Abstract
In utero and lactational exposure of mice to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) leads to cardiac hypertrophy and hydronephrosis in adulthood. We tested the hypothesis that perinatal TCDD exposure increases the susceptibility to cardiovascular disease when offspring are exposed to a common cardiovascular disease risk factor, angiotensin II (Ang II). Pregnant C57BL/6N mice were exposed to corn oil (control) or 6.0 µg/kg TCDD on gestation day 14.5. Male offspring were then exposed to a subpressor (0.1 mg/kg/d) or pressor (0.7 mg/kg/d) dose of Ang II at 3.5 mo and cardiac morphology and blood pressure analyzed, respectively. Perinatal TCDD exposure increased left ventricular cavity dilation during diastole, and wall thickness during diastole and systole. While Ang II stimulated an increase in wall thickness, the degree of increase was equivalent between control and TCDD offspring. In contrast, perinatal TCDD exposure did not alter basal blood pressure. However, Ang II increased systolic blood pressure more rapidly and to a greater degree in TCDD offspring. Further, Ang II stimulated renal myofibroblast differentiation and collagen deposition to a greater degree, and tended to increase procollagen I mRNA in TCDD offspring, compared to controls. These data suggest that perinatal TCDD exposure increases the susceptibility of offspring to renal fibrosis and hypertension in adulthood.
INTRODUCTION
Barker and others (1–3) have proposed that critical periods exist during fetal and neonatal development when tissues are plastic and responsive to environmental insults, but afterwards lose their plasticity and exhibit a fixed functional capacity. Thus, the Barker Hypothesis postulates that exposure to an exogenous insult during a critical period will be manifested later in life by increased susceptibility to chronic disease. In support of this hypothesis, for example, human epidemiology studies have shown that early insults occurring during the prenatal and perinatal periods increase the risk of a variety of cardiovascular diseases in adulthood, including hypertension, stroke, and coronary heart disease (4).
One physiological system that may play a key role in fetal programming of adult cardiovascular disease is the renin-angiotensin system (RAS). The primary biological active, humoral factor in the RAS is angiotensin II (Ang II). Ang II induces vasoconstriction, cardiac hypertrophy, and cardiac and renal fibrosis, the latter mediated in part by induction of transforming growth factor β1 and activation of matrix metalloproteinases (5). The RAS is commonly activated in human cardiovascular diseases and blockade of this pathway has lead to some of the most successful therapeutic approaches for reducing morbidity and mortality associated with cardiovascular disease (6). For example, ACE inhibitors, drugs that block the conversion of angiotensin I to Ang II by angiotensin converting enzyme (ACE), reduce the incidence of stroke, reverse cardiac hypertrophy, improve symptoms of congestive heart failure, and slow the progression of renal disease (7). Thus, an activated RAS is a common feature of cardiovascular disease in adult humans.
A number of animal studies have suggested that the normal homeostasis of the RAS can become reprogrammed during fetal development, leading to increased risk of cardiovascular disease later in life. For example, feeding a low protein diet to rats during gestation results in hypertension in the offspring, which can be normalized by treatment with an ACE inhibitor (8) and made significantly worse by infusion of Ang II (9). These effects may be mediated, in part, by changes in expression of Ang II receptors observed in the kidney, adrenal gland, and brain (9–11). In addition, a low protein diet during gestation results in reduced renal nephron number, which could increase the susceptibility to renal injury, particularly in the face of an activated RAS.
We have previously shown that in utero and lactational exposure of mice to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) results in changes in fetal cardiac gene expression, which persist into adulthood (12, 13). Further, many of the genes that are changed in expression cluster into a category associated with extracellular matrix turnover and remodeling, including matrix metalloproteinases and collagens. Importantly, these changes in gene expression are associated with cardiac hypertrophy and mild hydronephrosis in the adult offspring (13, 14). It has been demonstrated that hydronephrosis occurring during fetal development increases expression of renal Ang II receptors, activates the renal RAS, and promotes renal fibrosis (15, 16). Taken together these observations led us to test the hypothesis that in utero and lactational TCDD exposure would increase the sensitivity to Ang II-induced cardiac hypertrophy, renal fibrosis, and hypertension in adult offspring and would be associated with alterations in Ang II receptor expression.
MATERIALS AND METHODS
Chemicals
2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) was a gift from Dr. Richard E. Peterson (University of Wisconsin-Madison). Angiotensin II (Ang II) was purchased from American Peptide (Vista, CA). 2,2,2-Tribromoethanol (Avertin) and methyl green was purchased from Sigma-Aldrich (St. Louis, MO).
Developmental Exposure of Mice to TCDD
Six-to-eight week old male and female C57BL/6N mice were purchased from Harlan (Indianapolis, IN) and mated. Time-pregnant dams were dosed with corn oil (control, 5 µl/g body weight) or 6.0 µg TCDD/kg in corn oil by oral gavage on gestation day (GD) 14.5 (control, n=23; TCDD, n=24). We selected this dose because it represented the lowest dose that reduced fetal heart weight to the greatest degree (14). Dams were allowed to deliver and one male offspring from each litter was randomly assigned to one of two analysis groups at 3.5 mo of age.
Mice in group 1 (control, n=15; TCDD, n=15) were exposed to 0.9% NaCl (n=8/developmental exposure group) or a subpressor dose (0.1 mg/kg/d) of Ang II (n=7/developmental exposure group) for 14 d via an osmotic minipump. After 14 d, these mice were analyzed by echocardiography. Mice in group 2 (control, n=8; TCDD, n=9) were implanted with a radiotelemeter and baseline blood pressure values were recorded for 7 d (described in detail below). Following collection of baseline information, mice were exposed to 0.9% NaCl (n=4/developmental exposure group) or a pressor dose (0.7 mg/kg/d) of Ang II (n=4/developmental exposure group) for 11 d via an osmotic minipump and blood pressure continued to be recorded. One mouse from a TCDD-exposed litter died prior to minipump implantation. After 11 d, these mice were euthanized, the right kidney collected for histology and immunohistochemistry, and the left kidney frozen in RNAlater (Ambion) for mRNA analysis.
Echocardiography
Mice were anesthetized with 300 µl of Avertin (20 mg/ml) by i.p. injection. An Acuson Sequoia C512 with a 10V4 pediatric transducer was used to record a two dimensional, M-mode transthoracic echocardiogram. The M-mode echocardiogram was used to measure the internal diameter of the left ventricle and the thickness of the left ventricle posterior wall in diastole and systole.
Implantation of osmotic minipumps
Mice were anesthetized with isoflurane and, using aseptic technique, an osmotic minipump (model 1002, Alzet Osmotic Minipumps, Cupertino, CA) was placed into a subcutaneous pouch between the scapula. The pump was loaded to deliver 0.25 µl/hr for a maximum of 14 d.
