Abstract
Cardiometabolic syndrome occurs with obesity and consists of pathophysiological factors that increase the risk for cardiovascular events. Soluble epoxide hydrolase inhibition (sEHi) is a novel therapeutic approach that exerts renal and cardiovascular protection. Although sEHi as a therapeutic approach is promising, it could be more effective for the treatment of cardiometabolic syndrome when combined with peroxisome proliferator activated receptor γ (PPARγ) agonists. We hypothesized that the PPARγ agonist, rosiglitazone in combination with a sEHi (tAUCB) will provide synergistic actions to decrease blood pressure, improve vascular function, decrease inflammation, and prevent renal damage in spontaneously hypertensive obese rats (SHROB). SHROB were treated with rosiglitazone, tAUCB or the combination of tAUCB and rosiglitazone for four-weeks and compared with spontaneously hypertensive (SHR) and Wistar–Kyoto (WKY) rats. Blood pressure increased in SHROB (164 ±7 mmHg) and decreased 10 mmHg when treated with rosiglitazone, tAUCB, or tAUCB and rosiglitazone. Mesenteric artery dilation to the KATP channel opener pinacidil was attenuated in SHROB (EMax = 77 ±7%), compared with WKY (EMax = 115 ±19) and SHR (EMax = 93 ±12%). Vasodilation to pinacidil was improved by rosiglitazone (EMax = 92 ±14%) but not tAUCB. Renal macrophage infiltration increased in SHROB and significantly decreased with rosiglitazone or tAUCB and rosiglitazone treatment. Albuminuria was increased in SHROB (90 ±20 mg/d) and was significantly decreased by the combination of tAUCB and rosiglitazone (37 ±9 mg/d). Glomerular injury in SHROB was also significantly decreased by tAUCB and rosiglitazone. These results indicate that even though sEHi or PPARγ agonist have benefits when used individually, the combination is more beneficial for the multidisease features in cardiometabolic syndrome.
Keywords: epoxyeicosatrienoic acid, kidney, metabolic syndrome
Introduction
Cardiometabolic syndrome is a cluster of pathophysiological factors that has obesity associated with insulin resistance, hypertension, hyperlipidemia and inflammation, all of which increase the risk for cardiovascular disease contributing to progressive renal disease.1,2 Worldwide a vast adult population is classified as having cardiometabolic syndrome and more adults are expected to be afflicted with this disease as they age.2,3 Visceral obesity, the central component of cardiometabolic syndrome, results in insulin resistance and glucose intolerance that ultimately leads to type 2 diabetes. The exact factors that lead to end organ damage are unknown due to the multitude of pathophysiological factors involved in cardiometabolic syndrome. Coexistence of hypertension and diabetes accounts for 70% of patients with end-stage renal disease.4–6 Vascular dysfunction is also a predictor of cardiovascular events and the severity of renal injury in metabolic syndrome.7–9 Inflammation is another factor that has been associated with increased risks for cardiovascular events and renal injury in cardiometabolic syndrome.7,10,11 Hence, it is difficult to predict if treatment of one or more of these pathophysiological factors will ameliorate the progression of renal damage in cardiometabolic syndrome.
One therapeutic approach for patients with cardiometabolic syndrome is multiple pharmacological interventions to treat several components of the disease. Rosiglitazone is a thiazolidinedione that activates peroxisome proliferator-activator γ (PPARγ) and is used in the treatment of type 2 diabetes because it improves insulin sensitivity and lipid metabolism.12,13 PPARγ agonists are also reported to have potential benefits in treating chronic kidney diseases in patients and animal models, as well as, exert anti-inflammatory and antiproliferative effects.14–16
Another emerging therapeutic strategy that can affect multiple components of cardiometabolic syndrome is by using epoxyeicosatrienoic acid (EET) analogs or soluble epoxide hydrolase inhibitors (sEHi ).17–19 EETs are arachidonic acid metabolites important in maintaining renal and cardiovascular homeostasis.17,20 EETs are metabolized by sEH to its less active form dihydroxyeicosatrienoic acids. A decrease in EETs can impair endothelial dilator responses in obesity and diabetes.21,22 Inhibitors of sEH have been shown to elevate EET levels, decrease blood pressure and protect from renal injury in animal models of hypertension.19,23,24
Although PPARγ agonists have been demonstrated to be beneficial, there are actions on the kidney that may counteract these effects with long-term treatment.25,26 Sodium and water retention are frequently observed during PPARγ agonist treatment and this fluid retaining state could be detrimental to patients with congestive heart failure.25,26 Interestingly, sEHi and EETs are natriuretic and help to maintain fluid and electrolyte homeostasis.20,27 Therefore, the combination of rosiglitazone and sEHi could have less edema in humans and this therapeutic approach for cardio-metabolic syndrome have not been fully investigated. In this study we used an animal with a genetic predisposition to obesity and hypertension, the spontaneously hypertensive obese rat (SHROB), as a model of cardiometabolic syndrome. We hypothesized that the PPARγ agonist, rosiglitazone in combination with an sEHi, trans-4-(4-[3-adamantan-1-yl-ureido]-cyclohexyloxy)-benzoic acid (tAUCB) would provide synergistic actions to decrease blood pressure, improve vascular function, decrease inflammation and prevent renal damage in SHROB.
Materials and methods
Animal groups
The Medical College of Wisconsin Institutional Animal Care and Use Committee according to the National Institutes of Health Guidelines for Care and Use of Laboratory Animals approved all animal studies. Eight to nine-week-old male Wistar–Kyoto (WKY), spontaneously hypertensive rats (SHR) and SHROB were purchased from Charles River Laboratories (Wilmington, MA, USA). Animals were housed in the Biomedical Resource Center at Medical College of Wisconsin with a 12 h light–dark cycle and free access to tap water along with normal rat chow. SHR and WKY rats were used as control groups as a comparison for disease progression. SHROB rats were divided into four groups (n = 6–10). Group 1 received pudding as a vehicle, Groups 2–4 received the following drugs (10 mg/kg/d orally) for four weeks: PPARγ agonist – rosiglitazone, sEHi – tAUCB or rosiglitazone and tAUCB. Rosiglitazone and tAUCB doses are based on those previously reported.14,22,24 Rats were weighed and systolic blood pressure was measured by tail-cuff plethysmography every week.
Urine and plasma biochemical analysis
At the end of the four-week experimental period rat urine was collected from rats housed in metabolic cages for 24 h. Urinary biochemical analysis was done using commercially available ELISA kits; albumin and nephrin from Exocell (Philadelphia, PA, USA), kidney injury molecule-1 (KIM-1) from R&D Systems (Minneapolis, MN, USA) and monocyte chemoattractant protein-1 (MCP-1) from BD Biosciences (San Jose, CA, USA). Rats were anesthetized using isoflorane and plasma collected from the artery. Plasma biochemical analysis was done for leptin from Millipore Corporation (Billerica, MA, USA), triglycerides and cholesterol from Wako Chemicals (Richmond, VA, USA), and free fatty acids from Zen-Bio Inc. (Research Triangle Park, NC, USA). Blood glucose levels from the tail vein were measured using a glucometer.
