Abstract
Background
The interpretation of an adequate response to the unconjugated 23-valent pneumococcal vaccine for serotypes having high preimmunization titers remains challenging.
Objectives
We sought to determine whether high preimmunization titers preclude a 4-fold or greater response to vaccination. Moreover, we sought to determine the effect of the following covariates on this response: absolute preimmunization titer value, age, sex, serum IgG level, and serum IgG subclasses.
Methods
We conducted a retrospective analysis of patients who were seen in our immune disorders clinic between 2001 and 2007 who had received the unconjugated 23-valent pneumococcal vaccine. Logistic regression was used to estimate the effect of different covariates, including preimmunization titer values, age, sex, IgG levels, and IgG subclass values, on the odds of a 4-fold or greater antibody response.
Results
Per serotype, 10% to 40% of subjects with a high preimmunization titer attained at least a 4-fold response to vaccination. However, the odds of a 4-fold or greater response were found to decrease as a function of the absolute preimmunization titer value with an absolute value for each serotype beyond which the odds ratio approached zero.
Conclusion
High pneumococcal preimmunization titers do not necessarily preclude a 4-fold or greater response to vaccination. However, there appear to be serotype-specific preimmunization titer values, ranging from 4.4 to 10.3 μg/mL, above which a 4-fold or greater response would not be expected. This response does not seem to be significantly affected by age, sex, IgG level, or IgG subclass value.
Keywords: Pneumococcal vaccine, antibody, high preimmunization titer, fold response, age, sex, IgG, IgG subclass
Specific antibody deficiency is defined as an abnormal antibody response to polysaccharide antigens in the context of normal serum immunoglobulin concentrations.1,2 A vaccine containing polysaccharide antigens is administered, most commonly the un-conjugated 23-valent pneumococcal vaccine, to evaluate a patient suspected of having this immunodeficiency. Paired blood samples (before and after immunization) at least 4 weeks apart are drawn for evaluation of the IgG antibody response to each serotype. Depending on the laboratory used, various numbers of pneumococcal serotypes are evaluated. Although no definitive data are available, the recommendation of an expert panel is that an adequate response to 50% or 70% of serotypes tested is a normal response for children or adults, respectively.1,3
On an individual serotype basis, an adequate response is defined as a 4-fold or greater increase over baseline (ratio of postimmunization titer to preimmunization titer) or a postimmunization titer value of 1.3 μg/mL or greater.1 The 4-fold or greater increase as a requirement for an adequate response is based on the recommendation of the expert panel referenced above.1 This recommendation was recently validated in a population of HIV-infected children.3 The requirement that the postimmunization titer be 1.3 μg/mL or greater is based on the fact that this level is thought to be protective against infection with Streptococcus pneumoniae.1,4,5
Interpretation of the antibody response to the 23-valent pneumococcal vaccine is straightforward if the preimmunization titer for a given serotype is less than 1.3 μg/mL and the postimmunization titer is 1.3 μg/mL or greater. Interpretation of the antibody response when the preimmunization titer for a given serotype is already 1.3 μg/mL or greater is more controversial. One could argue that because the preimmunization titer is protective, the response to vaccination for that serotype is irrelevant. However, this ignores the purpose of vaccination in this case, which is to determine whether the immunologic machinery necessary for the response to polysaccharide antigens is currently intact. The issue is not whether the titer is protective but whether one can use the data to evaluate the current ability to mount an antibody response. This is seen in adult patients with HIV who have protective titers but whose ability to respond to new antigens decreases as their CD4 T-cell counts decrease.6-8 The debate then focuses on whether, for serotypes with preimmunization titers of 1.3 μg/mL or greater, one should expect a 4-fold or greater response to vaccination.
