression models were used to assess the association between admixture and self-reported history of hysterectomy with and without controlling for relevant covariates. Multinomial logistic regression models were used to assess the association between admixture and self-reported age of hysterectomy. We also considered other potential risk factors (adiposity, hypertension, and education) for hysterectomy accounting for admixture.
Results
African admixture was a strong risk factor after adjusting for multiple covariates (OR 1.85, P<.0001). The admixture risk for hysterectomy was highest for those performed in the 35–39 age range (OR 3.08, P<.0001) and least evident in oldest ages (45 or older). Our analyses also suggest that adiposity, hypertension and education were independently associated with hysterectomy in this population group.
Conclusion
These results suggest that higher African admixture is associated with higher frequencies of hysterectomy and that genetic studies specifically targeting African American women and diseases associated with hysterectomy may be especially useful in understanding the pathogenesis and underlying cause of this disparity in health outcome.
Keywords: ancestry, ancestry informative markers, fibroid tumors, Women’s Health Initiative
Introduction
Hysterectomy is the most common gynecological surgery in the United States.1 African American women have a higher risk of hysterectomy compared with other ethnic groups.2,3 Explanations for this higher risk include less access to more conservative treatments due to lower socioeconomic status (SES), higher population density in the southern United States where hysterectomy is more common, and psychosocial factors that affect patient and/or physician preferences towards hysterectomy over other treatments.3–6 However, the higher hysterectomy rates in African Americans may also reflect differences in the prevalence of diseases for which hysterectomy is performed. African Americans have a higher prevalence of uterine leiomyomas (fibroid tumors)3 although the etiology is not well understood.
In African Americans, 60–70% of hysterectomies are performed for fibroids compared to 29–33% for non-Hispanic whites.1,7,8 The odds of African Americans having fibroids as an indication for hysterectomy ranges from 2.7–9.4 compared to women without African ancestry.3,9–11 African Americans develop fibroids at younger ages, and have more rapid fibroid growth and larger tumor volumes than women of European descent.2 Although the effect of psychosocial or environmental factors should not be discounted, differences in fibroid behavior could also suggest an underlying biological etiology including genetic polymorphisms and/or expression.12,13 Some estimates place genetics as a primary factor for fibroids, with heritability estimates ranging between 26% to 80% with most estimates above 50%,14–16 and the diagnosis of fibroids is associated with several genetic loci in Japanese women.17
To explore the possible influence of genetics on the higher rate of hysterectomy in African Americans, we investigate whether the relative proportion of sub-Saharan African (referred to here as African) is associated with hysterectomy. We use a set of ancestry informative single nucleotide polymorphisms (SNPs)18,19 to accurately estimate admixture in >10,000 self-identified African American participants of the Women’s Health Initiative (WHI). In addition, we examine whether education, body mass index (BMI) and hypertension are associated with a history of hysterectomy (after adjusting for admixture) and whether these factors influence the admixture-associated risks for hysterectomy.
MATERIALS AND METHODS
This study is a cross-sectional analysis of self-identified African American participants in the WHI. WHI is the largest US health study of postmenopausal women aged 50–79 and includes over 160,000 women recruited between 1993 and 1998.20,21 Our study included 10,439 African American women for whom both DNA samples were genotyped, and baseline hysterectomy information and other study covariates were available. The WHI provided access to clinical data and DNA samples under appropriate Human Institutional Review Board (IRB) approval and the study design was approved by the NHLBI as part of a BAA for the WHI. The study was otherwise exempt from IRB approval.
We estimated the proportion of African and European admixture using a validated set of ancestry informative markers (AIMs)18,19 that included 92 SNPs enabling accurate estimation of admixture proportions in African Americans.18,19,22 Genotyping was performed by our group as previously described22 using the TaqMan® OpenArrays® system (https://products.appliedbiosystems.com/ab/en/US/adirect/ab?cmd=catNavigate2&catID=605783) (ABI, Foster City, CA, USA). The mean call rate was 97.1%. All AIM SNPs were in Hardy Weinberg equilibrium (P>.005) in parental populations, and the mean width of the 90% Bayesian confidence intervals (CIs) was 0.2 for admixture estimates in our studies of groups of African and Hispanic Americans.18 While the mean width of CIs are larger for smaller AIM sets, the admixture proportions estimated in individuals assessed with these highly selected AIMs18 are strongly correlated with larger AIMs sets. Correlations with larger sets of SNP AIMs (>500 SNPs) are >0.9 in testing with Mexican American and African American admixed populations (MFS, unpublished data). For the current study we did not include assessment of Amerindian admixture since the African American participants showed a very low frequency of Amerindian admixture(mean = 0.019).
