Abstract
Stroke is the leading cause of long-term disability. Understanding how people recover from stroke and other brain lesions remain one of the biggest conundrums in neuroscience. As a result, concerted efforts in recent years have focused on investigating the neurophysiological changes that occur in the brain after stroke, and in developing novel strategies to enhance motor recovery. In particular, transcranial magnetic stimulation (TMS) is a non-invasive tool that has been used to investigate the brain plasticity changes resulting from stroke and as a therapeutic modality to safely improve motor function. In this review, we discuss the contributions of TMS to understand how different motor areas, such as the ipsilesional hemisphere, secondary motor areas, and contralesional hemisphere are involved in motor recovery. We also consider recent studies using repetitive TMS (rTMS) in stroke patients to enhance upper extremity function. Although further studies are needed, these investigations provide an important starting point to understand the stimulation parameters and patient characteristics that may influence the optimal response to non-invasive brain stimulation. Future directions of rTMS are discussed in the context of post-stroke motor recovery.
Keywords: Transcranial magnetic stimulation, stroke, plasticity
1. Introduction
Stroke is the third leading cause of death in the United States and is the leading cause of long-term disability. Current estimates suggest that 795,000 new or recurrent cases of stroke occur each year (Lloyd-Jones et al., 2009) and stroke related disability is predicted to increase in the future as our elderly population expands (Kavanagh et al., 1999). Major progress over the past decades has been made in acute stroke management to reduce the number of deaths, leading to more people requiring rehabilitation services (Barker and Mullooly, 1997; Lloyd-Jones et al., 2009). Previously it was believed that the brain undergoes significant changes during development, but that after adulthood the CNS retains a relatively static organization. By this account, central nervous system damage would be irreversible, given that there would be no mechanism to replace or restore the lost neurons. Clinical observation has shown that after stroke most people with motor deficits recover a varying degree of motor function. Unfortunately, our limited understanding of this recovery process, combined with the time and financial constraints imposed in the rehabilitation setting, has shifted the focus of therapies in neurorehabiltation towards compensatory techniques to attain functional goals. This focus on compensatory approaches has limited the attention paid to reducing neurologic impairment, which in turn may promote learned non-use of affected limbs, with consequent persistence of functional limitations. Indeed, recent estimates suggest that nearly 80% of stroke patients continue to have impairments despite the use of traditional therapy approaches (Mayo et al., 1999). Fortunately, in recent years novel interventions in neurorehabilitation have been evolving. One of these is the application of transcranial magnetic stimulation (TMS), a non-invasive technique to investigate brain functionality in healthy subjects and in patients after stroke. Based on Faradays’ law of electromagnetic induction, TMS can be used to create magnetic field pulses, which in turn can induce electrical activity in focal brain areas. TMS of the primary motor cortex (M1) activates corticospinal neurons transsynaptically, eliciting volleys of neuronal output in the form of motor evoked potentials (MEP). It can also be used to study mixed populations of inhibitory and excitatory interneurons of various motor and nonmotor cortical regions within and across cerebral hemispheres (Reis et al., 2008). These physiological measurements have enabled researchers to characterize reorganizational changes in motor networks after stroke. As a therapeutic tool, TMS has also been used to safely enhance motor performance in stroke patients as well as study the way that adjuvant rehabilitation interventions may facilitate adaptive brain plasticity. In collaboration with behavioral and neuro-imaging studies, these applications of TMS have helped develop models of functional connectivity between different brain regions and test theories of neural mechanisms that underlie stroke recovery. Given the promising perspective of TMS in the field of neurorehabilitation, understanding its application in stroke patients may also aid in the development of future interventions to improve motor function.
2. Post-stroke reorganization
An integrated approach using the combination of animal, neuroimaging and physiological studies using TMS has enhanced our understanding of the different mechanisms that may contribute to functional recovery after stroke. Early recovery after the first few days post-stroke is likely due to resolution of edema and necrotic tissue as well as reperfusion of the ischemic penumbra through collateral circulation (Furlan et al., 1996). After this early period much of the recovery is likely due to neuronal mechanisms such as the recruitment of functionally homologous pathways, disinhibition of redundant neuronal connections, resolution of diaschisis and the formation of new neural networks to take over function of the damaged areas (Rossini et al., 2007). Patterns of plasticity in the motor system appear to correlate with the degree of damage to the corticospinal tracts and are presumed to optimize control of the remaining motor output (Hamzei et al., 2006; Stinear et al., 2007).
2.1. Local reorganization
One strategy for recovery may be for the surrounding surviving cortex to regain control over the function of the damaged motor area through somatotopic reorganization, a phenomenon called vicariation (Donoghue et al., 1990; Merzenich et al., 1984; Sanes et al., 1990). Studies in animals suggest that preservation of the periinfarct penumbra may provide a substrate for recovery after cortical lesions by unmasking redundant motor representations adjacent to the site of injury (Nudo and Milliken, 1996; Nudo et al., 1996). This form of periregional reorganization has also been demonstrated in healthy human subjects (Brasil-Neto et al., 1993) and in chronic stroke patients (Cramer et al., 1997; Cramer et al., 2000). In particular, the primary motor cortex may be capable of vicarious function after a limited ischemic lesion to this area (Jaillard et al., 2005).
2.2. Reorganization of secondary motor areas
When more extensive neuronal damage has occurred, there may not be enough surviving cortical neurons for somatotopic reorganization in the perilesional area. Another strategy may be for a functionally similar area that is not anatomically in immediate proximity to take over function of the damaged region. Well-recovered stroke patients continue to have reduced ipsilesional corticospinal excitability, evidenced by reduced MEP amplitudes (Byrnes et al., 2001), suggesting that alternative mechanisms such as cortical reorganization and increased activity of secondary motor areas may have contributed to their motor recovery. In particular, the premotor cortex (PM) and the supplementary motor area (SMA) may play a new and functionally relevant role in accessing alternative motor output pathways and creating alternative networks in patients with corticospinal system disruption (McNeal et al., 2010; Swayne et al., 2008;Ward et al., 2006). This is likely due to the similar somatotopic organization properties and efferent tracts that descend in parallel with those originating in the ipsilesional primary sensorimotor cortex (Chainay et al., 2004; Dum and Strick, 1991; Fries et al., 1993; He et al., 1993). Indeed, studies in non-human primate models of stroke have shown premotor reorganizational changes and formation of novel connections following discrete cortical lesions in M1 (Dancause et al., 2006; Frost et al., 2003). Similarly, a study in well-recovered stroke patients highlighted the role of PM areas in motor recovery by showing that single-pulse TMS of the ipsilesional dorsal PM cortex disrupted performance of the paretic hand (Fridman et al., 2004).
With respect to activity in the SMA proper, a study in stroke patients showed that lack of functional MRI (fMRI) activation was predictive of slow or poor recovery, whereas higher activation was associated with faster and better motor recovery (Loubinoux et al., 2003). In healthy subjects, the SMA is associated with execution of more complex tasks (Tanji, 1994), which suggests that recovering stroke patients appear to need additional resources for basic motor control (Aizawa et al., 1991). The SMA also seems to be important in accessing additional neural resources from the contralesional hemisphere in organizing movement of the impaired limb (Riecker et al., 2010).
2.3. Bihemispheric reorganization
Early imaging studies have shown that in some stroke patients motor network activity appears to expand to bilateral sensorimotor representations, in comparison to healthy individuals (Chollet et al., 1991; Weiller et al., 1992). Although activity in both hemispheres reflects some aspect of reorganization, it is unclear how these areas contribute to motor recovery. Customarily it is thought that activation of the ipsilesional hemisphere is associated with better motor recovery (Cao et al., 1998; Fridman et al., 2004), and those patients with poorer recovery have more bilateral activation (Ward and Cohen, 2004). For example, in a key press motor task in well-recovered stroke patients, a suprathreshold TMS stimulation to the ipsilesional M1 disrupted contralateral paretic hand movements, but contralesional stimulation did not (Werhahn et al., 2003). These findings can be explained by an anatomical model in which that hemispheric control is based primarily on contralateral spinal cord projections from cortical motor areas (Darian-Smith et al., 1999; Dum and Strick, 1996). In comparison, the role of the contralesional hemisphere in motor recovery continues to be debated.
There is significant evidence suggesting that continued activation of the contralesional side might not be entirely beneficial for motor recovery after stroke. For example, imaging studies have indicated an evolution in activation changes associated with concurrent recovery, originating in the contralesional side and transferring to the ipsilesional side (Calautti et al., 2001; Tombari et al., 2004). In this progression, poorly recovered stroke patients appear to show continued activation of the contralesional hemisphere (Calautti et al., 2007; Loubinoux et al., 2003). However, these investigations using imaging techniques cannot determine causality. For instance, is the bilateral activation the consequence of the poor performance (an epiphenomena) or is bilateral activation causing an imbalance of brain activity and therefore interfering with performance?