In this retrospective analysis we examined the antibody response to the unconjugated 23-valent pneumococcal vaccine in our patient population. For preimmunization titers of 1.3 μg/mL or greater, we looked at whether there is a relationship between absolute preimmunization titer value and the likelihood of a 4-fold or greater response. Our hypothesis was that a high preimmunization titer (≥1.3 μg/mL) for a given serotype need not preclude a 4-fold or greater response to vaccination. We also examined whether the likelihood (odds) of obtaining a 4-fold response for preimmunization titers of 1.3 μg/mL or greater was affected by factors such as age, sex, IgG level, or IgG subclass value.
This study was focused on assessing the antibody response to the 23-valent pneumococcal vaccine as a function of preimmunization titer value; it was not intended to answer the question of how to diagnose antibody deficiency. Moreover, this study was not intended to define the normal response to vaccination based on the percentage of serotypes tested having an adequate antibody response. It was also not intended to correlate antibody response to vaccination with clinical phenotype. This study was focused on examining an issue that frequently arises in the adult population, among which one is more likely to find high preimmunization titer levels.9
METHODS
This was a retrospective chart analysis of all of the patients who were seen in our immune disorders clinic between 2001 and 2007. Approval was obtained from the institutional review board. To be included in the study, patients had to have received the unconjugated 23-valent pneumococcal vaccine as part of their immune evaluation. Children were defined as being 16 years of age or younger. Serum antipneumococcal IgG titers were tested on paired samples drawn at least 4 weeks apart. To avoid interlaboratory variances, 1 inclusion criterion was that all the samples were analyzed by the same commercial laboratory for the following 14 pneumococcal serotypes: 1, 3, 4, 5, 6B, 7F, 8, 9N, 9V, 12F, 14, 18C, 19F, and 23F. Serum IgG, IgA, IgM, and IgE levels were collected; serum IgG subclass values were collected when available. Patients were excluded from the study if the 23-valent pneumococcal vaccination was not administered by our clinic, if the preimmunization and postimmunization antipneumococcal blood samples were not paired, or if the blood was not analyzed for the above 14 pneumococcal serotypes by the same commercial laboratory. The antibody titers were analyzed by using Multi-Analyte Fluorescence Detection, as previously described.10 The majority of the postimmunization samples were obtained between 4 and 6 weeks after immunization. Patients were also excluded if they had previously received intravenous immunoglobulin or if they had secondary immunodeficiencies, including HIV. On an individual serotype basis, paired preimmunization and postimmunization titers in which the preimmunization titers were above the limit of reporting (ie, reported as greater than a certain value) were excluded from analysis. For postimmunization titers above the limit of reporting, the postimmunization titer value was set equal to the limit of reporting (eg, if reported as ≥28 μg/mL, the postimmunization value was set equal to 28 μg/mL). If the fold increase was 4 or greater, then the data were accepted because we could demonstrate an adequate response; if the fold increase was less than 4, then the data were excluded because the true fold increase could not be determined. For this study, neither the clinical picture nor the ultimate immunologic diagnoses were considered, except when an attempt at determining the response in normal subjects was made. For this purpose, a normal subject was defined as a patient whose ultimate diagnosis did not include an overt immune deficiency.
Multivariable logistic regression was used to jointly estimate the effect of patient covariates, including preimmunization titer values, age, sex, IgG levels, and IgG subclass values, on the odds of the titer ratio (postimmunization vs preimmunization) being 4 or greater among subjects with a preimmunization titer value of 1.3 μg/mL or greater. Regression estimates were obtained by using the method of generalized estimating equations, with a compound symmetry correlation structure used to account for correlation among repeated titer measurements within patients.11 All statistical tests were 2-sided and carried out at the 5% level of significance with the SAS statistical software package (SAS Institute, Inc, Cary, NC).
RESULTS
Five hundred seventy-nine patients were eligible for this study after application of the inclusion and exclusion criteria. There were 40 children (≤16 years old) and 539 adults (Table I). Ages ranged from 4 to 87 years. The geometric mean age for the total population was 45 years, with an SD of 17 years. Among the children, there were 14 female and 26 male subjects, as compared with the adults, among whom there were 383 female and 156 male subjects (Table I). As shown in Table II, the arithmetic mean serum IgG levels for children and adults were 797 and 1005 mg/dL, respectively (P = .0001). As expected, IgG2 was the only IgG subclass that showed a statistically significant difference between children and adults (194 vs 324 mg/dL, P <.0001). The difference in IgG and IgG subclass levels for female versus male subjects was not statistically significant.