The African and European admixture contribution (a proportion ranging from 0% to 100%) to each self-identified African American woman was assessed using STRUCTURE (v2.3.3)23 analyses of genotyping results with AIMs as previously described.24 The analyses were performed under the assumption of two populations (K = 2) with 100,000 replicates and 100,000 burn-in cycles and representatives of parental population groups as previously described.24 The results were consistent with < 0.02 difference between each of three independent runs. We considered African admixture in data analyses because the results for European admixture mirror those for African admixture.
The primary outcome variable for all analyses was self-reported history of hysterectomy at baseline. We also investigated the association between African admixture and self-reported age at hysterectomy, comparing each age category with women who did not report hysterectomy. Age at hysterectomy was available in categories: “< 30”, “30–34”, “35–39”, “40–44”, “45–49”, “50–54”, “55–59”, and “60 or older”. We combined “50–54”, “55–59”, “60 or older” as “50 or older” to have a larger sample size for this category.
We considered the following baseline covariates: entry age, education, BMI, self-reported age at menarche, smoking history, alcohol intake, parity, and diastolic blood pressure. These covariates have been reported in previous studies as factors associated or potentially associated with the frequency of hysterectomy and/or fibroids.3–6,11,25,26 BMI was computed as measured weight (kg) divided by the square of measured height (m2). Parity included the following categories: never pregnant, no full-term pregnancy, 1 child, 2, 3, 4 children respectively and 5 or more children. We consider education with the following scale: high school or less, vocational, some college including associate degree and college degree (BA/BS and above). Alcohol intake had the following scale: non-drinker, past drinker, <1 drink/month, <1 drink/week, 1 to <7 drinks/week and 6, 7+ drinks/week. Smoking was categorized as: never smoker, < 5 years, 5–9 years, 10–19 years, 20–29 years, 30–39 years, 40–49 years, and 40–49 years. Diastolic hypertension status (hypertensive or normotensive) was determined based on the participants’ baseline diastolic blood pressure as previously defined.27
We conducted statistical analyses using SAS version 9.2 (SAS Institute, Cary, NC). All statistical tests were two sided and P<.05 was considered statistically significant. We used 10,439 participants for all analyses except when diastolic blood pressure was used as a covariate (N = 9,792) or for when we examined whether diastolic hypertension was associated with hysterectomy. This analysis included only individuals classified as hypertensive (blood pressure >90 mm Hg) or normotensive (blood pressure <80 mm Hg) (N = 7,593). Individuals with borderline blood pressures were not included in this analyses consistent with our previous study.27 Baseline characteristics of the women with and without hysterectomy were compared using chi-square tests for categorical variables and Student’s t tests for continuous variables. To investigate the association between hysterectomy status and African admixture proportion, we obtained odds ratios (ORs) and 95% CIs using logistic regression models. The ORs described the effect of 100% African admixture compared to 0% African admixture, which corresponds to comparing one parental population to another parental population. We considered the following analysis models: 1) African admixture; 2) African admixture and education; 3) African admixture, education and BMI; 4) African admixture, education, BMI and parity; 5) African admixture, education, BMI, parity, age at menarche, smoking, and alcohol intake; 6) African admixture, education, BMI, parity, age at menarche, smoking, alcohol intake and diastolic blood pressure. All models included entry age and BMI was divided by its standard deviation consistent with our previous studies.22,27–29
We studied the association between age at hysterectomy and admixture using multinomial logistic models where the response variable has seven categories: “women without a hysterectomy” which was used as the reference level, and “less than 30”, “30–34”, “35–39”, “40–44”, “45–49”, and “50 or older” for women with hysterectomy. Multinomial logistic models generalize logistic regression by allowing response variables with more than two levels, and comparing different levels of the response variables to the reference level.
We also investigated the associations between hysterectomy status and education, BMI, diastolic hypertension, respectively, with and without controlling for admixture and other covariates using logistic regression models.
RESULTS
In our African American sample, there were significant differences in age at menarche, BMI, education and parity among the women who had hysterectomy compared with those who did not (Table 1). BMI was significantly higher among women with hysterectomy (P<.0001). Among women with hysterectomy a higher frequency of women reported high school or below education levels than among women who did not report hysterectomy. Women who were never pregnant had a markedly higher percentage of hysterectomy than those without hysterectomy. More women who reported hysterectomy had a higher frequency of hypertension as measured at study entry. Although the mean age at menarche for the study was significantly lower for those with hysterectomy this difference was small.
