Abstract
The mouse heart is a target of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) during fetal development, and microarray analysis demonstrates significant changes in expression of cardiac genes involved in extracellular matrix (ECM) remodeling. We tested the hypothesis that developmental TCDD exposure would disrupt cardiac ECM expression and be associated with changes in cardiac morphology in adulthood. In one study, time-pregnant C57BL/6 mice were dosed with corn oil or 1.5, 3.0, or 6.0 µg TCDD/kg on gestation day (GD) 14.5 and sacrificed on GD 17.5, when changes in fetal cardiac mRNA expression were analyzed using quantitative PCR. TCDD induced mRNA expression of genes associated with ECM remodeling (matrix metalloproteinase 9 and 13, preproendothelin-1), cardiac hypertrophy (atrial natriuretic peptide, β-myosin heavy chain, osteopontin), and aryl hydrocarbon receptor (AHR) activation (cytochrome P4501A1, AHR repressor). Further, all TCDD-induced changes required the AHR since gene expression was not altered in AHR knockout fetuses. In a second study, time-pregnant mice were treated with corn oil or 6.0 µg TCDD/kg on GD 14.5, and male offspring were assessed for changes in cardiac gene expression and cardiac and renal morphology at 3 mo. All TCDD-induced changes in cardiac gene expression observed fetally, except for preproET-1, remained induced in the hearts of adult male offspring. Adult male offspring of TCDD-exposed dams also displayed cardiac hypertrophy, decreased plasma volume, and mild hydronephrosis. These results demonstrate that in utero and lactational TCDD exposure alters cardiac gene expression and cardiac and renal morphology in adulthood, which may increase the susceptibility to cardiovascular dysfunction.
INTRODUCTION
The developing cardiovascular system is a target of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), a potent environmental contaminant belonging to the halogenated aromatic hydrocarbon family of compounds. TCDD exposure during development results in cardiovascular malformations in fish and birds. In avian embryos TCDD exposure induces ventricular septal abnormalities, aortic arch anomalies (Cheung et al. 1981), and enlarged ventricles in the absence of a thickened myocardial wall (Walker et al. 1997; Walker and Catron 2000), while in piscine species, TCDD induces altered heart looping, resulting in a misshapen, elongated heart (Antkiewicz et al. 2005). Moreover, cardiac function also is impaired in TCDD-treated embryos, and exposed offspring display reduced heart rate, blood regurgitation, irregularities in ventricular rhythm, and impaired contractile responsiveness (Antkiewicz et al. 2005; Canga et al. 1988; Walker and Catron 2000).
Despite the extensive amount of research conducted in piscine and avian embryos, the extent of TCDD-induced developmental cardiotoxicity in mammals is relatively unknown. Recently, microarray analysis shows that cardiac gene expression is highly altered in gestation day (GD) 17.5 mouse fetuses from dams exposed to 1.5–6.0 µg/kg TCDD on GD 14.5. As expected, TCDD exposure significantly induces the expression of aryl hydrocarbon receptor (AHR)-regulated genes, such as cytochrome P4501A1 (CYP1A1), but one of the most striking findings is that TCDD highly dysregulates the expression of cardiac extracellular matrix (ECM) genes, including five procollagens and three matrix metalloproteinases (MMPs) (Thackaberry et al. 2005a). This is particularly notable since ECM remodeling plays a critical role in the pathogenesis of cardiac hypertrophy and dysfunction (D'Armiento 2002; Ju and Dixon 1996)
In addition to changes in gene expression, two studies suggest that cardiac morphology and function are also altered as a consequence of in utero and lactational TCDD exposure in the mouse. Following maternal exposure to TCDD, offspring initially exhibit smaller hearts, but as the offspring age they exhibit significant increases in heart weight, relative to body weight (Lin et al. 2001; Thackaberry et al. 2005b). Consistent with this observation, cardiac atrial natriuretic peptide (ANP) mRNA expression is induced in these offspring with enlarged hearts, suggestive of compensatory cardiac hypertrophy. Analogous to observations that have been made in other TCDD-exposed species, offspring from TCDD-exposed dams exhibit bradycardia (Thackaberry et al. 2005b).
The goals of the present study were to confirm the degree to which fetal TCDD exposure altered fetal cardiac mRNA expression of AHR-regulated, ECM, and hypertrophy marker genes, and establish whether these changes in expression required the AHR. Secondly, we sought to determine whether these TCDD-induced changes in fetal gene expression persisted into adulthood and whether they were associated with altered cardiac and renal morphology.
MATERIALS AND METHODS
Chemicals
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a gift from Dr. Richard E. Peterson (University of Wisconsin-Madison). Evan’s blue dye and Masson’s Trichrome stain were purchased from Sigma-Aldrich (St. Louis, MO).
Animals
Six-to-eight week old male and female AHR+/+ C57BL/6N mice (Harlan, Indianapolis, IN) were purchased, and AHR−/− mice were obtained from Dr. Frank Gonzalez (National Cancer Institute) (Fernandez-Salguero et al. 1995) and backcrossed 11 generations to C57BL/6N. Mice were individually housed in the the Animal Resource Facility at the University of New Mexico on a 12:12 hr light:dark cycle and provided standard mouse chow and water ad libitum. The University of New Mexico Institutional Animal Care and Use Committee approved all experiments.
Animal Exposures to TCDD
AHR+/+ mice were mated and time-pregnant dams were dosed with corn oil (vehicle control, N=12 litters) or 1.5, 3.0, or 6.0 µg TCDD/kg in corn oil by oral gavage on GD 14.5 (N=9, 10, and 8 litters for the three TCDD doses, respectively). For analysis of fetal cardiac gene expression, dams were sacrificed on GD 17.5, and fetal hearts were isolated, pooled within a litter, and frozen at −80°C. The number of fetuses per litter ranged from 6–11 and did not differ among treatment groups. However, when fetal numbers were small, we did not always have a sufficient amount of RNA from each litter to conduct the PCR analyses for all genes. The figure legends indicate the number of litters analyzed for each set of genes. For analysis of changes in adult offspring, dams exposed to corn oil or 6.0 µg TCDD/g on GD 14.5 were allowed to deliver and male offspring were analyzed for cardiac and hepatic gene expression (control, N=4–5 litters; TCDD, N=4–5 litters), renal and cardiac morphology (control, N= 7 litters; TCDD, N=5–10 litters), and plasma volume at ~ 3 mo (control, N=5 liters; TCDD, N=7 litters). To determine AHR dependent changes in fetal gene expression, AHR+/− male and female mice were mated, and time-pregnant dams were dosed with 6.0 µg TCDD/kg on GD 14.5 and sacrificed on GD 17.5. Fetuses were removed and genotyped as previously described (Fernandez-Salguero et al. 1995), and then hearts from AHR+/+ or AHR−/− fetuses were pooled within a litter (N=6 litters) and frozen at −80°C for mRNA isolation.