TABLE I.
Patient demographics
| Female (no. of subjects) | Male (no. of subjects) | Total (no. of subjects) | |
|---|---|---|---|
| Children | 14 | 26 | 40 |
| Adults | 383 | 156 | 539 |
| Total | 397 | 182 | 579 |
TABLE II.
IgG and IgG subclass data
| Children |
Adults |
Females |
Males |
|||
|---|---|---|---|---|---|---|
| Mean (mg/dL; SEM) | Mean (mg/dL; SEM) | P value | Mean (mg/dL; SEM) | Mean (mg/d; SEM) | P value | |
| IgG | 797 (43) | 1005 (16) | .0001 | 1001 (16) | 969 (35) | .7811 |
| IgG1 | 548 (44) | 609 (16) | .2008 | 605 (15) | 609 (35) | .9231 |
| IgG2 | 194 (25) | 324 (8) | <.0001 | 328 (9) | 294 (16) | .0685 |
| IgG3 | 60 (8) | 64 (3) | .6948 | 63 (2) | 65 (7) | .82 |
| IgG4 | 21 (5) | 29 (2) | .1161 | 28 (2) | 30 (4) | .7332 |
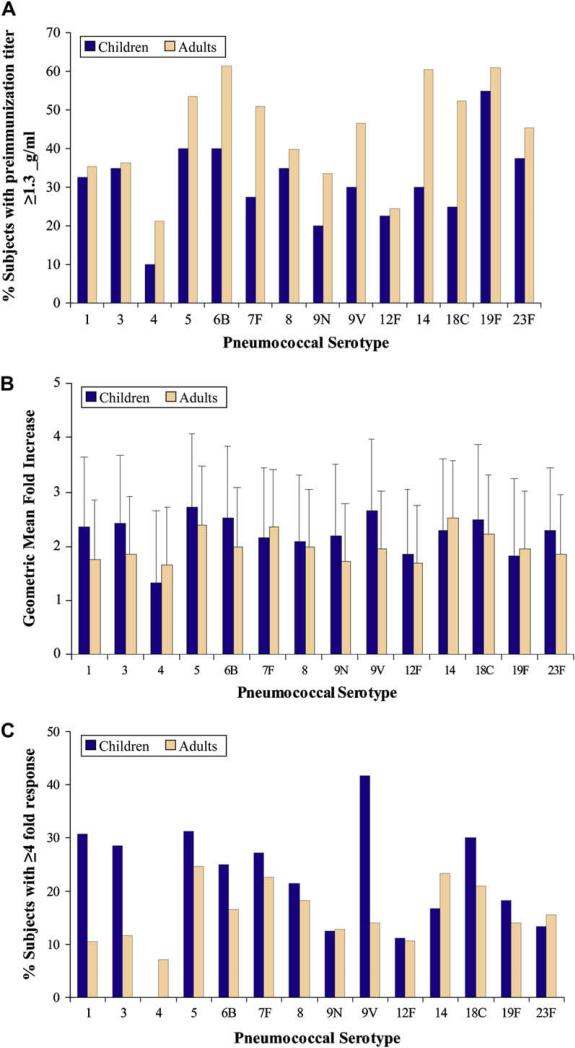
We had a significant number of subjects with a high preimmunization titer for 1 or more serotypes. The number of serotypes with high preimmunization titers varied from subject to subject. Subjects with high titers (≥ 1.3 μg/mL) for each serotype are shown in Fig 1, A. The mean fold increase in this subset for each serotype is shown in Fig 1, B. The percentage of subjects with at least a 4-fold increase over a preimmunization titer of 1.3 μg/mL or greater is shown in Fig 1, C. It is readily apparent, per serotype, that 10% to 40% of subjects with a high preimmunization titer attained at least a 4-fold response (with the exception of serotype 4 in children). We performed a more detailed statistical analysis to examine the effects of the following variables: absolute preimmunization titer value, age, sex, IgG level, and IgG subclass value. To obtain meaningful data, we examined the likelihood (odds) of a response as a function of a change per unit increase of a given variable.