Neurophysiological studies using TMS have attempted to discern this. Several studies have suggested that persistence of contralesional motor evoked responses to TMS over M1 are associated with a poor clinical outcome (Feydy et al., 2002; Netz et al., 1997; Turton et al., 1996). In addition, TMS applied to contralesional motor areas while poorly recovered patients perform a motor task disrupted behavior (Johansen-Berg et al., 2002).
In contrast, there is also other evidence suggesting that activation of the contralesional hemisphere during paretic limb movement may play an important role in post-stroke recovery. From a clinical perspective, some case reports have been shown that well-recovered stroke patients who have a second stroke in the healthy hemisphere, not only develop new contralateral hemiparesis but also an impairment of the recovered limb (Ago et al., 2003). Stroke patients may also show mild motor deficits in the hand ipsilateral to the stroke (Jones et al., 1989). This is further supported by findings in well-recovered patients with chronic striatocapsular motor strokes who show significant activity in contralesional motor areas (Weiller et al., 1992) and the presence of ipsilateral MEPs (Wassermann et al., 1994). These studies suggested that uncrossed corticospinal tracts possibly play a role given that approximately 10% of the corticospinal tract fibers stay on the ipsilateral side (Brus-Ramer et al., 2009; Davidoff, 1990). Alternatively, activation of the contralesional hemisphere for limb use may also represent recruitment of additional neural resources in response to the increased demands on a damaged motor system (Riecker et al., 2010). In line with this,Lotze et al. (2006) showed that repetitive TMS to contralesional motor areas in well-recovered stroke patients induced timing and accuracy deficits during the production of a complex sequential motor task.
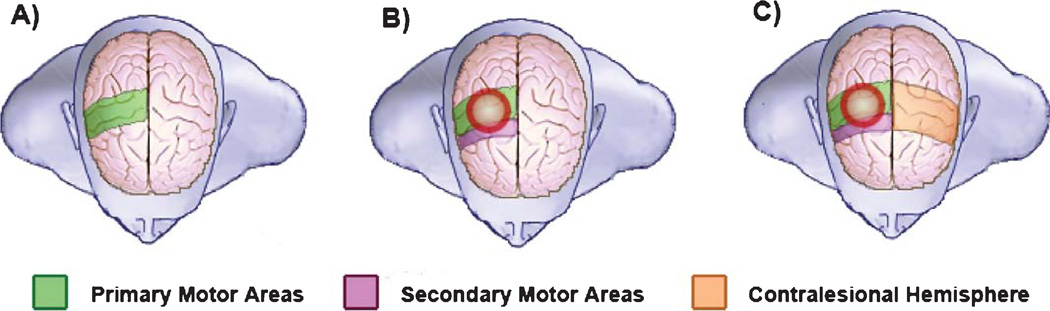
In summary, these findings highlight the complexity of defining a clear role for the contralesional hemisphere with respect to motor recovery and performance after stroke. Although ongoing investigations are addressing this question, current evidence suggests that the importance and role of contralesional neural activity is probably determined by variables such as the time since stroke (i.e., acute vs. chronic), the degree of damage to the motor system, and the complexity of the motor task (Fig. 1).
Fig. 1.
Schematic representation of brain activation areas during motor performance. A) Activation of primary motor areas in a healthy subject performing a simple motor task. B) Secondary motor areas in the ipsilesional hemisphere are activated in a well-recovered stroke patient performing a simple motor task. C) Activation in the contralesional hemisphere motor areas. The possible roles of this activation include the facilitation of: 1) Motor performance in a severely affected stroke patient in the acute or chronic phase, 2) Performance of a complex task, 3) Performance of a simple task in the acute phase.
2.4. Mechanisms of reorganization
In addition to the ability to measure changes in cortical excitability in different brain regions after stroke, TMS has also been used as a powerful tool to understand the underlying neurophysiologic mechanisms in brain plasticity. One important contribution has been the study of connectivity between intrahemispheric and interhemispheric motor areas (Reis et al., 2008). For example, recruitment of the contralesional hemisphere is presumably accomplished through unmasking of formerly silent polysynaptic cortical pathways, given that these changes can be observed within 15 minutes after contralateral cortical disruption with TMS (O’shea et al., 2007). The underlying mechanism of this process is likely down regulation of GABA-A mediated inhibitory networks (Buchkremer-Ratzmann et al., 1996; Buchkremer-Ratzmann and Witte, 1997), which can be studied directly using a paired pulse technique (Chen et al., 1998; Di Lazzaro et al., 2000). This method consists of a suprathreshold test pulse preceded by a subthreshhold conditioning stimulus (CS) at interstimulus intervals between 1 and 5ms (Kujirai et al., 1993).
Several studies have shown that at rest, stroke patients have decreased intracortical inhibition (ICI) in both the injured and healthy hemispheres (Butefisch et al., 2003; Butefisch et al., 2008; Liepert et al., 2000a; Liepert et al., 2000b) suggesting that modulation of intracortical disinhibition may be a strategy for motor recovery by facilitating the continued use of additional motor areas (Swayne et al., 2008). On the other hand, in the context of movement, even those stroke patients with better recovery seem to exhibit abnormal inhibitory activity within the motor network (Blicher et al., 2009; Hummel et al., 2009). Future longitudinal studies will need to assess the association between intracortical excitability and recovery after stroke. This will be fundamental to determine whether abnormalities in ICI are a consequence of brain lesions or a mechanism by which the brain attempts to compensate for and diminish motor impairment.
In evaluating interhemispheric changes, earlier studies in stroke patients examined the excitability of bilateral motor cortices reflected by differences inMEP sizes and cortical areas producing MEPs in the contralateral hand muscles (Cicinelli et al., 1997; Traversa et al., 1998). These investigations found relative hyperexcitability of the contralesional side in comparison to the ipsilesional side that normalized with time after stroke. This interhemispheric balance is thought to be partly mediated by inhibitory transcallosal glutamatergic connections acting on local inhibitory interneurons in the contralateral motor cortex (Daskalakis et al., 2002). Using a double pulse TMS protocol (Ferbert et al., 1992), these interhemispheric inhibitory interactions (IHI) have been directly studied while individuals performed a unimanual task (Duque et al., 2007; Murase et al., 2004). Indeed,Murase et al. (2004) showed that chronic stroke subjects had abnormally increased transcallosal inhibition from the healthy hemisphere onto the injured side relative to healthy subjects. This IHI imbalance was correlated with 2 simple measures of motor performance where the patients with more motor impairment had larger inhibition. These findings support the hypothesis that activation of the contralesional hemisphere may have a maladaptive negative modulatory effect on the ipsilesional side resulting in impaired function of the affected side. Therefore, strategies to enhance motor recovery may attempt to re-establish a normalized interhemispheric balance between the lesioned and healthy hemispheres. This general model of interhemispheric rivalry has served as the foundation for a number of neurorehabilitation interventions.
3. Enhancing motor recovery with repetitive transcranial magnetic stimulation
Recovery from hemiparesis likely involves motor learning processes (Krakauer, 2006). At the cellular and molecular level, learning motor skills is associated with neural plasticity mediated in part by long- term potentiation (LTP) and long-term depression (LTD) (Buonomano and Merzenich, 1998). LTP is defined as long lasting synaptic enhancement, whereas LTD by the decrease of synaptic activity, both mediated by AMPA and/or NMDA receptors (seeHuerta et al. (2009) for review). LTP and LTD like changes can be induced in healthy and stroke patients using various TMS protocols. For example, application of low frequency trains of repetitive transcranial magnetic stimulation (rTMS) can lead to a significant decrease in cortical excitability that lasts beyond the time of stimulation (Chen et al., 1997). In comparison, high frequency stimulation of rTMS can lead to significant facilitation of cortical excitability (Maeda et al., 2000). Theta Burst Stimulation (TBS) is another novel form of rTMS that employs very high frequency but low stimulation intensity (i.e., trains of 50 Hz with 80% of active motor threshold intensity) resulting in modulation of motor cortical excitability possibly through LTP and LTD like mechanisms (Huang et al., 2005). Interestingly, rTMS may also exert long-lasting effects through gene induction linked to neuroplastic changes (Brunoni et al., 2008). Thus, in the context of post-stroke reorganization, modulation of motor networks with TMS may serve a complementary role to traditional therapies in enhancing motor recovery post-stroke. In this section we consider recent rTMS trials in stroke patients assessing upper extremity function, and the various factors that may influence treatment outcomes.
