Abstract
Rationale
Depression is associated with medical comorbidities, particularly cardiovascular disease. However, mechanisms linking depression and cardiovascular disease remain unclear.
Objectives
This study investigated whether the rat resident–intruder model of social stress would elicit behavioral dysfunctions and autonomic changes characteristic of psychiatric/cardiovascular comorbidity. Furthermore, the efficacy of the corticotropin-releasing factor-1 (CRF1) receptor antagonist, NBI-30775 (NBI), or the tricyclic antidepressant, desipramine (DMI), to prevent social stress-induced behavioral, neuroendocrine, and cardiovascular changes were evaluated.
Methods
Adult male rats were exposed to resident–intruder stress (seven consecutive days) and systemically administered NBI (10 mg/kg/7 days), DMI (10 mg/kg/14 days), or vehicle. The efficacy of NBI and DMI to alter the behavioral and neuroendocrine responses to social stress was assessed. Furthermore, their effects on stress-induced forced swim behavior (FST), bladder and adrenal weight, and heart rate variability (HRV) were examined.
Results
NBI, but not DMI, increased time spent in an upright, defensive posture and the latency to submit to the resident. Additionally, only NBI reduced social stress-induced adrenocorticotropic hormone and corticosterone release. Social stress increased FST immobility, caused bladder and adrenal hypertrophy, and decreased HRV. Both NBI and DMI blocked stress-induced increases in immobility during the FST. However, only NBI inhibited social stress-induced adrenal and bladder hypertrophy and decreases in heart rate variability.
Conclusions
Rat resident–intruder stress paradigm models aspects of psychiatric/medical comorbidity. Furthermore, the CRF system may contribute to both the behavioral response during social stress and its behavioral and autonomic consequences, offering insight into potential therapy to treat these comorbid conditions.
Keywords: Resident–intruder, Heart rate variability, NBI-30775, CRH, Corticotropin
Introduction
Depression is a leading cause of disability and places a major financial burden on society of more than $100 billion annually (Greenberg et al. 2003; Kessler et al. 2005a, b). In addition to the debilitating neurobehavioral consequences of depression, there is a strong association between depression and serious medical disorders including diabetes, irritable bowel syndrome, and cardiovascular disease (Campayo et al. 2011; Fenton and Stover 2006; Folks 2004). Importantly, depression significantly increases the risk of cardiac morbidity and mortality (Rugulies 2002; Surtees et al. 2008; Van Der Kooy et al. 2007). Despite the clinical relevance of an association between depression and cardiovascular disease, little is known of the pathophysiology or mediators underlying this comorbidity.
Because stress is a common risk factor for both depression and cardiovascular disease, investigating the neural pathways or substrates that mediate the stress response may provide clues as to the shared pathophysiology that links depression and cardiovascular disease. Social stress is one of the most common stressors encountered by humans and has been implicated in both depression and cardiovascular disease (Albus 2010; Bjorkqvist 2001; Friedmann et al. 2006). The resident–intruder model is an ethologically relevant animal model of psychosocial stress in rats that involves subjecting a male rat (intruder) to aggressive threats by a larger, unfamiliar male rat (resident) by placing it in the resident’s home cage. Rodents exposed to daily intermittent social stress exhibit behavioral changes often associated with depressive disorders such as decreased motivation, increased behavioral despair (indicated by increased immobility in the Porsolt forced swim test), anhedonia, and decreased social interactions (Becker et al. 2008; Rygula et al. 2005; Von Frijtag et al. 2000; Wood et al. 2010). It has also been reported that 5 weeks of chronic social stress exposure had distinct effects compared with four intermittent exposures, and chronic exposure resulted in anhedonia (Miczek et al. 2011). Like psychosocial stress in humans, social stress in rats has been well characterized as engaging the sympathetic nervous system (Sgoifo et al. 1996, 1999), and brief intermittent exposure to social stress results in long-lasting changes in normal circadian heart rate rhythmicity (Sgoifo et al. 1999, 2001, 2002; Tornatzky and Miczek 1993). Additionally, using this model, we and others demonstrated that social stress can cause severe urinary dysfunctions and bladder pathology (Chang et al. 2009; Wood et al. 2009a). Therefore, the resident–intruder model of social stress represents a useful animal model to study neural systems and substrates involved in the comorbidity of affective and medical dysfunctions seen in depression and may be used to identify novel therapies to treat this condition.
While many systems are acutely activated during stress and contribute to a healthy, adaptive stress response, dysfunction of the hypothalamic–pituitary–adrenal (HPA) stress axis is thought to occur during depression (Checkley 1996; Gold and Chrousos 2002; Nemeroff et al. 1984; Raadsheer et al. 1994). Corticotropin-releasing factor (CRF) is the initiating hormone in the HPA axis and acts as a neurotransmitter outside of the HPA axis to coordinate autonomic and behavioral aspects of the stress response with HPA activation (Dunn and Swiergiel 2008; Holsboer and Ising 2008; Vale et al. 1981; Wood and Woods 2007). Increased CRF function at the level of both the HPA and extrahypothalamic regions has been hypothesized to occur in depression and accounts for abnormal glucocorticoid levels and responses seen in some patients, decreases in motivation, and anxiogenesis that characterizes depression (Checkley 1996; Gold and Chrousos 2002; Nemeroff et al. 1984; Raadsheer et al. 1994; Raison and Miller 2003; Wong et al. 2000). Because CRF elicits both behavioral and autonomic aspects of the stress response, excessive activation of the CRF system represents a putative mechanism that could contribute to comorbid psychopathology and autonomic disorders, including cardiovascular disease.
The current study sought to determine whether repeated social stress generates persistent changes in autonomic function that could contribute to cardiovascular disease in combination with behavioral dysfunction in rodents. Particularly, we examined heart rate variability (HRV) in this model because reduced HRV is a common cardiovascular finding in depressed patients that is thought to contribute to increased cardiac risk (Udupa et al. 2007). The ability of a CRF1 receptor antagonist, NBI-30775, or a classical antidepressant agent, desipramine hydrochloride (DMI), to attenuate the behavioral and/or cardiovascular consequences of social stress was assessed. Additionally, these studies sought to determine the efficacy of these agents to reverse social stress-induced bladder hypertrophy.
Materials and methods
Animals
Male Sprague Dawley rats (275–300 g) were used as controls or intruders, and male Long–Evans retired breeders (650–850 g) served as residents (Charles River, Wilmington, MA). Rats were singly housed with a 12-h light–dark cycle (lights on at 0700 hours) in a climate-controlled room. Food and water were available ad libitum. All studies were approved by the Children’s Hospital of Philadelphia Institutional Animal Care and Use Committee and followed the Principles of Laboratory Animal Care and the Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. All experimentation was conducted between 0930 and 1300 hours.