RNA Isolation and Quantitative PCR
Total RNA was isolated from fetal and adult hearts using Trizol reagent (Invitrogen, Carlsbad, CA) or an RNeasy Mini Kit (Qiagen, Valencia, CA). RNA integrity was verified using an Agilent 2100 bioanalyzer (Agilent Technologies, Foster City, CA) and samples were deemed of sufficient quality by the presence of clear rRNA bands and a 260/280 ratio of > 1.9. No samples were excluded due to poor quality. cDNA was synthesized from total RNA using 6 mU reverse transcriptase (Promega, Madison, WI) and 5.0 ng oligo dT primer (Promega). mRNA transcript levels were quantified with the BioRad I-Cycler (BioRad, Hercules, CA) using gene-specific primers (Sigma-Genosys, The Woodlands, TX; Table 1). Standard curves were used to assess the efficiency of each primer set, and usable primer sets were 90–100% efficient. Quantitative PCR reactions used 500 nM primers, 250 ng of cDNA, and SYBR Green Supermix (BioRad). Reactions were run in triplicate for the target genes and phosphoglycerate kinase 1 (PGK-1), which was used as the internal normalization control. Differences between CT values were computed, and values converted to mean relative expression using Q-gene software (Simon 2003) and expressed as a percent of control.
Table 1.
Mouse-specific primer sequences used for quantitative PCR analysis.
| mRNA Target | Forward primer (5’ to 3’) | Reverse Primer (5’ to 3’) |
|---|---|---|
| Aryl hydrocarbon receptor repressor (AHRR) | GTTGGATCCTGTAGGGAGCA | AGTCCAGAGGCTCACGCTTA |
| Atrial natriuretic peptide (ANP) | GCCGAACTGATAACTTTAAAAGGG | ACCCTCCCCATTCTGTCACT |
| β-Myosin heavy chain (βMHC) | GCCATCATGCACTTTGGAAACA | CCATGAGGTAGGCTGATTTGTCA |
| Cytochrome P4501A1 (CYP1A) | TGAGTCAGCAGTATGGGGACG | AGGGCCTGCTTGATGGTGTT |
| Matrix metalloproteinase 2 (MMP2) | ACCAGGTGAAGGATGTGAAGC | ACCAGGTGAAGGAGAAGGCT |
| MMP9 | GACAGGCACTTCACCGGCTA | CCCGACACACAGTAAGC |
| MMP13 | CACCTTCTTCTTGTTGAGCTGGA | TCTTCCTCAGACAGGTCATCATCA |
| Osteopontin (OPN) | TCCAATGAAAGCCATGACCAC | TCAGATTCATCCGAGTCCACA |
| Phosphoglycerate kinase 1 (PGK-1) | CAAGCTACTGTGGCCTCTGGTA | CGGCATATTTCTTGCTGCTCTCA |
| Preproendothelin-1 (preproET-1) | AAGACCATCTGTGTGGTTCTAC | CAGCCTTTCTTGGAATGTTTGGAT |
| Procollagen I (proCol1) | CGCTGGCAAGAATGGCGATC | TCTCACCCTTDTCACCACGG |
Plasma Volume
Adult offspring were anesthetized with isoflurane, and plasma volume was determined by infusing 50 µL of 1% Evan’s Blue dye into a jugular catheter. Blood was collected by cardiac puncture 5 min after dye infusion and plasma was separated by centrifugation (4°C, 15 min, 3500 × g). Plasma was diluted 1:50 with saline and absorbance determined at 620 nm. Plasma volume was calculated according to the formula plasma volume = [injected dye (g) ÷ plasma dye (g/l)] − injection volume (Maas et al. 2005).
Tissue Collection
Adult offspring were anesthetized via IP injection of ketamine/xylazine (80 mg/kg ketamine, 4 mg/kg xylazine) and euthanized by exsanguination via cardiac puncture. Plasma was immediately separated by centrifugation and stored at −20°C. Body, liver, lung, aorta, kidney, and heart weights were measured, and then the left and right ventricles were dissected and weighed. The liver, lung, aorta, left kidney, and upper half of the left ventricle (including septum) were frozen at −80°C, while the lower half of the left ventricle (including septum) and right kidney were fixed in 10% neutral buffered formalin overnight, permeabilized in 4% sucrose, and embedded in paraffin.
Analysis of Renal Morphology
To assess the incidence and severity of hydronephrosis, the right kidney was cut along the transverse plane and severity of hydronephrosis scored according to the scale published previously (Bryant et al. 2001). Paraffin-embedded kidney sections were stained with Masson’s trichrome and the presence of fibrosis was determined using light microscopy and Magna Fire computer software (Silver Spring, MD).
Residual TCDD Analysis
TCDD was measured in livers from offspring of control and TCDD-exposed dams by a previously reported method based on EPA Method 1613 (Tetra-through Octa-Chlorinated Dioxins and Furans by Isotope Dilution HRGC/HRMS, 1994) (Huwe and Smith 2005). All chemical standards used for the analysis were purchased from Wellington Laboratories (Guelph, ON). Briefly, liver samples (1 g) were ground with Celite (6 g), spiked with 13C-labeled recovery standards, and extracted in an Accelerated Solvent Extractor (Dionex, Sunnyvale, CA) using isopropanol:hexane:methylene chloride (35:30:35). The extract was exchanged into hexane and subsequently washed with 20% potassium hydroxide, water, concentrated sulfuric acid, and water. Samples were then applied to a PowerPrep automated dioxin cleanup instrument (Fluid Management Systems, Waltham, MA) for chromatography on tri-phasic silica, basic alumina, and carbon cartridges. 13C-Labeled internal standards were added prior to high resolution gas chromatography/high resolution mass spectrometry (HRGC/HRMS) analysis on an Autospec Ultima mass spectrometer (Waters, Milford, MA) coupled to an Agilent 6890 gas chromatograph. The limit of detection for this analysis was 0.06 pg/g.
Statistics
Data are expressed as a mean ± SEM. An unpaired Student’s t test was used for statistical comparison of two treatment groups. One-way analysis of variance (ANOVA) was used for statistical analysis of multiple treatment groups. A Fisher’s Exact test was used to determine significance for hydronephrotic incidence and severity. A value of p < 0.05 was considered statistically significant in all cases.
RESULTS
Fetal cardiac gene expression
Quantitative PCR was used to determine fetal cardiac expression of AHR-regulated, ECM modulators, and cardiac hypertrophy markers on GD 17.5, following in utero exposure to corn oil control or TCDD for 3 d. mRNA expression of both AHR repressor (AHRR) and CYP1A1 were up regulated in a dose-dependent manner, and maternal doses as low as 1.5 and 3.0 µg TCDD/kg significantly increased expression, respectively (Fig. 1A and 1B). Further, fetal cardiac AHRR and CYP1A1 mRNA were induced up to 10- and 17-fold, respectively, by the highest maternal dose of TCDD. Finally, TCDD-induced expression required the AHR as evidenced by the inability of maternal TCDD exposure to increase CYP1A1 or AHRR mRNA in hearts of AHR−/− fetuses, compared to the induction observed in fetal AHR+/+ littermate controls (Fig. 1C and 1D).
Figure 1.