Implantation of blood pressure radiotelemeters and blood pressure measurements
Mice were given a s.q. injection of buprenorphine (0.1 mg/kg) prior to surgery as an analgesic followed by anesthesia with isoflurane. Using aseptic technique, the catheter of the telemeter (PhysioTel PA-C10, Data Sciences International, St. Paul, MN) was inserted into left carotid artery, while the body of the telemeter was placed subcutaneously to the left of the abdomen. Seven days after surgery, diastolic, systolic, and mean arterial blood pressure; and heart rate and activity were recorded every 15 minutes for 10 seconds (Dataquest ART 3.0, Data Sciences International, St. Paul, MN). Baseline telemetry values were recorded for 7 d prior to the implantation of an osmotic minipump, which contained 0.9% NaCl or a pressor dose of Ang II. Following minipump implantation, telemetry values were recorded for 11 d and then mice were euthanized and kidneys collected for histological and gene expression analyses.
Renal Immunohistochemistry
Kidneys were fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned at 6 µm. Slides were dewaxed, rehydrated, and stained with picrosirius red to detect collagen deposition or with anti-alpha smooth muscle actin (A5228, Sigma-Aldrich) to identify the presence of myofibroblasts. For antibody staining, endogenous peroxidase activity was blocked with 2% hydrogen peroxide in phosphate buffered saline (PBS) and then tissue sections were blocked with 10% goat serum in Tris-buffered saline, 0.1% Tween 20 for 1 hr at room temperature. Sections were incubated with 1/400 dilution of the primary antibody for 1 hr at room temperature, washed three times with PBS containing 1% bovine serum albumin and 0.5% Tween 20, and then incubated with 1/50 dilution of goat anti-mouse IgG-horse radish peroxidase (HRP) (Southern Biotech, Birmingham, Alabama) for 1 hr at room temperature. After washing, HRP was developed using a peroxidase substrate kit (Vector Laboratories, Burlingame, CA) and counterstained with methyl green.
Renal RAS and collagen gene expression
Total RNA was isolated from kidneys of saline- and Ang II-treated adult mice from control and TCDD-exposed litters with RNeasy Mini Kit (Qiagen, GmbH, Germany) (n=4/minipump treatment/developmental exposure). cDNA was synthesized using iScript Select cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA) with random primers and 250 ng RNA. PCR amplification was performed using an iCycler (Bio-Rad Laboratories) with a reaction mixture comprised of iQ SYBR Green Supermix (Bio-Rad Laboratories) with 500 µM forward and reverse primers (Table 1). Cycle threshold data and amplification efficiency for the target and reference gene, polymerase (RNA) II (DNA directed) polypeptide A (Polr2a), were used to calculate mean normalized expression as previously described (17).
Table 1.
Real time PCR primer sequences.
| mRNA Target | Forward primer (5’ to 3’) | Reverse Primer (5’ to 3’) |
|---|---|---|
| Angiotensinogen | CACTCATTTGTTCAGAGCCTGG | GTTCATCTTCCACCCTGTCACA |
| Ang II receptor 1a | GGGCGTCATCCATGACTGTAAA | TTCCCCAGAAAGCCGTAAAACA |
| Ang II receptor 1b | CCAATGAAGTCTCGCCTCCG | ACATTTCGGTGGATGACGGC |
| Ang II receptor 2 | GCAGCCGTCCTTTTGATAATCTC | ATCTGATGGTTTGTGTGAGCAATT |
| gp91phox | CACCCATTCACACTGACCTCTG | CTTATCACAGCCACAAGCATTGAA |
| Procollagen I | CGCTGGCAAGAATGGCGATC | TCTCACCCTTDTCACCACGG |
| RNA polymerase II 1 | TGACTCACAAACTGGCTGACA | TACATCTTCTGCTATGACATGG |
| Renin | ATGAATATGTTGTGAGCTGTAGCC | GTCTCTCCTGTTGGGATACTGTAG |
Statistics
Data are expressed as a mean ± SEM. One-way or two-way analysis of variance (ANOVA) was used for statistical analysis of multiple treatment groups. A two-way repeated measures ANOVA was used to compare blood pressure changes assessed by radiotelemetry. A value of p<0.05 was considered statistically significant.
RESULTS
We investigated the degree to which developmental TCDD exposure increased the susceptibility to cardiac hypertrophy and hypertension following a exposure to a common cardiovascular risk factor in adulthood. In this study following perinatal TCDD exposure, the adult offspring were exposed to either a subpressor or pressor dose of Ang II, and then analyzed by echocardiography to assess cardiac morphology or by radiotelemetry to evaluate blood pressure regulation, respectively.
Adult cardiac morphology assessed by echocardiography
Adult offspring from control- or TCDD-exposed litters were challenged at 3.5 mo of age with a dose of Ang II that is not associated with increases in blood pressure, but rather with cardiac hypertrophy and fibrosis (18) and echocardiography was used to assess cardiac morphology after 14 days of exposure. The internal diameter of the left ventricular cavity in diastole was increased in TCDD offspring, but treatment with Ang II did not result in a further increase in the size of the cavity in either control or TCDD animals (Fig. 1A). In addition, there was a trend for an increase in the internal diameter of the left ventricular cavity in systole in TCDD offspring (one-way ANOVA, p<0.06), but it did not reach statistical significance (Fig. 1B). Further, Ang II treatment had no effect on this measurement in either control or TCDD animals.
Figure 1.
Effects of a subpressor dose of Ang II on left ventricular morphology of offspring from control- and TCDD-exposed litters as assessed by echocardiography. Pregnant C57BL/6N mice were exposed to corn oil (control) or 6.0 µg/kg TCDD on GD 14.5 by oral gavage and their offspring raised to 3.5 months of age. Offspring were then implanted with an osmotic minipump containing either 0.9% NaCl or 0.1 mg/kg/day Ang II for 14 d. M-mode echocardiography was used to measure left ventricular internal dimension in diastole (A) and systole (B), as well as left ventricular posterior wall thickness in diastole (C) and systole (D). One-way ANOVA, p<0.05 for TCDD treatment in panels A, C, and D. *p<0.05, post hoc comparisons between control and TCDD; #p<0.05, post hoc comparisons between saline and Ang II treatment. Control - saline (n=8); TCDD - saline (n=8); control - Ang II (n=7); TCDD + Ang II (n=7).
In addition to measuring the left ventricular cavity size, we also assessed left ventricular posterior wall thickness by echocardiography, which is considered one of the most reliable assessments of cardiac hypertrophy. An increased posterior wall thickness was observed during diastole in TCDD offspring (Fig. 1C). While treatment with Ang II further increased the left ventricular wall thickness in TCDD adult offspring, it also significantly increased the left ventricular wall thickness of control offspring. This was reflected in the two-way ANOVA by a significant effect resulting from the TCDD exposure (p<0.004) and from the Ang II treatment (p<0.008), but no significant interaction between the two variables. Similarly, the thickness of the left ventricular wall in systole was increased in offspring from TCDD-exposed litters and Ang II stimulated an increase in wall thickness in systole in both the control and TCDD offspring (Fig. 1D). These results suggest that developmental TCDD exposure by itself induces cardiac hypertrophy in adult offspring, as we have reported previously (13, 14), but that it does not increase the sensitivity to cardiac hypertrophy induced by Ang II.