Surgery
Telemetric ECG transmitters (models TA11CTA-F40, Data Sciences, Transoma Medical, Inc., St. Paul, MN, USA) were implanted into the peritoneal cavity of all rats as previously reported (Wood et al. 2009b). Rats were singly housed and allowed 6–7 days to recover before testing. Rats that were administered DMI and their vehicle controls were also implanted subcutaneously with iPRECIO programmable microinfusion pumps generously donated by Primetech Corporation (Tokyo, Japan) and Data Sciences International (St. Paul, MN, USA).
Social stress
The social stress utilized in these studies was modified from the resident–intruder model originally developed by Miczek et al. (1979). Rats were randomly assigned to either a social stress or control exposure for 30 min on seven consecutive days identical to Wood et al. (2010). Social stress exposure resulted in intruder subordination, termed defeat, and was operationally defined by the intruder assuming a supine posture that was held for approximately 3 s. Latency to exhibit a supine posture was recorded and averaged over the seven exposures. Controls were placed in a novel cage behind a partition for 30 min daily. Rats were returned to their home cage after each session. Body weights were recorded on days 1 and 8. Bladders and adrenal glands were removed and weighed postmortem at 48 or 72 h after the seventh social stress or control exposure in the NBI-30775 and DMI groups, respectively.
Drug treatments
NBI-30775
Rats were treated on days 1–7 of social stress with either vehicle (10% solution of 0.1 M tartaric acid in water, n=15) or NBI-30775 (n=11, 10 mg/kg, s.c.) 60 min prior to exposure to the resident–intruder stress. Control rats were treated with either vehicle (n=8) or NBI-30775 (n=5, 10 mg/kg, s.c.) 60 min prior to the control exposure. The single dose and treatment regimen of NBI-30775 was selected based on studies indicating that it occupies 80% of brain CRF1 receptors (Gutman et al. 2003; Heinrichs et al. 2002) and blocks stress-induced ACTH release (Jutkiewicz et al. 2005) and social defeat-induced avoidance behavior (Erhardt et al. 2009).
Desipramine HCl
The iPRECIO Pumps were set to dispense either vehicle (sterile water, n=9) or 10 mg/kg/day of DMI (n=9) for 14 days, and infusion rates increased every second day to account for increases in body weight. The resident–intruder stress occurred on days 8–14 of treatment. Pumps were programmed to turn off on the last day of social stress. Control rats (n=6) received a sham pump implantation and therefore were untreated. The DMI dose and treatment regimen were selected based on studies reporting the antidepressant efficacy of 10 mg/kg/day DMI in rats (Lopez-Rubalcava and Lucki 2000). DMI was dissolved in sterile water. The volume remaining in the pumps was measured postmortem to confirm that the pumps dispensed an accurate dose during treatment.
Hormone analysis
Tail vein blood was collected immediately following the first exposure to social stress (30 min) and assayed for adrenocorticotropic hormone (ACTH) and corticosterone levels using commercially available RIA kits (MP Biomedicals) as previously described (Grissom et al. 2008; Wood et al. 2010).
Behavioral analysis
Social stress behavior
The first and fourth exposures to social stress were video recorded for 15 randomly selected rats in the NBI group (n=7 vehicle, n=8 NBI) and all socially stressed rats in the DMI group (n=9 vehicle, n=9 DMI). An observer blinded to the conditions quantified the duration of time spent in an upright posture. A behavior was classified as a defensive upright posture if the intruder rat was facing the resident with both forepaws elevated above the cage floor.
Forced swim test
The modified Porsolt forced swim test (FST) was conducted and analyzed as previously published (Jutkiewicz et al. 2005). Briefly, rats were exposed to 40 cm of 25°C water for 15 and 5 min on two consecutive days. The subject’s behavior was videotaped during each swim session, and a trained observer blinded to the treatment condition classified the rat’s behavior every 5 s as immobility, swimming, or climbing (Detke et al. 1995). Total behavioral counts for each rat were averaged within each treatment group. Rats included in the NBI-30775 or DMI study were exposed to the FST 24 and 48 h or 48 and 72 h, respectively, after the seventh and final social stress or control exposure to allow adequate time for the drug to be metabolized.
Cardiovascular data acquisition and analysis
ECG activity was monitored in freely moving rats using the telemetric acquisition system Dataquest ART 4.2 (Data Sciences, Transoma Medical, St. Paul, MN, USA). The Ponemah Physiology Platform 4.80 software (Data Sciences) was used to analyze the ECG complexes. RR interval and heart rate were calculated on a beat-to-beat basis, and manual editing of RR data was performed to ensure correct identification of every QRS complex. Data were exported in 5-s bins, and the following time domain indices of HRV were calculated: the standard deviation of normal RR intervals (SDNN) and the root mean square of the standard deviations of adjacent normal RR intervals (rMSSD) within 3-min bins of data were calculated. Heart rate variability measures fluctuations in autonomic input to the heart. RMSSD reflects vagal input to the heart, and SDNN reflects the sympathovagal balance such that reductions in variability represent an increase in sympathetic activity and/or parasympathetic withdrawal (Pagani et al. 1986; Ramaekers et al. 2002). Two to three determinations of SDNN and rMSSD were averaged for each rat under resting home cage conditions on the first and seventh day of social stress (after drug treatment) and 24 h (NBI group) or 48 h (DMI group) after the final social stress or control exposure (in the absence of drug treatment) in NBI-and DMI-treated rats. Spectral analysis of HRV has revealed that enhanced sympathetic drive to the heart is associated with an increased low frequency (LF) and decreased high-frequency (HF) component of the power spectrum, resulting in a marked increase in the LF/HF ratio (Pagani et al. 1986; Ramaekers et al. 2002). Frequency domain analysis of HRV was conducted using Dataquest ART 4.2 Analysis. Two to three 30-s segments of ECG waveforms were selected from the baseline, resting condition 24 h (NBI group) or 48 h (DMI group) after the final social stress or control exposure. The segments chosen for analysis were free of movement and arrhythmia artifacts. Fast Fourier transformation of these segments of data was performed, and spectral power was quantified within the following frequency bands: LF power, 0.04 to 1.00 Hz, and HF power, 1.00 to 3.00 Hz (Kuwahara et al. 1994). The average of 2–3 LF/HF ratios were quantified for each rat under resting, drug treatment-free conditions.