Table 1.
Descriptive Statistics of Variables by Hysterectomy Status
| Variable | No Hysterectomy (N = 4637, 44.4%) | Hysterectomy (N = 5802, 55.6%) | p* |
|---|---|---|---|
| Entry age (y) | 61.7±7.0 | 61.5±7.2 | 0.09 |
| Age at menarche (y) | 12.7±1.6 | 12.6±1.6 | <.0001 |
| BMI (kg/m2) | 30.8±6.5 | 31.4±6.5 | <.0001 |
| DBP (mm Hg)+ | 79.3±9.9 | 80.0±10.0 | 0.0009 |
| Education | <.0001 | ||
| high school or below | 1154 (24.9) | 1500 (25.9) | |
| Vocational | 562 (12.1) | 749 (12.9) | |
| some college | 1161 (25.0) | 1601 (27.6) | |
| college degree | 1760 (38.0) | 1952 (33.6) | |
| Parity | <.0001 | ||
| Never Pregnant | 269 (5.8) | 506 (8.7) | |
| No term pregnancy | 254 (5.5) | 366 (6.3) | |
| 1 Child | 682 (14.7) | 887 (15.3) | |
| 2 Children | 1087 (23.4) | 1315 (22.7) | |
| 3 Children | 827 (17.8) | 1048 (18.1) | |
| 4 Children | 624 (13.5) | 687 (11.8) | |
| 5 Children or more | 894 (19.3) | 993 (17.1) | |
| Smoke | 0.51 | ||
| Never smoker | 2373 (51.2) | 2949 (50.8) | |
| Less than 5 years | 273 (5.9) | 343 (5.9) | |
| 5–9 years | 182 (3.9) | 258 (4.5) | |
| 10–19 years | 493 (10.6) | 612 (10.6) | |
| 20–29 years | 533 (11.5) | 710 (12.2) | |
| 30–39 years | 483 (10.4) | 560 (9.7) | |
| 40–49 years | 222 (4.8) | 290 (5.0) | |
| 50 or more years | 78 (1.7) | 80 (1.4) | |
| Alcohol intake | 0.27 | ||
| Non drinker | 809 (17.5) | 995 (17.2) | |
| Past drinker | 1530 (33.0) | 1981 (34.1) | |
| <1 drink per month | 612 (13.2) | 750 (12.9) | |
| <1 drink per week | 840 (18.1) | 1044 (18.0) | |
| 1 to <7 drinks per week | 663 (14.3) | 764 (13.2) | |
| 7+ drinks per week | 183 (4.0) | 268 (4.6) | |
| HTN | <.0001 | ||
| No | 2120 (62.9) | 2445 (57.9) | |
| Yes | 1248 (37.1) | 1780 (42.1) |
BMI, body mass index; DBP: diastolic blood pressure; smoke: years of a regular smoker; HTN: hypertension status based on baseline diastolic blood pressure.
Two-sample t-tests were used for continuous variables; Chi-squared tests were used for BMI.
N = 4363, 44.6% for no hysterectomy, N = 5429, 55.4% for hysterectomy.
Data are mean ± standard deviation, or n (%). For education, some college included associate degrees and college degree includes BA/BS and above.
The hysterectomies were primarily performed during three decades: 21.9% in the 1960’s; 40.2% in the 1970’s; and 26.3% in the 1980’s.
We examined the effect of admixture with and without adjusting for covariates (Table 2). African admixture was associated with hysterectomy when adjusting for entry age alone (OR 2.10, 95% CI 1.57–2.80). This association remained highly significant when controlling for education albeit with a slightly reduced OR (1.98, 95% CI 1.47–2.65). The ORs were similar when including BMI, parity, age at menarche, smoking and alcohol intake in the logistic regression model, but decreased to 1.85 when further adjusting for diastolic blood pressure. The admixture-hysterectomy association was highly significant in all models (P<.0001). In the full model, the association was also observed when adjusting for the recruitment region or when only women from southern recruitment centers were considered.
Table 2.