Adult blood pressure assessed by radiotelemetry
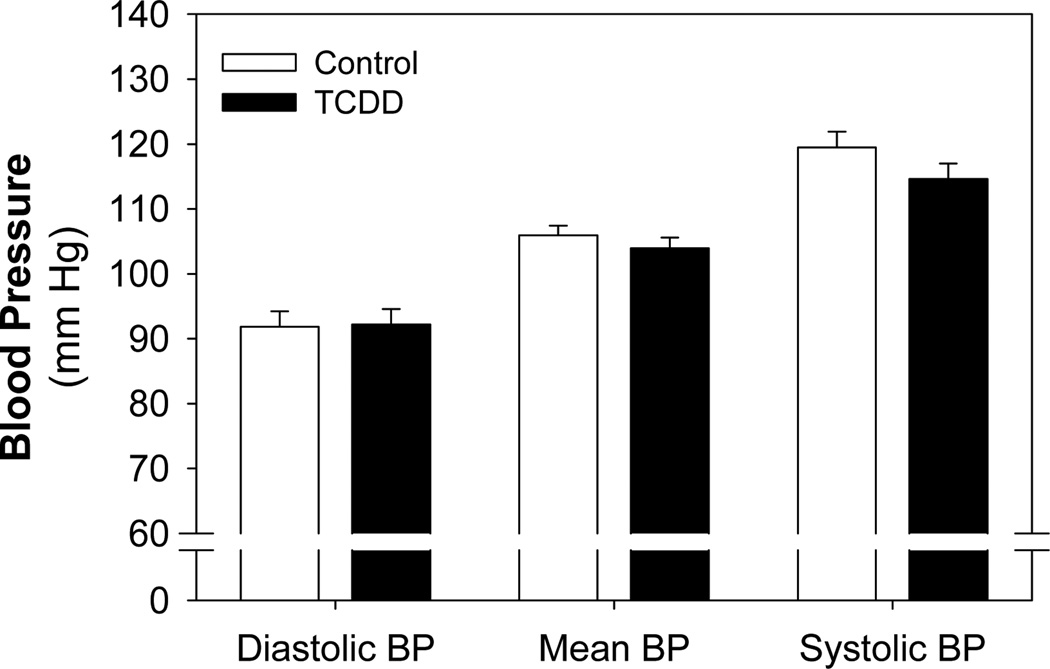
Blood pressure was assessed in adult offspring from control- or TCDD-exposed litters by radiotelemetry at 3.5 mo of age. We found that neither mean, diastolic, nor systolic blood pressure at baseline were affected by developmental TCDD exposure (Fig. 2). After collecting this baseline information, these adult offspring were challenged with a dose of Ang II that has been shown to increase blood pressure in mice (19) and blood pressure was continuously monitored during the 11 days of exposure.
Figure 2.
Effect of perinatal TCDD exposure on blood pressure in adulthood. Pregnant C57BL/6N mice were exposed to corn oil (control, n=8) or 6.0 µg/kg TCDD (n=9) on GD 14.5 by oral gavage and their offspring raised to 3.5 months of age. Offspring were then implanted with radiotelemeters, allowed to recover from surgery, and then mean, diastolic, and systolic blood pressure recorded for 7 d.
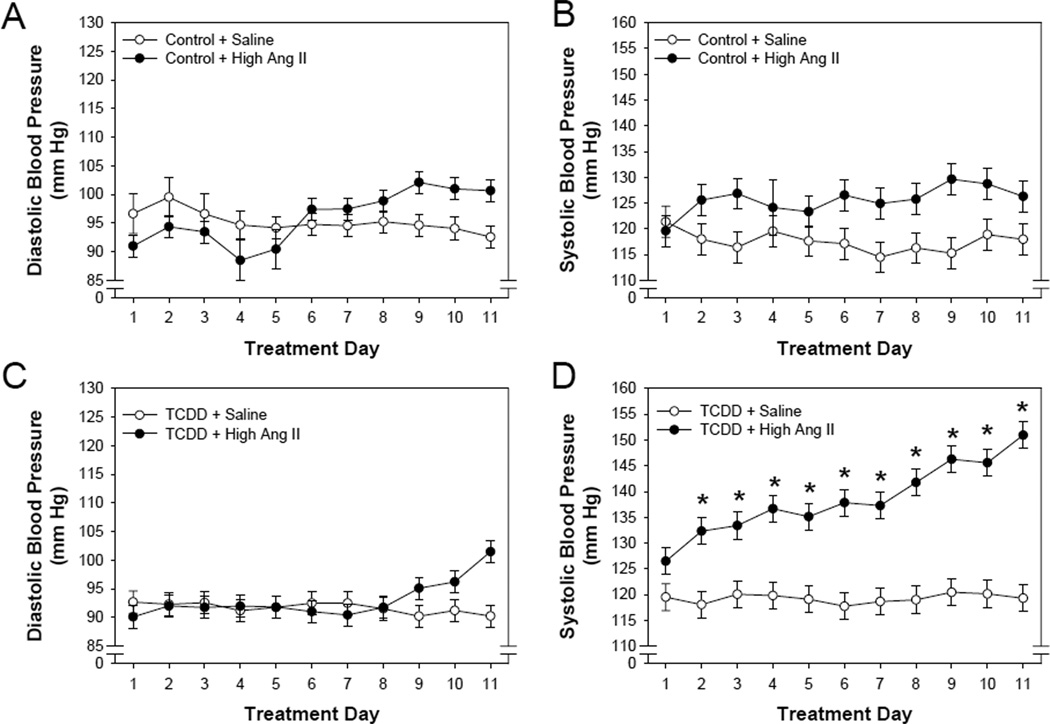
The time-dependent changes in diastolic and systolic blood pressure were compared within the control and TCDD treatment groups and between saline and Ang II exposure groups. Ang II did not significantly alter diastolic blood pressure in offspring from either the control or TCDD perinatal exposure groups (Fig. 3 A, C). However, Ang II significantly increased systolic blood pressure in both groups of offspring, although the time-dependent changes differed (Fig. 3 B, D). The increase in systolic blood pressure in the control offspring did not exhibit a time-dependent increase, rather systolic blood pressure increased with the first days of exposure and remained at approximately the same level for the entire 11 days of exposure. In contrast, the increase in systolic blood pressure in the TCDD offspring exhibited a significant time-dependent effect where systolic blood pressure increased over the first few days and continued to increase throughout the entire 11 days of exposure. This resulted in both a significant effect of Ang II and Ang II-day interaction.
Figure 3.