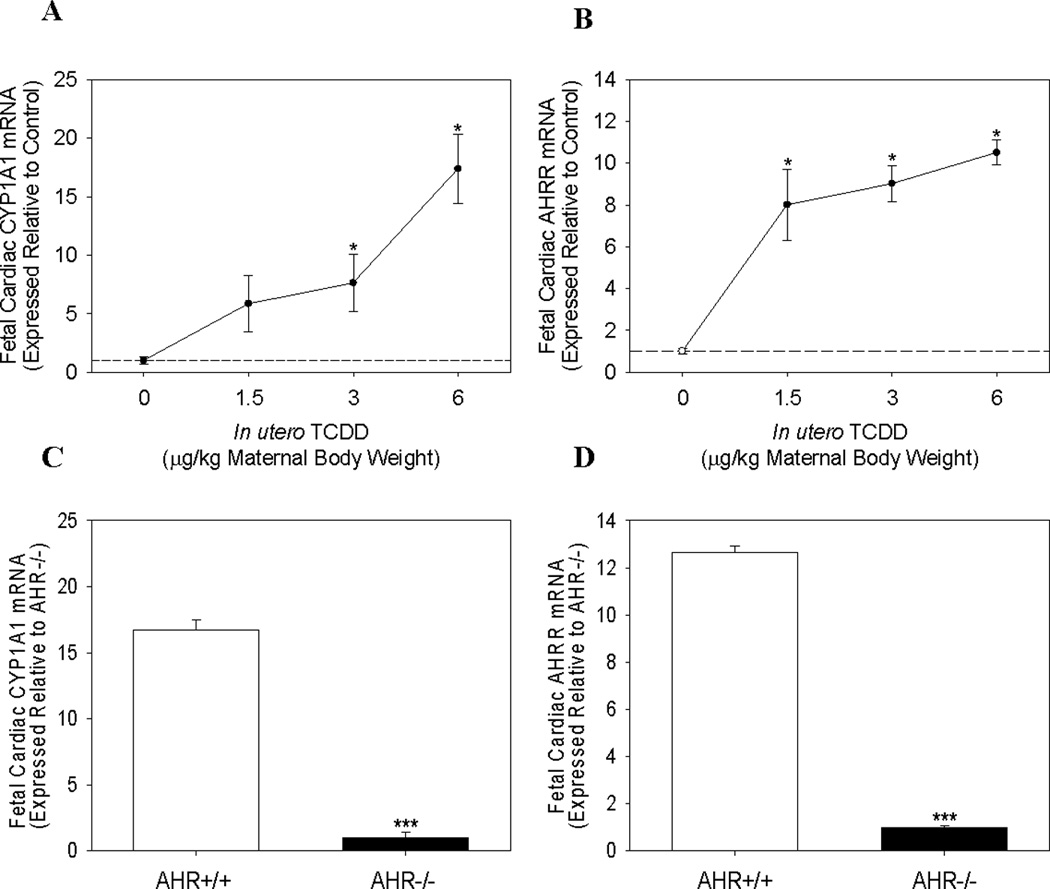
Cardiac mRNA expression of AHR-regulated genes in AHR+/+ and AHR−/− fetuses following maternal TCDD exposure. Cardiac mRNA expression of (A) CYP1A1 and (B) AHRR, in AHR+/+ fetuses on GD 17.5, following maternal exposure on GD 14.5 to corn oil or 1.5–6.0 µg TCDD/kg body weight. Fetal cardiac mRNA expression of (C) CYP1A1 and (D) AHRR in AHR+/+ and AHR−/− littermates on GD 17.5, following maternal exposure to 6.0 µg TCDD/kg body weight. Data represent mean ± SEM of mRNA expression, normalized to PGK-1 mRNA, and expressed relative to control; *p < 0.05; ***p<0.001. 0.0 µg/kg TCDD (Control, N=12); 1.5 µg/kg TCDD (N=9); 3.0 µg/kg TCDD (N=10); 6.0 µg/kg TCDD (N=8); AHR+/+ and AHR−/− (N=6).
Next, we examined the mRNA expression of genes involved in ECM regulation. Both MMP9 and MMP13 were significantly induced ~5 fold following maternal exposure to 6.0 µg TCDD/kg, although TCDD was more potent in inducing MMP13 mRNA with a significant increase also observed at a maternal dose of 3.0 µg TCDD/kg (Fig. 2A and 2B). Additionally, fetal cardiac mRNA expression of prepro-endothelin-1 (ET-1), a vasoactive peptide known to stimulate ECM turnover in the heart, was significantly induced 3.7- and 5.5-fold subsequent to maternal exposure to 3.0 and 6.0 µg TCDD/kg, respectively (Fig. 2C). As was observed for CYP1A1 and AHRR induction, MMP9, MMP13, and preproET-1 mRNA induction by maternal TCDD exposure required the AHR, since none of these genes were induced in the hearts of AHR−/− fetuses (data not shown). Lastly, mRNA expression of two genes involved in ECM regulation and remodeling, MMP2 and procollagen 1 (proCOL1), was not detected in either the control or TCDD-exposed fetal heart.
Figure 2.
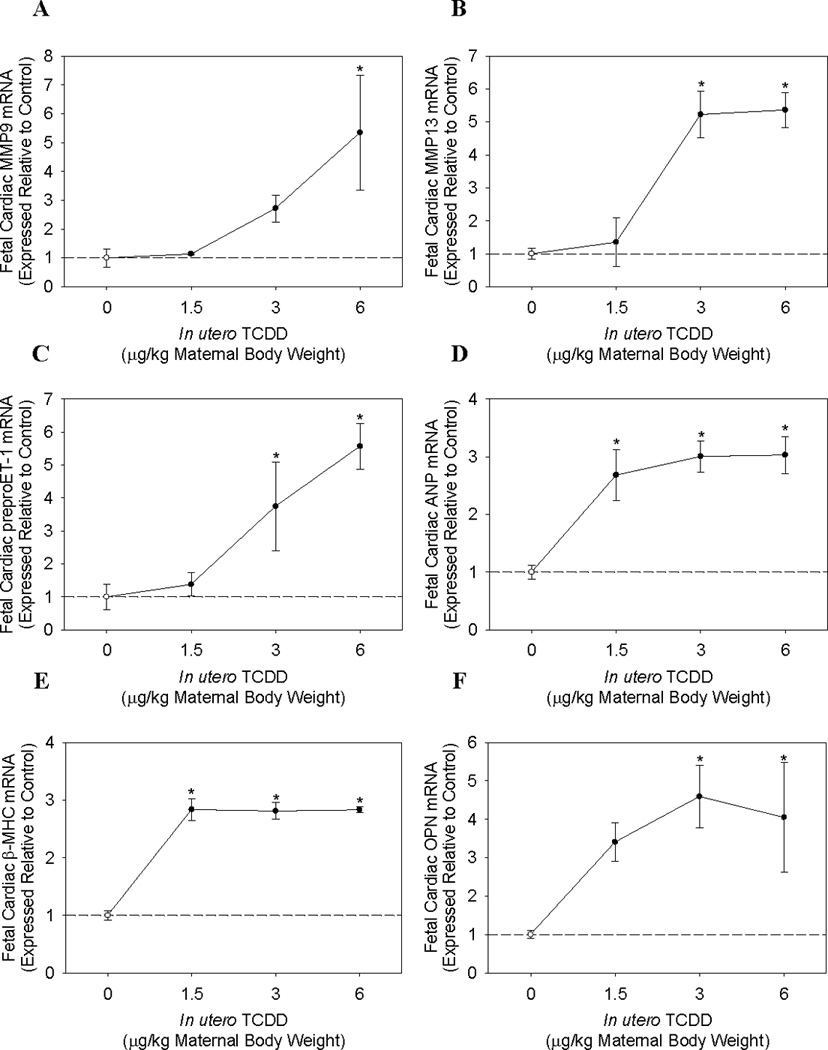
Cardiac mRNA expression of ECM and cardiac hypertrophy marker genes in AHR+/+ fetuses following maternal TCDD exposure. Cardiac mRNA expression of (A) MMP9, (B) MMP13, (C) preproET-1, (D) ANP, (E) β-MHC, and (F) OPN in AHR+/+ fetuses on GD 17.5, following maternal exposure on GD 14.5 to corn oil or 1.5–6.0 µg TCDD/kg body weight. Data represent mean ± SEM of mRNA expression, normalized to PGK-1 mRNA, and expressed relative to control; *p < 0.05. 0.0 µg/kg TCDD (Control, N=4); 1.5 µg/kg TCDD (N=4); 3.0 µg/kg TCDD (N=4); 6.0 µg/kg TCDD (N=3).