3.1. Which hemisphere should we stimulate?
As we previously described some studies suggest that using rTMS to normalize interhemispheric imbalances in stroke patients may be an effective strategy to improve motor function. Thus, the alternative strategies include inhibiting the healthy hemisphere or enhancing excitability in the ipsilesional side (seeNowak et al. (2009) for review).
In healthy subjects, inhibitory 1-Hz rTMS over the non-performing M1 has been shown to improve performance of a motor task of the ipsilateral hand (Kobayashi et al., 2004). This suggests that inhibitory rTMS may improve motor performance of a hand by indirectly enhancing cortical excitability of the contralateral M1, possibly via decreased interhemispheric inhibition from the stimulated hemisphere towards the contralateral side (Grefkes et al., 2010). Indeed, inhibitory 1-Hz rTMS over the right M1 results in an increase of regional cerebral blood flow in left M1 during right hand movement, as detected by positron emission tomography (PET) (Conchou et al., 2009). Since chronic stroke patients appear to have increase inhibitory tone during movement preparation from the healthy side to the ipsilesional hemisphere (Duque et al., 2005), one proposed therapeutic strategy has been to reduce the excitability of the healthy hemisphere using inhibitory rTMS. The first sham stimulation-controlled, double-blind study applied inhibitory Hz rTMS to the unaffected hemisphere in 8 mild to moderate patients within 1 year of their stroke and found a modest improvement in motor function (Mansur et al., 2005). These behavioral findings are in line with other controlled studies which found that inhibitory contralesional rTMS caused improvement in motor function in the paretic hand without significant side effects (see Table 1).
Table 1.
Summary of dosage parameters of previous studies investigating rTMS over the primary motor cortex to enhance upper extremity motor function after stroke
| Study reference | Intensity | Freq (Hz) | Quantity of pulses | No. of stimulation sessions |
|---|---|---|---|---|
| Contralesional stimulation | ||||
| Mansur et al. (2005) | 100% rMT | 1 Hz | Continuous train of 600 pulses | 1 |
| Takeuchi et al. (2005) | 90% rMT | 1 Hz | Continuous train of 25 min duration (1500 pulses) | 1 |
| Boggio et al. (2006) | 100% rMT | 1 Hz | Continuous train of 1200 pulses | 1 |
| Fregni et al. (2006) | 100% rMT | 1 Hz | Continuous train of 1200 pulses | 5 |
| Liepert et al. (2007) | 90% rMT | 1 Hz | Continuous train of 1200 pulses | 1 |
| Talelli et al. (2007) | 80% aMT | 50 Hz | Continuous train of 3 pulses at 50 Hz given at a freq of 5 Hz, 300 total pulses. | 1 |
| Dafotakis et al. (2008) | 100% rMT | 1 Hz | Continuous train of 10 min duration (600 pulses) | 1 |
| Nowak et al. (2008) | 100% rMT | 1 Hz | Continuous train of 10 min duration (600 pulses) | 1 |
| Takeuchi et al. (2008) | 90% rMT | 1 Hz | Continuous train of 25 min duration (1500 pulses) | 1 |
| Khedr et al. (2009) | 100% rMT | 1 Hz | Continuous train of 900 pulses | 5 |
| Ackerley et al. (2010) | 90% aMT | 50 Hz | 40 second continuous train consisting of 3 pulses at 50 Hz given at a freq of 5 Hz, repeated every 10 seconds, a total of 600 pulses | 1 |
| Emara et al. (2010) | 110–120% rMT | 1 Hz | Continuous train of 150 pulses | 10 |
| Kakuda et al. (2010) | 90% rMT | 1 Hz | Continuous train of 1200 pulses | 22 |
| Ipsilesional stimulation | ||||
| Khedr et al. (2005) | 120% rMT | 3 Hz | 10 trains of 30 pulses, 50 seconds between trains, a total of 300 pulses | 10 |
| Kim et al. (2006) | 80% rMT | 10 Hz | 8 trains of 20 pulses, 58 second intertrain interval, interspersed by finger movement task, a total of 160 pulses | 1 |
| Malcolm et al. (2007) | 90% rMT | 20 Hz | 50 trains of 40 pulses, intertrain interval of 28 seconds, a total of 2000 pulses | 10 |
| Pomeroy et al. (2007) | 120% rMT | 1 Hz | 5 trains of 40 pulses, 3 minute intertrain intervals, a total of 200 pulses | 1 |
| Talelli et al. (2007) | 80% aMT | 50 Hz | 20 trains consisting of 3 pulses at 50 Hz given at a freq of 5 Hz, intertrain interval of 8 seconds, a total of 600 pulses | 1 |
| Ameli et al. (2009) | 80% rMT | 10 Hz | 20 trains of 50 pulses, intertrain interval of 25 seconds, a total of 1000 pulses | 1 |
| Khedr et al. (2009) | 130% rMT | 3 Hz | 30 trains of 30 pulses, intertrain interval of 2 seconds, a total of 900 pulses | 5 |
| Yozbatiran et al. (2009) | 90% rMT | 20 Hz | 40 trains of 40 pulses, intertrain interval of 28 seconds, a total of 1600 pulses | 1 |
| Ackerley et al. (2010) | 90% aMT | 50 Hz | 2 second trains consisting of 3 pulses at 50 Hz given at a freq of 5 Hz , repeated every 10 seconds, a total of 600 pulses | 1 |
| Chang et al. (2010) | 90% rMT | 10 Hz | 50 trains of 50 pulses, intertrain interval of 55 seconds, a total of 1000 pulses | 10 |
| Emara et al. (2010) | 80–90% rMT | 5 Hz | Continuous train of 750 pulses | 10 |
| Khedr et al. (2010) | 3 Hz: 130% rMT; 10 Hz: 100% rMT | 3 Hz vs. 10 Hz | 3 Hz: 50 trains of 15 pulses, a total of 750 pulses 10 Hz: 37 trains, 20 pulses, a total of 750 pulses | 5 |
In addition to 1Hz rTMS, other stimulation parameters can be used to cause long lasting inhibition to the contralesional hemisphere such as continuous TBS (cTBS) (Ackerley et al., 2010; Di Lazzaro et al., 2008; Talelli et al., 2007) or priming 1Hz rTMS with intermittent 6-Hz rTMS (Carey et al., 2008; Carey et al., 2010). One important element to keep in mind in these studies, relative to other forms of non-invasive brain stimulation (Vines et al., 2008), is that inhibitory rTMS does not appear to deteriorate motor performance in the non-affected hand contralateral to the stimulated hemisphere (Fregni et al., 2006; Liepert et al., 2007). This is of great relevance to rehabilitation given that disrupting the healthy hand would be detrimental if the goal is to enhance motor learning in the context of bimanual tasks (McCombe Waller and Whitall, 2008).
An alternative approach, is to enhance activity of the ipsilesional hemisphere given that (1) this may help augment the normal activity going on in the area stimulated, (2) vicarious changes may have occurred in the affected side and (3) this may help to counterbalance excessive interhemispheric inhibition from the unaffected hemisphere (Ameli et al., 2009; Kim et al., 2006; Talelli et al., 2007). For instance, in healthy subjects, high frequency rTMS to the motor cortex has been shown to improve finger sequence performance in the contralateral hand (Kim et al., 2004). In stroke patients, the first controlled study performed byKhedr et al. (2005) showed that excitatory 3-Hz rTMS over ipsilesional M1 for 10 days could improve motor function in acute stroke patients. Interestingly, the behavioral effect at 10 days post-stroke was comparable to the effects observed after thrombolytic therapy (Alonso-Alonso et al., 2007). Subsequent rTMS studies in patients have used different stimulation parameters to enhance motor function via facilitation of the ipsilesional motor cortex (i.e., 3 Hz, 10z, 20 Hz, and intermittent trains of TBS (iTBS); see Table 1). Importantly, the results of these studies also suggest that ipsilesional rTMS can be applied safely (Yozbatiran et al., 2009).
Altogether, these studies in stroke patients suggest that using rTMS may be an effective strategy to improve motor functional outcomes. However, it remains unclear which hemisphere is the optimal target for stimulation, or whether a combination of both stimulation sites should be considered. Results from recent studies have begun to address this question.Khedr et al. (2009) compared 1Hz rTMS over the unaffected hemisphere, 3Hz rTMS over the affected hemisphere, and sham stimulation in 36 acute stroke patients. They found that patients receiving contralesional 1Hz rTMS showed more improvement relative to the other interventions on simple motor tasks (keyboard tapping and nine hole pegboard task), stroke impairment and disability. Similarly,Takeuchi et al. (2009) compared three different forms of rTMS in combination with motor training on thirty chronic stroke patients. The authors applied inhibitory 1Hz rTMS contralesionally, excitatory 10 Hz rTMS ipsilesionally, and a combination of bilateral rTMS to both hemispheres. They found that only contralesional inhibitory rTMS, and bilateral rTMS produced a significant improvement in motor training that lasted one week. Finally,Emara et al. (2010) recently compared 5Hz ipsilesional stimulation with 1Hz contralesional stimulation over ten days and found that both groups had improvement in motor function and disability scales that lasted up to 12 weeks post-intervention. Together, these studies suggest that inhibitory rTMS over the contralesional hemisphere may be a more effective method of enhancing paretic limb function (Matz and Brainin, 2009), although ipsilesional stimulation is still beneficial.