Effects of a pressor dose of Ang II and day of treatment on blood pressure of offspring from control- and TCDD-exposed litters as assessed by radiotelemetry. Pregnant C57BL/6N mice were exposed to corn oil (control, n=8) or 6.0 µg/kg TCDD (n=9) on GD 14.5 by oral gavage and their offspring raised to 3.5 months of age. Offspring were then implanted with radiotelemeters at 3.5 months with an osmotic minipump containing either 0.9% NaCl or 0.7 mg/kg/day Ang II (n=4/treatment group) at 4 months of age. Radiotelemetry was used to continuously record diastolic (A, C) and systolic (B,D) blood pressure during an 11 d exposure. Two-way repeated measure ANOVA, Ang II effect on systolic blood pressure of offspring from control (panel B, p<0.018) and TCDD litters (panel D, p<0.003); treatment day effect on systolic blood pressure of offspring from TCDD litters (panel D, p<0.001); and an Ang II-day interaction on systolic blood pressure of offspring from TCDD litters (panel D, p<0.002). *p<0.05, post hoc comparisons between saline and Ang II treatment on individual days.
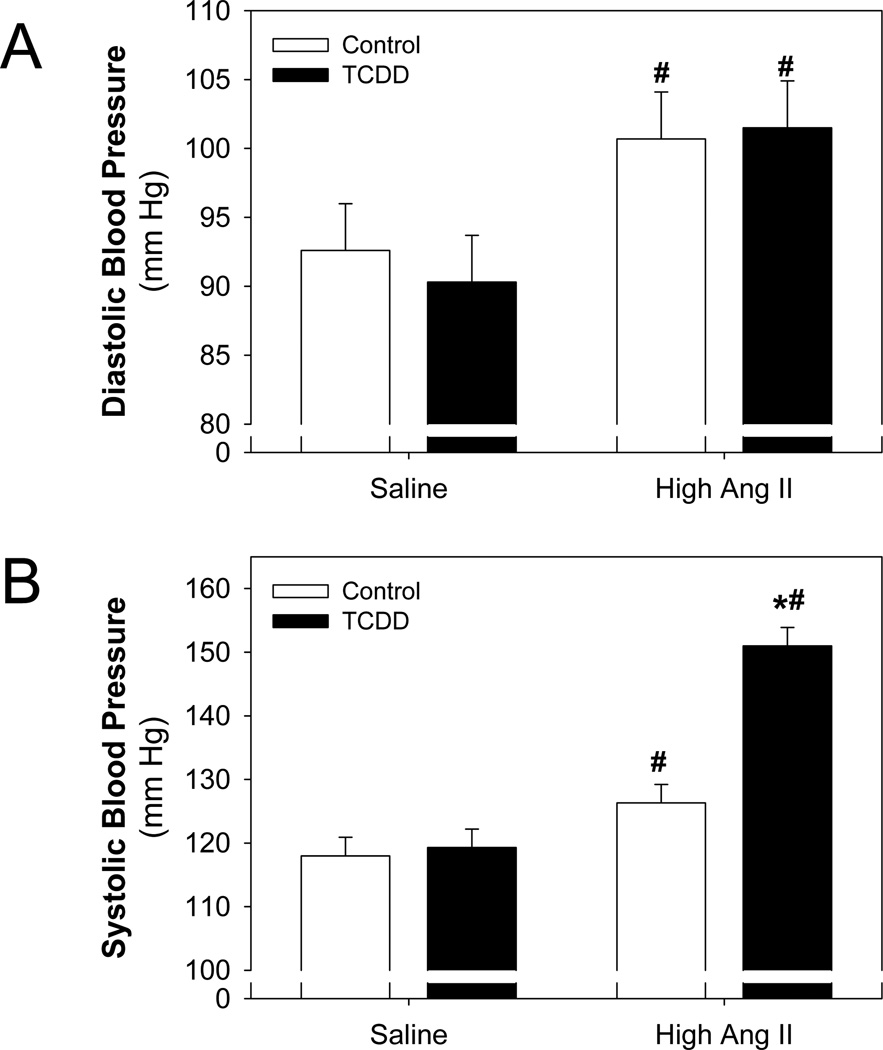
The diastolic and systolic blood pressures were then compared at the end of the 11 day exposure between the perinatal treatment groups and minipump exposure groups. This analysis showed that Ang II significantly increased diastolic blood pressure in offspring from both the control and TCDD groups (Fig. 4A). However, while Ang II significantly increased systolic blood pressure in both groups of offspring, the degree of increase differed. In this case, Ang II increased systolic blood pressure in the TCDD offspring to a significantly greater degree, compared to the control offspring (Fig. 4B). These data demonstrate that following perinatal TCDD exposure offspring are sensitized to the pressor response of Ang II.
Figure 4.
Effect of perinatal TCDD exposure on Ang II-induced increases in blood pressure. Radiotelemetry was used to record diastolic (A) and systolic (B) blood pressure at the end of the 11 d exposure to Ang II. Two-way ANOVA, significant Ang II effect on diastolic blood pressure (panel A, p<0.02) and systolic blood pressure (panel B, p<0.001); significant TCDD effect on systolic blood pressure (panel B, p<0.002); and a significant Ang II-TCDD interaction on systolic blood pressure (panel B, p<0.004). *p<0.05, post hoc comparisons between control and TCDD; #p<0.05, post hoc comparisons between saline and Ang II treatment.
Adult renal myofibroblasts and collagen deposition
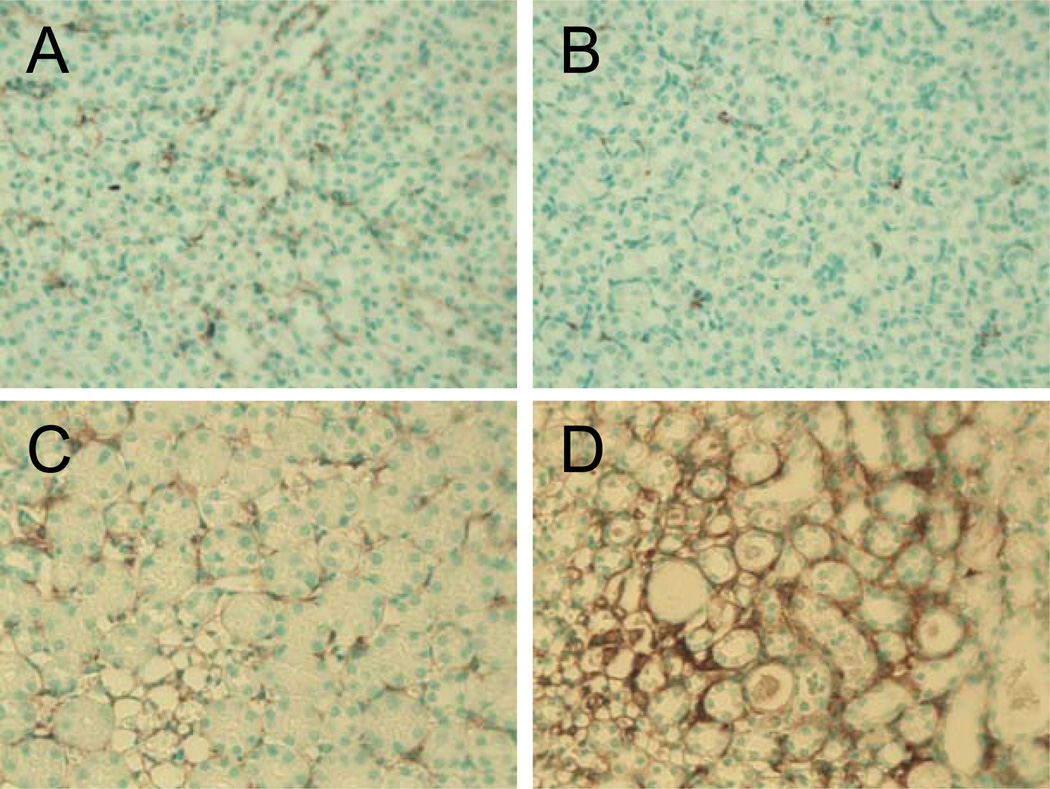
To assess whether the pressor dose of Ang II had a differential effect on renal fibrosis between control and TCDD offspring, kidney sections from male offspring exposed to saline or pressor Ang II were stained for the presence of myofibroblasts, using an anti-smooth muscle alpha actin antibody. There was no evidence for myofibroblast differentiation in the kidneys from either perinatal exposure group when exposed to saline in adulthood (Fig. 5 A, B). While Ang II stimulated a small degree of myofibroblast differentiation in the renal medulla of control offspring, a much greater degree was detected in the renal medulla of TCDD offspring (Fig. 5 C, D).