Isolated mesenteric resistance artery preparation
Second-order mesenteric arteries were excised and segments were suspended between two cannulae in a pressure myograph system (Danish Myo Technology model 111P, Aarhus, Denmark). The bath was oxygenated in 95% O2/5% CO2 Krebs physiological salt solution (119.0 mmol/L NaCl, 25.0 mmol/L NaHCO3, 4.6 mmol/L KCL, 1.2 mmol/L KH2PO4, 1.2 mmol/L MgSO4, 1.8 mmol/L CaCl2, 11.0 mm glucose) at pH 7.4 and 37°C. Under no-flow conditions, the vessel was pressurized from 10 to 60 mmHg in increments of 10 mmHg every three minutes. The vessel was then pressurized to 65 mmHg for 30 min for equilibration and kept at 65 mmHg for the remainder of the experiment. One vessel segment was used per experiment. Lumen diameter measurements were acquired and logged using the myoview 1.2P user interface. The control lumen diameter was measured as a mean over the last minute of the 30 min equilibration period. After being constricted with U46619, a thromboxane mimetic, the diameter was measured as a mean over the last five minutes of a 15 min period. Following U46619 constriction, vessel diameter responses to the KATP channel activator, pinacidil (0.001 μmol/L – 30 μmol/L) and the BKCa channel activator, NS1619 (0.01 μmol/L-100 μmol/L) were assessed. Relaxation responses were plotted as a percentage of relaxation from the maximum contraction.
Kidney histology and immunohistochemical analysis
For histological analysis kidneys were excised, longitudinally sectioned, immersion-fixed in 10% neutral buffered for-malin and paraffin embedded. The kidney sections were embedded and cut into 4 μm slices for use in histology and immunohistochemistry protocols. For histology, formalin-fixed paraffin-embedded kidney slices were depar-affinized, re-hydrated and stained with hematoxylin–eosin, or with Periodic Acid-Schiff. Glomerulosclerosis and mesangial matrix expansion were blindly scored from kidney sections to determine glomerular damage using the following numeric scale: 0= no damage; +1 = very mild; +2 = mild; +3 = moderate and +4 = severe). Two observers in a blinded fashion conducted histological analysis. Tubules that contain proteinaceous casts were determined at magnification of ×100 using Nikon NIS Elements Software (Nikon Instruments Inc., Melville, NY, USA). The percentage area positive for cast was calculated from the mean of eight cortical and five medullary fields for each animal. Sections were also immunostained with anti-CD68 from Serotec (Raleigh, NC, USA) to determine macrophage infiltration. Cells positive for CD68 were counted square millimeter of tissue area and the numbers obtained from each treatment group were averaged.
Statistical analysis
Data are expressed as mean ±SE. Data were analyzed using one-way analysis of variance followed by Tukey’s post hoc test for multiple group comparisons using Prizm version 4.0 by GraphPad Software Inc. (La Jolla, CA, USA). The levels of significance were considered statistically significant with P < 0.05.
Results
Body weight, blood pressure and blood glucose
Mean body weight was measured in all experimental groups at baseline and throughout the study. Body weight, systolic blood pressure and blood glucose are shown in Table 1. At the end of the four-week study body weights were significantly higher in the SHROB compared with the SHR. Body weight in SHROB treated with rosiglitazone or rosiglitazone and tAUCB gained approximately 40 g more than vehicle treated SHROB. It should be noted that the increased body weight in rosiglitazone treated rats could be due to fluid retention caused by rosiglitazone, which is a thiazolidinedione and fluid retention is a well-defined side-effect of thiazolidinediones.26,28,29 Treatment with tAUCB had a similar body weight as vehicle-treated SHROB at the end of the four-week period. Systolic blood pressure in the treatment groups, rosiglitazone, tAUCB or rosiglitazone and tAUCB were approximately 10 mmHg lower than vehicle treated SHROB. SHROB have an increase in non-fasting blood glucose compared with the SHR. The elevated non-fasting blood glucose was reduced in SHROB treated with rosiglitazone, tAUCB or the combination of rosiglitazone and tAUCB.
Table 1.
Metabolic parameters in different experimental groups
| WKY | SHR | SHROB | SHROB RGZ | SHROB tAUCB | SHROB RGZ and tAUCB | |
|---|---|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 119 ±6 | 166 ±9 | #164 ±7 | 153 ±5 | 155 ±6 | 156 ±4 |
| Blood glucose (mg/dL) | 97 ±9 | 127 ±12 | #154 ±13 | *128 ±4 | *131 ±8 | *127 ±6 |
| Body weight (g) | 305 ±12 | 271 ±3 | #428 ±28 | 470 ±11 | 433 ±6 | 467 ±11 |
Wistar–Kyoto rats (WKY), spontaneously hypertensive rats (SHR), spontaneous hypertensive obese rats (SHROB), rosiglitazone (RGZ), trans-4-(4-[3-adamantan-1-yl-ureido]-cyclohexyloxy)-benzoic acid (tAUCB). Data are mean ±SEM; n = 6–8 animals/group
P < 0.05 versus corresponding control WKY
P < 0.05 versus corresponding untreated SHROB
Plasma leptin, triglyceride, cholesterol and free fatty acids
Table 2 displays the results of plasma biochemical analysis for leptin, triglycerides, cholesterol and free fatty acids. SHROB have leptin receptor deficient signaling that results in elevated leptin concentration and hyperphagia.30 Drug treatments with rosiglitazone, tAUCB or rosiglitazone tAUCB did not alter plasma leptin levels in SHROB. Triglycerides, cholesterol and free fatty acid levels were significantly increased in the vehicle-treated SHROB compared with the normotensive WKY and hypertensive SHR groups. SHROB had significant improvement of triglycerides when treated with rosiglitazone (−68%) or rosiglitazone and tAUCB (−49%). This improvement of triglycerides in SHROB was primarily due to the PPARγ agonist since the sEHi, tAUCB (−39%) was less effective in lowering these plasma levels. Cholesterol levels in rosiglitazone, tAUCB or rosiglitazone and tAUCB-treated SHROB groups decreased over the four-week treatment period. Plasma free fatty acid levels decreased in SHROB-treated with rosiglitazone (−38%), tAUCB (−45%) or rosiglitazone and tAUCB (−54%).
Table 2.