In summary, in recent years trials in small patient groups started to provide important initial hints to determine the best stimulation site to apply rTMS to improve motor function. However, significant limitations remain. For instance, most investigations have been done in chronic stroke patients making it difficult to extrapolate the findings to the acute setting. Also, it is difficult to determine the consistency of stimulation when applied over the ipsilesional side given that the shape of brain lesions may affect the distribution of electrical current induced by TMS (Wagner et al., 2006). Moreover, the majority of the studies have only assessed the effects of rTMS on simple motor tasks performance. Therefore, further investigation with larger sample sizes, more global motor tasks and at different stages of the recovery process (i.e., acute, subacute or chronic) are needed to make more definitive conclusions.
3.2. What are the plastic changes induced by TMS?
To better understand the mechanisms by which TMS affects behavior in stroke patients many investigations have examined the physiological and or fMRI activation changes resulting from the interventions. For instance, excitatory rTMS and intermittent TBS over the ipsilesional hemisphere, an important neural substrate for motor recovery (Stinear et al., 2007; Ward et al., 2006), appear to produce lasting changes in excitability (Di Lazzaro et al., 2008; Fregni et al., 2006; Khedr et al., 2010; Kim et al., 2006; Talelli et al., 2007). Similarly, contralesional inhibitory stimulation also appears to increase ipsilesional excitability in stroke patients (Fregni et al., 2006; Khedr et al., 2009; Nowak et al., 2008), while decreasing cortical excitability in the unaffected hemisphere (Khedr et al., 2009; Nowak et al., 2008) and decreasing interhemispheric inhibition towards the ipsilesional side (Takeuchi et al., 2005). Similarly, a study using fMRI described that 1-Hz rTMS to the contralesional M1 decreased activity in this region and enhanced activation in the ipsilesional motor areas. These changes were associated with better movement kinematics of the affected hand (Nowak et al., 2008). These findings argue in favor of the interhemispheric competition theory as a relevant process responsible, at least in part, in the recovery of motor function following stroke. However, it is important to be cautious before settling on one theory given that there is not always a consistent relationship between physiological and clinical outcomes. For instance,Khedr et al. (2005) found no correlation between clinical recovery after stroke and MEP changes after ipsilesional rTMS, whereasKim et al. (2006) found a significant correlation between MEP amplitude changes and accuracy of paretic arm movements.
3.3. What is the contribution of motor training?
Motor learning and task-specific practice appear to be essential for neural and functional changes to occur (Lotze et al., 2003; Plautz et al., 2000). One of the reasons why changes in cortical excitability in response to rTMS may not result in behavioral benefits may be the lack of coupling between the stimulation and task-specific training. For example, several studies that applied rTMS alone with no other exercises did not find behavioral improvements when assessing pinch force or grip strength (Khedr et al., 2009; Liepert et al., 2007; Takeuchi et al., 2005; Talelli et al., 2007). On the other hand, delivery of TMS at the appropriate time during movement execution enhances the use-dependent plasticity resulting from the training (Butefisch et al., 2004), and improves pinch force (Takeuchi et al., 2008; Takeuchi et al., 2009). The benefit of coupling brain stimulation with physical practice may rely on Hebbian principles of synaptic plasticity (Buonomano and Merzenich, 1998), which capitalize on the temporal relationship of the interventions facilitating the formation of durable synaptic connections. Importantly though, some studies found that rTMS did not provide additive benefits to motor training (Fregni et al., 2006; Malcolm et al., 2007). These negative results may be due to ceiling effects of the behavioral outcome measures, the complexity of the task being modulated (Fregni et al., 2006; Gerloff et al., 1998) or neural homeostatic properties of the stimulated region (Ziemann and Siebner, 2008). Taken together, the data suggest that if the goal is to modulate behavior, the brain stimulation must be coupled with some form of training. However, further research is required to determine the optimal manner to deliver TMS to modulate the effects of motor training.
3.4. What is the dose of rTMS?
In order for rTMS to be considered as an adjunct to other neurorehabilitation practices it is crucial to understand the relationship between dose and therapeutic effect (seeHiscock et al. (2008) for review). To date it remains unclear what the optimal dose of magnetic stimulation is to affect behavior (i.e., what is the best intensity, frequency, total amount of stimulation, and or number of stimulation sessions?). So far, most studies have used TMS parameters known to induce corticomotor excitability changes with the hope they translate to behavioral modifications. For instance, many rTMS studies targeting the contralesional hemisphere used intensities near or at resting motor threshold at 1Hz frequency because these parameters lead to reductions in cortical excitability (Chen et al., 1997). In comparison, a variety of stimulation frequencies have been used over the ipsilesional hemisphere, although most of the studies applied sub-threshold intensities due to concerns of increased seizure risk (see Table 1 for a summary). Very few studies to date have compared different stimulation parameters. For example, Kedhr et al. compared 3Hz and 10 Hz rTMS in different groups of stroke patients undergoing rehabilitation and found that the 3Hz group had a trend toward better response relative to the 10 Hz group. This difference may be accounted for by the fact that the 3Hz group was stimulated at an intensity of 130% resting motor threshold (RMT), whereas the 10 Hz group was stimulated at 100% RMT due to safety concerns.
Another aspect of discrepancy has been the number of stimulation pulses applied per session or the duration of the TMS trains. This is important given that these differences may lead to different durations of cortical excitability changes (Gershon et al., 2003). For example, in healthy subjects, extending stimulation of 5Hz rTMS from 900 to 1800 pulses resulted in an increase of cortical excitability from a few minutes to 30–40 minutes (Peinemann et al., 2004). This dosing effect may explain why some studies found enhanced ipsilesional excitability after 600 pulses of cTBS to the contralesional hemisphere (Di Lazzaro et al., 2008), but others did not observe that change when using 300 pulses of cTBS (Talelli et al., 2007). This difference may be due to the fact that cTBS-600 has been shown to induce depression of corticospinal excitability more significantly than cTBS-300 (Stefan et al., 2008). Nonetheless, we must emphasize again that caution should be used in inferring a direct effect between physiological responses and behavioral measures. For example, in a study byTakeuchi et al. (2009) one week after bilateral rTMS subjects continued to have improvement in motor function, while physiological measures of interhemispheric interactions had already returned to baseline.
Lastly, recent studies in stroke patients have begun exploring the advantages of multiple sessions of rTMS. This strategy may provide additional benefits over a single session through cumulative effects resulting in increased facilitation to subsequent stimulations (Baumer et al., 2003; Valero-Cabre et al., 2008). Studies in acute stroke patients indicate that multiple sessions of ipsilesional excitatory rTMS can lead to improvements in motor function that last 3 months and even up to a year post-intervention (Chang et al., 2010; Khedr et al., 2010). However, these enduring effects may have been due to the normal process of recovery, which could have been “jump started” by the application of rTMS. These findings speak to the potential for long-lasting benefits of rTMS in the context of neurorehabilitation.
3.5. Which patients benefit more?
An understanding of how different patient characteristics affect response to rTMS would be helpful to match the patient to the particular treatment intervention. Currently, it remains largely unknown what characteristics (i.e., stroke location, size, chronicity) determines the individual response to rTMS. This may be in part due to most studies focusing in subcortical strokes (Dafotakis et al., 2008; Di Lazzaro et al., 2008; Liepert et al., 2007; Nowak et al., 2008; Takeuchi et al., 2008; Takeuchi et al., 2009). A recent study byAmeli et al. (2009) compared ipsilesional 10 Hz rTMS in patients with subcortical lesions with patients with both subcortical and cortical damage. They found that subcortical patients had improvement in movement kinematics whereas the cortical patients showed either no change or in some cases worsening in movement kinematics. The authors conjectured that this difference may be due to the effects of cortical damage and decreased GABA-ergic cortical inhibition causing an enhancement of glutamatergic activity, resulting in a pathological propagatation of motor network excitability. This is supported by findings of decreased interhemispheric inhibition in cortical strokes relative to subcortical strokes (Boroojerdi et al., 1996). However, the lack of effects could also be due simply to stroke size decreasing the amount of healthy tissue available to sustain changes that impact behavior in a meaningful manner. For example, one study showed that patients with the largest lesions failed to show any benefit to 3Hz ipsilesional rTMS for 10 consecutive days (Khedr et al., 2005). In comparison, inhibitory contralesional rTMS was shown to be superior over sham stimulation in restoring partial hand motor function in a severely affected chronic stroke patient with no prior movement in the affected hand (Boggio et al., 2006). This suggests that even highly dysfunctional cortical activity can sustain benefits from brain stimulation.