Statistical analysis
The effects of NBI and DMI on social stress-induced behavior and ACTH and corticosterone release were quantified using separate unpaired Student’s t tests that compared socially stressed rats treated with vehicle versus drug (Table 1). To determine whether the behavioral response to social stress was related to the latency to exhibit a supine defeat posture, average defeat latency was correlated with the duration of upright posture on day 4. The effects of drug treatment on stress-induced deficits in body weight gain, adrenal and bladder hypertrophy, HR, and HRV were evaluated using two-way ANOVAs (stress × treatment) followed by Holm–Sidak’s multiple comparison method for the NBI group and one-way ANOVAs followed by Tukey’s multiple comparison tests for the DMI group to evaluate differences between control rats and socially stressed rats treated with vehicle and those treated with drug (Tables 2 and 3, p Fig. 2). Two-way ANOVAs followed by Holm–Sidak’s multiple comparison method were used within the NBI group to identify stress × treatment effects on immobility, swimming, and climbing behavior during the FST (Fig. 1). Separate one-way ANOVAs followed by Tukey’s multiple comparison test were also used to identify differences in immobility, swimming, and climbing behavior during the FST between DMI-treatment groups. In addition, to determine whether the cardiovascular repercussions of social stress were related to the latency to exhibit a defeat posture, average defeat latency was correlated with the LF/HF ratio. For all analyses, two-tailed values of <0.05 were considered significant.
Table 1.
Effect of NBI and DMI on behavioral response to social stress and stress-induced ACTH and corticosterone
| Treatment | Average defeat latency (s) | Change in upright posture (s; day 4–day 1) | ACTH (pg/ml) (day 1 post-stress) | Cort (ug/dL) (day 1 post-stress) |
|---|---|---|---|---|
| Stress/NBI-vehicle (n=8) | 423±41 | (−)67±50 | 316±21 | 31.5±1.5 |
| Stress/NBI-30775 (n=8) | 560±38* | 96±67* | 228±27* | 16.7±2.1** |
| Stress/DMI-vehicle (n=10) | 539±67 | (−)80±70 | 315±22 | 25.1±2.7 |
| Stress/DMI (n=10) | 512±90 | (−)12±42 | 288±36 | 29.5±3.9 |
p<0.05;
p=0.0001; Student’s t test vs. stress/vehicle
Table 2.
Effect of NBI and DMI on social stress-induced physiological changes
| Treatment | Daily body weight gain (g) | Adrenal weight/100 g body wt | Bladder/body weight |
|---|---|---|---|
| Control/NBI-vehicle (n=10) | 6.9±0.3 | 11.6±0.5 | 0.35±0.02 |
| Control/NBI-30775 (n=5) | 5.4±0.5 | 14.5±0.8 | 0.38±0.02 |
| Stress/NBI-vehicle (n=17) | 4.3±0.3* | 14.0±0.7*,**** | 0.40±0.02*****,**** |
| Stress/NBI-30775 (n=15) | 4.8±0.3* | 11.3±0.4 | 0.31±0.02 |
| Control/sham (n=6) | 5.8±0.5 | 11.7±1.0 | 0.35±0.03 |
| Stress/DMI-vehicle (n=10) | 4.8±0.3** | 13.1±0.8 | 0.40±0.03 |
| Stress/DMI (n=10) | 3.2±0.4*** | 12.6±1.0 | 0.44±0.03****** |
p<0.01 vs. control/NBI-vehicle;
p<0.05 vs. stress/DMI;
p<0.01 vs. control/sham;
p<0.01 vs. stress/NBI-30775;
p=0.09 vs. control/NBI-vehicle;
p<0.05, t test vs. control/sham
Table 3.
Effect of NBI and DMI on resting cardiovascular parameters
| Treatment | 7th day of social defeat (+ treatment)
|
24–48 h after defeat (no treatment)
|
||||
|---|---|---|---|---|---|---|
| SDNN | rMSSD | HR | SDNN | rMSSD | HR | |
| Control/NBI-vehicle (n=7) | 5.8±0.3 | 4.7±0.1 | 363±7 | 5.7±0.5 | 5.1±0.7 | 369±5 |
| Control/NBI-30775 (n=5) | 6.5±0.4 | 5.2±0.6 | 360±11 | 7.1±0.8 | 7.0±0.9* | 350±15 |
| Stress/NBI-vehicle (n=15) | 4.7±0.3*,** | 4.4±0.3 | 371±7 | 4.2±0.3*,** | 4.5±0.3 | 365±5 |
| Stress/NBI-30775 (n=11) | 6.2±0.6 | 5.1±0.4 | 376±8 | 6.7±0.4 | 5.3±0.3 | 366±5 |
| Control/sham (n=6) | 8.0±0.5 | 6.0±0.3 | 350±12 | 6.5±0.5 | 5.3±0.3 | 370±16 |
| Stress/DMI-vehicle (n=10) | 5.7±0.6*** | 5.8±0.6 | 359±7 | 5.2±0.4 | 5.7±0.4 | 367±7 |
| Stress/DMI (n=10) | 5.7±0.9*** | 5.0±0.4 | 393±11****, ***** | 4.7±0.5**** | 3.9±0.5****, ***** | 414±9****,***** |
p<0.05 vs. control/NBI-vehicle;
p=0.01 vs. stress/NBI-30775;
p<0.57, one-way ANOVA;
p<0.05 vs. control/sham;
p<0.05 vs. stress/DMI-vehicle
Fig. 2.
A representative power spectrum from each treatment group 24 h (NBI-30775 group) or 48 h (DMI group) after the final social stress exposure and drug treatment; a1 control rat treated with the NBI vehicle; a2 social stress rat treated with NBI vehicle; a3 social stress rat treated with NBI-30775; b1 control rat with a sham pump surgery; b2 social stress rat treated with DMI vehicle; b3 social stress rat treated with DMI. The power spectrum from each control rat shows two peaks, one at the low-frequency end (0.04–1 Hz, LF) and a higher frequency (1–3 Hz). The high-frequency peak is absent in stressed rats treated with vehicle (a2, b2), while prior treatment with NBI-30775 (a3), but not DMI (b3), blocks this effect. a4 and b4 represent the ratio of the two components of the power spectral density of heart rate variability for each group. The relative LF/HF ratios indicate that social stress (n=15) increases the sympathovagal balance as compared with vehicle-treated controls (n=6), and prior treatment with NBI-30775 (n=8) blocks this effect (a4). Socially stressed rats treated with the DMI vehicle (n =8) also exhibit an increased LF/HF ratio compared with controls (n=5). However, DMI treatment (n=7) had no effect on these social stress-induced changes (b4). ap<0.05 vs. control/vehicle; bp<0.05 vs. stress/NBI-30775; cp<0.05 vs. control/sham
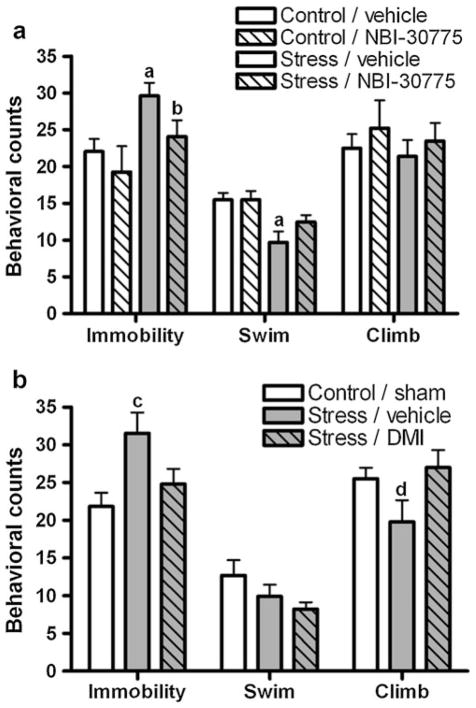
Fig. 1.