Plasma leptin and lipid levels in different experimental groups
| WKY | SHR | SHROB | SHROB RGZ | SHROB tACUB | SHROB RGZ and tAUCB | |
|---|---|---|---|---|---|---|
| Leptin (ng/mL) | 3.4 ±0.7 | 3.0 ±0.6 | #58.0 ±3.0 | 62.0 ±3.0 | 59.0 ±0.6 | 60.0 ±2.0 |
| Triglycerides (mg/dL) | 34.0 ±5.0 | 27.0 ±4.0 | #386.0 ±50.0 | *125.0 ±50.0 | 234.0 ±28.0 | *196.0 ±45.0 |
| Cholesterol (mg/dL) | 69.0 ±8.5 | 57.0 ±6.0 | #132.0 ±16.0 | 86.0 ±11.0 | 111.0 ±11.0 | 113.0 ±7.0 |
| Free fatty acids (μmol/L) | 185.0 ±16.0 | 188.0 ±21.0 | #740.0 ±97.0 | 479.0 ±102.0 | *407.0 ±34.0 | *339.0 ±57.0 |
Wistar–Kyoto rats (WKY), spontaneously hypertensive rats (SHR), spontaneous hypertensive obese rats (SHROB), rosiglitazone (RGZ), trans-4-(4-[3-adamantan-1-yl-ureido]-cyclohexyloxy)-benzoic acid (tAUCB). Data are mean ±SEM; n = 6–8 animals/group
P < 0.05 versus corresponding control WKY
P < 0.05 versus corresponding untreated SHROB
K+ channel opener-induced mesenteric resistance artery relaxation
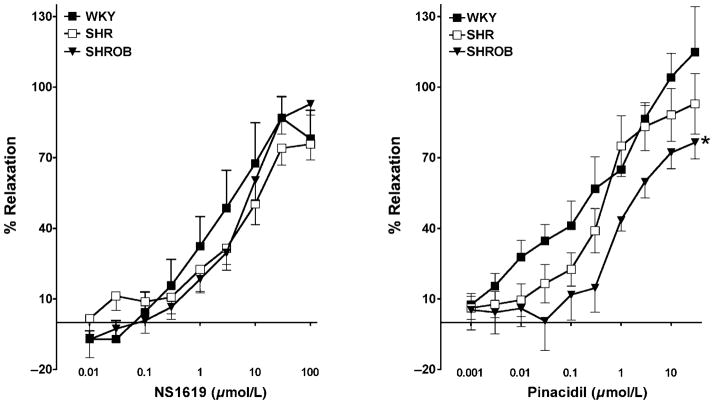
Mesenteric resistance artery dilation to KATP and BKCa channel openers was assessed in WKY, SHR and SHROB. Mesenteric resistance artery diameter was not different between groups averaging 266 ±10 μm and decreasing to 148 ±9 μm in response to U46619. Concentration-dependent mesenteric resistance artery relaxation to pinacidil is attenuated in SHROB compared with WKY with an increase in EC50 (SHROB = 1.098 ±0.03; WKY = 0.649 ± 0.07) and a decrease in EMax (SHROB = 76.5 ±7.0; WKY = 114.9 ±19.3) (Figure 1). In contrast, concentration-dependent vasorelaxation to the BKCa channel activator, NS1619 was not altered between the groups (Figure 1). Treatment with rosiglitazone or rosiglitazone and tAUCB but not tAUCB alone restored the vasodilatory response to pinacidil in SHROB, with a decrease in EC50 (rosiglitazone = 0.04019 ±0.09; rosiglitazone and tAUCB = 0.2531±0.027) and an increase in EMax (rosiglitazone = 91.71 ±13.79; rosiglitazone and tAUCB = 89.56 ±6.46) (Figure 2). These data demonstrate that PPARγ agonist but not sEHi improved KATP opener mesenteric resistance artery vasodilation in SHROB.
Figure 1.
Mesenteric resistance artery dilation to K+ channel openers. Left panel: Concentration-dependent mesenteric resistance artery dilation to pinacidil in Wistar–Kyoto rats (WKY), spontaneously hypertensive rats (SHR) and spontaneous hypertensive obese rats (SHROB) (n = 5–7). Right panel: concentration-dependent mesenteric resistance artery dilation to NS1619 in WKY, SHR and SHROB (n = 5–8). Values are expressed as mean ±SEM. *P < 0.05 considered significant versus corresponding WKY
Figure 2.
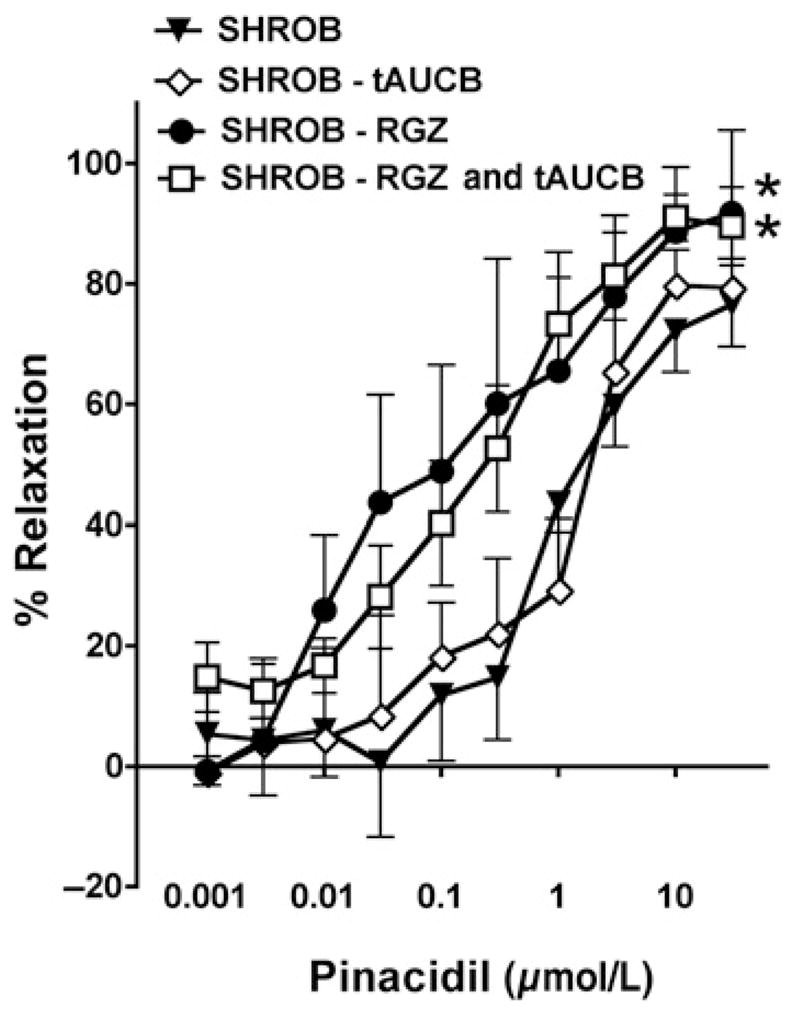
Mesenteric resistance artery dilation to KATP opener in PPARγ and sEHi treatment. Concentration-dependent mesenteric resistance artery dilation to pinacidil in spontaneous hypertensive obese rats (SHROB) treated with rosiglitazone (RGZ), trans-4-(4-[3-adamantan-1-yl-ureido]-cyclohexyloxy)-benzoic acid (t-AUCB) or RGZ and tAUCB (n = 5–6). *P < 0.05 considered significant versus corresponding vehicle SHROB
Kidney injury biomarkers: albumin, nephrin and KIM-1
Urinary albumin, nephrin and KIM-1 excretion levels were analyzed to assess the degree of renal injury in SHROB (Figure 3). Albumin excretion significantly increased in the SHROB compared with WKY and hypertensive SHR groups. Rosiglitazone decreased albumin levels by 25% in SHROB. Urinary albumin excretion was significantly decreased by 40% in tAUCB treated SHROB. The combination of rosiglitazone and tAUCB resulted in a further decrease (59%) in albumin excretion in SHROB. Likewise, nephrin did not significantly decrease in SHROB treated with rosiglitazone or tAUCB; however, neprhin excretion was reduced by approximately 48% in rosiglitazone and tAUCB treated SHROB. The pattern of reduced urinary excretion of KIM-1 in the SHROB treatment groups was similar to that observed for albumin and nephrin excretion. KIM-1 excretion decreased by 30% in SHROB treated with rosiglitazone. The sEHi tAUCB had a greater effect on decreasing KIM-1 excretion (49%) in SHROB. Treatment of SHROB with rosiglitazone and tAUCB resulted in a further decline (61%) in KIM-1 excretion. These data suggest that sEHi had a slightly greater effect on decreasing renal injury in SHROB and that the combination of a sEHi and PPARγ was dramatically more effective.