Another important clinical variable that may affect patients’ response to rTMS may be the chronicity of the stroke. Early modulation of cortical excitability with rTMS may be most beneficial by preventing the development of maladaptive reorganization (Nowak et al., 2008) resulting in greater motor improvement (Khedr et al., 2009). However, only few studies have so far applied rTMS in the acute period. Most of the investigations, showing improvement of motor performance, have targeted chronic (>6 months) stroke patients, suggesting that behavioral changes can be facilitated by rTMS even after maximal potential motor recovery.
Lastly, patient age may be another factor that can affect response to rTMS protocols in stroke patients. Studies in healthy subjects suggest that younger subjects have greater potential to undergo plastic changes resulting from training and TMS protocols (Rogasch et al., 2009; Sawaki et al., 2003; Todd et al., 2010; Celnik et al., 2006). Importantly, two studies in stroke patients also found that younger age was a strong predictor of response to rTMS (Khedr et al., 2010; Yozbatiran et al., 2009). In contrast,Fregni et al. (2006) found no correlation between motor function improvement and the age of the stroke subjects.
In summary, the previous described studies suggest that rTMS can still be considered in all patients with stroke related motor impairments. However, more research is needed to understand whether specific stroke sub-populations benefit more than others when receiving brain stimulation.
3.6. Future directions for rTMS studies
Together these studies represent important first steps in understanding how rTMS can be used to modulate the reorganization of motor areas of stroke patients. However, much work is still required to optimize the way rTMS is utilized to affect motor recovery, performance and learning in stroke patients. These investigations will also need to address many different aspects from patient characteristics to rTMS dosing. For example, strategies to normalize interhemispheric imbalances have been shown to be beneficial, yet the optimal site of stimulation is not settled. It is very possible that patients will need to be assessed prior to application of brain stimulation to understand how their brain is allowing them to perform motor tasks and or dealing with the injury. In this manner, the stimulation strategy will be customized to the particular mechanism the patient is using at the time of the intervention. Importantly, given most investigations have primarily focused on stimulating M1, future applications of rTMS should also examine the effects of its application to other regions involved in motor control processes. For instance, motor imagery studies have revealed an altered organization of connectivity between primary motor and regions upstream, such as the prefrontal cortex (Sharma et al., 2009). This suggests that in addition to strategies which focus on balancing the interhemispheric relationships between contralateral M1’s, other cortical regions should also be considered as potential entry ways to enhancing motor recovery (Celnik and Hillis, 2009; Grefkes et al., 2010). Another benefit deriving from the use of TMS in stroke patients is a better understanding of the mechanisms underlying motor recovery. Indeed, based on proposed models of recovery (Ward and Cohen, 2004), other adjuvant strategies have been investigated to facilitate motor function in stroke patients. For example, transcranial direct current stimulation (tDCS), another method to modulate brain excitability in humans non-invasively, has been shown to enhance performance in stroke patients (Fregni et al., 2005; Hummel et al., 2005; Lindenberg et al., 2010). Similarly, somatosensory stimulation, in the form of peripheral nerve stimulation (PNS), has also been used to enhance motor cortical excitability (Celnik et al., 2007; Kaelin-Lang et al., 2002) resulting in improved motor performance in stroke patients (Celnik et al., 2007; Conforto et al., 2002). Perhaps more interesting, we have recently shown that combining strategies, in this case tDCS with PNS to the paretic hand, enhanced the effects of motor training beyond levels reached by either intervention alone (Celnik et al., 2009). This suggests that combining other adjuvant interventions with rTMS may represent a better approach than using rTMS alone to modulate motor behavior in neurorehabilitation.
4. Conclusions
The use of transcranial magnetic stimulation has helped improve our understanding of the mechanisms underlying recovery of motor function after stroke. This in turn has opened the opportunity to test repetitive TMS, as well as other interventions, to affect motor behavior. At this point, it is clear that it is possible to modulate motor function in stroke patients. However, more studies are needed to continue advancing our knowledge of the recovery processes after brain lesions, to determine optimal stimulation parameters and to understand how different patient characteristics influence response to non-invasive brain stimulation. Undoubtedly, TMS has become an invaluable tool to understand neurophysiological processes in healthy individuals and patients with brain lesions. Likely in the near future TMS will also become a therapeutic strategy, either alone or combined with other interventions, to enhance functional recovery.
Acknowledgments
Erik Hoyer is supported by the Rehabilitation Medicine Scientist Training Program (RMSTP; 5K12HD001097). Pablo Celnik is supported by the NICHD, NIH (R01 HD053793 and R21 HD060169) and the Brain Science Institute of Johns Hopkins University.
References
- Ackerley SJ, Stinear CM, Barber PA, Byblow WD. Combining theta burst stimulation with training after subcortical stroke. Stroke. 2010;41(7):1568–1572. doi: 10.1161/STROKEAHA.110.583278. [DOI] [PubMed] [Google Scholar]
- Ago T, Kitazono T, Ooboshi H, et al. Deterioration of preexisting hemiparesis brought about by subsequent ipsilateral lacunar infarction. J Neurol Neurosurg Psychiatry. 2003;74(8):1152–1153. doi: 10.1136/jnnp.74.8.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizawa H, Inase M, Mushiake H, Shima K, Tanji J. Reorganization of activity in the supplementary motor area associated with motor learning and functional recovery. Exp Brain Res. 1991;84(3):668–671. doi: 10.1007/BF00230980. [DOI] [PubMed] [Google Scholar]
- Alonso-Alonso M, Fregni F, Pascual-Leone A. Brain stimulation in post-stroke rehabilitation. Cerebrovasc Dis. 2007;24(Suppl 1):157–166. doi: 10.1159/000107392. [DOI] [PubMed] [Google Scholar]
- Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol. 2009;66(3):298–309. doi: 10.1002/ana.21725. [DOI] [PubMed] [Google Scholar]
- Barker WH, Mullooly JP. Stroke in a defined elderly population: 1967–1985. A less lethal and disabling but no less common disease. Stroke. 1997;28(2):284–290. doi: 10.1161/01.str.28.2.284. [DOI] [PubMed] [Google Scholar]
- Baumer T, Lange R, Liepert J, et al. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage. 2003;20(1):550–560. doi: 10.1016/s1053-8119(03)00310-0. [DOI] [PubMed] [Google Scholar]
- Blicher JU, Jakobsen J, Andersen G, Nielsen JF. Cortical excitability in chronic stroke and modulation by training: A TMS study. Neurorehabil Neural Repair. 2009;23(5):486–493. doi: 10.1177/1545968308328730. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Alonso-Alonso M, Mansur CG, et al. Hand function improvement with low-frequency repetitive transcranial magnetic stimulation of the unaffected hemisphere in a severe case of stroke. Am J Phys Med Rehabil. 2006;85(11):927–930. doi: 10.1097/01.phm.0000242635.88129.38. [DOI] [PubMed] [Google Scholar]
- Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144(1–2):160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- Brasil-Neto JP, Valls-Sole J, Pascual-Leone A, et al. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116(Pt 3):511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Boggio PS, Fregni F. Can the ‘yin and yang’ BDNF hypothesis be used to predict the effects of rTMS treatment in neuropsychiatry? Med Hypotheses. 2008;71(2):279–282. doi: 10.1016/j.mehy.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Brus-Ramer M, Carmel JB, Martin JH. Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J Neurosci. 2009;29(19):6196–6206. doi: 10.1523/JNEUROSCI.5852-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, August M, Hagemann G, Witte OW. Electrophysiological transcortical diaschisis after cortical photothrombosis in rat brain. Stroke. 1996;27(6):1105–1109. doi: 10.1161/01.str.27.6.1105. discussion 1109–1111. [DOI] [PubMed] [Google Scholar]