Treatment with NBI-30775 and DMI during social stress blocked subsequent stress-induced changes in Porsolt FST behavior. a Stress/vehicle rats (solid gray bar, n=13) displayed increases in immobility indicative of a depressive-like behavioral response and a reduced incidence of swimming behaviors compared with control/vehicle (open bars, n=15) or control/NBI-30775 (hashed open bar, n=5). Interestingly, stressed rats that were treated with NBI-30775 (hashed gray bar, n=11) during the seven exposures to social stress did not exhibit stress-induced behavioral changes. b Similarly, social stress-induced increases in immobility observed in stress/vehicle rats (gray bar, n=10) as compared with control/sham (open bar, n=6) were also inhibited by prior treatment with DMI (gray hashed bar, n=10). ap<0.01 vs. control/vehicle, bp<0.05 vs. stress/vehicle, cp<0.05 vs. control/sham, dp<0.05 vs. stress/DMI
Results
Effect of drug treatments on behavioral responses to social stress
Chronic NBI-30775 (stress/NBI-30775) increased the average latency to exhibit a submissive, supine posture (defeat latency) compared with stress/vehicle rats (Table 1). NBI-treated rats also spent more time engaged in an upright, defensive posture during the social stress exposure (Table 1). Interestingly, the average defeat latency of all treated and untreated rats was found to be positively correlated with the duration of upright posture expressed on day 4 (Pearson r=0.48, p=0.012). In contrast to NBI, chronic DMI (stress/DMI) had no effect on the average defeat latency as compared with stress/vehicle rats (p=0.4) (Table 1). DMI also did not change the time spent engaged in upright postures during the social stress exposure (p=0.4) (Table 1). It should also be noted that there was no significant difference between vehicle-treated groups (p=0.13).
Effect of drug treatments on hormonal responses to social stress
ACTH release in response to the first social stress exposure was blunted in rats treated with NBI-30775 as compared with vehicle-treated rats (Table 1). Similarly, social stress-induced corticosterone release was also blunted in rats treated with NBI-30775 as compared with vehicle treatment (Table 1). These results confirm the efficacy of a 10-mg/kg dose of NBI-30775 to effectively block CRF1 receptors during social stress. In contrast, DMI did not affect social stress-induced ACTH (p=0.5) or corticosterone (p=0.4) release as compared with vehicle-treated rats.
Effect of drug treatments on physiological consequences of social stress
Table 2 shows the effects of social stress and drug treatments on body and organ weights. There was a significant stress × treatment interaction for body weight gain within the NBI group (F(1,46) =7.3, p =0.01). Social stress/vehicle treatment resulted in impairment in body weight gain as compared with the control/vehicle. However, this was unaffected by treatment with NBI-30775 (Table 2). There was also a significant stress × treatment interaction for adrenal gland weight (F(1,43) =14.1, p<0.001). Post hoc analysis revealed increased adrenal weights in stress/vehicle vs. control/vehicle, and this was blocked by treatment with NBI-30775 (Table 2). A two-way ANOVA also revealed a significant stress × treatment interaction for bladder/body weight (F(1,44)=6.9, p =0.012). There was a trend for increased bladder/body weight in stress/vehicle as compared with control/vehicle (p=0.09) as previously reported in untreated socially stressed rats (Wood et al. 2010). NBI-30775 treatment prevented this effect (Table 2).
A one-way ANOVA revealed a reduction in body weight gain in stress/vehicle rats that was exacerbated by treatment with DMI (F(2,26)=10.7, p=0.0005; Table 2). Due to high variability, the effects of social stress on adrenal weights in the vehicle- and drug-treatment groups were not significantly different from control rats. In addition, the increases in bladder weight observed in the stressed rats treated with vehicle and DMI were below the level of significance (F(2,25)=2.4, p=0.1). However, it is clear that DMI treatment did not block social stress-induced bladder hypertrophy as an unpaired Student’s t test revealed that stress/DMI-treated rats have significantly greater bladder weight compared with the control/sham (Table 2).
Effect of drug treatments on behavioral consequences of social stress
There was a significant effect of stress (F(1,40) =9.4, p =0.004) and NBI-30775 treatment (F(1,40)= 4.7, p=0.04) on immobility during the FST (Fig. 1a). Stress/vehicle-treated rats exhibited an increase in the incidence of immobility as compared with the control/vehicle. Treatment with NBI (stress/NBI) blocked the development of this social stress-induced increase in passive, immobile behavior (Fig. 1a). There was also a significant effect of stress on swimming behavior (F(1,40) =9.3, p =0.004). Social stress (stress/vehicle) produced a decrease in swimming behaviors compared with the control/vehicle (Fig. 1a). NBI treatment (stress/NBI) did not significantly block this effect. There were no significant effects of stress (p=0.12) or treatment (p=0.27) on climbing behavior.
In the DMI study, a one-way ANOVA revealed a significant effect of social stress on FST immobility (F(2,23) =4.2, p=0.029; Fig. 1b); stress/vehicle-treated rats exhibited greater immobility as compared with the control/sham (Fig. 1b). This effect was absent in stress/DMI-treated rats (Fig. 1b). There were no significant differences in swimming behavior between treatment groups. However, there was a significant effect of stress on climbing behaviors (F(2,23)= 4.5, p=0.02); stress/vehicle-treated rats exhibited less climbing behaviors than stress/DMI (Fig. 1b).