Figure 3.
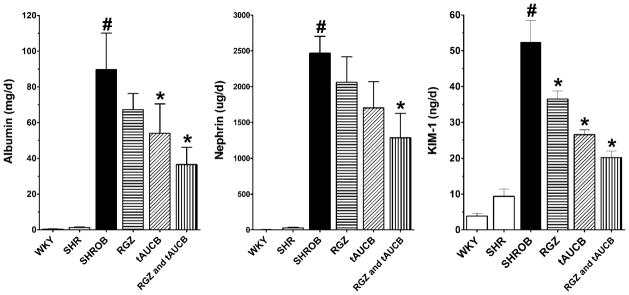
Kidney injury biomarkers. Urinary biochemical analysis of albumin (left graph), nephrin (middle graph) and kidney injury molecule-1 (KIM-1, right graph) is presented for Wistar–Kyoto rats (WKY), spontaneously hypertensive rats (SHR) and spontaneous hypertensive obese rats (SHROB). SHROB were treated with rosiglitazone (RGZ), trans-4-(4-(3-adamantan-1-yl-ureido)-cyclohexyloxy)-benzoic acid (tAUCB) or RGZ and tAUCB. Values are mean ±SEM. n = 5–7 animals/ group with #P < 0.05 versus corresponding control WKY. *P < 0.05 versus corresponding untreated SHROB
Renal injury and inflammation
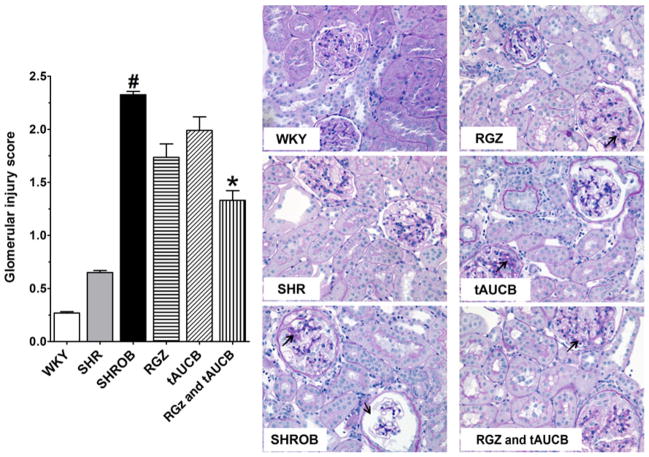
Renal injury was further evaluated semiquantitatively by scoring a sampling of 100 glomeruli per kidney from histological sections (Figure 4). Glomerular injury was minimal in the WKY and SHR whereas vehicle-treated SHROB demonstrate a significantly higher glomerular injury score. The vehicle treated SHROB demonstrated mesangial expansion and damage to the glomerular basement membrane that is consistent with the albumin and nephrin excretion rates. SHROB treated with rosiglitazone and tACUB demonstrated the greatest reduction in glomerular injury. Additionally, rosiglitazone and tACUB but not tAUCB or rosiglitazone alone significantly reduced intratubular proteinaceous cast formation in the cortical and medullary areas of the kidney (Figure 5). Taken together, these findings demonstrate that the combination of rosiglitazone and tAUCB had a greater effect to decrease glomerular injury in SHROB.
Figure 4.
Glomerular injury. Glomerular injury was determined in Wistar-Kyoto rats (WKY), spontaneously hypertensive rats (SHR), and spontaneous hypertensive obese rats (SHROB). SHROB were treated with rosiglitazone (RGZ), trans-4-(4-[3-adamantan-1-yl-ureido]-cyclohexyloxy)-benzoic acid (tAUCB) or RGZ and tAUCB. Values are mean ±SEM; n = 4–8 animals/group with #P < 0.05 versus corresponding control WKY. *P < 0.05 versus corresponding untreated SHROB. Black arrows indicate the damaged area. (A color version of this figure is available in the online journal)
Figure 5.
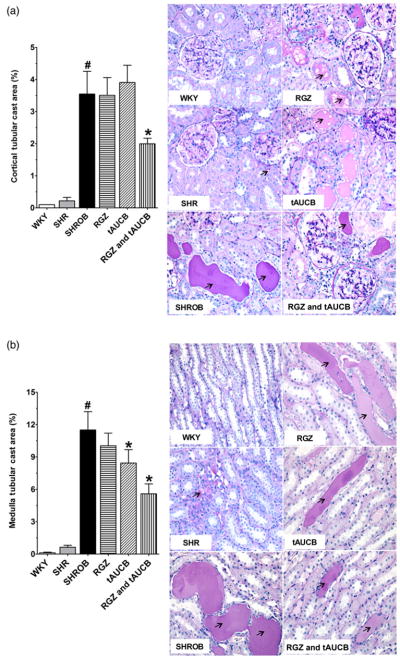
Kidney proteinacious cast area. A colour is available onlineKidney cortical (a) and medullary (b) protenacious cast area was determined in Wistar–Kyoto rats (WKY), spontaneously hypertensive rats (SHR) and spontaneous hypertensive obese rats (SHROB). SHROB were treated with rosiglitazone (RGZ), trans-4-(4-[3-adamantan-1-yl-ureido]-cyclohexyloxy)-benzoic acid (tAUCB) or RGZ and tAUCB. Values are mean ±SEM; n = 4–8 animals/group with #P < 0.05 versus corresponding control WKY. *P < 0.05 versus corresponding untreated SHROB. Black arrows indicating the proteinacious cast area. (A color version of this figure is available in the online journal)
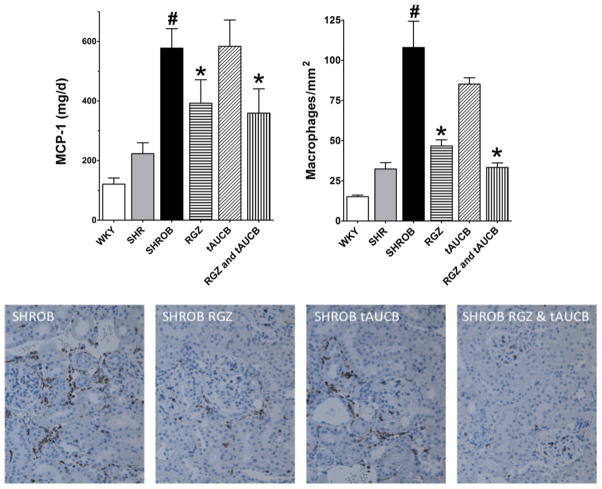
Figure 6 presents urinary MCP-1 excretion and representative analysis and pictures of macrophage infiltration in the kidney sections immunostained with anti-CD68, a glyco-protein that is expressed in monocytes and macrophages. MCP-1 levels were significantly elevated three-to four-fold in SHROB compared with SHR and WKY groups. Rosiglitazone or the combination of rosiglitazone & tAUCB decreased urinary MCP-1 excretion in SHROB by 35–40%; however, the sEHi tAUCB failed to decrease MCP-1 excretion. Consistent with the MCP-1 data, the SHROB vehicle group had a 4–5-fold increase in renal cortical macrophage infiltration compared with WKY and SHR groups. SHROB treated with rosiglitazone or rosiglitazone and tAUCB had a significant reduction in macrophage infiltration while tAUCB treatment resulted in a smaller decrease in macrophage infiltration. These data indicate that PPARγ treatment reduced inflammation to a greater extent in SHROB.