FIG 1.
Antibody response interpreted as fold increase (postimmunization vs preimmunization) in titer on a per-serotype basis. A, The percentage of subjects per serotype with a preimmunization titer of 1.3 μg/mL or greater. B, Geometric mean fold increase associated with preimmunization titers of 1.3 μg/mL or greater. C, The percentage of subjects with preimmunization titers of 1.3 μg/mL or greater who achieved at least a 4-fold increase over baseline.
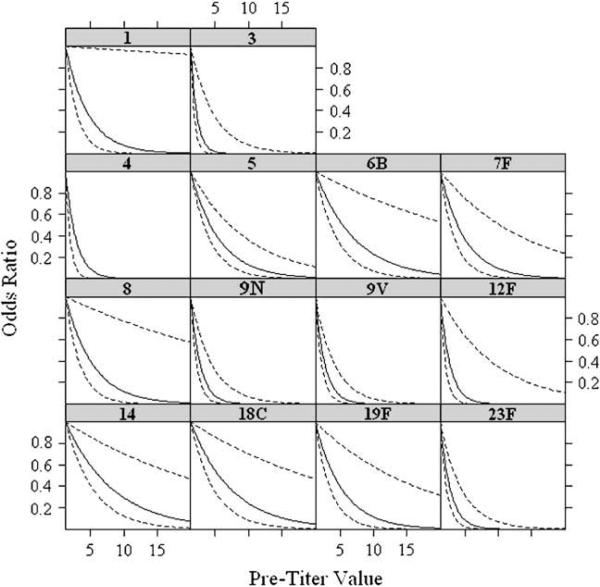
We used logistic regression to estimate and compare the effect of the absolute preimmunization titer value (for values 1.3 ≥ μg/mL) on the odds of the response being greater than or equal to 4 while controlling for the effects of age, sex, and IgG level. Fig 2 shows plots of the odds ratio for a 4-fold or greater response as a function of the absolute preimmunization titer value (relative to a value of 1.3 μg/mL). The plots demonstrate that the odds of a 4-fold or greater response decrease as a function of the absolute preimmunization titer value.
FIG 2.
Results from multivariable logistic regression analysis of antibody response as a function of preimmunization titer adjusted for age, sex, and IgG level. The data shown represent the odds ratio of a 4-fold or greater increase in antibody titer as a function of the absolute preimmunization titer, starting at the lower limit of 1.3 μg/mL. As the absolute preimmunization titer value increases, the odds ratio of a 4-fold or greater response approaches zero for all serotypes. The solid line represents the mean value, and the dashed lines represent the 95% CIs.
There seemed to be an inflection point beyond which the probability of a response approached zero. The inflection point was calculated as the preimmunization titer value at which the slope of the plot was –45°. The absolute value of this inflection point varied for each serotype, ranging from 4.4 μg/mL for serotype 3 to 10.3 μg/mL for serotype 14 (Table III). The overall result is that there are serotype-specific absolute preimmunization titer values above which a 4-fold or greater response to immunization would not be expected. When the odds ratio of obtaining a 4-fold or greater response and postimmunization titer of 1.3 μg/mL or greater was plotted as a function of all preimmunization titer values (ie, those values <1.3 μg/mL, as well as values ≥ 1.3 μg/mL), identical results were obtained with similar inflection points (data not shown).
TABLE III.