- Buchkremer-Ratzmann I, Witte OW. Extended brain disinhibition following small photothrombotic lesions in rat frontal cortex. Neuroreport. 1997;8(2):519–522. doi: 10.1097/00001756-199701200-00028. [DOI] [PubMed] [Google Scholar]
- Buonomano DV, Merzenich MM. Cortical plasticity: From synapses to maps. Annu Rev Neurosci. 1998:21149–21186. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Khurana V, Kopylev L, Cohen LG. Enhancing encoding of a motor memory in the primary motor cortex by cortical stimulation. J Neurophysiol. 2004;91(5):2110–2116. doi: 10.1152/jn.01038.2003. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V. Remote changes in cortical excitability after stroke. Brain. 2003;126(Pt 2):470–481. doi: 10.1093/brain/awg044. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Wessling M, Netz J, Seitz RJ, Homberg V. Relationship between interhemispheric inhibition and motor cortex excitability in subacute stroke patients. Neurorehabil Neural Repair. 2008;22(1):4–21. doi: 10.1177/1545968307301769. [DOI] [PubMed] [Google Scholar]
- Byrnes ML, Thickbroom GW, Phillips BA, Mastaglia FL. Long-term changes in motor cortical organisation after recovery from subcortical stroke. Brain Res. 2001;889(1–2):278–287. doi: 10.1016/s0006-8993(00)03089-4. [DOI] [PubMed] [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Marie RM, Baron JC. Sequential activation brain mapping after subcortical stroke: Changes in hemispheric balance and recovery. Neuroreport. 2001;12(18):3883–3886. doi: 10.1097/00001756-200112210-00005. [DOI] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, et al. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage. 2007;34(1):322–331. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Cao Y, D’Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after post-stroke hemiparesis. Stroke. 1998;29(1):112–122. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- Carey JR, Anderson DC, Gillick BT, Whitford M, Pascual-Leone A. 6-Hz primed low-frequency rTMS to contralesional M1 in two cases with middle cerebral artery stroke. Neurosci Lett. 2010;469(3):338–342. doi: 10.1016/j.neulet.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey JR, Evans CD, Anderson DC, et al. Safety of 6- Hz primed low-frequency rTMS in stroke. Neurorehabil Neural Repair. 2008;22(2):185–192. doi: 10.1177/1545968307305458. [DOI] [PubMed] [Google Scholar]
- Celnik P, Hillis AE. Reconnecting the dots after stroke. Ann Neurol. 2009;66(5):570–571. doi: 10.1002/ana.21811. [DOI] [PubMed] [Google Scholar]
- Celnik P, Hummel F, Harris-Love M, Wolk R, Cohen LG. Somatosensory stimulation enhances the effects of training functional hand tasks in patients with chronic stroke. Arch Phys Med Rehabil. 2007;88(11):1369–1376. doi: 10.1016/j.apmr.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Celnik P, Paik NJ, Vandermeeren Y, Dimyan M, Cohen LG. Effects of combined peripheral nerve stimulation and brain polarization on performance of a motor sequence task after chronic stroke. Stroke. 2009;40(5):1764–1771. doi: 10.1161/STROKEAHA.108.540500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celnik P, Stefan K, Hummel F, Duque J, Classen J, Cohen LG. Encoding a motor memory in the older adult by action observation. Neuroimage. 2006;29(2):677–684. doi: 10.1016/j.neuroimage.2005.07.039. [DOI] [PubMed] [Google Scholar]
- Chainay H, Krainik A, Tanguy ML, Gerardin E, Le Bihan D, Lehericy S. Foot, face and hand representation in the human supplementary motor area. Neuroreport. 2004;15(5):765–769. doi: 10.1097/00001756-200404090-00005. [DOI] [PubMed] [Google Scholar]
- Chang WH, Kim YH, Bang OY, Kim ST, Park YH, Lee PK. Long-term effects of rTMS on motor recovery in patients after subacute stroke. J Rehabil Med. 2010;42(8):758–764. doi: 10.2340/16501977-0590. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48(5):1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. J Neurosci. 1998;18(9):3443–3450. doi: 10.1523/JNEUROSCI.18-09-03443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans:Astudy with positron emission tomography. Ann Neurol. 1991;29(1):63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- Cicinelli P, Traversa R, Rossini PM. Post-stroke reorganization of brain motor output to the hand: A 2–4 month follow-up with focal magnetic transcranial stimulation. Electroencephalogr. Clin Neurophysiol. 1997;105(6):438–450. doi: 10.1016/s0924-980x(97)00052-0. [DOI] [PubMed] [Google Scholar]
- Conchou F, Loubinoux I, Castel-Lacanal E, et al. Neural substrates of low-frequency repetitive transcranial magnetic stimulation during movement in healthy subjects and acute stroke patients. A PET study. Hum Brain Mapp. 2009;30(8):2542–2557. doi: 10.1002/hbm.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conforto AB, Kaelin-Lang A, Cohen LG. Increase in hand muscle strength of stroke patients after somatosensory stimulation. Ann Neurol. 2002;51(1):122–125. doi: 10.1002/ana.10070. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Moore CI, Finklestein SP, Rosen BR. A pilot study of somatotopic mapping after cortical infarct. Stroke. 2000;31(3):668–671. doi: 10.1161/01.str.31.3.668. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, et al. A functionalMRIstudy of subjects recovered from hemiparetic stroke. Stroke. 1997;28(12):2518–2527. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- Dafotakis M, Grefkes C, Eickhoff SB, Karbe H, Fink GR, Nowak DA. Effects of rTMS on grip force control following subcortical stroke. Exp Neurol. 2008;211(2):407–412. doi: 10.1016/j.expneurol.2008.02.018. [DOI] [PubMed] [Google Scholar]
- Dancause N, Barbay S, Frost SB, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006;96(6):3506–3511. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- Darian-Smith I, Burman K, Darian-Smith C. Parallel pathways mediating manual dexterity in the macaque. Exp Brain Res. 1999;128(1–2):101–108. doi: 10.1007/s002210050824. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Fitzgerald PB, Roshan L, Chen R. The mechanisms of interhemispheric inhibition in the human motor cortex. J Physiol. 2002;543(Pt 1):317–326. doi: 10.1113/jphysiol.2002.017673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidoff RA. The pyramidal tract. Neurology. 1990;40(2):332–339. doi: 10.1212/wnl.40.2.332. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, et al. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000;111(5):794–799. doi: 10.1016/s1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, et al. Modulating cortical excitability in acute stroke: A repetitive TMS study. Clin Neurophysiol. 2008;119(3):715–723. doi: 10.1016/j.clinph.2007.11.049. [DOI] [PubMed] [Google Scholar]
- Donoghue JP, Suner S, Sanes JN. Dynamic organization of primary motor cortex output to target muscles in adult rats. II. Rapid reorganization following motor nerve lesions. Exp Brain Res. 1990;79(3):492–503. doi: 10.1007/BF00229319. [DOI] [PubMed] [Google Scholar]
- Dum RP, Strick PL. Spinal cord terminations of the medial wall motor areas in macaque monkeys. J Neurosci. 1996;16(20):6513–6525. doi: 10.1523/JNEUROSCI.16-20-06513.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, Strick PL. The origin of corticospinal projections from the premotor areas in the frontal lobe. J Neurosci. 1991;11(3):667–689. doi: 10.1523/JNEUROSCI.11-03-00667.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque J, Hummel F, Celnik P, Murase N, Mazzocchio R, Cohen LG. Transcallosal inhibition in chronic subcortical stroke. Neuroimage. 2005;28(4):940–946. doi: 10.1016/j.neuroimage.2005.06.033. [DOI] [PubMed] [Google Scholar]
- Duque J, Murase N, Celnik P, et al. Intermanual Differences in movement-related interhemispheric inhibition. J Cogn Neurosci. 2007;19(2):204–213. doi: 10.1162/jocn.2007.19.2.204. [DOI] [PubMed] [Google Scholar]
- Emara TH, Moustafa RR, Elnahas NM, et al. Repetitive transcranial magnetic stimulation at 1Hz and 5Hz produces sustained improvement in motor function and disability after ischaemic stroke. Eur J Neurol. 2010;17(9):1203–1209. doi: 10.1111/j.1468-1331.2010.03000.x. [DOI] [PubMed] [Google Scholar]
- Ferbert A, Priori A, Rothwell JC, Day BL, Colebatch JC, Marsden CD. Interhemispheric inhibition of the human motor cortex. J Physiol. 1992:453525–453546. doi: 10.1113/jphysiol.1992.sp019243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby-Brami A, et al. Longitudinal study of motor recovery after stroke: Recruitment and focusing of brain activation. Stroke. 2002;33(6):1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, et al. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16(14):1551–1555. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Valle AC, et al. A sham-controlled trial of a 5-day course of repetitive transcranial magnetic stimulation of the unaffected hemisphere in stroke patients. Stroke. 2006;37(8):2115–2122. doi: 10.1161/01.STR.0000231390.58967.6b. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG. Reorganization of the human ipsilesional premotor cortex after stroke. Brain. 2004;127(Pt 4):747–758. doi: 10.1093/brain/awh082. [DOI] [PubMed] [Google Scholar]