Figure 6.
Renal inflammation. Urinary monocyte chemoattractant protein-1 (MCP-1) levels (left graph) and CD-68 positive cells (right graph) is presented for Wistar–Kyoto rats (WKY), spontaneously hypertensive rats (SHR) and spontaneous hypertensive obese rats (SHROB). SHROB were treated with rosiglitazone (RGZ), trans-4-(4-[3-adamantan-1-yl-ureido]-cyclohexyloxy)-benzoic acid (tAUCB) or RGZ and tAUCB. Values are mean ±SEM. n = 5–7 animals/group with #P < 0.05 versus corresponding control WKY. *P < 0.05 versus corresponding untreated SHROB. (A color version of this figure is available in the online journal)
Discussion
The current study determined if the PPARγ agonist rosiglitazone in combination with the sEHi tAUCB would improve vascular function, decrease inflammation and prevent renal damage in a rat model of cardiometabolic syndrome. The studies determined that rosiglitazone had greater effects to decrease plasma triglycerides, improve vascular function and decrease inflammation when compared with tAUCB. Furthermore, the combination of rosiglitazone and tAUCB was more effective overall in preventing renal damage in the SHROB, an animal model of cardio-metabolic syndrome.
PPARγ agonists and sEHi have been demonstrated to decrease blood pressure and lower plasma lipids in various animal models of obesity, insulin resistance and hypertension.12,17,18,21 The current study found that rosiglitazone, tAUCB or rosiglitazone and tACUB had a mild blood pressure lowering effect in SHROB. The three treatment regimens also lowered blood glucose levels to a similar extent. In contrast, rosiglitazone had a much greater effect on plasma triglycerides and tAUCB was more effective in lowering free fatty acid levels. The blood pressure lowering effect of sEHi is consistent with the findings of a number of studies demonstrating antihypertensive actions.17,19 Although PPARγ agonists were developed to treat diabetes due to their insulin-sensitizing actions, they have also been demonstrated to lower blood pressure in the obese Zucker rats.31,32 With regard to plasma triglycerides, the reduction of triglycerides by rosiglitazone is similar to that previously reported in fructose-enriched-diet fed rats, obese and diabetic animal models.33,34 No synergistic action was found in the combination treatment implying that the significant reduction in triglycerides is due mainly to the action of rosiglitazone. Rosiglitazone has also been demonstrated to lower cholesterol and free fatty acids in several studies.13,33,34 In the current study, tAUCB had a greater effect to lower free fatty acids; however, the combination of rosiglitazone and tAUCB demonstrated superior lowering of free fatty acids. On the whole, the combination of rosiglitazone and tAUCB treatment on blood pressure, blood glucose, plasma lipids and free fatty acids was superior to either treatment when used alone.
Hypertension and obesity have been linked to vascular and endothelial function which proceeds renal inflammation and injury.7,10 Impaired vasodilation to K+ channel openers has been demonstrated in obese, diabetic and hypertension animal models as well as humans with type 2 diabetes.35–40 Experimental studies in obese Zucker and Zucker diabetic fatty rats have demonstrated impaired vascular BKCa and KATP channel-mediated vasodilation.35,36,38 Acetylcholine dilation of aorta and mesenteric resistance arteries has also been demonstrated to be impaired in SHROB rats.41 The findings of the current study determined that mesenteric resistance artery dilation to the BKCa channel opener NS-1619 was unaltered in SHROB rats. On the other hand, mesenteric artery dilation to the KATP channel opener pinacidil was significantly attenuated in the SHROB model of cardiometabolic syndrome. Furthermore, rosiglitazone and rosiglitazone and tAUCB treatment improved the mesenteric artery dilator response to pinacidil. These findings are in agreement with previous reports demonstrating improved endothelial function in rosiglitazone-treated SHR, obese and type 2 diabetic rat models.41–44 Even short-term incubation with rosiglitazone improved acetylcholine mesenteric artery dilation in SHROB.41 Interestingly, elevated circulating free fatty acid levels in insulin-resistant patients causes endothelial dysfunction and rosiglitazone has been demonstrated to lower free fatty acid levels and prevent endothelial dysfunction in patients infused with triglyceride/heparin to increase free fatty acids.45,46 Rosiglitazone has also been demonstrated to recover the function of KATP channels in human vascular smooth muscle cells exposed to high glucose.44 Previous studies have also determined that sEHi improve vascular and endothelial function in animal models of obesity, hypertension and diabetes.17,22,23 These previous studies focused on afferent arteriolar and mesenteric resistance artery dilation to acetylcholine and did not assess K+ channel dilator responses. The findings of the current study failed to demonstrate improved mesenteric artery dilation to pinacidil in sEHi-treated SHROB. Taken together, the findings of this study demonstrate PPARγ agonist but not sEHi improved the mesenteric artery dilation to the KATP channel activator pinacidil in SHROB cardiometabolic syndrome rats.
Increasing evidence demonstrates that the development of endothelial dysfunction and renal injury is associated with increased levels of circulating pro-inflammatory cytokines in obesity and hypertension.47–49 Animal models of obesity and hypertension such as the obese Zucker rat have been demonstrated to have endothelial dysfunction and develop albuminuria and progressive glomerulosclerosis.21,50 The present study demonstrates significant renal inflammation and injury in the SHROB kidney as assessed by increased urinary MCP-1, nephrin, KIM-1 and albumin excretion. Increased renal inflammation in SHROB was further demonstrated by kidney immunohistological analysis. In the current study rosiglitazone or rosiglitazone and tAUCB decreased MCP-1 levels and renal inflammation in SHROB. On the other hand, tAUCB or rosiglitazone and tAUCB decreased renal injury to a greater extent in SHROB. These findings suggest that the PPARγ agonist had a greater effect in decreasing inflammation whereas the sEHi was more effective in preventing renal injury in SHROB. It is also clear that the combination of rosiglitazone tAUCB was the most effective treatment to decrease inflammation and renal injury in SHROB.