Finally, we assessed the fetal cardiac mRNA expression of three molecular markers of cardiac hypertrophy; ANP, β-myosin heavy chain (β-MHC), and osteopontin (OPN). Strikingly, maternal TCDD exposure exhibited the greatest potency in the induction of two of these three genes. Fetuses displayed significant increases of ~3 fold in both cardiac ANP and β-MHC mRNA expression following exposure to a maternal dose of 1.5 µg/kg TCDD, while increasing doses of TCDD did not increase mRNA expression further (Fig. 2D and 2E). Fetal cardiac OPN mRNA expression was increased at a maternal dose of 1.5 µg/kg TCDD, although the increase was not significant, while maternal doses of 3.0 and 6.0 µg/kg TCDD did significantly induce OPN expression by ~4-fold (Fig. 2F). As observed for AHR-regulated genes and ECM modulators, induction of fetal cardiac hypertrophy marker genes required the AHR (data not shown).
Adult cardiac gene expression
To determine if changes in fetal cardiac mRNA expression persisted in adulthood, pregnant dams exposed to corn oil control or 6.0 µg/kg TCDD on GD 14.5 were allowed to deliver and male offspring were analyzed for cardiac gene expression changes at ~3 mo of age. Cardiac mRNA expression of AHR-regulated genes, CYP1A1 and AHRR, remained significantly up regulated in offspring from TCDD-exposed litters, albeit at much lower levels than observed fetally. Adult cardiac CYP1A1 and AHRR mRNA expression was significantly increased by 2- and 3-fold, respectively from TCDD-exposed litters, compared to control litters (Fig. 3A and 3B). To determine how this degree of induction compared to the adult liver, we analyzed hepatic CYP1A1 and AHRR mRNA expression in the same mice. We found that adult hepatic CYP1A1 and AHRR mRNA expression was significantly induced ~3-fold for both genes in offspring of TCDD-exposed litters (Fig. 3C and 3D).
Figure 3.
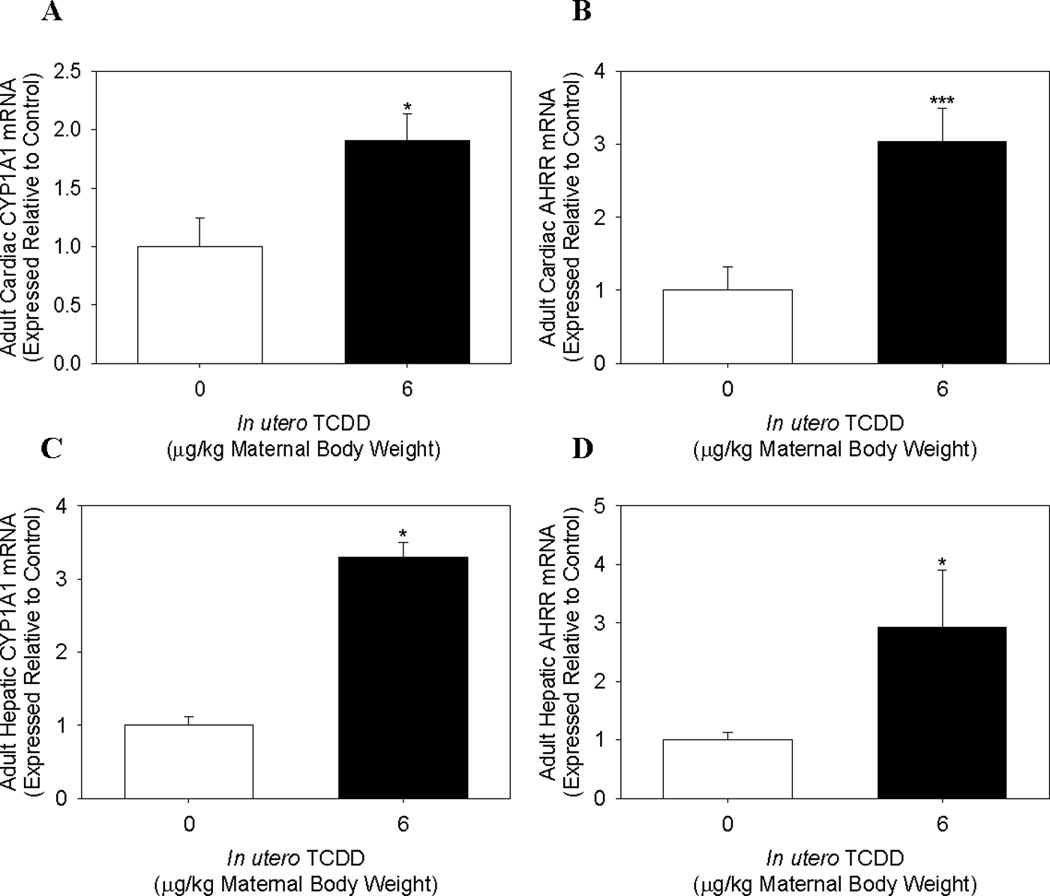
Cardiac and hepatic mRNA expression of AHR-regulated genes in adult male offspring exposed to TCDD in utero and via lactation. Cardiac (A) CYP1A1 and (B) AHRR, and hepatic (C) CYP1A1 and (D) AHRR mRNA expression in 3 mo old adult male offspring following maternal exposure to corn oil or 6.0 µg TCDD/kg body weight on GD 14.5. Data represent mean ± SEM of mRNA expression, normalized to PGK-1 mRNA, and expressed relative to control; *p < 0.05; ***p<0.001. Cardiac mRNA expression: 0.0 µg/kg TCDD (Control, N=4); 6.0 µg/kg TCDD (N=5). Hepatic mRNA expression: 0.0 µg/kg TCDD (Control, N=5); 6.0 µg/kg TCDD (N=3).
The MMPs, which were significantly induced in the fetal heart, also remained significantly elevated in the adult heart (Table 2). MMP9 and MMP13 mRNA were up regulated 3.4- and 2.6-fold, respectively, compared to control. MMP2, a gene commonly up regulated in parallel fashion with other MMPs, was not detected in the fetal heart, but was expressed in adult heart samples and was significantly induced 1.5-fold in offspring from TCDD-exposed dams. Similarly, proCOL1 mRNA was also undetected in the fetal heart, but was measurable in adult hearts and was significantly induced 2.2-fold in offspring from TCDD-exposed dams. It is notable that the only cardiac mRNA that was induced in the fetal heart but not in the adult offspring was preproET-1. As previously described, fetal mRNA expression of cardiac hypertrophy markers, ANP, β-MHC, and OPN, was significantly induced subsequent to TCDD in utero exposure. Likewise, these significant alterations persisted in adulthood.
Table 2.