Per pneumococcal serotype, preimmunization titer values associated with the inflection point, at which the odds ratio of achieving a 4-fold or greater response approaches zero
| Serotype | OR | Preimmunization titer value (μg/mL) |
|---|---|---|
| 1 | 0.13 | 8.0 |
| 3 | 0.04 | 4.4 |
| 4 | 0.06 | 5.7 |
| 5 | 0.17 | 8.8 |
| 6B | 0.25 | 9.9 |
| 7F | 0.15 | 8.5 |
| 8 | 0.16 | 8.7 |
| 9N | 0.06 | 5.4 |
| 9V | 0.06 | 5.4 |
| 12F | 0.06 | 5.4 |
| 14 | 0.29 | 10.3 |
| 18C | 0.25 | 9.9 |
| 19F | 0.16 | 8.7 |
| 23F | 0.06 | 5.5 |
OR, odds ratio.
We next looked at the effect of other covariates on the response to 23-valent pneumococcal vaccination in all subjects. These included age, sex, IgG level, and IgG subclass value (Table IV). The effect of each covariate on the response to vaccination was consistent across all serotypes and held regardless of preimmunization titer value. The odds ratios presented for each covariate therefore are inclusive of all serotypes tested. Age, sex, IgG levels, and IgG1 and IgG3 values had no statistically significant effect on the outcome (Table IV). IgG2 and IgG4 values were statistically significant but not likely to be clinically relevant because the odds ratios were 1.002 and 1.005, respectively, for a 1 mg/dL increase in value.
TABLE IV.
The effect of patient covariates on the odds ratio of achieving a 4-fold or greater response (for preimmunization titers ≥1.3 μg/mL) per unit increase in value
| Covariate | Unit increase | OR | 95% CI | P value |
|---|---|---|---|---|
| Age | 1 y | 0.9922 | 0.9816-1.0030 | .1548 |
| Sex | Female vs male | 1.3600 | 0.9136-2.0246 | .1298 |
| IgG | 1 mg/dL | 1.0004 | 1.0000-1.0008 | .0658 |
| IgG1 | 1 mg/dL | 1.0001 | 0.9995-1.0007 | .6743 |
| IgG2 | 1 mg/dL | 1.0020 | 1.0006-1.0034 | .0084 |
| IgG3 | 1 mg/dL | 1.0013 | 0.9982-1.0044 | .4082 |
| IgG4 | 1 mg/dL | 1.0053 | 1.0014-1.0093 | .0066 |
The odds ratios were calculated in relation to each 1-year increase in age, as a function of sex, or in relation to a 1 mg/dL increase in IgG or IgG subclass value. The odds ratios hold for any pneumococcal serotype.
OR, Odds ratio.
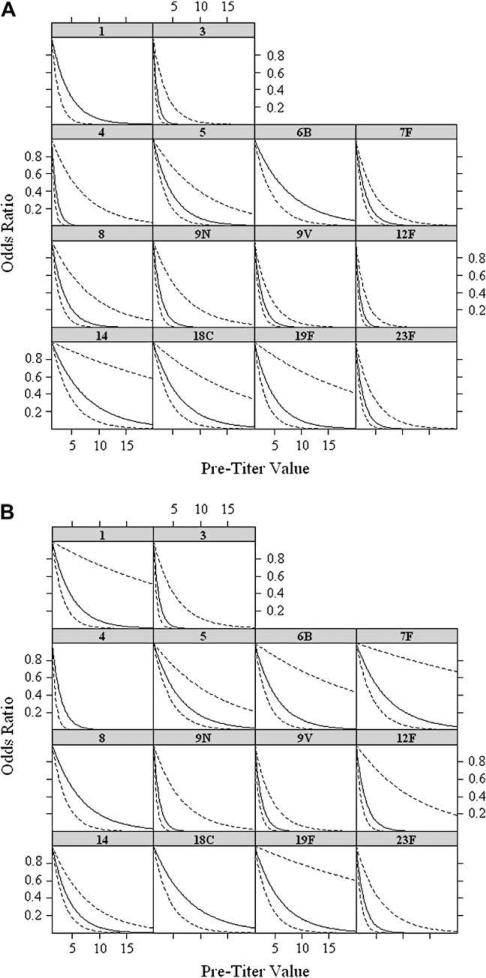
Because all of our patients were those reporting to an immunodeficiency clinic, it remained possible that a different pattern might emerge if one were to examine healthy individuals. In the absence of such a population, we broke down our patient population into those who eventually were given a diagnosis of an immunodeficiency and those who eventually were found not to have any major immunodeficiency. The latter group, which contained 223 individuals, was designated as the healthy subject group. As shown in Fig 3, the odds ratios and the inflection points were identical for both groups; indeed, the 2 figures are virtually superimposable.