Figure 5.
Effect of perinatal TCDD exposure on Ang II-induced increase in renal myofibroblasts. Adult offspring from control and TCDD-exposed litters were exposed for 11 d to 0.9% NaCl or a pressor dose of Ang II. Renal myofibroblasts were identified by staining with anti-alpha smooth muscle actin from control (A, C) and TCDD offspring (B, D), which had been treated with saline (A, B) or a pressor dose of Ang II (C,D).
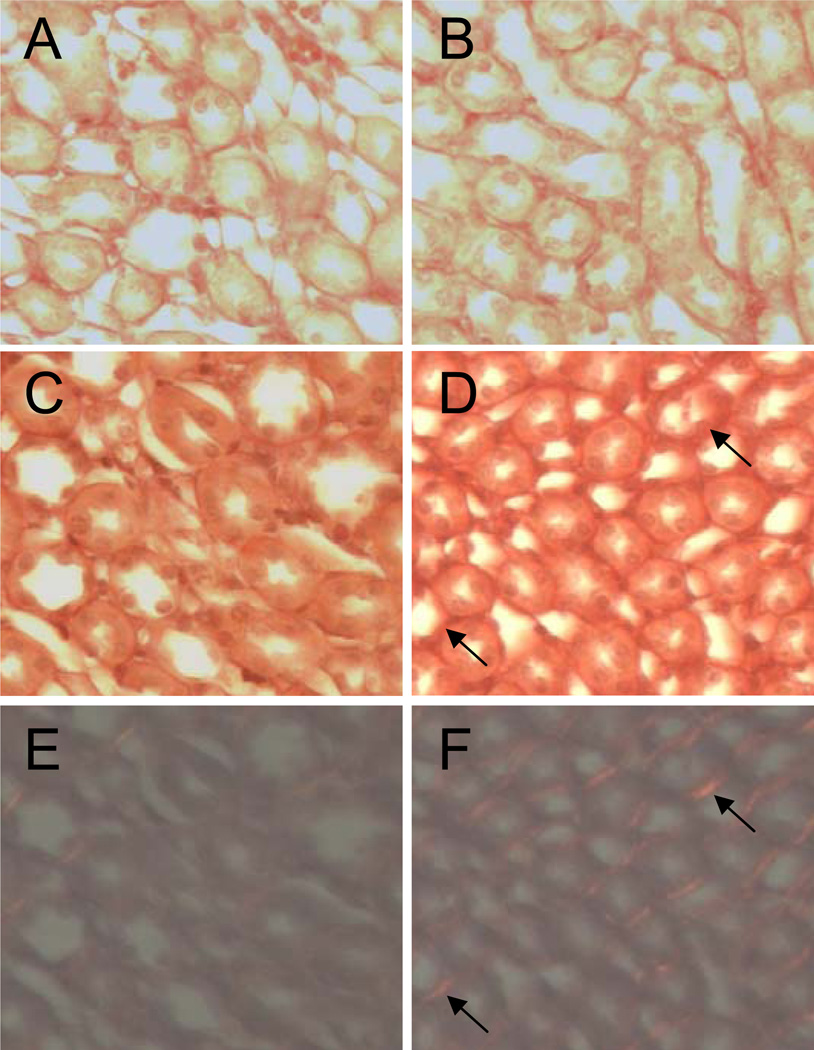
To determine whether this increase in renal myofibroblasts was associated with an increase in renal fibrosis, kidney sections from male offspring exposed to saline or pressor Ang II were stained with picrosirus red to detect collagen deposition. There was an equal degree of collagen deposition evident in the renal medulla from both perinatal exposure groups when exposed to saline in adulthood (Fig. 6 A, B). While Ang II stimulated collagen deposition in the renal medulla of control offspring, a much greater degree was detected in the renal medulla of TCDD offspring (Fig. 6 C, D). This was further evident when the sections were exposed to polarizing light, revealing more bifringence in the renal medulla of TCDD offspring (Fig. 6 E, F).
Figure 6.
Effect of perinatal TCDD exposure and Ang II on renal collagen deposition. Adult offspring from control and TCDD-exposed litters were exposed for 11 d to 0.9% NaCl or a pressor dose of Ang II. Collagen deposition as detected by picrosirius red staining from control (A, C, E) and TCDD offspring (B, D, F), which had been treated with saline (A, B) or a pressor dose of Ang II (C–F). Visualization of collagen deposition using polarizing light from control (E) and TCDD (F) offspring treated with a pressor dose of Ang II.