Comparison of cardiac mRNA expression of ECM regulators and cardiac hypertrophy markers in fetuses on GD 17.5 and adult offspring after exposure of pregnant dams to 6.0 µg/kg TCDD on GD 14.5.
| mRNA | Fetal Cardiac Expression | Adult Offspring Cardiac Expression | ||
|---|---|---|---|---|
| Fold Changea | p value | Fold Changeb | p value | |
| MMP2 | Not detected | NA | 1.5 ± 0.1 | 0.042 |
| MMP9 | 5.3 ± 2.0 | 0.004 | 3.4 ± 0.5 | 0.004 |
| MMP13 | 5.4 ± 0.5 | < 0.001 | 2.6 ± 0.5 | 0.027 |
| preproET-1 | 5.6 ± 0.7 | < 0.001 | No change | NA |
| proCol1 | Not detected | NA | 2.2 ± 0.1 | 0.001 |
| ANP | 3.0 ± 0.3 | < 0.001 | 1.7 ± 0.3 | 0.029 |
| β-MHC | 2.8 ± 0.05 | < 0.001 | 2.1 ± 0.4 | 0.031 |
| OPN | 4.1 ± 1.4 | 0.022 | 2.3 ± 0.2 | 0.008 |
Expressed relative to control fetuses; Control, N=4 litters; TCDD, N=3 litters.
Expressed relative to control adult offspring; Control, N=4 litters; TCDD, N=5 litters.
Hepatic TCDD concentration in adult offspring
To evaluate the degree to which adult offspring from TCDD-exposed dams were still exposed to TCDD, we measured hepatic TCDD concentrations in 3 mo old offspring of control and TCDD-exposed dams. We found that the offspring from TCDD-exposed dams exhibited detectable TCDD concentrations in the liver (range = 5.35 – 8 pg/g; 6.8 ± 1.9 pg/g, N=4) versus very low levels in the offspring from control dams (range = 0 – 0.25 pg/g; 0.2 ± 0.2 pg/g, N=4) with a limit of detection of 0.06 pg/g. The percent lipid in the liver samples did not differ between the control and TCDD offspring (control, 6.4 ± 0.3%; TCDD, 6.8 ± 0.2%; p > 0.3).
Assessment of body and organ weights
Three month old adult offspring of TCDD-exposed dams failed to exhibit any significant changes in body weight or organ weights of liver, kidney, or intact heart (Table 3). However, when the heart was dissected into right ventricle and left ventricle plus septum, the left ventricle portion tended to be larger and exhibited a significant increase in weight, when expressed relative to body weight.
Table 3.
Body and tissue weights of 3 mo old male offspring after exposure of pregnant dams to corn oil (control) or 6.0 µg/kg TCDD on GD 14.5.
| Control | TCDD | |
|---|---|---|
| Body weight(g) | 30.70 ± 1.19 | 28.15 ± 1.15 |
| Liver (g) | 1.505 ± 0.059 | 1.370 ± 0.103 |
| Liver-to-body weight (%) | 4.91 ± 0.08 | 4.96 ± 0.45 |
| Kidney (g) | 0.353 ± 0.009 | 0.337± 0.010 |
| Kidney-to-body weight (%) | 1.16 ± 0.05 | 1.17 ± 0.08 |
| Heart (g) | 0.141 ± 0.006 | 0.137± 0.004 |
| Heart-to-body weight (%) | 0.478 ± 0.018 | 0.510 ± 0.016 |
| Left Ventricle + septum (g) | 0.097 ± 0.002 | 0.103 ± 0.005 |
| Left ventricle + septum-to-body weight (%) | 0.329 ± 0.010 | 0.382 ± 0.017* |
| Right ventricle (g) | 0.025 ± 0.002 | 0.024 ± 0.001 |
| Right ventricle-to-body weight (%) | 0.084 ± 0.004 | 0.089 ± 0.005 |
p < 0.05, Control; N=7 litters; TCDD, N=5 litters
Assessment of renal histology and function
Since it is well established that in utero and/or lactational TCDD exposure induces hydronephrosis, we assessed renal histology in 3 mo old adult offspring of control and TCDD-exposed dams. Developmental TCDD exposure resulted in a 90% litter incidence of hydronephrosis, while there was no evidence of these renal lesions in any adult offspring from control dams (Table 4). However, the severity of the hydronephrosis, based on a graded scale of 0–4 (Bryant et al. 2001), was mild. Additionally, Masson’s trichrome staining of kidney sections revealed the presence of fibrosis immediately surrounding the renal lesions in TCDD offspring (Fig. 4).
Table 4.
Hydronephrotic incidence and severity in the right kidney of 3 mo old male offspring after exposure of pregnant dams corn oil (control) or 6.0 µg/kg TCDD on GD 14.5.
| Litters | Affected litters | Hydronephrotic incidence (%) | Hydronephrotic severity | |
|---|---|---|---|---|
| Control | 7 | 0 | 0 | N/A |
| TCDD | 10 | 9 | 90* | 1.70 ± 0.95 |
p < 0.001 versus control
Figure 4.
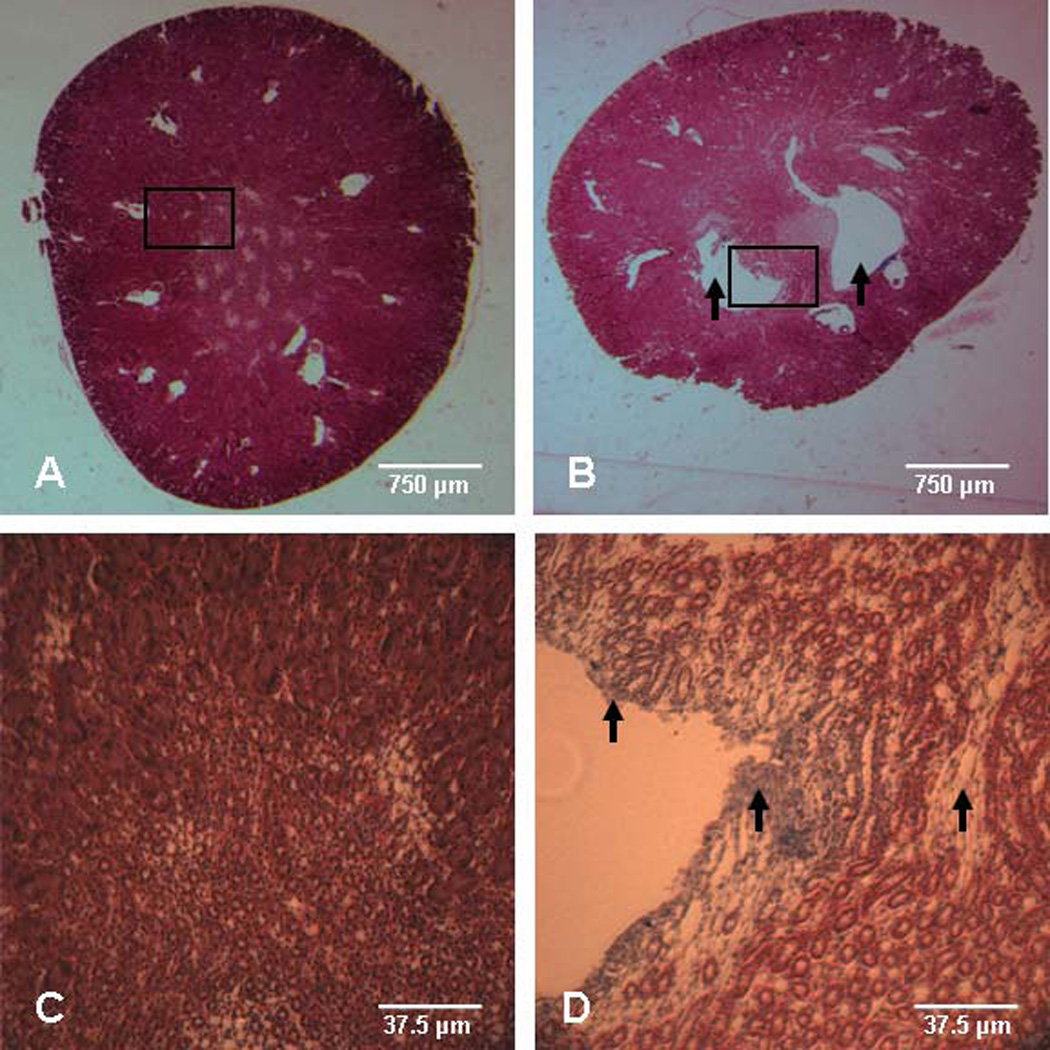
Representative renal sections of adult male offspring exposed to TCDD in utero and via lactation. Masson’s trichrome staining of right kidney from 3 mo old adult male offspring following maternal exposure to (A,C) corn oil control or (B,D) 6.0 µg TCDD/kg body weight. Micrographs in panels (C) and (D) are 20x magnification of the boxed areas shown in (A) and (B), respectively. The presence of mild hydronephrosis is apparent by the open spaces in the offspring exposed to TCDD (panel B, arrows) and fibrosis is noted by light blue staining adjacent to the hydronephrotic lesion in the offspring exposed to TCDD (panel D, arrow heads).