Previous reports have demonstrated that PPARγ agonists decrease inflammation in animal models of hypertension and obesity as well as humans with type 2 diabetes and cardiometabolic syndrome.14,39,51,52 Interestingly, rosiglitazone treatment improved endothelial function and lowered C-reactive protein levels in type 2 diabetic patients.51 The PPARγ agonist pioglitazone has been demonstrated to decrease fibrosis in the human kidney.53,54 Similar to the current findings, greater decreases in renal inflammation compared with glomerular injury have also been determined in diabetic rats treated with rosiglitazone.14 Likewise, sEHi have been demonstrated to decrease inflammation and renal injury in a number of animal models of obesity, diabetes and hypertension.17,18,22,23,55 There is evidence to support the notion that the combination of rosiglitazone and tAUCB would have a greater effectiveness compared with either treatment alone. PPARγ activation decreases sEH expression in cardiomyocytes suggesting that this could account for a portion of the therapeutic benefits ascribed to PPARγ agonists.56 It is further demonstrated that EETs are capable of binding and activating PPARγ to exert an antiinflammatory effect.55 This previous finding suggests that sEHi and the subsequent increase in EETs could have actions to enhance PPARγ activity to prevent renal injury in SHROB. As observed in the present study, the antiinflammatory and the associated kidney protective activity of tAUCB can also be explained by EETs ability to inhibit NF-κB activation. Indeed, EETs are anti-inflammatory, at least in part via inhibition of NF-κB inflammatory signaling.56 The key step in NF-κB activation is the phosphorylation of IκB by the active phospho-IKK. In a recent study, we have demonstrated that deletion of soluble epoxide hydrolase gene (Ephx2) or sEH inhibition could reduce renal injury in diabetes via inhibition of phospho-IKK-derived NF-κB activation.57 We have also demonstrated that sEHi reduced macrophage infiltration, renal NF-κB activity, and MCP-1 excretion in salt-sensitive hypertensive diabetic rats.58 These previous reports and our current findings provide support to the notion that the antiinflammatory actions and associated renal protective effects of tAUCB could be related to PPARγ activation as well as inhibitory effects on NF-κB inflammatory signaling.
In conclusion, the beneficial effects of rosiglitazone and tAUCB drug treatment in cardiometabolic syndrome were the most effective in preventing kidney damage. Comparison of PPARγ agonist and sEHi treatments demonstrated that rosiglitazone was more effective in decreasing plasma lipids and free fatty acids, improving KATP-mediated vasodilation, and decreasing inflammation in SHROB. In contrast, treatment with tAUCB was more effective in preventing renal injury in SHROB. Rosiglitazone and tAUCB had similar actions to lower blood pressure and blood glucose in SHROB that were not additive with combined rosiglitazone and tAUCB treatment. Overall, the results of the current study indicate that even though sEHi or PPARγ agonist have benefits when used individually, the combination is more beneficial for the multidisease features in cardiometabolic syndrome.
Acknowledgments
These studies were supported by NIH grants HL59699, UL1RRO31973, and DK38226 and Advancing a Healthier Wisconsin. Partial support was provided from NIEHS R01 ES002710. Bruce D Hammock is a George and Judy Marcus Senior Fellow of the American Asthma Society.
Footnotes
Author contributions: J DI designed the experimental studies, assisted with data analysis and wrote and edited manuscript text. K AW conducted vascular studies, assisted with other experimental aspects, assisted with data analysis and assisted with writing and editing manuscript text. MdAH K assisted with histological analysis, assisted with data analysis, and assisted with writing and editing manuscript text. T N conducted histological studies, assisted with other experimental aspects, assisted with data analysis and assisted with editing manuscript text. M C-S conducted biochemical analysis, assisted with other experimental aspects, assisted with data analysis and assisted with writing and editing manuscript text. SMS assisted with biochemical analysis, assisted with other experimental aspects, assisted with data analysis and assisted with editing manuscript text. BDH assisted with experimental design and assisted with writing and editing manuscript text.
References
- 1.Lastra G, Manrique C, Sowers JR. Obesity, cardiometabolic syndrome, and chronic kidney disease: the weight of the evidence. Adv Chronic Kidney Dis. 2006;13:365–73. doi: 10.1053/j.ackd.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Kirk EP, Klein S. Pathogenesis and pathophysiology of the cardiometabolic syndrome. J Clin Hypertens (Greenwich) 2009;11:761–5. doi: 10.1111/j.1559-4572.2009.00054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ford ES. Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the US. Diabetes Care. 2005;28:2745–9. doi: 10.2337/diacare.28.11.2745. [DOI] [PubMed] [Google Scholar]
- 4.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–7. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 5.Kambham N, Markowitz GS, Valeri AM, Lin J, D’Agati VD. Obesity-related glomerulopathy: an emerging epidemic. Kidney Int. 2001;59:1498–509. doi: 10.1046/j.1523-1755.2001.0590041498.x. [DOI] [PubMed] [Google Scholar]
- 6.Lyon CJ, Law RE, Hsueh WA. Minireview: adiposity, inflammation, and atherogenesis. Endocrinology. 2003;144:2195–200. doi: 10.1210/en.2003-0285. [DOI] [PubMed] [Google Scholar]
- 7.Knight SF, Quigley JE, Yuan J, Roy SS, Elmarakby A, Imig JD. Endothelial dysfunction and the development of renal injury in spontaneously hypertensive rats fed a high-fat diet. Hypertension. 2008;51:352–9. doi: 10.1161/HYPERTENSIONAHA.107.099499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochodnicky P, Vettoretti S, Henning RH, Buikema H, Van Dokkum RP, de Zeeuw D. Endothelial dysfunction in chronic kidney disease: determinant of susceptibility to end-organ damage and therapeutic response. J Nephrol. 2006;19:246–58. [PubMed] [Google Scholar]
- 9.Picchi A, Gao X, Belmadani S, Potter BJ, Focardi M, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in the prediabetic metabolic syndrome. Circ Res. 2006;99:69–77. doi: 10.1161/01.RES.0000229685.37402.80. [DOI] [PubMed] [Google Scholar]