Association of African Admixture With Hysterectomy Status
| Model* | Odds Ratio | 95% CI | P | |
|---|---|---|---|---|
| Lower | Upper | |||
| AFR | 2.10 | 1.57 | 2.80 | <.0001 |
| AFR, EDU | 1.98 | 1.47 | 2.65 | <.0001 |
| AFR, EDU, BMI | 1.90 | 1.42 | 2.55 | <.0001 |
| AFR, EDU, BMI, parity | 1.92 | 1.43 | 2.58 | <.0001 |
| AFR, EDU, BMI, parity, menarche, smoke, alcohol | 1.94 | 1.44 | 2.62 | <.0001 |
| AFR, EDU, BMI, parity, menarche, smoke, alcohol, DBP | 1.85 | 1.36 | 2.52 | <.0001 |
| Full Model, Region^ | 1.86 | 1.36 | 2.54 | <.0001 |
| Full Model (Southern)& | 2.02 | 1.26 | 3.25 | 0.004 |
CI: confidence interval; AFR: African admixture; EDU: education; BMI: body mass index; menarche: age at menarche; smoke: years of a regular smoker; alcohol: alcohol intake; DBP: diastolic blood pressure.
Logistic regression models were used to study the association between African admixture and hysterectomy status. All models included entry age. The odds ratios attributed to the difference in 0% and 100% African admixture.
Full model (AFR; EDU, BMI, age at menarche, smoke, alcohol and DBP) was also adjusted for region of recruitment (South, East, Midwest, and Northwest).
Analyses restricted to only South recruitment region (no hysterectomy, 1870 women; hysterectomy, 2642 women).
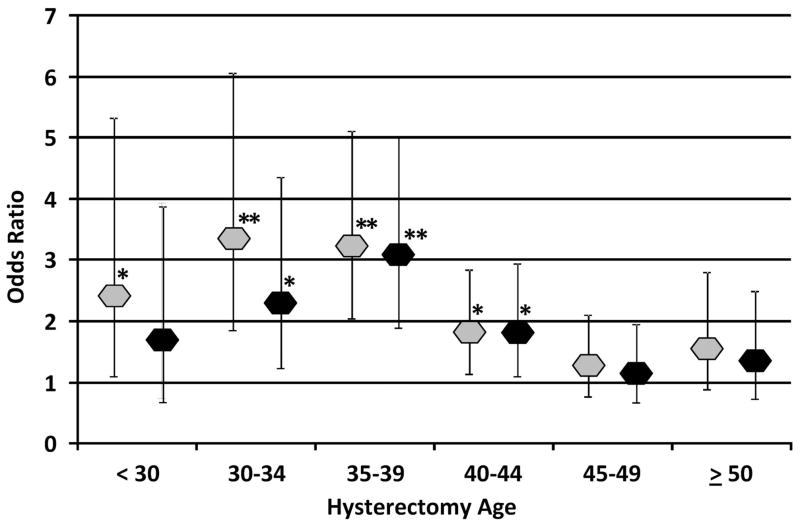
African admixture was significantly associated with age at hysterectomy with and without controlling for education, BMI, parity, age at menarche, smoking, alcohol intake and diastolic blood pressure (P=.0002 and <.0001, respectively). The ORs of African admixture for age at hysterectomy and the corresponding 95% CIs are presented in Figure 1. African admixture is significantly associated with hysterectomy at a younger age (< 45 years old). This association is especially strong for hysterectomies performed in 30–34 (adjusted OR 2.31, 95% CI 1.90–5.01) and 35–39 year-old women (adjusted OR 3.08, 95% CI 1.90–5.01).
Figure 1. Association of African Admixture with Hysterectomy Age.
African admixture odds ratios and 95% confidence intervals for hysterectomy are shown for different age at hysterectomy groups based on the multinormial logistic regression model adjusting for entry age (grey diamonds) and for the fully adjusted model (black diamonds). Significant P values as indicated at P<.05 (*) and at P<.0001 (**). The fully adjusted model included the following covariates: entry age, education, parity, body mass index, age at menarche, smoking, alcohol intake, and diastolic blood pressure (see Methods for details). The frequency of the reference group and each hysterectomy defined age group is as below for the two models respectively: women without a hysterectomy: 4637, 4363; < 30: 414, 394; 30–34: 808, 748; 35–39: 1459, 1383; 40–44: 1352, 1255; 45–49: 1020, 943; ≥ 50: 719, 680.