Effects of drug treatments on cardiovascular consequences of social stress
To identify the persistent effects that social stress has on autonomic cardiovascular function, time domain indices of HRV were quantified. The rMSSD and SDNN values reflect vagal input to the heart and the sympathovagal balance, respectively. Reductions in the variability of these measures indicate an increase in sympathetic activity and/or parasympathetic withdrawal. First, it was confirmed that there were no differences in resting SDNN or heart rate (HR) between rats assigned to all four NBI-30775 groups 20 min before the first social stress or control exposure (data not shown). There was a significant effect of treatment on resting rMSSD (F(1,41)=6.1, p=0.02) prior to the first stress exposure. Rats treated with NBI exhibited slightly elevated rMSSD as compared with vehicle-treated rats which is indicative of increased vagal tone (control/vehicle, 4.6±0.3; control/NBI, 5.7±0.5; stress/vehicle, 4.1±0.3; stress/NBI, 5.4±0.3). Interestingly, assessment of resting HRV after rats had experienced six social defeat exposures revealed a persistent sympathomimetic effect that was blocked by treatment with NBI (treatment effect, F(1,41) =6.7; p =0.01; Table 3). In particular, 20 min before the seventh exposure to social stress, post hoc analysis revealed that resting SDNN was significantly lower in stress/vehicle rats compared with the control/vehicle (Table 3). This effect was blocked by treatment with NBI-30775. There were no significant effects of treatment or stress on rMSSD or HR on the seventh day of stress. On day 8 (24 h after the final social stress and treatment), there was a persistent treatment effect on resting SDNN (F(1,41) =12.8, p<0.001). SDNN was still reduced in stress/vehicle rats as compared with control/vehicle rats and prior to treatment with NBI-30775 blocked the sympathomimetic effect of social stress despite the absence of drug treatment on day 8 (Table 3). There was also an overall significant effect of stress (F(1,42)=5.5, p=0.02) and treatment (F(1,42)=8.1, p=0.007) on rMSSD on day 8. Post hoc comparisons also revealed a significant effect of treatment on rMSSD within control rats comparable to the NBI-30775-induced increase in vagal tone observed on day 1. There were no differences in HR on day 8.
In addition to time domain indices of HRV, spectral analysis also revealed enhanced sympathetic drive to the heart. Figure 2a shows representative power spectra of ECG waveforms from a control/vehicle rat, a stress/vehicle rat, and a stress/NBI-30775 rat. The power spectrum from the control rat shows two peaks, a relatively high-amplitude peak at the low-frequency end (LF, 0.04–1 Hz) and a smaller peak within the high-frequency range (HF, 1–3 Hz; Fig. 2, a1). Exaggerated sympathetic activity is associated with an increased LF and decreased HF component of the power spectrum, resulting in a marked increase in the LF/HF ratio. Spectral analysis of HRV 24 h after the final social stress exposure revealed a significant stress × treatment interaction (F(1,32) =4.3, p=0.048). There was a slight increase in LF and a decrease in the HF peak resulting in an increased LF/HF ratio in social stress/vehicle rats as compared with control/vehicle (Fig. 2, a4). This effect was blocked by prior treatment with NBI-30775 (Fig. 2, a4).
Resting HRV parameters were also evaluated in rats included in the DMI study prior to the first social stress or control exposure. There were no differences in resting SDNN (p=0.3), rMSSD (p=0.2), or HR (p=0.4) between control/vehicle, stress/vehicle, or stress/DMI rats prior to the first exposure to social stress or control conditions (data not shown). DMI treatment began 7 days prior to the first stress or control exposure, and therefore, these data indicate that 7 days of DMI treatment has no independent cardiovascular effects. Consistent with the NBI study results, 20 min before the seventh exposure to social stress, resting SDNN was reduced in stress/vehicle rats (one-way ANOVA just below the level of significance: F(2,21)=3.3, p=0.057; Table 3). However, unlike NBI-30775, DMI treatment did not block this effect (Table 3). There were no differences in rMSSD between groups (p=0.4). Heart rate, on the other hand, was elevated in stress/DMI-treated rats (F(2,23)=5.1, p=0.02). Similar to day 7 results, there was a trend for resting SDNN to be reduced in stress/vehicle rats on day 9 (48 h after the final social stress and treatment) and significantly reduced in stress/DMI rats (F(2,23) =3.7, p=0.04). rMSSD was also significantly lower in stress/DMI rats compared with stress/vehicle rats despite the absence of drug treatment on day 9 (F(2,23)=5.0, p=0.02). A significantly greater HR also persisted in stress/DMI rats despite lack of treatment on day 9 (F(2,32)=7.0, p=0.004; Table 3).
Figure 2b shows representative power spectra of ECG waveforms from a control/sham rat, a stress/vehicle rat, and a stress/DMI rat. The power spectrum from this control rat also shows two peaks, one at the LF range (0.04–1 Hz) and one within the HF range (1–3 Hz, Fig. 2, b1). Consistent with the time domain indices of heart rate variability, 48 h after the final social stress exposure, there was a decrease in the high-frequency peak so that the LF/HF ratio increased in stress/vehicle-treated rats as compared with control/sham rats (F(2,19) =4.1, p =0.036; Fig. 2, b4). Unlike NBI-30775, DMI treatment did not prevent stress-induced changes in HRV; stress/DMI rats exhibited an increase in LF/HF compared with the controls (Fig. 2, b4).
Individual differences in cardiovascular consequences of social stress
To determine whether a submissive phenotype was related to the severity of cardiovascular repercussions, spectral analysis of HRV was correlated with defeat latency in vehicle-treated rats. There was a trend for LF/HF to be negatively correlated with defeat latency (p=0.06, r=−0.47). Furthermore, classifying rats as exhibiting a short latency (SL) or long latency (LL) phenotype as previously published (Wood et al. 2010) reveals an exaggerated increase in the LF/HF ratio associated with the passive SL phenotype (one-way ANOVA: F(2,20)=5.9, p=0.011). The LF/HF ratio was significantly greater in the SL rats (20.6±4.2) as compared with control (6.6±1.1, p<0.01) and LL rats (11.9±1.8, p<0.05).
Discussion
This study demonstrated that social stress in rodents concurrently elicits persistent depressive-like behaviors and cardiovascular changes consistent with exaggerated sympathetic drive as evidenced by decreased HRV and increased LF/HF. The cardiovascular consequences of social stress in the rodent model correspond well with clinical literature indicating that depression is associated with a reduction in HRV (Kemp et al. 2010; Udupa et al. 2007). Importantly, this results in an increased risk of cardiac morbidity and mortality in depressed patients (Carney et al. 2001). The present findings highlight the ability of the resident–intruder stress paradigm to model this psychiatric/cardiovascular comorbidity. Clinical studies suggest that despite successfully alleviating the behavioral symptoms of depression, traditional antidepressants have not demonstrated efficacy towards treating the autonomic dysfunction (Kemp et al. 2010). The current results agree with this, as treatment with a tricyclic antidepressant (DMI) blocked the development of the behavioral consequences of social stress but did not prevent the changes in heart rate variability. Importantly, the finding that NBI-30775 selectively blocked the development of the behavioral and cardiovascular repercussions of social stress implicates CRF as a link between stress-related psychiatric and cardiovascular pathology and as a novel target for the treatment of these comorbid pathologies.