- 10.Elmarakby AA, Imig JD. Obesity is the major contributor to vascular dysfunction and inflammation in high-fat diet hypertensive rats. Clin Sci (Lond) 2010;118:291–301. doi: 10.1042/CS20090395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishimatsu H, Suzuki E, Takeda R, Takahashi M, Oba S, Kimura K, Nagano T, Hirata Y. Blockade of endogenous proinflammatory cytokines ameliorates endothelial dysfunction in obese Zucker rats. Hypertens Res. 2008;31:737–43. doi: 10.1291/hypres.31.737. [DOI] [PubMed] [Google Scholar]
- 12.Rangwala SM, Lazar MA. Peroxisome proliferator-activated receptor gamma in diabetes and metabolism. Trends Pharmacol Sci. 2004;25:331–6. doi: 10.1016/j.tips.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Boden G, Cheung P, Mozzoli M, Fried SK. Effect of thiazolidinediones on glucose and fatty acid metabolism in patients with type 2 diabetes. Metabolism. 2003;52:753–9. doi: 10.1016/s0026-0495(03)00055-6. [DOI] [PubMed] [Google Scholar]
- 14.Fogo AB. PPARgamma and chronic kidney disease. Pediatr Nephrol. 2010;26:347–51. doi: 10.1007/s00467-010-1602-2. [DOI] [PubMed] [Google Scholar]
- 15.Setti G, Hayward A, Dessapt C, Barone F, Buckingham R, White K, Bilous R, Hiroshi K, Gruden G, Viberti G, Gnudi L. Peroxisome proliferator-activated receptor-γ agonist rosiglitazone prevents albuminuria but not glomerulosclerosis in experimental diabetes. Am J Nephrol. 2010;32:393–402. doi: 10.1159/000320129. [DOI] [PubMed] [Google Scholar]
- 16.Mohanty P, Aljada A, Ghanim H, Hofmeyer D, Tripathy D, Syed T, Al-Haddad W, Dhindsa S, Dandona P. Evidence for a potent antiinflammatory effect of rosiglitazone. J Clin Endocrinol Metab. 2004;89:2728–35. doi: 10.1210/jc.2003-032103. [DOI] [PubMed] [Google Scholar]
- 17.Imig JD, Hammock BD. Soluble epoxide hydrolase as a therapeutic target for cardiovascular diseases. Nat Rev Drug Discov. 2009;8:794–805. doi: 10.1038/nrd2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luria A, Bettaieb A, Xi Y, Shieh GJ, Liu HC, Inoue H, Tsai HJ, Imig JD, Haj FG, Hammock BD. Soluble epoxide hydrolase deficiency alters pancreatic islet size and improves glucose homeostasis in a model of insulin resistance. Proc Natl Acad Sci USA. 2011;108:9038–43. doi: 10.1073/pnas.1103482108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Imig JD. Cardiovascular therapeutic aspects of soluble epoxide hydrolase inhibitors. Cardiovasc Drug Rev. 2006;24:169–88. doi: 10.1111/j.1527-3466.2006.00169.x. [DOI] [PubMed] [Google Scholar]
- 20.Imig JD. Epoxides and soluble epoxide hydrolase in cardiovascular physiology. Physiol Rev. 2012;92:101–30. doi: 10.1152/physrev.00021.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao X, Dey A, Romanko OP, Stepp DW, Wang MH, Zhou Y, Jin L, Pollock JS, Webb RC, Imig JD. Decreased epoxygenase and increased epoxide hydrolase expression in the mesenteric artery of obese Zucker rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R188–96. doi: 10.1152/ajpregu.00018.2004. [DOI] [PubMed] [Google Scholar]
- 22.Elmarakby AA, Faulkner J, Al-Shabrawey M, Wang MH, Maddipati KR, Imig JD. Deletion of soluble epoxide hydrolase gene improves renal endothelial function and reduces renal inflammation and injury in streptozotocin-induced type 1 diabetes. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1307–17. doi: 10.1152/ajpregu.00759.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao X, Yamamoto T, Newman JW, Kim IH, Watanabe T, Hammock BD, Stewart J, Pollock JS, Pollock DM, Imig JD. Soluble epoxide hydrolase inhibition protects the kidney from hypertension-induced damage. J Am Soc Nephrol. 2004;15:1244–53. [PubMed] [Google Scholar]
- 24.Manhiani M, Quigley JE, Knight SF, Tasoobshirazi S, Moore T, Brands MW, Hammock BD, Imig JD. Soluble epoxide hydrolase gene deletion attenuates renal injury and inflammation with DOCA-salt hypertension. Am J Physiol Renal Physiol. 2009;297:F740–748. doi: 10.1152/ajprenal.00098.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang WH, Maroo A. PPARγ agonists: safety issues in heart failure. Diabetes Obes Metab. 2007;9:447–54. doi: 10.1111/j.1463-1326.2006.00616.x. [DOI] [PubMed] [Google Scholar]
- 26.Yang T, Soodvilai S. Renal and vascular mechanisms of thiazolidinedione-induced fluid retention. PPAR Res. 2008;2008:1–8. doi: 10.1155/2008/943614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Capdevila JH, Falck JR, Imig JD. Roles of the cytochrome P450 arachidonic acid monooxygenases in the control of systemic blood pressure and experimental hypertension. Kidney Intl. 2007;72:683–9. doi: 10.1038/sj.ki.5002394. [DOI] [PubMed] [Google Scholar]
- 28.Kalaitzidis RG, Sarafidis PA, Bakris GL. Effects of thiazolidinediones beyond glycaemic control. Curr Pharm Des. 2009;15:529–36. doi: 10.2174/138161209787315693. [DOI] [PubMed] [Google Scholar]
- 29.Tolman KG. The safety of thiazolidinediones. Expert Opin Drug Saf. 2011;10:419–28. doi: 10.1517/14740338.2011.534982. [DOI] [PubMed] [Google Scholar]
- 30.Ernsberger P, Koletsky RJ, Kline DD, Bedol DM, Friedman JE. The SHROB model of syndrome X: effects of excess dietary sucrose. Ann N Y Acad Sci. 1999;892:315–8. doi: 10.1111/j.1749-6632.1999.tb07806.x. [DOI] [PubMed] [Google Scholar]
- 31.Khan O, Riazi S, Hu X, Song J, Wade JB, Ecelbarger CA. Regulation of the renal thiazide-sensitive Na-Cl cotransporter, blood pressure, and natriuresis in obese Zucker rats treated with rosiglitazone. Am J Physiol Renal Physiol. 2005;289:F442–450. doi: 10.1152/ajprenal.00335.2004. [DOI] [PubMed] [Google Scholar]
- 32.Walker AB, Chattington PD, Buckingham RE, Williams G. The thiazolidinedione rosiglitazone (BRL-49653) lowers blood pressure and protects against impairment of endothelial function in Zucker fatty rats. Diabetes. 1999;48:1448–53. doi: 10.2337/diabetes.48.7.1448. [DOI] [PubMed] [Google Scholar]
- 33.Ackerman Z, Oron-Herman M, Pappo O, Peleg E, Safadi R, Schmilovitz-Weiss H, Grozovski M. Hepatic effects of rosiglitazone in rats with the metabolic syndrome. Basic Clin Pharmacol Toxicol. 2010;107:663–8. doi: 10.1111/j.1742-7843.2010.00553.x. [DOI] [PubMed] [Google Scholar]
- 34.Chaput E, Saladin R, Silvestre M, Edgar AD. Fenofibrate and rosiglitazone lower serum triglycerides with opposing effects on body weight. Biochem Biophys Res Commun. 2000;271:445–50. doi: 10.1006/bbrc.2000.2647. [DOI] [PubMed] [Google Scholar]
- 35.Burnham MP, Johnson IT, Weston AH. Reduced Ca2+-dependent activation of large-conductance Ca2+-activated K+ channels from arteries of Type 2 diabetic Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2006;290:H1520–1527. doi: 10.1152/ajpheart.00827.2005. [DOI] [PubMed] [Google Scholar]