Adult renal mRNA expression of RAS components, gp91phox, and procollagen I
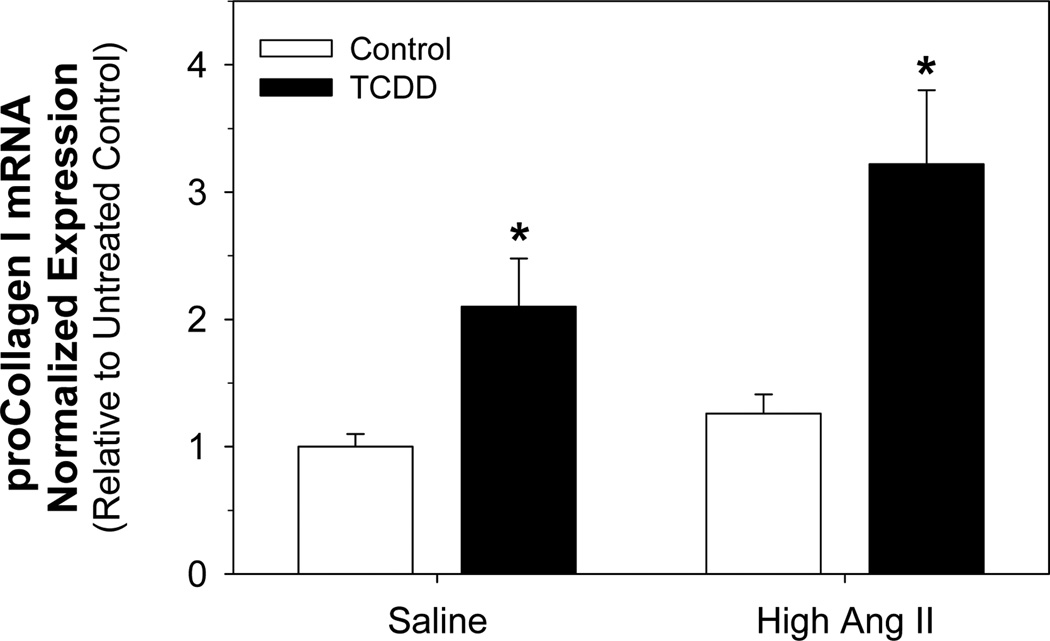
We then determined whether the increase in renal fibrosis and increased sensitivity to Ang II-induced hypertension in TCDD offspring was associated with alterations in the renal mRNA expression of RAS components or downstream targets. Thus, total RNA was isolated from kidneys of male offspring exposed to saline or pressor Ang II and analyzed for expression of angiotensinogen (upstream precursor to Ang II), renin (rate limiting enzyme in formation of Ang II), Ang II receptors (AT1a, AT1b, AT2), gp91phox (membrane-bound catalytic subunit of NADPH oxidase and Ang II downstream target), and procollagen I. We did not find any alterations in mRNA expression of any RAS components or the Ang II target, gp91phox (data not shown). However, renal procollagen I mRNA expression was significantly increased in kidneys from TCDD offspring in both the saline and Ang II treatment groups (Fig. 7). While the pressor dose of Ang II did not increase renal procollagen I mRNA in the control offspring, it did show a trend for increasing expression further in the TCDD offspring.
Figure 7.
Effect of perinatal TCDD exposure and Ang II on renal procollagen I mRNA expression. Adult offspring from control and TCDD-exposed litters were exposed for 11 d to 0.9% NaCl or a pressor dose of Ang II. proCollagen I mRNA expression was analyzed by real time PCR and was normalized to the reference gene, polymerase (RNA) II (DNA directed) polypeptide A (Polr2a). Two-way ANOVA, significant effect of TCDD exposure (p<0.001) and a trend for an Ang II effect (p<0.065). *p<0.05, post hoc comparisons between control and TCDD groups.
DISCUSSION
We have shown previously that in utero and lactational exposure of mice to TCDD induces changes in fetal cardiac gene expression that persistent into adulthood and which are associated with cardiac hypertrophy and mild hydronephrosis in the adult offspring (12–14). As in the previous study, the pregnant dam was dosed with TCDD resulting in a very small percentage of the total TCDD dose being delivered to the fetus in utero (20). More significant TCDD exposure occurred during lactation when the dam transferred a large percentage of her body burden to the pups (21). Previously, we showed that the residual TCDD remaining in the adult offspring was extremely low, such that studies of adult offspring assess how early life exposure to TCDD alters their susceptibility to other diseases (13). The results of the current study demonstrate that this same perinatal TCDD exposure predisposes offspring to renal fibrosis and hypertension in adulthood when challenged with a common cardiovascular risk factor, Ang II.
The most significant finding of this research is that perinatal TCDD exposure sensitizes offspring to Ang II-induced hypertension. Infusion of Ang II increases systolic blood pressure more rapidly and to a greater degree in TCDD offspring, compared to control offspring. Human epidemiology and laboratory animal studies have suggested that developmental reprogramming, leading to activation of the RAS, has been implicated as one mediator of hypertension later in life. However, the specific mechanisms that contribute to this reprogramming remain unclear. One study in rats fed a low protein diet during gestation shows that multiple components of the RAS are altered in expression, including increases in angiotensinogen mRNA in the liver, renin mRNA in the kidney, and Ang II receptor, AT1, mRNA and protein in the adrenal gland (10). Interestingly, increases in AT1 expression are associated with epigenetic changes, specifically reduced methylation of the proximal promoter (10). In contrast to these results, we did not find any changes in mRNA expression of any of the RAS components in the kidney. It is possible that the increased sensitivity to Ang II-induced hypertension results from changes in expression of RAS components in other tissues; however, the kidney is a logical target for analysis since it is a primary target for Ang II-mediated responses that plays a fundamental role in Ang II production, blood pressure regulation by the RAS, and is a known target of TCDD during perinatal development.
An alternative explanation for the increased sensitivity of TCDD offspring to Ang II-induced hypertension is the presence of hydronephrosis. The ability of TCDD to induce hydronephrosis following perinatal exposure is well established (22–27). Our previous work shows that litter incidence of hydronephrosis in perinatal TCDD-exposed offspring is 100%, although the relative severity is mild and does not alter renal filtration as assessed by blood urea nitrogen and creatinine levels (13). Despite the lack of overt alterations in renal function, other studies have shown that hydronephrosis is an underlying risk factor for development of hypertension (28, 29). Blood pressure of rats is highly correlated with their degree of hydronephrosis induced by unilateral partial ureteral obstruction (29). While severe hydronephrosis significantly increases blood pressure, mild hydronephrosis results in a much smaller increase. In addition, the hypertension associated with hydronephrosis is salt sensitive, compared to control rats, and correlates with the severity of renal injury. Similar results have been reported in mice (28). These results are very consistent with our observations that Ang II infusion increases the degree of hypertension in mildy hydronephrotic animals. Ang II not only induces vasoconstriction but also stimulates sodium retention and blood volume expansion that resemble, in part, the renal responses induced by a high salt diet.
In addition to TCDD offspring being more sensitive to Ang II-induced hypertension, we found that TCDD offspring were more sensitive to Ang II-induced renal fibrosis. Infusion of Ang II stimulates myofibroblast differentiation and collagen deposition in the kidney of TCDD offspring, compared to controls. In addition, perinatal TCDD exposure increases renal collagen I mRNA expression and Ang II tends to increase this expression further. These changes are not associated with changes in renal Ang II receptor mRNA expression nor in another Ang II target gene in the kidney, gp91phox. These data suggest that there is not a general increase in Ang II signaling in the kidney, but rather previous TCDD exposure enhances the sensitivity of renal tissue to extracellular matrix (ECM) remodeling and collagen deposition. Numerous studies show that TCDD exposure is associated with significant changes in genes that regulate ECM remodeling. TCDD significantly alters the intensity and spatial expression of ECM proteins in the developing kidney (23) as well as various collagen isoforms during zebrafish fin regeneration, a process that heavily relies on precise ECM regulation (30). TCDD also increases collagen, fibronectin, and laminin expression in the marmoset heart (31). Thus, the increased sensitivity to Ang II-induced myofibroblast differentiation and collagen deposition may result from the combination of both TCDD and Ang II stimulating pathways that mediate renal fibrosis. The degree to which the renal fibrosis contributes to Ang II-induced hypertension remains to be determined; however, renal ECM remodeling can play a critical role in Ang II-induced hypertension (32).