Behavioral repercussions of social stress in rodents have previously been reported and bear semblance to symptoms of human depression. For example, rats exposed to social stress exhibit anhedonia, motivational deficits, and increased immobility in the Porsolt FST (Rygula et al. 2005; Wood et al. 2010). Furthermore, these behavioral changes are reversed upon antidepressant treatment (Rygula et al. 2006a, b). The current findings are in agreement with this literature. This study is unique in demonstrating that social stress elicits persistent changes in resting autonomic function in addition to depressive-like behaviors.
Long-lasting shifts in circadian rhythm of heart rate have been reported as a consequence of intermittent exposure to social stress (Tornatzky and Miczek 1993). However, this study is the first to report a shift in resting spectral analysis of HRV. Based on these indices, the present study revealed that exposure to social stress increases resting sympathetic drive and/or reduces parasympathetic contribution. Surprisingly, these changes in HRV did not result in a change in resting heart rate in stressed rats. While this is unexpected, these data could be affected by our short 20-min resting recording segment. Overall, the present data reveal a shift in autonomic function consistent with greater sympathetic activity in socially stressed rats and correlate with clinical observations in depressed patients.
It is important to note that the response to stress (Gomez et al. 1989; Sgoifo et al. 1996, 1999; Wood et al. 2010) and consequences of stress exposure (Grippo et al. 2004; Meerlo et al. 1999; Stefanski 1998; Wood et al. 2010) occur with substantial variation. We previously reported that rats exhibiting a submissive coping strategy resulting in an average defeat latency of less than 300 s (termed SL) were more susceptible to developing HPA dysfunction and exaggerated immobility in the FST as compared with rats exhibiting an active coping strategy and a long latency phenotype (LL, >300 s). In agreement with our findings, Meerlo et al. (1999) identified a negative relationship between the number of counterattacks during social stress and subsequent reduction in heart rate circadian rhythmicity. In the present study, there was a trend for LF/HF ratio to be negatively correlated with defeat latency. Furthermore, classification of socially stressed rats as exhibiting the SL or LL phenotype revealed that SL rats exhibited greater increases in LF/HF than both controls and LL rats. These results indicate that a submissive behavioral coping strategy may be related to greater cardiovascular disease risk and underlies the importance of studying individual differences in vulnerability to stress-related pathologies.
Rats treated with NBI-30775 during exposure to social stress were not only resistant to developing depressive-like behaviors in the forced swim test but also remained resistant to the cardiac autonomic changes both before the seventh stress exposure (in the presence of NBI) and 24 h later (in the absence of NBI treatment). CRF has been linked to the anxiogenic effects of stress (Stenzel-Poore et al. 1996; Zorrilla and Koob 2004; Zorrilla et al. 2002), and daily CRF1 receptor antagonism was recently shown to prevent the development of social stress-induced locomotor sensitization to a cocaine challenge (Boyson et al. 2011). Furthermore, CRF1 receptor activation in select brain regions is also known to contribute to stress-induced sympathetic activation (Brown et al. 1982; Chu et al. 2004; Kurosawa et al. 1986; Nijsen et al. 2000). The dampening of sympathetic activation together with the para-sympathomimetic effect of CRF1 antagonist treatment has been previously reported to be centrally mediated and likely contributes to the observed cardioprotective effects of NBI-30775 (Nijsen et al. 2000; Wood et al. 2006). Therefore the present study confirms our supposition that treatment with a centrally acting CRF1 antagonist during exposure to social stress would prevent the development of stress-induced behavioral and autonomic dysfunction.
In contrast, DMI treatment produced a behavioral antidepressant-like effect but did not exhibit a protective effect over the stress-induced autonomic dysfunction. DMI has been reported to exhibit acute sympathomimetic effects, and therefore resting cardiovascular parameters were evaluated 48 h after the final social stress and DMI treatment. The resting cardiovascular parameters measured both in the presence and absence of DMI indicated that socially stressed rats still exhibited exaggerated sympathetic drive as evidenced by a decrease in SDNN, increase in LF/HF ratio, and tachycardia, despite treatment with the antidepressant during exposure to social stress. Together, these results indicate that CRF1 receptor activation may contribute to stress-induced cardiovascular dysfunction. While the traditional tricyclic DMI did not prevent the cardiovascular repercussions of social stress, CRF1 antagonists represent a potential novel therapy for depressed patients with comorbid cardiovascular pathology.
In addition to the current findings that social stress results in cardiovascular dysfunction, we previously reported the negative impact it has on neuroendocrine and urodynamic function (Wood et al. 2009a, 2010). Therefore, in the present study, we also measured adrenal and bladder weights to identify the effect of antidepressant treatment on these stress-sensitive systems. NBI-30775 treatment reduced stress-induced HPA activity as indicated by ACTH and corticosterone levels after the first social stress exposure and blocked the development of adrenal hypertrophy evident in socially stressed rats. We also reported that exaggerated CRF may be related to bladder hypertrophy and urinary dysfunction in socially stressed rats (Wood et al. 2009a). Interestingly, in the present study, socially stressed rats treated with NBI-30775 have significantly lower bladder weights than those treated with vehicle, further supporting the role of CRF in social stress-induced bladder dysfunction. DMI, on the other hand, did not have comparable effects. As such, CRF is also a potential neuromodulator that underlies psychiatric/urologic comorbidity and is a novel target for the treatment of this comorbid medical pathology.
These data clearly indicate that NBI-30775 affects the consequences of the resident–intruder stress. Furthermore, NBI-30775, but not DMI, also changed the behavioral response to social stress as it shifted the defensive stress response from one that was submissive to one that was more aggressive. This was indicated by an increase in the time spent in an upright posture that was correlated to increased average latency to exhibit a submissive, supine posture (defeat latency). These findings implicate CRF1 in the acute behavioral response to the stressor, as well as in the consequences of stress. We previously demonstrated that more rapid submission was related to vulnerability to the consequences of social stress (Wood et al. 2010). Consistent with this, the ability of NBI-30775 to shift the defensive strategy to a more active mode was associated with a general resilience to the consequences of social stress.
The present study supports the use of the resident–intruder paradigm in modeling the comorbidity between stress-induced affective disorders and cardiovascular disease. Furthermore, the present results highlight the potential contribution of the CRF system in behavioral responses to social stress and behavioral and physiological consequences of social stress. Although these studies revealed the ability of NBI-30775 to block the development of social stress-induced behavioral and cardiovascular dysfunction, future studies should determine whether CRF antagonist treatment will be effective in reversing these repercussions. Overall, these studies implicate exaggerated CRF activity as a contributing factor to affective-cardiovascular disease comorbidity and offer insight into potential therapy to treat this condition.
Acknowledgments
These studies were supported by the following grants: MH06751 to SB, ARO-58077LS DPR, MH40008, MH58250, and AHA0825572D.