FIG 3.
The patients were divided in 2 categories: those who were eventually given a diagnosis of an immune abnormality and those who were not found to have any major immunodeficiency. The latter group was considered normal subjects (n 5 223). The values for the normal subject (A) and patient (B) groups were reanalyzed, and the odds ratio was calculated as in Fig 2.
DISCUSSION
This retrospective analysis demonstrated that a high (≥ 1.3 μg/mL) preimmunization titer value for a given serotype does not necessarily preclude a 4-fold or greater response to the unconjugated 23-valent pneumococcal vaccine. Instead, the data clearly show that the extent of an antibody response to immunization depends on the absolute preimmunization titer value. We present evidence that the likelihood of a 4-fold or greater response decreases as the preimmunization titer value increases. This seemed to hold true for all serotypes examined. Our data also suggest an approximate preimmunization titer value at which the likelihood of a response approaches zero. This inflection point varied for each serotype but ranged from 4.4 to 10.3 μg/mL (Table III). Interestingly, the fold response to vaccination for all serotypes, both for children and adults, seems to hover around 2-fold (Fig 1, B). This is consistent with our previous meta-analysis of fold-response12 of a normal population.
Increasing age has been shown to be an important factor in the response to the pneumococcal vaccine. Sorenson et al9 found that in patients with recurrent respiratory tract infections, the geometric mean preimmunization and postimmunization antibody titers for all serotypes increased as children aged. Therefore older patients might be more likely to have high preimmunization titers. They also found that postimmunization specific antibody titers in adults were higher than in any pediatric age group. Some have wondered whether adults lose their ability to respond to a vaccination as they age. However, studies have shown that older patients can still mount an immunologic response to the pneumococcal vaccine, albeit that certain subsets might be nonresponders.13,14 Although our study population consisted of 90% adults and 10% children, we looked at age as a continuum. We found that age had no significant effect on the outcome. This is important when evaluating the response to the pneumococcal vaccine in older adults. The data regarding the effect of sex differences on response to the pneumococcal vaccine is conflicting. In the elderly population male subjects have been found to have higher preimmunization and postimmunization titers compared with female subjects.15,16 Gleeson et al,17 though, found that the response of elite swimmers and healthy age-matched control subjects to the pneumococcal vaccine did not differ based on sex. We found that sex had no effect on the likelihood of achieving a 4-fold or greater response to the pneumococcal vaccine.
With regard to the serum IgG level, Ko et al18 found that in patients with common variable immunodeficiency, those with a lower median serum IgG level had a decreased response to pneumococcal vaccination. However, a separate study looking at patients who had completed treatment for Hodgkin's disease and healthy control subjects did not find a correlation between mean postimmunization pneumococcal antibody concentration and IgG level.19 We found that the serum IgG level did not have a statistically significant effect on the likelihood of achieving a 4-fold or greater response to vaccination.
The response to the pneumococcal vaccine has been shown to correlate with the serum IgG2 level but not with any other IgG subclass value.19-21 In our population the serum IgG2 value did have a statistically significant effect on the outcome, but the odds ratio was 1.002, which is likely too small to be of clinical significance. The same held true for the serum IgG4 value, with an odds ratio of 1.005. Serum IgG1 and IgG3 values had no effect on the outcome.