The possibility that TCDD-induced effects on kidney during perinatal development may contribute to the increased sensitivity to Ang II-induced hypertension is supported by other studies showing that TCDD-induced hydronephrosis is associated with changes in renal gene expression that could have a major impact on renal function. In a recent study, hydronephrosis associated with lactational TCDD exposure significantly reduces mRNA expression of two key sodium and potassium transporters in the kidney as well as induces renal cyclooxygenase-2 (COX-2) and prostaglandin E2 (33). It is not apparent how these changes might contribute to increases in Ang II-induced hypertension or renal fibrosis; however, COX-2 and COX-2 metabolites are associated with regulating renal function and may mediate both acute and chronic renal injury. Thus, these TCDD-induced changes in the neonate could increase the susceptibility of the kidney to additional injury and dysfunction later in life.
A final important finding of our research is that perinatal TCDD exposure by itself is a risk factor for cardiac hypertrophy and diastolic dilation. Perinatal TCDD exposure increases left ventricular cavity dilation during diastole, and wall thickness during diastole and systole. We and others have previously reported that perinatal TCDD exposure increases heart weight in postnatal and adult mice (12, 13, 34). The specific mechanisms that mediate this effect remain unclear. In utero TCDD exposure reduces cardiomyocyte proliferation, which may contribute to the decrease observed in fetal heart weight (14). The reduction in heart size at birth may subsequently induce a compensatory cardiomyocyte hypertrophy in order to maintain sufficient cardiac output. This notion is supported by the evidence that the cardiac enlargement that occurs postnatally is associated with an increase in expression of the hypertrophy marker gene, atrial natruiretic factor (14). New evidence from the current study suggests that the cardiac hypertrophy also is associated with left ventricle cavity dilation during diastole, but that these changes occur in the absence of systemic hypertension. This physiological and/or pathological mechanisms underlying the cardiac hypertrophy and cavity dilation remain to be determined.
In summary, these results demonstrate that perinatal TCDD exposure induces cardiac hypertrophy and dilation, and increases the risk of hypertension when exposed to a common cardiovascular risk factor in adulthood. Human cardiovascular disease, including hypertension, is multifactorial and rarely is one single risk factor responsible for disease etiology. Studies suggest that there is a fetal basis for adult cardiovascular disease risk, including exposure to environmental agents (35). Results from our laboratory animal study suggest that exposure to a prototypical halogenated aromatic hydrocarbon may increase the susceptibility to cardiovascular disease, specifically, hypertension later in life.
ACKNOWLEDGEMENTS
We thank Phillip G. Kopf for his technical support and expertise. This work was supported by grant funding from NIH (ES012335 and HL078914) to M.K.W. and (ES012335-02S1) to A.C.A.
REFERENCES
- 1.Eriksson JG, Forsen T, Tuomilehto J, Osmond C, Barker DJ. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ. Health Perspect. 2000;108:545–553. doi: 10.1289/ehp.00108s3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thornburg KL. In: Fetal Origins of Cardiovascular and Lung Disease. Barker DJ, editor. New York: Marcel Dekker; 2000. pp. 97–139. [Google Scholar]
- 4.Jarvelin MR, Sovio U, King V, Lauren L, Xu B, McCarthy MI, Hartikainen AL, Laitinen J, Zitting P, Rantakallio P, et al. Early life factors and blood pressure at age 31 years in the 1966 northern Finland birth cohort. Hypertension. 2004;44:838–846. doi: 10.1161/01.HYP.0000148304.33869.ee. [DOI] [PubMed] [Google Scholar]
- 5.Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am. J. Physiol Cell Physiol. 2007;292:C82–C97. doi: 10.1152/ajpcell.00287.2006. [DOI] [PubMed] [Google Scholar]
- 6.Volpe M, Savoia C, De PP, Ostrowska B, Tarasi D, Rubattu S. The renin-angiotensin system as a risk factor and therapeutic target for cardiovascular and renal disease. J. Am. Soc. Nephrol. 2002;13(Suppl 3):S173–S178. doi: 10.1097/01.asn.0000032549.36050.78. [DOI] [PubMed] [Google Scholar]
- 7.Bohm M. Angiotensin receptor blockers versus angiotensin-converting enzyme inhibitors: where do we stand now? Am. J. Cardiol. 2007;100:38J–44J. doi: 10.1016/j.amjcard.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem. Physiol A Physiol. 1995;110:223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 9.McMullen S, Gardner DS, Langley-Evans SC. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br. J. Nutr. 2004;91:133–140. doi: 10.1079/bjn20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bogdarina I, Welham S, King PJ, Burns SP, Clark AJ. Epigenetic modification of the renin-angiotensin system in the fetal programming of hypertension. Circ. Res. 2007;100:520–526. doi: 10.1161/01.RES.0000258855.60637.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, Nuyt AM. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr. Res. 2004;55:1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 12.Thackaberry EA, Jiang Z, Johnson CD, Ramos KS, Walker MK. Toxicogenomic profile of 2,3,7,8-tetrachlorodibenzo-p-dioxin in the murine fetal heart: modulation of cell cycle and extracellular matrix genes. Toxicol. Sci. 2005;88:231–241. doi: 10.1093/toxsci/kfi301. [DOI] [PubMed] [Google Scholar]
- 13.Aragon AC, Kopf PG, Campen MJ, Huwe JK, Walker MK. In utero and lactational 2,3,7,8-tetrachlorodibenzo-p-dioxin exposure: effects on fetal and adult cardiac gene expression and adult cardiac and renal morphology. Toxicol. Sci. 2008;101:321–330. doi: 10.1093/toxsci/kfm272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thackaberry EA, Nunez B, Ivnitski-Steele ID, Friggens MM, Walker MK. Effects of 2,3,7,8-tetrachlorodibenzo-p-dixoin (TCDD) on the fetal murine heart II: TCDD alters fetal and postnatal cardiac growth, and postnatal cardiac chronotrophy. Toxicol. Sci. 2005;88:242–249. doi: 10.1093/toxsci/kfi302. [DOI] [PubMed] [Google Scholar]