We also investigated the association of education, BMI and hypertension with hysterectomy, respectively, with and without adjusting for admixture and other covariates (Table 3). For education, college degree was associated with decreased risk for hysterectomy when adjusting for entry age alone (OR 0.85, P=.001). The strength of the association was reduced after adjusting for African admixture (OR 0.87, P=.01), and when also including BMI in the model (OR 0.90, P=.04), but increased when adding parity and other covariates to the model (OR 0.81, P<.0001). BMI had a significant association with hysterectomy with or without adjusting for African admixture and other covariates (ORs ranged from 1.09 to 1.11, P<.0001) (Table 3). Diastolic hypertension was also positively related to hysterectomy with or without controlling for other covariates including African admixture (ORs ranged from 1.20 to 1.24, P<.0005) (Table 3).
Table 3.
Association of Education, BMI and Hypertension With Hysterectomy Status
| Model* | Odds Ratio | 95% CI
|
P | |
|---|---|---|---|---|
| Lower | Upper | |||
| EDU+ | 0.85 | 0.76 | 0.93 | 0.001 |
| EDU, AFR | 0.87 | 0.79 | 0.97 | 0.01 |
| EDU, AFR, BMI | 0.90 | 0.81 | 1.00 | 0.04 |
| EDU, AFR, BMI, parity | 0.82 | 0.74 | 0.91 | 0.0003 |
| EDU, AFR, BMI, parity, menarche, smoke, alcohol | 0.81 | 0.72 | 0.90 | <.0001 |
| BMI# | 1.11 | 1.06 | 1.15 | <.0001 |
| BMI, AFR | 1.10 | 1.05 | 1.14 | <.0001 |
| BMI, AFR, EDU | 1.09 | 1.05 | 1.13 | <.0001 |
| BMI, AFR, EDU, parity | 1.10 | 1.05 | 1.14 | <.0001 |
| BMI, AFR, EDU, parity, menarche, smoke, alcohol | 1.09 | 1.04 | 1.13 | <.0001 |
| HTN | 1.24 | 1.13 | 1.36 | <.0001 |
| HTN, AFR | 1.23 | 1.12 | 1.35 | <.0001 |
| HTN, AFR, BMI | 1.22 | 1.11 | 1.34 | <.0001 |
| HTN, AFR, EDU, BMI, parity | 1.20 | 1.09 | 1.32 | 0.0002 |
| HTN, AFR, EDU, BMI, parity, menarche, smoke, alcohol | 1.20 | 1.09 | 1.32 | 0.0002 |
CI: confidence interval; EDU: education; AFR: African admixture; BMI: body mass index; menarche: age at menarche; smoke: years of a regular smoker; alcohol: alcohol intake; HTN: hypertension status based on baseline diastolic blood pressure.
All models included entry age. BMI was divided by its standard deviation.
Logistic regression models were used to study the association between education, BMI and hypertension and hysterectomy status. The odds ratio and P value are presented for the education category “college degree BA/BS and above” versus “high school and below”. Results for “vocational school” and “some college including associate degree” versus “high school and below” are not significant.
BMI was divided by its standard deviation.
COMMENT
The most important finding of the current study is that, amongst African American women, we found that a higher percentage of African admixture is associated with a higher frequency of hysterectomy. This association was significant with only a modest reduction in the odds ratio after controlling for socioeconomic and environmental covariates including education level, adiposity, and hypertension. Previous studies comparing rates of hysterectomy between African Americans and other race/ethnicities found higher rates of hysterectomy in African American women.2,3 In a cross-sectional study of women aged 33–45, the adjusted frequency of hysterectomy in African Americans was estimated to be nearly four-fold higher frequency than in the non-Hispanic white population.3 Our study, using a quantitative assessment of African admixture within an African American sample, suggests that hysterectomy is associated with African ancestry.
A critical consideration of the underlying explanation for these results is whether they have been influenced by societal factors including psychosocial and environmental factors. Education level although inversely associated with risk for hysterectomy (see below) does not account for the admixture association and is also a strong surrogate measure for socio-economic status. In addition, socio-economic status (measured by geocoding) showed almost identical results (data not shown), albeit this measured current rather than lifetime status. However, we cannot completely discount the possibility that unmeasured environmental factors and either patient or physician attitudes towards treatment options for underlying medical conditions could influence the current findings. However, it should be noted that in general there is some evidence for a physician bias against surgical intervention in ethnic minorities.30 Moreover, other studies have found that adjusting for SES factors and many other variables such as knowledge of alternatives, physician practice preferences, and cultural factors, were not sufficient to eliminate the racial difference in hysterectomy rates.3,10,31–33
Our study had several important limitations including the lack of information on the underlying indication(s) for hysterectomy or whether oopherectomies were performed. There was also no information on whether other treatments including myomectomies or medical therapy were available. All information on hysterectomy including age at hysterectomy was self-reported. Given this restriction it is not possible to exclude recall bias that may have influenced results, however, the recall bias for a major surgical event as hysterectomy would be small. In the WHI dataset, another self-reported phenotype (diabetes) has been validated to be sufficiently accurate34, which may also suggest low recall bias for the self-reported outcome. Another limitation is that the measurements of both hypertension and BMI were not contemporaneous with the time of hysterectomy but rather at WHI enrollment (at ages 55–75). Thus, we cannot distinguish whether these associations observed in our study are predisposing factors to, or a consequence of hysterectomy.