The authors would like to thank Data Sciences International and Primetech Corporation for their generous donation of the iPRECIO infusion pumps used in this study. The authors also thank Catherine S. Lee and Sandra Luz for their technical assistance. All experiments complied with the current laws of animal research in the USA.
Footnotes
Conflict of interest None.
The authors have nothing to disclose.
Contributor Information
Susan K. Wood, Email: susankwood2@gmail.com, Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA. 3615 Civic Ctr Blvd, ARC suite 402E, Philadelphia, PA 19104, USA
Kile V. McFadden, Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA
Dimitri Grigoriadis, Neurocrine Biosciences, Inc., San Diego, CA 92130, USA.
Seema Bhatnagar, Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA.
Rita J. Valentino, Department of Anesthesiology and Critical Care Medicine, The Children’s Hospital of Philadelphia, University of Pennsylvania School of Medicine, Philadelphia, PA 19104, USA
References
- Albus C. Psychological and social factors in coronary heart disease. Ann Med. 2010;42:487–494. doi: 10.3109/07853890.2010.515605. [DOI] [PubMed] [Google Scholar]
- Becker C, Zeau B, Rivat C, Blugeot A, Hamon M, Benoliel JJ. Repeated social defeat-induced depression-like behavioral and biological alterations in rats: involvement of cholecystokinin. Mol Psychiatry. 2008;13:1079–1092. doi: 10.1038/sj.mp.4002097. [DOI] [PubMed] [Google Scholar]
- Bjorkqvist K. Social defeat as a stressor in humans. Physiol Behav. 2001;73:435–442. doi: 10.1016/s0031-9384(01)00490-5. [DOI] [PubMed] [Google Scholar]
- Boyson CO, Miguel TT, Quadros IM, Debold JF, Miczek KA. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl) 2011;218:257–269. doi: 10.1007/s00213-011-2266-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MR, Fisher LA, Spiess J, Rivier C, Rivier J, Vale W. Corticotropin-releasing factor: actions on the sympathetic nervous system and metabolism. Endocrinology. 1982;111:928–931. doi: 10.1210/endo-111-3-928. [DOI] [PubMed] [Google Scholar]
- Campayo A, Gomez-Biel CH, Lobo A. Diabetes and depression. Curr Psychiatry Rep. 2011;13:26–30. doi: 10.1007/s11920-010-0165-z. [DOI] [PubMed] [Google Scholar]
- Carney RM, Blumenthal JA, Stein PK, Watkins L, Catellier D, Berkman LF, Czajkowski SM, O’Connor C, Stone PH, Freedland KE. Depression, heart rate variability, and acute myocardial infarction. Circulation. 2001;104:2024–2028. doi: 10.1161/hc4201.097834. [DOI] [PubMed] [Google Scholar]
- Chang A, Butler S, Sliwoski J, Valentino R, Canning D, Zderic S. Social stress in mice induces voiding dysfunction and bladder wall remodeling. Am J Physiol Renal Physiol. 2009;297:F1101–F1108. doi: 10.1152/ajprenal.90749.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checkley S. The neuroendocrinology of depression and chronic stress. Br Med Bull. 1996;52:597–617. doi: 10.1093/oxfordjournals.bmb.a011570. [DOI] [PubMed] [Google Scholar]
- Chu CP, Qiu DL, Kato K, Kunitake T, Watanabe S, Yu NS, Nakazato M, Kannan H. Central stresscopin modulates cardiovascular function through the adrenal medulla in conscious rats. Regul Pept. 2004;119:53–59. doi: 10.1016/j.regpep.2003.12.007. [DOI] [PubMed] [Google Scholar]
- Detke MJ, Rickels M, Lucki I. Active behaviors in the rat forced swimming test differentially produced by serotonergic and noradrenergic antidepressants. Psychopharmacology (Berl) 1995;121:66–72. doi: 10.1007/BF02245592. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of acute and chronic stressors and CRF in rat and mouse tests for depression. Ann N Y Acad Sci. 2008;1148:118–126. doi: 10.1196/annals.1410.022. [DOI] [PubMed] [Google Scholar]
- Erhardt A, Muller MB, Rodel A, Welt T, Ohl F, Holsboer F, Keck ME. Consequences of chronic social stress on behaviour and vasopressin gene expression in the PVN of DBA/2OlaHsd mice–influence of treatment with the CRHR1-antagonist R121919/NBI 30775. J Psychopharmacol. 2009;23:31–39. doi: 10.1177/0269881108089813. [DOI] [PubMed] [Google Scholar]
- Fenton WS, Stover ES. Mood disorders: cardiovascular and diabetes comorbidity. Curr Opin Psychiatry. 2006;19:421–427. doi: 10.1097/01.yco.0000228765.33356.9f. [DOI] [PubMed] [Google Scholar]
- Folks DG. The interface of psychiatry and irritable bowel syndrome. Curr Psychiatry Rep. 2004;6:210–215. doi: 10.1007/s11920-004-0066-0. [DOI] [PubMed] [Google Scholar]
- Friedmann E, Thomas SA, Liu F, Morton PG, Chapa D, Gottlieb SS. Relationship of depression, anxiety, and social isolation to chronic heart failure outpatient mortality. Am Heart J. 2006;152(940):e1–e8. doi: 10.1016/j.ahj.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Gold PW, Chrousos GP. Organization of the stress system and its dysregulation in melancholic and atypical depression: high vs low CRH/NE states. Mol Psychiatry. 2002;7:254–275. doi: 10.1038/sj.mp.4001032. [DOI] [PubMed] [Google Scholar]
- Gomez RE, Pirra G, Cannata MA. Open field behavior and cardiovascular responses to stress in normal rats. Physiol Behav. 1989;45:767–769. doi: 10.1016/0031-9384(89)90292-8. [DOI] [PubMed] [Google Scholar]
- Greenberg PE, Kessler RC, Birnbaum HG, Leong SA, Lowe SW, Berglund PA, Corey-Lisle PK. The economic burden of depression in the United States: how did it change between 1990 and 2000? J Clin Psychiatry. 2003;64:1465–1475. doi: 10.4088/jcp.v64n1211. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, Johnson AK. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286:H619–H626. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- Grissom N, Kerr W, Bhatnagar S. Struggling behavior during restraint is regulated by stress experience. Behav Brain Res. 2008;191:219–226. doi: 10.1016/j.bbr.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutman DA, Owens MJ, Skelton KH, Thrivikraman KV, Nemeroff CB. The corticotropin-releasing factor1 receptor antagonist R121919 attenuates the behavioral and endocrine responses to stress. J Pharmacol Exp Ther. 2003;304:874–880. doi: 10.1124/jpet.102.042788. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Ising M. Central CRH system in depression and anxiety–evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583:350–357. doi: 10.1016/j.ejphar.2007.12.032. [DOI] [PubMed] [Google Scholar]