- 15.Ayan S, Roth JA, Freeman MR, Bride SH, Peters CA. Partial ureteral obstruction dysregulates the renal renin-angiotensin system in the fetal sheep kidney. Urology. 2001;58:301–306. doi: 10.1016/s0090-4295(01)01156-6. [DOI] [PubMed] [Google Scholar]
- 16.Gobet R, Park JM, Nguyen HT, Chang B, Cisek LJ, Peters CA. Renal renin-angiotensin system dysregulation caused by partial bladder outlet obstruction in fetal sheep. Kidney Int. 1999;56:1654–1661. doi: 10.1046/j.1523-1755.1999.00732.x. [DOI] [PubMed] [Google Scholar]
- 17.Simon P. Q-Gene: processing quantitative real-time RT-PCR data. Bioinformatics. 2003;19:1439–1440. doi: 10.1093/bioinformatics/btg157. [DOI] [PubMed] [Google Scholar]
- 18.Li Y, Kishimoto I, Saito Y, Harada M, Kuwahara K, Izumi T, Takahashi N, Kawakami R, Tanimoto K, Nakagawa Y, et al. Guanylyl cyclase-A inhibits angiotensin II type 1A receptor-mediated cardiac remodeling, an endogenous protective mechanism in the heart. Circulation. 2002;106:1722–1728. doi: 10.1161/01.cir.0000029923.57048.61. [DOI] [PubMed] [Google Scholar]
- 19.Vecchione C, Fratta L, Rizzoni D, Notte A, Poulet R, Porteri E, Frati G, Guelfi D, Trimarco V, Mulvany MJ, et al. Cardiovascular influences of alpha1b-adrenergic receptor defect in mice. Circulation. 2002;105:1700–1707. doi: 10.1161/01.cir.0000012750.08480.55. [DOI] [PubMed] [Google Scholar]
- 20.Weber H, Birnbaum LS. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) and 2,3,7,8-tetrachlorodibenzofuran (TCDF) in pregnant C57BL/6N mice: distribution to the embryo and excretion. Arch. Toxicol. 1985;57:159–162. doi: 10.1007/BF00290880. [DOI] [PubMed] [Google Scholar]
- 21.Nau H, Bass R, Neubert D. Transfer of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) via placenta and milk, and postnatal toxicity in the mouse. Arch. Toxicol. 1986;59:36–40. doi: 10.1007/BF00263955. [DOI] [PubMed] [Google Scholar]
- 22.Abbott BD, Birnbaum LS. Effects of TCDD on embryonic ureteric epithelial EGF receptor expression and cell proliferation. Teratology. 1990;41:71–84. doi: 10.1002/tera.1420410108. [DOI] [PubMed] [Google Scholar]
- 23.Abbott BD, Morgan KS, Birnbaum LS, Pratt RM. TCDD alters the extracellular matrix and basal lamina of the fetal mouse kidney. Teratology. 1987;35:335–344. doi: 10.1002/tera.1420350308. [DOI] [PubMed] [Google Scholar]
- 24.Abbott BD, Birnbaum LS, Pratt RM. TCDD-induced hyperplasia of the ureteral epithelium produces hydronephrosis in murine fetuses. Teratology. 1987;35:329–334. doi: 10.1002/tera.1420350307. [DOI] [PubMed] [Google Scholar]
- 25.Couture-Haws L, Harris MW, Lockhart AC, Birnbaum LS. Evaluation of the persistence of hydronephrosis induced in mice following in utero and/or lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 1991;107:402–412. doi: 10.1016/0041-008x(91)90304-w. [DOI] [PubMed] [Google Scholar]
- 26.Couture-Haws L, Harris MW, McDonald MM, Lockhart AC, Birnbaum LS. Hydronephrosis in mice exposed to TCDD-contaminated breast milk: identification of the peak period of sensitivity and assessment of potential recovery. Toxicol. Appl. Pharmacol. 1991;107:413–428. doi: 10.1016/0041-008x(91)90305-x. [DOI] [PubMed] [Google Scholar]
- 27.Couture LA, Harris MW, Birnbaum LS. Characterization of the peak period of sensitivity for the induction of hydronephrosis in C57BL/6N mice following exposure to 2,3,7, 8-tetrachlorodibenzo-p-dioxin. Fund. Appl. Toxicol. 1990;15:142–150. doi: 10.1016/0272-0590(90)90171-f. [DOI] [PubMed] [Google Scholar]
- 28.Carlstrom M, Sallstrom J, Skott O, Larsson E, Wahlin N, Persson AE. Hydronephrosis causes salt-sensitive hypertension and impaired renal concentrating ability in mice. Acta Physiol (Oxf) 2007;189:293–301. doi: 10.1111/j.1748-1716.2006.01637.x. [DOI] [PubMed] [Google Scholar]
- 29.Carlstrom M, Wahlin N, Sallstrom J, Skott O, Brown R, Persson AE. Hydronephrosis causes salt-sensitive hypertension in rats. J. Hypertens. 2006;24:1437–1443. doi: 10.1097/01.hjh.0000234126.78766.00. [DOI] [PubMed] [Google Scholar]
- 30.Andreasen EA, Mathew LK, Lohr CV, Hasson R, Tanguay RL. Aryl hydrocarbon receptor activation impairs extracellular matrix remodeling during zebra fish fin regeneration. Toxicol. Sci. 2007;95:215–226. doi: 10.1093/toxsci/kfl119. [DOI] [PubMed] [Google Scholar]
- 31.Nottebrock C, Riecke K, Kruse M, Shakibaei M, Stahlmann R. Effects of 2,3,7,8-tetrachloro-dibenzo-p-dioxin on the extracellular matrix of the thymus in juvenile marmosets (Callithrix jacchus) Toxicology. 2006;226:197–207. doi: 10.1016/j.tox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Zhao W, Chen SS, Chen Y, Ahokas RA, Sun Y. Kidney fibrosis in hypertensive rats: role of oxidative stress. Am. J. Nephrol. 2008;28:548–554. doi: 10.1159/000115289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nishimura N, Matsumura F, Vogel CF, Nishimura H, Yonemoto J, Yoshioka W, Tohyama C. Critical role of cyclooxygenase-2 activation in pathogenesis of hydronephrosis caused by lactational exposure of mice to dioxin. Toxicol. Appl. Pharmacol. 2008 doi: 10.1016/j.taap.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 34.Lin TM, Ko K, Moore RW, Buchanan DL, Cooke PS, Peterson RE. Role of the aryl hydrocarbon receptor in the development of control and 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed male mice. J. Toxicol. Environ. Health. 2001;64:327–342. doi: 10.1080/152873901316981312. [DOI] [PubMed] [Google Scholar]
- 35.Geerts CC, Grobbee DE, van der Ent CK, de Jong BM, van der Zalm MM, van Putte-Katier N, Kimpen JL, Uiterwaal CS. Tobacco smoke exposure of pregnant mothers and blood pressure in their newborns: results from the wheezing illnesses study Leidsche Rijn birth cohort. Hypertension. 2007;50:572–578. doi: 10.1161/HYPERTENSIONAHA.107.091462. [DOI] [PubMed] [Google Scholar]