Interpretation of the response to the 23-valent pneumococcal vaccine remains an important part of the immunologic evaluation of patients with suspected antibody deficiency. In adults in particular, the question often arises of how to assess the response for serotypes when the preimmunization titers are 1.3 μg/mL or greater. We have shown that for such titers, the geometric mean response is around 2-fold (Fig 1, B). Although a 4-fold or greater response can occur, there are preimmunization titer values for each serotype above which this response would not be expected (Table III). For such serotypes, our data suggest that the response would be uninterpretable. In addition, it appears that the response to vaccination is not affected by age, sex, IgG level, or IgG1 or IgG3 values. The effect of IgG2 and IgG4 value is not clinically significant.
In summary, we have identified an absolute preimmunization titer for each of 14 pneumococcal serotypes beyond which the probability of a 4-fold or greater response approaches zero. This inflection point varied for each serotype (Table III) and ranged from 4.4 to 10.3 μg/mL. This inflection point was identical regardless of whether the analysis was done for all preimmunization titers or was restricted to titers of 1.3 μg/mL or greater (the putative protective level). We believe that these results should help guide the interpretation of antibody response in the clinic.
Clinical implications.
Interpretation of antibody response to pneumococcal immunization is often difficult. Our results establish an absolute preimmunization titer for each pneumococcal serotype at which no significant response is expected.
Acknowledgments
Dr Smith is supported by National Cancer Institute grant P30 CA086862-09. Dr Ballas is supported by VA Merit and National Institutes of Health grant AA014418-06.
Footnotes
Disclosure of potential conflict of interest: B. J. Smith has received research support from the National Institutes of Health. Z. K. Ballas has consulting arrangements with Novartis DSMB (National Institute of Allergy and Infectious Diseases) and has received research support from Talecris Biotherapeutics, VA Merit Review, and the National Institutes of Health. N. D. Hare has declared that he has no conflict of interest.
REFERENCES
- 1.Bonilla FA, Bernstein IL, Khan DA, Ballas ZK, Chinen J, Frank MM, et al. Practice parameter for the diagnosis and management of primary immunodeficiency. Ann Allergy Asthma Immunol. 2005;94(suppl 1):S1–63. doi: 10.1016/s1081-1206(10)61142-8. [DOI] [PubMed] [Google Scholar]
- 2.Ballow M. Primary immunodeficiency disorders: antibody deficiency. J Allergy Clin Immunol. 2002;109:581–91. doi: 10.1067/mai.2002.122466. [DOI] [PubMed] [Google Scholar]
- 3.Kamchaisatian W, Wanwatsuntikul W, Sleasman JW, Tangsinmankong N. Validation of current joint American Academy of Allergy, Asthma & Immunology and American College of Allergy, Asthma and Immunology guidelines for antibody response to the 23-valent pneumococcal vaccine using a population of HIV-infected children. J Allergy Clin Immunol. 2006;118:1336–41. doi: 10.1016/j.jaci.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Siber GR, Ransil BJ, Schiffman G. Graphical method for evaluating antibody response to vaccines. Infect Immun. 1980;28:641–4. doi: 10.1128/iai.28.2.641-644.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landesman SH, Schiffman G. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev Infect Dis. 1981;3(suppl):S184–97. doi: 10.1093/clinids/3.supplement_1.s184. [DOI] [PubMed] [Google Scholar]
- 6.Ammann AJ, Schiffman G, Abrams D, Volberding P, Ziegler J, Conant M. B-cell immunodeficiency in acquired immune deficiency syndrome. JAMA. 1984;251:1447–9. [PubMed] [Google Scholar]