We assessed kidney function by measuring plasma creatinine, blood urea nitrogen, and total plasma volume. Since the kidney filters most of the creatinine and urea from the blood, the concentrations in the blood are excellent indicators of renal filtering capability. The kidney also is an important regulator of total plasma volume and a reduction in volume may reflect an increased excretion of water. We found that plasma creatinine levels were undetectable and blood urea nitrogen was normal (data not shown), suggesting that renal filtration function was normal. However, plasma volume, quantified using a dye dilution method, was significantly reduced in offspring of TCDD-exposed dams, compared to controls (controls, 41.0 ± 3.2 µl/g body weight; TCDD, 32.1 ± 1.8 µl/g body weight; p<0.05), suggesting increased renal diuresis.
DISCUSSION
While the developing cardiovascular system in mammals has not been considered a major target of TCDD-induced teratogenicity previously, emerging evidence suggests that TCDD induces significant changes in mouse fetal cardiac gene expression which are associated with subtle changes in cardiac morphology (Lin et al. 2001; Thackaberry et al. 2005b) Our study extends these observations by showing that induction of fetal cardiac genes related to AHR activation, ECM remodeling, and cardiac hypertrophy are AHR mediated, are sustained into adulthood, and are associated with cardiac hypertrophy in adulthood.
A striking finding of our study was that nearly all TCDD-induced increases in fetal cardiac mRNA expression were sustained into adulthood, albeit at lower levels of induction. There are a number of possible explanations for this sustained induction, which are not mutually exclusive. It is possible that the residual TCDD body burden in adulthood is sufficient to continue AHR activation. This seems plausible for those genes known to be directly regulated by AHR, including CYP1A1 and AHRR. However, the presence of extremely low levels of residual TCDD (~ 7 pg/g) detected in the liver of adult offspring would not be expected to be sufficient to induce CYP1A1 (DeVito et al. 2000; DeVito et al. 1997; Diliberto et al. 2001). Further, based on tissue distribution studies in adult mice (Diliberto et al. 2001), considerably less TCDD would be expected to be present in the heart and thus the relative induction of CYP1A1 and AHRR mRNA would be expected to be different. This comparison, however, is complicated by the relative expression levels of AHR, induction of CYP1A2, and the percent lipid in the tissues. Nonetheless, the sustained induction of CYP1A1 and AHRR at similar levels in the adult offspring heart and liver, suggest that mechanisms other than the TCDD residue may be important in their sustained expression.
Another possible explanation for the sustained induction of these genes in the adult heart includes changes in epigenetic regulation. Studies in human keratinocytes and mouse preimplantation embryos show that TCDD alters the methylation status of genes, and as a result, gene transcription is altered (Ray and Swanson 2004; Wu et al. 2004). Further, neonatal and adult offspring subjected to undernutrition in utero display altered mRNA and protein expression of components of the renin-angiotensin system, and these alterations are linked to abnormal methylation status of the genes (Bogdarina et al. 2007). Thus, developmental exposure of the mouse to TCDD may induce irregular methylation patterns in some of these genes, permanently altering their expression.
Finally, changes in cardiac morphology and function induced by developmental TCDD exposure may lead to compensatory physiological changes. Fetal TCDD exposure significantly decreases fetal heart weight and heart-to-body weight ratio (Thackaberry et al. 2005b). However, the reduced heart size does not persist, but rather the heart hypertrophies as the offspring age (Lin et al. 2001; Thackaberry et al. 2005b). Thus, the cardiac hypertrophy observed in our study and those of others in TCDD-exposed offspring may represent a compensatory response to changes induced developmentally, and these compensatory responses induce a battery of changes in gene expression. Consistent with this idea is the sustained induction of cardiac hypertrophy marker genes, ANP, β-MHC, and OPN, into adulthood. These genes normally are expressed during fetal heart development and down regulate postnatally. However, during the pathogenesis of cardiac hypertrophy and dysfunction, these fetal genes are re-expressed (Carrero et al. 2000; Morkin 2000; Okamoto 2007). Thus, the persistent expression of fetal cardiac hypertrophy markers in offspring of TCDD-exposed dams may represent gene expression changes associated with the very early development of cardiac disease.
As expected, in utero TCDD exposure significantly induces mRNA expression of two known AHR-regulated genes, CYP1A1 and AHRR, in GD 17.5 fetal hearts, and this induction is dependent on the AHR. Notably, however, TCDD-induced increases in mRNA expression of all ECM modulators and cardiac hypertrophy marker genes in the fetal heart require the AHR. Although the transcriptional regulation of CYP1A1 and AHRR is controlled by the AHR binding to dioxin response elements (DRE) in their promoters (Lusska et al. 1991; Mimura et al. 1999), our results do not imply that the changes in mRNA expression of these other targets result from direct transcriptional regulation via DREs. It is more likely that up regulation of many of these fetal heart genes result from secondary changes induced downstream of AHR-mediated effects. For example, preproET-1 is up regulated in fetal hearts exposed to TCDD, and overexpression of ET-1 can induce ECM proteins in the heart (Schwarz et al. 2002) and accelerate the transcription of OPN (Okamoto 2007). Nonetheless, future studies investigating the time course of TCDD-induced changes in fetal cardiac gene expression are needed to reveal those changes that may be primary responses versus those that are secondary.
One unexpected result of our studies is that induction of fetal cardiac AHRR mRNA is the most sensitive indicator of in utero TCDD exposure, exhibiting an 8 fold increase at the lowest dose of TCDD, in comparison to CYP1A1 mRNA which increases 6 fold at this same dose. In adult mice, the heart expresses some of the highest levels of AHRR mRNA, compared to other tissues, but AHRR is un-inducible by acute exposure to benzo(a)pyrene, compared to a nearly 10 fold induction of cardiac CYP1A1 mRNA by the same exposure (Bernshausen et al. 2006). This is in complete contrast to the relative inducibility of AHRR and CYP1A1 mRNA by TCDD in the mouse liver, where induction of CYP1A1 mRNA is 20 times higher than that for AHRR (Tijet et al. 2006). Our results highlight the tissue-dependent differences in the relative sensitivity of genes in the AHR gene battery to TCDD induction and suggest that these differences may contribute to mechanistic differences in TCDD-induced toxicity among different tissues.