It is important to note that the highest proportion (40%) of the hysterectomies in our study participants occurred during the 1970’s as a function of enrollment age and age at hysterectomy. Thus, our results predominantly reflect treatment decisions for this decade. In addition, ~50% of the participants in our study were enrolled from recruitment centers in southern U.S.A. Although our data showed a higher frequency of hysterectomy in the southern group (58.5%) compared to all other participants (52.8%) consistent with previous studies,6 analyses adjusting for regional recruitment and those restricted to only southern participants showed a similar admixture association with hysterectomy (Table 2).
The current study also suggests association between hysterectomy and adiposity (BMI), education and hypertension. Although BMI measurements were obtained at WHI enrollment and not at the time of surgery, our results showed a significant association similar to findings from previous studies of hysterectomy rates where BMI at multiple time points before hysterectomy were examined.25
The lower rate of hysterectomy associated with higher education levels in our study are in accord with previous studies, suggesting that SES may play a role in the higher rate of hysterectomy among African Americans.3–6,35 It has been hypothesized that women with lower SES might be more likely to delay care due to poorer knowledge about alternatives or less access to care. Such delay could result in a more serious condition at diagnosis precluding more conservative treatment. The decreased risk of hysterectomy associated with higher education levels was weakened when BMI was included in the analysis, consistent with our previous studies of adiposity in the WHI population group.22
For hypertension, our findings are consistent with others showing association of diastolic blood pressure and hysterectomy although more studies are needed.26,36 The association between history of hysterectomy and hypertension was still observed in our study after inclusion of admixture as a covariate, which has not been considered in other studies. This is important since our previous investigation has shown an association of hypertension with African admixture.27 We note that the analysis of systolic hypertension showed nearly identical results as diastolic hypertension (data not shown).
Although our study could not ascertain the indications for hysterectomy, our results suggest the possible association between African admixture and fibroids. In African Americans, fibroid tumors have been reported as the main indication for hysterectomy in 60–65% of cases compared to only 28–29% of cases in white women.7 Studies have reported a higher prevalence of fibroid tumors in African Americans with younger age of onset, more rapid growth, a greater number of tumors, and larger tumor volume than in European Americans.2,10, 11 The association we found between hysterectomy and African admixture may be a proxy for a genetic relationship between African admixture and fibroids.
Age at hysterectomy and indication for surgery vary by ethnicity.2,3 In the early reproductive years, hysterectomies are most commonly performed for symptomatic endometriosis and menstrual disorders, with little difference in ethnic distribution. In the mid and late reproductive years, symptomatic fibroid tumors predominate as the major indication for hysterectomy, with higher rates in African Americans than in non-Hispanic white population groups. After menopause, fibroid tumors generally decrease in size and become asymptomatic. Rates of hysterectomy also drop with prolapse and cancer becoming the predominant indications for hysterectomy, and rates are higher in white women than in African Americans.8 In our study, African admixture has the strongest association for hysterectomies in 35–39 year-olds (adjusted OR 3.08) where fibroids are more likely to be the primary indication for hysterectomies. The association between African admixture and hysterectomy is lower in the 45–49 (adjusted OR 1.14) and 50 or above groups (adjusted OR 1.35) (Figure 1) as other indications for hysterectomy begin to increase.
The admixture association with hysterectomy in the adjusted model is not significant when hysterectomies are performed at less than 30 years of age and shows a more moderate effect for hysterectomies performed before 35 years of age. This suggests that environmental or psychosocial factors may be more important for these younger women where indications for hysterectomy are predominantly endometriosis and women are potentially less informed about their health care choices.
The association between hysterectomy and African admixture amongst African American women reported here has an important implication. Our findings suggest that there is genetic variation within ethnic categories that should be more fully explored. Admixture mapping37 and genetic association studies focusing on African Americans may identify specific genetic variations that more fully identify women at risk for hysterectomy, thus stimulating investigations to better define disease pathways that may be important in the etiology for ethnic disparities in hysterectomy rates.