- Fries W, Danek A, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke. Role of descending pathways from multiple motor areas. Brain. 1993;116(Pt 2):369–382. doi: 10.1093/brain/116.2.369. [DOI] [PubMed] [Google Scholar]
- Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury:Apotential substrate for stroke recovery. J Neurophysiol. 2003;89(6):3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- Furlan M, Marchal G, Viader F, Derlon JM, Baron JC. Spontaneous neurological recovery after stroke and the fate of the ischemic penumbra. Ann Neurol. 1996;40(2):216–226. doi: 10.1002/ana.410400213. [DOI] [PubMed] [Google Scholar]
- Gerloff C, Corwell B, Chen R, Hallett M, Cohen LG. The role of the human motor cortex in the control of complex and simple finger movement sequences. Brain. 1998;121(Pt 9):1695–1709. doi: 10.1093/brain/121.9.1695. [DOI] [PubMed] [Google Scholar]
- Gershon AA, Dannon PN, Grunhaus L. Transcranial magnetic stimulation in the treatment of depression. Am J Psychiatry. 2003;160(5):835–845. doi: 10.1176/appi.ajp.160.5.835. [DOI] [PubMed] [Google Scholar]
- Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. Neuroimage. 2010;50(1):233–242. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: An exploratory study with fMRI and TMS. Neuroimage. 2006;31(2):710–720. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- He SQ, Dum RP, Strick PL. Topographic organization of corticospinal projections from the frontal lobe: Motor areas on the lateral surface of the hemisphere. J Neurosci. 1993;13(3):952–980. doi: 10.1523/JNEUROSCI.13-03-00952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscock A, Miller S, Rothwell J, Tallis RC, Pomeroy VM. Informing dose-finding studies of repetitive transcranial magnetic stimulation to enhance motor function: A qualitative systematic review. Neurorehabil Neural Repair, 2008;22(3):228–249. doi: 10.1177/1545968307307115. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Volpe BT. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J Neuroeng Rehabil. 2009:67. doi: 10.1186/1743-0003-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel F, Celnik P, Giraux P, et al. Effects of noninvasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128(Pt 3):490–499. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Steven B, Hoppe J, et al. Deficient intracortical inhibition (SICI) during movement preparation after chronic stroke. Neurology. 2009;72(20):1766–1772. doi: 10.1212/WNL.0b013e3181a609c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jailard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? A longitudinal fMRI stroke study. Brain. 2005;128(Pt 5):1122–1138. doi: 10.1093/brain/awh456. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci U S A. 2002;99(22):14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RD, Donaldson IM, Parkin PJ. Impairment and recovery of ipsilateral sensory-motor function following unilateral cerebral infarction. Brain. 1989;112(Pt 1):113–132. doi: 10.1093/brain/112.1.113. [DOI] [PubMed] [Google Scholar]
- Kaelin-Lang A, Luft AR, Sawaki L, Burstein AH, Sohn YH, Cohen LG. Modulation of human corticomotor excitability by somatosensory input. J Physiol. 2002;540(Pt 2):623–633. doi: 10.1113/jphysiol.2001.012801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuda W, Abo M, Kobayashi K, et al. Low-frequency repetitive transcranial magnetic stimulation and intensive occupational therapy for post-stroke patients with upper limb hemiparesis: Preliminary study of a 15-day protocol. Int J Rehabil Res. 2010 doi: 10.1097/MRR.0b013e32833cdf10. [DOI] [PubMed] [Google Scholar]
- Kavanagh S, Knapp M, Patel A. Costs and disability among stroke patients. J Public Health Med. 1999;21(4):385–394. doi: 10.1093/pubmed/21.4.385. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Abdel-Fadeil MR, Farghali A, Qaid M. Role of 1 and 3Hz repetitive transcranial magnetic stimulation on motor function recovery after acute ischaemic stroke. Eur J Neurol. 2009;16(12):1323–1330. doi: 10.1111/j.1468-1331.2009.02746.x. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Ahmed MA, Fathy N, Rothwell JC. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology. 2005;65(3):466–468. doi: 10.1212/01.wnl.0000173067.84247.36. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Etraby AE, Hemeda M, Nasef AM, Razek AA. Long-term effect of repetitive transcranial magnetic stimulation on motor function recovery after acute ischemic stroke. Acta Neurol Scand. 2010;121(1):30–37. doi: 10.1111/j.1600-0404.2009.01195.x. [DOI] [PubMed] [Google Scholar]
- Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett. 2004;367(2):181–185. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Kim YH, You SH, Ko MH, et al. Repetitive transcranial magnetic stimulation-induced corticomotor excitability and associated motor skill acquisition in chronic stroke. Stroke. 2006;37(6):1471–1476. doi: 10.1161/01.STR.0000221233.55497.51. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Hutchinson S, Theoret H, Schlaug G, Pascual-Leone A. RepetitiveTMSof the motor cortex improves ipsilateral sequential simple finger movements. Neurology. 2004;62(1):91–98. doi: 10.1212/wnl.62.1.91. [DOI] [PubMed] [Google Scholar]
- Krakauer JW. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993:471501–471519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000a;23(11):1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Liepert J, Storch P, Fritsch A, Weiller C. Motor cortex disinhibition in acute stroke. Clin Neurophysiol. 2000b;111(4):671–676. doi: 10.1016/s1388-2457(99)00312-0. [DOI] [PubMed] [Google Scholar]
- Liepert J, Zittel S, Weiller C. Improvement of dexterity by single session low-frequency repetitive transcranial magnetic stimulation over the contralesional motor cortex in acute stroke:Adouble-blind placebo-controlled crossover trial. Restor. Neurol Neurosci. 2007;25(5–6):461–465. [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75(24):2176–2184. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics-2009 update: A report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- Lotze M, Braun C, Birbaumer N, Anders S, Cohen LG. Motor learning elicited by voluntary drive. Brain. 2003;126(Pt 4):866–872. doi: 10.1093/brain/awg079. [DOI] [PubMed] [Google Scholar]
- Lotze M, Markert J, Sauseng P, Hoppe J, Plewnia C, Gerloff C. The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J Neurosci. 2006;26(22):6096–6102. doi: 10.1523/JNEUROSCI.4564-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubinoux I, Carel C, Pariente J, et al. Correlation between cerebral reorganization and motor recovery after subcortical infarcts. Neuroimage. 2003;20(4):2166–2180. doi: 10.1016/j.neuroimage.2003.08.017. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clin Neurophysiol. 2000;111(5):800–805. doi: 10.1016/s1388-2457(99)00323-5. [DOI] [PubMed] [Google Scholar]
- Malcolm MP, Triggs WJ, Light KE, et al. Repetitive transcranial magnetic stimulation as an adjunct to constraintinduced therapy: An exploratory randomized controlled trial. Am J Phys Med Rehabil. 2007;86(9):707–715. doi: 10.1097/PHM.0b013e31813e0de0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansur CG, Fregni F, Boggio PS, et al. A sham stimulation-controlled trial of rTMS of the unaffected hemisphere in stroke patients. Neurology. 2005;64(10):1802–1804. doi: 10.1212/01.WNL.0000161839.38079.92. [DOI] [PubMed] [Google Scholar]
- Matz K, Brainin M. Neurostimulation in ischaemic stroke - down with the healthy hemisphere! Eur J Neurol. 2009;16(12):1253–1254. doi: 10.1111/j.1468-1331.2009.02785.x. [DOI] [PubMed] [Google Scholar]
- Mayo NE, Wood-Dauphinee S, Ahmed S, et al. Disablement following stroke. Disabil Rehabil. 1999;21(5–6):258–268. doi: 10.1080/096382899297684. [DOI] [PubMed] [Google Scholar]
- McCombe Waller S, Whitall J. Bilateral arm training: Why and who benefits? NeuroRehabilitation. 2008;23(1):29–41. [PMC free article] [PubMed] [Google Scholar]
- McNeal DW, Darling WG, Ge J, et al. Selective longterm reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010;518(5):586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merzenich MM, Nelson RJ, Stryker MP, Cynader MS, Schoppmann A, Zook JM. Somatosensory cortical map changes following digit amputation in adult monkeys. J Comp Neurol. 1984;224(4):591–605. doi: 10.1002/cne.902240408. [DOI] [PubMed] [Google Scholar]