Our results are consistent with the growing body of evidence that TCDD toxicity is associated with significant changes in genes that regulate the ECM turnover and remodeling. AHR activation in melanoma and prostate cancer cell lines increases expression and activity of MMP2, MMP9, and MMP1, the human equivalent of murine MMP13 (Haque et al. 2005; Villano et al. 2006). TCDD increases expression of pro-MMP3 and 7 in endometrial cocultures of human endometrial stromal and epithelial cells (Igarashi et al. 2005). Developmental TCDD exposure significantly alters the intensity and spatial expression of ECM proteins in the kidney (Abbott et al. 1987b). Further, AHR activation alters the mRNA expression of MMPs as well as various collagen isoforms during zebrafish fin regeneration, a process that heavily relies on precise ECM regulation (Andreasen et al. 2007), and TCDD-exposed marmosets exhibit alterations in collagen, fibronectin, and laminin in the thymus (Nottebrock et al. 2006). The degree to which altered expression of ECM regulators in the heart contributes to cardiac hypertrophy and dysfunction in offspring of TCDD-exposed dams remains to be determined; however, ECM remodeling plays a critical role in the pathogenesis of these processes (D'Armiento 2002; Ju and Dixon 1996).
It is well established that in utero and/or lactational TCDD exposure induces hydronephrosis (Abbott et al. 1987a), and this lesion persists to at least ~2 mo of age (Couture-Haws et al. 1991). Our results extend this observation to show that TCDD-induced hydronephrosis persists further into adulthood. Although the mild severity of hydronephrosis is not associated with major changes in renal filtration, as evidenced by normal blood urea nitrogen and plasma creatinine, it is associated with reduced plasma volume. The physiological impact of mild hydronephrosis is not well studied, but bilateral ureteral obstruction could lead to a decrease in plasma volume, since hydronephrosis can reduce the ability to concentrate urine resulting in increased urine volume and reduced plasma volume (Kim et al. 2001). Furthermore, we cannot rule out the possibility that TCDD-induced changes in renal morphology and function contribute to the observed changes in cardiac hypertrophy; however, a reduction in plasma volume would not be consistent with the etiology of cardiac hypertrophy. More specifically, the significant increase in left ventricle plus septum weight likely reflects compensatory changes associated with systemic vascular dysfunction, such as pressure or volume overload. Thus, we have conducted additional studies to investigate cardiac function and blood pressure regulation in adult offspring of control and TCDD-exposed dams to further reveal the physiological mechanisms associated with this cardiac hypertrophy and these will be reported in a follow-up paper.
In conclusion, our results demonstrate that developmental TCDD exposure in the mouse results in non-overtly teratogenic effects on the developing heart, including significant changes in gene expression that persist into adulthood and which are associated with changes in cardiac morphology. These results suggest that, in contrast to piscine and avian species where developmental TCDD exposure induces cardio-teratogenic effects associated with mortality, effects of developmental TCDD exposure on the mouse heart could lead to cardiovascular dysfunction in adulthood. Our future studies will assess the potential for increased susceptibility to cardiovascular dysfunction in offspring following developmental TCDD exposure.
ACKNOWLEDGEMENTS
We thank Bethany Nunez for her technical support. This work was supported by grant funding from NIH (ES012335) to M.K.W. and (ES012335-0281) to A.C.A.
Footnotes
The use of trade, firm, or corporation names in this publication is for the information and convenience of the reader. Such use does not constitute an official endorsement or approval by the United States Department of Agriculture or the Agricultural Research Service of any product or service to the exclusion of others that may be suitable.
Contributor Information
Andrea C. Aragon, Email: acaragon@salud.unm.edu.
Phillip G. Kopf, Email: pkopf@salud.unm.edu.
Matthew J. Campen, Email: mcampen@lrri.org.
Janice K. Huwe, Email: Janice.Huwe@ars.usda.gov.
Mary K. Walker, Email: mwalker@salud.unm.edu.
REFERENCES
- Abbott BD, Birnbaum LS, Pratt RM. TCDD-induced hyperplasia of the ureteral epithelium produces hydronephrosis in murine fetuses. Teratology. 1987a;35(3):329–334. doi: 10.1002/tera.1420350307. [DOI] [PubMed] [Google Scholar]
- Abbott BD, Morgan KS, Birnbaum LS, Pratt RM. TCDD alters the extracellular matrix and basal lamina of the fetal mouse kidney. Teratology. 1987b;35(3):335–344. doi: 10.1002/tera.1420350308. [DOI] [PubMed] [Google Scholar]
- Andreasen EA, Mathew LK, Lohr CV, Hasson R, Tanguay RL. Aryl hydrocarbon receptor activation impairs extracellular matrix remodeling during zebra fish fin regeneration. Toxicol. Sci. 2007;95(1):215–226. doi: 10.1093/toxsci/kfl119. [DOI] [PubMed] [Google Scholar]
- Antkiewicz DS, Burns CG, Carney SA, Peterson RE, Heideman W. Heart malformation is an early response to TCDD in embryonic zebrafish. Toxicol. Sci. 2005;84(2):368–377. doi: 10.1093/toxsci/kfi073. [DOI] [PubMed] [Google Scholar]
- Bernshausen T, Jux B, Esser C, Abel J, Fritsche E. Tissue distribution and function of the Aryl hydrocarbon receptor repressor (AhRR) in C57BL/6 and Aryl hydrocarbon receptor deficient mice. Arch. Toxicol. 2006;80(4):206–211. doi: 10.1007/s00204-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007;100(4):520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant PL, Schmid JE, Fenton SE, Buckalew AR, Abbott BD. Teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice lacking the expression of EGF and/or TGF-alpha. Toxicol. Sci. 2001;62(1):103–114. doi: 10.1093/toxsci/62.1.103. [DOI] [PubMed] [Google Scholar]
- Canga L, Levi R, Rifkind AB. Hearts as a target organ in 2,3,7,8-tetrachlorodibenzo-p-dioxin toxicity: decreased B-adrenergic responsiveness and evidence of increased intracellular calcium. PNAS. 1988;85:905–909. doi: 10.1073/pnas.85.3.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrero P, Okamoto K, Coumailleau P, O'Brien S, Tanaka H, Poellinger L. Redox-regulated recruitment of the transcriptional coactivators CREB-binding protein and SRC-1 to hypoxia-inducible factor 1alpha. Mol. Cell. Biol. 2000;20(1):402–415. doi: 10.1128/mcb.20.1.402-415.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung MO, Gilbert EF, Peterson RE. Cardiovascular teratogenicity of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the chick embryo. Toxicol. Appl. Pharmacol. 1981;61:197–204. doi: 10.1016/0041-008x(81)90409-9. [DOI] [PubMed] [Google Scholar]
- Couture-Haws L, Harris MW, Lockhart AC, Birnbaum LS. Evaluation of the persistence of hydronephrosis induced in mice following in utero and/or lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 1991;107(3):402–412. doi: 10.1016/0041-008x(91)90304-w. [DOI] [PubMed] [Google Scholar]