Acknowledgments
The WHI Publication and Presentation Committee reviewed and approved the manuscript for submission. The NHLBI was not otherwise involved in the design and conduct of the study or preparation of the manuscript.
This work was supported by the National Institutes of Health NHLBI BAA contract no. HHSN268200764319C, and the National Cancer Institute (5K07CA136969) to HMO-B. The WHI program is funded by NHLBI through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Footnotes
The authors report no conflict of interest.
Reprints will not be available.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wu JM, Wechter ME, Geller EJ, Nguyen TV, Visco AG. Hysterectomy rates in the United States, 2003. Obstetrics and gynecology. 2007;110:1091–5. doi: 10.1097/01.AOG.0000285997.38553.4b. [DOI] [PubMed] [Google Scholar]
- 2.Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. American journal of obstetrics and gynecology. 2010;202:514–21. doi: 10.1016/j.ajog.2010.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bower JK, Schreiner PJ, Sternfeld B, Lewis CE. Black-White differences in hysterectomy prevalence: the CARDIA study. Am J Public Health. 2009;99:300–7. doi: 10.2105/AJPH.2008.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dharmalingam A, Pool I, Dickson J. Biosocial determinants of hysterectomy in New Zealand. Am J Public Health. 2000;90:1455–8. doi: 10.2105/ajph.90.9.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Powell LH, Meyer P, Weiss G, et al. Ethnic differences in past hysterectomy for benign conditions. Womens Health Issues. 2005;15:179–86. doi: 10.1016/j.whi.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 6.Kjerulff K, Langenberg P, Guzinski G. The socioeconomic correlates of hysterectomies in the United States. Am J Public Health. 1993;83:106–8. doi: 10.2105/ajph.83.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lepine LA, Hillis SD, Marchbanks PA, et al. Hysterectomy surveillance--United States, 1980–1993. MMWR CDC Surveill Summ. 1997;46:1–15. [PubMed] [Google Scholar]
- 8.Kjerulff KH, Guzinski GM, Langenberg PW, Stolley PD, Moye NE, Kazandjian VA. Hysterectomy and race. Obstetrics and gynecology. 1993;82:757–64. [PubMed] [Google Scholar]
- 9.Day Baird D, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. American journal of obstetrics and gynecology. 2003;188:100–7. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 10.Marshall LM, Spiegelman D, Barbieri RL, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstetrics and gynecology. 1997;90:967–73. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 11.Faerstein E, Szklo M, Rosenshein N. Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol. 2001;153:1–10. doi: 10.1093/aje/153.1.1. [DOI] [PubMed] [Google Scholar]
- 12.Huyck KL, Panhuysen CI, Cuenco KT, et al. The impact of race as a risk factor for symptom severity and age at diagnosis of uterine leiomyomata among affected sisters. Am J Obstet Gynecol. 2008;198:168, e1–e9. doi: 10.1016/j.ajog.2007.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan Q, Luo X, Chegini N. Genomic and proteomic profiling I: leiomyomas in African Americans and Caucasians. Reprod Biol Endocrinol. 2007;5:34. doi: 10.1186/1477-7827-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snieder H, MacGregor AJ, Spector TD. Genes control the cessation of a woman’s reproductive life: a twin study of hysterectomy and age at menopause. J Clin Endocrinol Metab. 1998;83:1875–80. doi: 10.1210/jcem.83.6.4890. [DOI] [PubMed] [Google Scholar]
- 15.Kurbanova M, Koroleva AG, Sergeev AS. Genetic-epidemiologic analysis of uterine myoma: assessment of repeated risk. Genetika. 1989;25:1896–8. [PubMed] [Google Scholar]
- 16.Luoto R, Kaprio J, Rutanen EM, Taipale P, Perola M, Koskenvuo M. Heritability and risk factors of uterine fibroids--the Finnish Twin Cohort study. Maturitas. 2000;37:15–26. doi: 10.1016/s0378-5122(00)00160-2. [DOI] [PubMed] [Google Scholar]
- 17.Cha PC, Takahashi A, Hosono N, et al. A genome-wide association study identifies three loci associated with susceptibility to uterine fibroids. Nat Genet. 2011;43:447–50. doi: 10.1038/ng.805. [DOI] [PubMed] [Google Scholar]