- 7.Rodriguez-Barradas MC, Musher DM, Lahart C, Lacke C, Groover J, Watson D, et al. Antibody to capsular polysaccharides of Streptococcus pneumoniae after vaccination of human immunodeficiency virus-infected subjects with 23-valent pneumococcal vaccine. J Infect Dis. 1992;165:553–6. doi: 10.1093/infdis/165.3.553. [DOI] [PubMed] [Google Scholar]
- 8.Janoff EN, O'Brien J, Thompson P, Ehret J, Meiklejohn G, Duvall G, et al. Streptococcus pneumoniae colonization, bacteremia, and immune response among persons with human immunodeficiency virus infection. J Infect Dis. 1993;167:49–56. doi: 10.1093/infdis/167.1.49. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen RU, Leiva LE, Javier FC, 3rd, Sacerdote DM, Bradford N, Butler B, et al. Influence of age on the response to Streptococcus pneumoniae vaccine in patients with recurrent infections and normal immunoglobulin concentrations. J Allergy Clin Immunol. 1998;102:215–21. doi: 10.1016/s0091-6749(98)70089-2. [DOI] [PubMed] [Google Scholar]
- 10.Pickering JW, Martins TB, Greer RW, Schroder MC, Astill ME, Litwin CM, et al. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am J Clin Pathol. 2002;117:589–96. doi: 10.1309/lmch-c4q2-vfl9-3t1a. [DOI] [PubMed] [Google Scholar]
- 11.Liang K-Y, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrics. 1986;73:13–22. [Google Scholar]
- 12.Go ES, Ballas ZK. Anti-pneumococcal antibody response in normal subjects: a meta-analysis. J Allergy Clin Immunol. 1996;98:205–15. doi: 10.1016/s0091-6749(96)70244-0. [DOI] [PubMed] [Google Scholar]
- 13.Artz AS, Ershler WB, Longo DL. Pneumococcal vaccination and revaccination of older adults. Clin Microbiol Rev. 2003;16:308–18. doi: 10.1128/CMR.16.2.308-318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rubins JB, Puri AK, Loch J, Charboneau D, MacDonald R, Opstad N, et al. Magnitude, duration, quality, and function of pneumococcal vaccine responses in elderly adults. J Infect Dis. 1998;178:431–40. doi: 10.1086/515644. [DOI] [PubMed] [Google Scholar]
- 15.Brandão AP, de Oliveira TC, de Cunto Brandileone MC, Gonçalves JE, Yara TI, Simonsen V. Persistence of antibody response to pneumococcal capsular polysaccharides in vaccinated long term-care residents in Brazil. Vaccine. 2004;23:762–8. doi: 10.1016/j.vaccine.2004.07.024. [DOI] [PubMed] [Google Scholar]
- 16.Sankilampi U, Isoaho R, Bloigu A, Kivelä SL, Leinonen M. Effect of age, sex and smoking habits on pneumococcal antibodies in an elderly population. Int J Epidemiol. 1997;26:420–7. doi: 10.1093/ije/26.2.420. [DOI] [PubMed] [Google Scholar]
- 17.Gleeson M, Pyne DB, McDonald WA, Clancy RL, Cripps AW, Horn PL, et al. Pneumococcal antibody responses in elite swimmers. Clin Exp Immunol. 1996;105:238–44. doi: 10.1046/j.1365-2249.1996.d01-752.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ko J, Radigan L, Cunningham-Rundles C. Immune competence and switched memory B cells in common variable immunodeficiency. Clin Immunol. 2005;116:37–41. doi: 10.1016/j.clim.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Siber GR, Schur PH, Aisenberg AC, Weitzman SA, Schiffman G. Correlation between serum IgG-2 concentrations and the antibody response to bacterial polysaccharide antigens. N Engl J Med. 1980;303:178–82. doi: 10.1056/NEJM198007243030402. [DOI] [PubMed] [Google Scholar]
- 20.Edwards E, Razvi S, Cunningham-Rundles C. IgA deficiency: clinical correlates and responses to pneumococcal vaccine. Clin Immunol. 2004;111:93–7. doi: 10.1016/j.clim.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosino DM, Schiffman G, Gotschlich EC, Schur PH, Rosenberg GA, DeLange GG, et al. Correlation between G2m(n) immunoglobulin allotype and human antibody response and susceptibility to polysaccharide encapsulated bacteria. J Clin Invest. 1985;75:1935–42. doi: 10.1172/JCI111909. [DOI] [PMC free article] [PubMed] [Google Scholar]