- 36.Lu T, Wang XL, He T, Zhou W, Kaduce TL, Katusic ZS, Spector AA, Lee HC. Impaired arachidonic acid-mediated activation of large-conductance Ca2+-activated K+ channels in coronary arterial smooth muscle cells in Zucker Diabetic Fatty rats. Diabetes. 2005;54:2155–63. doi: 10.2337/diabetes.54.7.2155. [DOI] [PubMed] [Google Scholar]
- 37.Haddock RE, Grayson TH, Morris MJ, Howitt L, Chadha PS, Sandow SL. Diet-induced obesity impairs endothelium-derived hyperpolarization via altered potassium channel signaling mechanisms. PLoS One. 2011;6:e16423. doi: 10.1371/journal.pone.0016423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hodnett BL, Xiang L, Dearman JA, Carter CB, Hester RL. K(ATP)-mediated vasodilation is impaired in obese Zucker rats. Microcirculation. 2008;15:485–94. doi: 10.1080/10739680801942240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang TD, Chen WJ, Lin JW, Chen MF, Lee YT. Effects of rosiglitazone on endothelial function, C-reactive protein, and components of the metabolic syndrome in nondiabetic patients with the metabolic syndrome. Am J Cardiol. 2004;93:362–5. doi: 10.1016/j.amjcard.2003.10.022. [DOI] [PubMed] [Google Scholar]
- 40.Irat AM, Aslamaci S, Karasu C, Ari N. Alteration of vascular reactivity in diabetic human mammary artery and the effects of thiazolidinediones. J Pharm Pharmacol. 2006;58:1647–53. doi: 10.1211/jpp.58.12.0012. [DOI] [PubMed] [Google Scholar]
- 41.Mendizabal Y, Llorens S, Nava E. Reactivity of the aorta and mesenteric resistance arteries from the obese spontaneously hypertensive rat: effects of glitazones. Am J Physiol Heart Circ Physiol. 2011;301:H1319–30. doi: 10.1152/ajpheart.01280.2010. [DOI] [PubMed] [Google Scholar]
- 42.Mendizabal Y, Llorens S, Nava E. Effects of pioglitazone and rosiglitazone on vascular function of mesenteric resistance arteries in rat genetic hypertension. Pharmacology. 2011;88:72–81. doi: 10.1159/000330092. [DOI] [PubMed] [Google Scholar]
- 43.Lu X, Guo X, Karathanasis SK, Zimmerman KM, Onyia JE, Peterson RG, Kassab GS. Rosiglitazone reverses endothelial dysfunction but not remodeling of femoral artery in Zucker diabetic fatty rats. Cardiovasc Diabetol. 2010;9:19. doi: 10.1186/1475-2840-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kinoshita H, Azma T, Iranami H, Nakahata K, Kimoto Y, Dojo M, Yuge O, Hatano Y. Synthetic peroxisome proliferator-activated receptor-gamma agonists restore impaired vasorelaxation via ATP-sensitive K+ channels by high glucose. J Pharmacol Exp Ther. 2006;318:312–8. doi: 10.1124/jpet.106.100958. [DOI] [PubMed] [Google Scholar]
- 45.Steinberg HO, Tarshoby M, Monestel R, Hook G, Cronin J, Johnson A, Bayazeed B, Baron AD. Elevated circulating free fatty acid levels impair endothelium-dependent vasodilation. J Clin Invest. 1997;100:1230–9. doi: 10.1172/JCI119636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mittermayer F, Schaller G, Pleiner J, Krzyzanowska K, Kapiotis S, Roden M, Wolzt M. Rosiglitazone prevents free fatty acid-induced vascular endothelial dysfunction. J Clin Endocrinol Metab. 2007;92:2574–80. doi: 10.1210/jc.2006-2130. [DOI] [PubMed] [Google Scholar]
- 47.Cindik N, Baskin E, Agras PI, Kinik ST, Turan M, Saatci U. Effect of obesity on inflammatory markers and renal functions. Acta Paediatr. 2005;94:1732–7. doi: 10.1111/j.1651-2227.2005.tb01845.x. [DOI] [PubMed] [Google Scholar]
- 48.Eardley KS, Zehnder D, Quinkler M, Lepenies J, Bates RL, Savage CO, Howie AJ, Adu D, Cockwell P. The relationship between albuminuria, MCP-1/CCL2, and interstitial macrophages in chronic kidney disease. Kidney Int. 2006;69:1189–97. doi: 10.1038/sj.ki.5000212. [DOI] [PubMed] [Google Scholar]
- 49.Chow FY, Nikolic-Paterson DJ, Ma FY, Ozols E, Rollins BJ, Tesch GH. Monocyte chemoattractant protein-1-induced tissue inflammation is critical for the development of renal injury but not type 2 diabetes in obese db/db mice. Diabetologia. 2007;50:471–80. doi: 10.1007/s00125-006-0497-8. [DOI] [PubMed] [Google Scholar]
- 50.Coimbra TM, Janssen U, Grone HJ, Ostendorf T, Kunter U, Schmidt H, Brabant G, Floege J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000;57:167–82. doi: 10.1046/j.1523-1755.2000.00836.x. [DOI] [PubMed] [Google Scholar]
- 51.Kelly AS, Thelen AM, Kaiser DR, Gonzalez-Campoy JM, Bank AJ. Rosiglitazone improves endothelial function and inflammation but not asymmetric dimethylarginine or oxidative stress in patients with type 2 diabetes mellitus. Vasc Med. 2007;12:311–8. doi: 10.1177/1358863X07084200. [DOI] [PubMed] [Google Scholar]
- 52.Ji Y, Liu J, Wang Z, Liu N, Gou W. PPARgamma agonist, rosiglitazone, regulates angiotensin II-induced vascular inflammation through the TLR4-dependent signaling pathway. Lab Invest. 2009;89:887–902. doi: 10.1038/labinvest.2009.45. [DOI] [PubMed] [Google Scholar]
- 53.Takano H, Hasegawa H, Zou Y, Komuro I. Pleiotropic actions of PPAR gamma activators thiazolidinediones in cardiovascular diseases. Curr Pharm Des. 2004;10:2779–86. doi: 10.2174/1381612043383719. [DOI] [PubMed] [Google Scholar]
- 54.Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA. Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol. 2005;16:638–45. doi: 10.1681/ASN.2004040278. [DOI] [PubMed] [Google Scholar]
- 55.Iyer A, Kauter K, Alam MA, Hwang SH, Morisseau C, Hammock BD, Brown L. Pharmacological inhibition of soluble epoxide hydrolase ameliorates diet-induced metabolic syndrome in rats. Exp Diabetes Res. 2012;2012 doi: 10.1155/2012/758614. ID758614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pang W, Li N, Ai D, Niu XL, Guan YF, Zhu Y. Activation of peroxisome proliferator-activated receptor-gamma downregulates soluble epoxide hydrolase in cardiomyocytes. Clin Exp Pharmacol Physiol. 2011;38:358–64. doi: 10.1111/j.1440-1681.2011.05492.x. [DOI] [PubMed] [Google Scholar]
- 57.Imig JD. Eicosanoids and renal damage in cardiometabolic syndrome. Expert Opin Drug Metab Toxicol. 2008;4:165–74. doi: 10.1517/17425255.4.2.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Y, Zhang Y, Schmelzer K, Lee TS, Fang X, Zhu Y, Spector AA, Gill S, Morisseau C, Hammock BD, Shyy JY. The anti-inflammatory effect of laminar flow: the role of PPARgamma, epoxyeicosatrienoic acids, and soluble epoxide hydrolase. Proc Natl Acad Sci USA. 2005;102:16747–52. doi: 10.1073/pnas.0508081102. [DOI] [PMC free article] [PubMed] [Google Scholar]