- D'Armiento J. Matrix metalloproteinase disruption of the extracellular matrix and cardiac dysfunction. Trends in Cardiovascular Medicine. 2002;12(3):97–101. doi: 10.1016/s1050-1738(01)00160-8. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Diliberto JJ, Ross DG, Menache MG, Birnbaum LS, Nessel CS, Amoruso MA, Umbreit TH, Meeker RJ, Gallo MA, Calvert GM, Sweeney MH, Morris JA, Fingerhut MA, Hornung RW, Halperin WE, Dey A, Westphal H, Nebert DW, Domin BA, Devereux TR, Philpot RM, Tuteja N, Gonzalez FJ, Nebert DW, Gasiewicz TA, Ness WC, Rucci G, Allen JR, Barsotti DA, Van Miller JP, Abrahamson LJ, Lalich JJ. Dose-response relationships for polyhalogenated dioxins and dibenzofurans following subchronic treatment in mice. I. CYP1A1 and CYP1A2 enzyme activity in liver, lung, and skin. Toxicol. Appl. Pharmacol. 1997;147(2):267–280. doi: 10.1006/taap.1997.8261. [DOI] [PubMed] [Google Scholar]
- DeVito MJ, Menache MG, Diliberto JJ, Ross DG, Birnbaum LS. Dose-response relationships for induction of CYP1A1 and CYP1A2 enzyme activity in liver, lung, and skin in female mice following subchronic exposure to polychlorinated biphenyls. Toxicol. Appl. Pharmacol. 2000;167(3):157–172. doi: 10.1006/taap.2000.9010. [DOI] [PubMed] [Google Scholar]
- Diliberto JJ, DeVito MJ, Ross DG, Birnbaum LS. Subchronic Exposure of [3H]- 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female B6C3F1 mice: relationship of steady-state levels to disposition and metabolism. Toxicol. Sci. 2001;61(2):241–255. doi: 10.1093/toxsci/61.2.241. [DOI] [PubMed] [Google Scholar]
- Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268(5211):722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- Haque M, Francis J, Sehgal I. Aryl hydrocarbon exposure induces expression of MMP-9 in human prostate cancer cell lines. Cancer Lett. 2005;225(1):159–166. doi: 10.1016/j.canlet.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Huwe JK, Smith DJ. Laboratory and on-farm studies on the bioaccumulation and elimination of dioxins from a contaminated mineral supplement fed to dairy cows. J. Agric. Food Chem. 2005;53:2362–2370. doi: 10.1021/jf0480997. [DOI] [PubMed] [Google Scholar]
- Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, Osteen KG. Reduced expression of progesterone receptor-B in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil. Steril. 2005;84(1):67–74. doi: 10.1016/j.fertnstert.2005.01.113. [DOI] [PubMed] [Google Scholar]
- Ju H, Dixon IM. Extracellular matrix and cardiovascular diseases. Canadian Journal of Cardiology. 1996;12(12):1259–1267. [PubMed] [Google Scholar]
- Kim SW, Lee J, Park JW, Hong JH, Kook H, Choi C, Choi KC. Increased expression of atrial natriuretic peptide in the kidney of rats with bilateral ureteral obstruction. Kidney Int. 2001;59(4):1274–1282. doi: 10.1046/j.1523-1755.2001.0590041274.x. [DOI] [PubMed] [Google Scholar]
- Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS, Peterson RE. Role of the aryl hydrocarbon receptor in the development of control and 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed male mice. J. Toxicol. Environ. Health. 2001;64(4):327–342. doi: 10.1080/152873901316981312. [DOI] [PubMed] [Google Scholar]
- Lusska AE, Jones KW, Elferink CJ, Wu L, Shen ES, Wen LP, Whitlock JP., Jr 2,3,7,8-Tetrachlorodibenzo-p-dioxin induces cytochrome P450IA1 enzyme activity by activating transcription of the corresponding gene. Adv. Enzyme Regul. 1991;31:307–317. doi: 10.1016/0065-2571(91)90019-i. [DOI] [PubMed] [Google Scholar]
- Maas M, Stapleton M, Bergom C, Mattson DL, Newman DK, Newman PJ. Endothelial cell PECAM-1 confers protection against endotoxic shock. Am. J. Physiol Heart Circ. Physiol. 2005;288(1):H159–H164. doi: 10.1152/ajpheart.00500.2004. [DOI] [PubMed] [Google Scholar]
- Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13(1):20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morkin E. Control of cardiac myosin heavy chain gene expression. Microsc. Res. Tech. 2000;50(6):522–531. doi: 10.1002/1097-0029(20000915)50:6<522::AID-JEMT9>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Nottebrock C, Riecke K, Kruse M, Shakibaei M, Stahlmann R. Effects of 2,3,7,8-tetrachloro-dibenzo-p-dioxin on the extracellular matrix of the thymus in juvenile marmosets (Callithrix jacchus) Toxicology. 2006;226(2–3):197–207. doi: 10.1016/j.tox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Okamoto H. Osteopontin and cardiovascular system. Mol. Cell Biochem. 2007;300(1–2):1–7. doi: 10.1007/s11010-006-9368-3. [DOI] [PubMed] [Google Scholar]
- Ray SS, Swanson HI. Dioxin-induced immortalization of normal human keratinocytes and silencing of p53 and p16INK4a. J. Biol. Chem. 2004;279(26):27187–27193. doi: 10.1074/jbc.M402771200. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Godes M, Thone-Reineke C, Theuring F, Bauer C, Neumayer HH, Hocher B. Tissue-dependent expression of matrix proteins in human endothelin-1 transgenic mice. Clin. Sci. (Lond) 2002;103(Suppl 48):39S–43S. doi: 10.1042/CS103S039S. [DOI] [PubMed] [Google Scholar]
- Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19(11):1439–1440. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: modulation of cell cycle and extracellular matrix genes. Toxicol. Sci. 2005a;88(1):231–241. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- Thackaberry EA, Nunez B, Ivnitski-Steele ID, Friggens MM, Walker MK. Effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) on the fetal murine heart II: TCDD alters fetal and postnatal cardiac growth, and postnatal cardiac chronotrophy. Toxicol. Sci. 2005b;88:242–249. doi: 10.1093/toxsci/kfi302. [DOI] [PubMed] [Google Scholar]
- Tijet N, Boutros PC, Moffat ID, Okey AB, Tuomisto J, Pohjanvirta R. Aryl hydrocarbon receptor regulates distinct dioxin-dependent and dioxin-independent gene batteries. Mol. Pharmacol. 2006;69(1):140–153. doi: 10.1124/mol.105.018705. [DOI] [PubMed] [Google Scholar]
- Villano CM, Murphy KA, Akintobi A, White LA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) induces matrix metalloproteinase (MMP) expression and invasion in A2058 melanoma cells. Toxicol. Appl. Pharmacol. 2006;210(3):212–224. doi: 10.1016/j.taap.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Walker MK, Catron TF. Characterization of cardiotoxicity induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin and related chemicals during early chick embryo development. Toxicol. Appl. Pharmacol. 2000;167(3):210–221. doi: 10.1006/taap.2000.8992. [DOI] [PubMed] [Google Scholar]
- Walker MK, Pollenz RS, Smith SM. Expression of the aryl hydrocarbon receptor (AhR) and AhR nuclear translocator during chick cardiogenesis is consistent with 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced heart defects. Toxicol. Appl. Pharmacol. 1997;143(2):407–419. doi: 10.1006/taap.1996.8068. [DOI] [PubMed] [Google Scholar]
- Wu Q, Ohsako S, Ishimura R, Suzuki JS, Tohyama C. Exposure of mouse preimplantation embryos to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) alters the methylation status of imprinted genes H19 and Igf2. Biol. Reprod. 2004;70(6):1790–1797. doi: 10.1095/biolreprod.103.025387. [DOI] [PubMed] [Google Scholar]