- Murase N, Duque J, Mazzocchio R, Cohen LG. Influence of interhemispheric interactions on motor function in chronic stroke. Ann Neurol. 2004;55(3):400–409. doi: 10.1002/ana.10848. [DOI] [PubMed] [Google Scholar]
- Netz J, Lammers T, Homberg V. Reorganization of motor output in the non-affected hemisphere after stroke. Brain. 1997;120(Pt 9):1579–1586. doi: 10.1093/brain/120.9.1579. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Ameli M, Fink GR. Interhemispheric competition after stroke: Brain stimulation to enhance recovery of function of the affected hand. Neurorehabil Neural Repair. 2009;23(7):641–656. doi: 10.1177/1545968309336661. [DOI] [PubMed] [Google Scholar]
- Nowak DA, Grefkes C, Dafotakis M, et al. Effects of low-frequency repetitive transcranial magnetic stimulation of the contralesional primary motor cortex on movement kinematics and neural activity in subcortical stroke. Arch Neurol. 2008;65(6):741–747. doi: 10.1001/archneur.65.6.741. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J.Neurophysiol. 1996;75(5):2144–2149. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272(5269):1791–1794. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- O’Shea J, Johansen-Berg H, Trief D, Gobel S, Rushworth MF. Functionally specific reorganization in human premotor cortex. Neuron. 2007;54(3):479–490. doi: 10.1016/j.neuron.2007.04.021. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Reimer B, Loer C, et al. Long-lasting increase in corticospinal excitability after 1800 pulses of subthreshold 5Hz repetitiveTMSto the primary motor cortex. Clin Neurophysiol. 2004;115(7):1519–1526. doi: 10.1016/j.clinph.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Plautz EJ, Milliken GW, Nudo RJ. Effects of repetitive motor training on movement representations in adult squirrel monkeys: Role of use versus learning. Neurobiol. Learn Mem. 2000;74(1):27–55. doi: 10.1006/nlme.1999.3934. [DOI] [PubMed] [Google Scholar]
- Pomeroy VM, Cloud G, Tallis RC, Donaldson C, Nayak V, Miller S .Transcranial magnetic stimulation and muscle contraction to enhance stroke recovery: A randomized proof of- principle and feasibility investigation. Neurorehabil Neural Repair. 2007;21(6):509–517. doi: 10.1177/1545968307300418. [DOI] [PubMed] [Google Scholar]
- Reis J, Swayne OB, Vandermeeren Y, et al. Contribution of transcranial magnetic stimulation to the understanding of cortical mechanisms involved in motor control. J Physiol. 2008;586(2):325–351. doi: 10.1113/jphysiol.2007.144824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecker A, Groschel K, Ackermann H, Schnaudigel S, Kassubek JR, Kastrup A. The role of the unaffected hemisphere in motor recovery after stroke. Hum Brain Mapp. 2010;31(7):1017–1029. doi: 10.1002/hbm.20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogasch NC, Dartnall TJ, Cirillo J, Nordstrom MA, Semmler JG. Corticomotor plasticity and learning of a ballistic thumb training task are diminished in older adults. J Appl Physiol. 2009;107(6):1874–1883. doi: 10.1152/japplphysiol.00443.2009. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Altamura C, Ferreri F, et al. Neuroimaging experimental studies on brain plasticity in recovery from stroke. Eura Medicophys. 2007;43(2):241–254. [PubMed] [Google Scholar]
- Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult ratsILong-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res. 1990;79(3):479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- Sawaki L, Yaseen Z, Kopylev L, Cohen LG. Agedependent changes in the ability to encode a novel elementary motor memory. Ann Neurol. 2003;53(4):521–524. doi: 10.1002/ana.10529. [DOI] [PubMed] [Google Scholar]
- Sharma N, Baron JC, Rowe JB. Motor imagery after stroke: Relating outcome to motor network connectivity. Ann Neurol. 2009;66(5):604–616. doi: 10.1002/ana.21810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Gentner R, Zeller D, Dang S, Classen J. Theta-burst stimulation: Remote physiological and local behavioral after-effects. Neuroimage. 2008;40(1):265–274. doi: 10.1016/j.neuroimage.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130(Pt 1):170–180. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- Swayne OB, Rothwell JC, Ward NS, Greenwood RJ. Stages of motor output reorganization after hemispheric stroke suggested by longitudinal studies of cortical physiology. Cereb Cortex. 2008;18(8):1909–1922. doi: 10.1093/cercor/bhm218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi N, Chuma T, Matsuo Y, Watanabe I, Ikoma K. Repetitive transcranial magnetic stimulation of contralesional primary motor cortex improves hand function after stroke. Stroke. 2005;36(12):2681–2686. doi: 10.1161/01.STR.0000189658.51972.34. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Toshima M, Chuma T, Matsuo Y, Ikoma K. Inhibition of the unaffected motor cortex by 1 Hz repetitive transcranical magnetic stimulation enhances motor performance and training effect of the paretic hand in patients with chronic stroke. J.Rehabil Med. 2008;40(4):298–303. doi: 10.2340/16501977-0181. [DOI] [PubMed] [Google Scholar]
- Takeuchi N, Tada T, Toshima M, Matsuo Y, Ikoma K. Repetitive transcranial magnetic stimulation over bilateral hemispheres enhances motor function and training effect of paretic hand in patients after stroke. J Rehabil Med. 2009;41(13):1049–1054. doi: 10.2340/16501977-0454. [DOI] [PubMed] [Google Scholar]
- Talelli P, Greenwood RJ, Rothwell JC. Exploring Theta Burst Stimulation as an intervention to improve motor recovery in chronic stroke. Clin Neurophysiol. 2007;118(2):333–342. doi: 10.1016/j.clinph.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Tanji J. The supplementary motor area in the cerebral cortex. Neurosci Res. 1994;19(3):251–268. doi: 10.1016/0168-0102(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Todd G, Kimber TE, Ridding MC, Semmler JG. Reduced motor cortex plasticity following inhibitory rTMS in older adults. Clin Neurophysiol. 2010;121(3):441–447. doi: 10.1016/j.clinph.2009.11.089. [DOI] [PubMed] [Google Scholar]
- Tombari D, Loubinoux I, Pariente J, et al. A longitudinal fMRI study: In recovering and then in clinically stable subcortical stroke patients. Neuroimage. 2004;23(3):827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- Traversa R, Cicinelli P, Pasqualetti P, Filippi M, Rossini PM. Follow-up of interhemispheric differences of motor evoked potentials from the ‘affected’ and ‘unaffected’ hemispheres in human stroke. Brain Res. 1998;803(1–2):1–8. doi: 10.1016/s0006-8993(98)00505-8. [DOI] [PubMed] [Google Scholar]
- Turton A, Wroe S, Trepte N, Fraser C, Lemon RN. Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr Clin Neurophysiol. 1996;101(4):316–328. doi: 10.1016/0924-980x(96)95560-5. [DOI] [PubMed] [Google Scholar]
- Valero-Cabre A, Pascual-Leone A, Rushmore RJ. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMSmediated behavioural disruptions. Eur J Neurosci. 2008;27(3):765–774. doi: 10.1111/j.1460-9568.2008.06045.x. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair D, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci. 2008;28(8):1667–1673. doi: 10.1111/j.1460-9568.2008.06459.x. [DOI] [PubMed] [Google Scholar]
- Wagner T, Fregni F, Eden U, et al. Transcranial magnetic stimulation and stroke: A computer-based human model study. Neuroimage. 2006;30(3):857–870. doi: 10.1016/j.neuroimage.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Ward NS, Cohen LG. Mechanisms underlying recovery of motor function after stroke. Arch Neurol. 2004;61(12):1844–1848. doi: 10.1001/archneur.61.12.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Newton JM, Swayne OB, et al. Motor system activation after subcortical stroke depends on corticospinal system integrity. Brain. 2006;129(Pt 3):809–819. doi: 10.1093/brain/awl002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassermann EM, Pascual-Leone A, Hallett M. Cortical motor representation of the ipsilateral hand and arm. Exp Brain Res. 1994;100(1):121–132. doi: 10.1007/BF00227284. [DOI] [PubMed] [Google Scholar]
- Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31(5):463–472. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- Werhahn KJ, Conforto AB, Kadom N, Hallett M, Cohen LG. Contribution of the ipsilateral motor cortex to recovery after chronic stroke. Ann Neurol. 2003;54(4):464–472. doi: 10.1002/ana.10686. [DOI] [PubMed] [Google Scholar]
- Yozbatiran N, Alonso-Alonso M, See J, et al. Safety and behavioral effects of high-frequency repetitive transcranial magnetic stimulation in stroke. Stroke. 2009;40(1):309–312. doi: 10.1161/STROKEAHA.108.522144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann Ulf, Siebner Hartwig R. Modifying motor learning through gating and homeostatic metaplasticity. Brain Stimulation. 2008;1(1):60–66. doi: 10.1016/j.brs.2007.08.003. [DOI] [PubMed] [Google Scholar]