- 18.Kosoy R, Nassir R, Tian C, et al. Ancestry informative marker sets for determining continental origin and admixture proportions in common populations in America. Hum Mutat. 2009;30:69–78. doi: 10.1002/humu.20822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nassir R, Kosoy R, Tian C, et al. An ancestry informative marker set for determining continental origin: validation and extension using human genome diversity panels. BMC Genet. 2009;10:39. doi: 10.1186/1471-2156-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hays J, Hunt JR, Hubbell FA, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;9:S18–77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 21.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 22.Nassir R, Qi L, Kosoy R, et al. Relationship between adiposity and admixture in African-American and Hispanic-American women. Int J Obes (Lond) 2012;36:304–13. doi: 10.1038/ijo.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falush D, Stephens M, Pritchard JK. Inference of population structure using multilocus genotype data: linked loci and correlated allele frequencies. Genetics. 2003;164:1567–87. doi: 10.1093/genetics/164.4.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nassir R, Qi L, Kosoy R, et al. Relationship between adiposity and admixture in African-American and Hispanic-American women. Int J Obes (Lond) 2012;36:304–313. doi: 10.1038/ijo.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper R, Hardy R, Kuh D. Is adiposity across life associated with subsequent hysterectomy risk? Findings from the 1946 British birth cohort study. Bjog. 2008;115:184–92. doi: 10.1111/j.1471-0528.2007.01569.x. [DOI] [PubMed] [Google Scholar]
- 26.Boynton-Jarrett R, Rich-Edwards J, Malspeis S, Missmer SA, Wright R. A prospective study of hypertension and risk of uterine leiomyomata. Am J Epidemiol. 2005;161:628–38. doi: 10.1093/aje/kwi072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kosoy R, Qi L, Nassir R, et al. Relationship between hypertension and admixture in post-menopausal African American and Hispanic American women. J Hum Hypertens. 2012;26:365–73. doi: 10.1038/jhh.2011.52. [DOI] [PubMed] [Google Scholar]
- 28.Nassir R, Qi L, Kosoy R, Garcia L, Robbins J, Seldin MF. Relationship Between Gallbladder Surgery and Ethnic Admixture in African American and Hispanic American Women. Am J Gastroenterol. 2012;107:930–40. doi: 10.1038/ajg.2012.46. [DOI] [PubMed] [Google Scholar]
- 29.Qi L, Nassir R, Kosoy R, et al. Relationship between diabetes risk and admixture in postmenopausal African-American and Hispanic-American women. Diabetologia Diabetologia. 2012;55:1329–37. doi: 10.1007/s00125-012-2486-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jha AK, Fisher ES, Li Z, Orav EJ, Epstein AM. Racial trends in the use of major procedures among the elderly. N Engl J Med. 2005;353:683–91. doi: 10.1056/NEJMsa050672. [DOI] [PubMed] [Google Scholar]
- 31.Geller SE, Burns LR, Brailer DJ. The impact of nonclinical factors on practice variations: the case of hysterectomies. Health services research. 1996;30:729–50. [PMC free article] [PubMed] [Google Scholar]
- 32.De Gelder R, Richters A, Peters L. The integration of a woman’s perspective in hysterectomy decisions. Journal of psychosomatic obstetrics and gynaecology. 2005;261:53–62. doi: 10.1080/01674820400023309. [DOI] [PubMed] [Google Scholar]
- 33.Williams RD, Clark AJ. A qualitative study of women’s hysterectomy experience. Journal of women’s health & gender-based medicine. 2000;9:S15–25. doi: 10.1089/152460900318731. [DOI] [PubMed] [Google Scholar]
- 34.Margolis KL, Lihong Q, Brzyski R, et al. Validity of diabetes self-reports in the Women’s Health Initiative: comparison with medication inventories and fasting glucose measurements. Clin Trials. 2008;5:240–7. doi: 10.1177/1740774508091749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer JR, Rao RS, Adams-Campbell LL, Rosenberg L. Correlates of hysterectomy among African-American women. Am J Epidemiol. 1999;150:1309–15. doi: 10.1093/oxfordjournals.aje.a009962. [DOI] [PubMed] [Google Scholar]
- 36.Radin RG, Rosenberg L, Palmer JR, Cozier YC, Kumanyika SK, Wise LA. Hypertension and risk of uterine leiomyomata in US black women. Hum Reprod. 2012;27:1504–9. doi: 10.1093/humrep/des046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seldin MF, Pasaniuc B, Price AL. New approaches to disease mapping in admixed populations. Nat Rev Genet. 2011;12:523–8. doi: 10.1038/nrg3002. [DOI] [PMC free article] [PubMed] [Google Scholar]