- Jutkiewicz EM, Wood SK, Houshyar H, Hsin LW, Rice KC, Woods JH. The effects of CRF antagonists, antalarmin, CP154,526, LWH234, and R121919, in the forced swim test and on swim-induced increases in adrenocorticotropin in rats. Psychopharmacology (Berl) 2005;180:215–223. doi: 10.1007/s00213-005-2164-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM. Impact of depression and antidepressant treatment on heart rate variability: a review and meta-analysis. Biol Psychiatry. 2010;67:1067–1074. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005b;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa M, Sato A, Swenson RS, Takahashi Y. Sympathoadrenal medullary functions in response to intracerebroventricularly injected corticotropin-releasing factor in anesthetized rats. Brain Res. 1986;367:250–257. doi: 10.1016/0006-8993(86)91599-4. [DOI] [PubMed] [Google Scholar]
- Kuwahara M, Yayou K, Ishii K, Hashimoto S, Tsubone H, Sugano S. Power spectral analysis of heart rate variability as a new method for assessing autonomic activity in the rat. J Electrocardiol. 1994;27:333–337. doi: 10.1016/s0022-0736(05)80272-9. [DOI] [PubMed] [Google Scholar]
- Lopez-Rubalcava C, Lucki I. Strain differences in the behavioral effects of antidepressant drugs in the rat forced swimming test. Neuropsychopharmacology. 2000;22:191–199. doi: 10.1016/S0893-133X(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Meerlo P, Sgoifo A, De Boer SF, Koolhaas JM. Long-lasting consequences of a social conflict in rats: behavior during the interaction predicts subsequent changes in daily rhythms of heart rate, temperature, and activity. Behav Neurosci. 1999;113:1283–1290. doi: 10.1037//0735-7044.113.6.1283. [DOI] [PubMed] [Google Scholar]
- Miczek KA. A new test for aggression in rats without aversive stimulation: differential effects of d-amphetamine and cocaine. Psychopharmacology (Berl) 1979;60:253–259. doi: 10.1007/BF00426664. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Nikulina EM, Shimamoto A, Covington HE., 3rd Escalated or suppressed cocaine reward, tegmental BDNF, and accumbal dopamine caused by episodic versus continuous social stress in rats. J Neurosci. 2011;31:9848–9857. doi: 10.1523/JNEUROSCI.0637-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, Kilts CD, Loosen PT, Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Croiset G, Stam R, Bruijnzeel A, Diamant M, de Wied D, Wiegant VM. The role of the CRH type 1 receptor in autonomic responses to corticotropin-releasing hormone in the rat. Neuropsychopharmacology. 2000;22:388–399. doi: 10.1016/S0893-133X(99)00126-8. [DOI] [PubMed] [Google Scholar]
- Pagani M, Lombardi F, Guzzetti S, Rimoldi O, Furlan R, Pizzinelli P, Sandrone G, Malfatto G, Dell’Orto S, Piccaluga E, et al. Power spectral analysis of heart rate and arterial pressure variabilities as a marker of sympatho-vagal interaction in man and conscious dog. Circ Res. 1986;59:178–193. doi: 10.1161/01.res.59.2.178. [DOI] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Ramaekers D, Beckers F, Demeulemeester H, Aubert AE. Cardiovascular autonomic function in conscious rats: a novel approach to facilitate stationary conditions. Ann Noninvasive Electrocardiol. 2002;7:307–318. doi: 10.1111/j.1542-474X.2002.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugulies R. Depression as a predictor for coronary heart disease. a review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U. Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res. 2005;162:127–134. doi: 10.1016/j.bbr.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Domenici E, Hiemke C, Fuchs E. Effects of fluoxetine on behavioral deficits evoked by chronic social stress in rats. Behav Brain Res. 2006a;174:188–192. doi: 10.1016/j.bbr.2006.07.017. [DOI] [PubMed] [Google Scholar]
- Rygula R, Abumaria N, Flugge G, Hiemke C, Fuchs E, Ruther E, Havemann-Reinecke U. Citalopram counteracts depressive-like symptoms evoked by chronic social stress in rats. Behav Pharmacol. 2006b;17:19–29. doi: 10.1097/01.fbp.0000186631.53851.71. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, de Boer SF, Haller J, Koolhaas JM. Individual differences in plasma catecholamine and corticosterone stress responses of wild-type rats: relationship with aggression. Physiol Behav. 1996;60:1403–1407. doi: 10.1016/s0031-9384(96)00229-6. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Koolhaas JM, Musso E, De Boer SF. Different sympathovagal modulation of heart rate during social and nonsocial stress episodes in wild-type rats. Physiol Behav. 1999;67:733–738. doi: 10.1016/s0031-9384(99)00134-1. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Koolhaas J, Alleva E, Musso E, Parmigiani S. Social stress. Acute and long-term effects on physiology and behavior. Physiol Behav. 2001;73:253–254. doi: 10.1016/s0031-9384(01)00544-3. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Pozzato C, Meerlo P, Costoli T, Manghi M, Stilli D, Olivetti G, Musso E. Intermittent exposure to social defeat and open-field test in rats: acute and long-term effects on ECG, body temperature and physical activity. Stress. 2002;5:23–35. doi: 10.1080/102538902900012387. [DOI] [PubMed] [Google Scholar]
- Stefanski V. Social stress in loser rats: opposite immunological effects in submissive and subdominant males. Physiol Behav. 1998;63:605–613. doi: 10.1016/s0031-9384(97)00492-7. [DOI] [PubMed] [Google Scholar]
- Stenzel-Poore MP, Duncan JE, Rittenberg MB, Bakke AC, Heinrichs SC. CRH overproduction in transgenic mice: behavioral and immune system modulation. Ann N Y Acad Sci. 1996;780:36–48. doi: 10.1111/j.1749-6632.1996.tb15110.x. [DOI] [PubMed] [Google Scholar]
- Surtees PG, Wainwright NW, Luben RN, Wareham NJ, Bingham SA, Khaw KT. Depression and ischemic heart disease mortality: evidence from the EPIC-Norfolk United Kingdom prospective cohort study. Am J Psychiatry. 2008;165:515–523. doi: 10.1176/appi.ajp.2007.07061018. [DOI] [PubMed] [Google Scholar]
- Tornatzky W, Miczek KA. Long-term impairment of autonomic circadian rhythms after brief intermittent social stress. Physiol Behav. 1993;53:983–993. doi: 10.1016/0031-9384(93)90278-n. [DOI] [PubMed] [Google Scholar]
- Udupa K, Sathyaprabha TN, Thirthalli J, Kishore KR, Lavekar GS, Raju TR, Gangadhar BN. Alteration of cardiac autonomic functions in patients with major depression: a study using heart rate variability measures. J Affect Disord. 2007;100:137–141. doi: 10.1016/j.jad.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394–1397. doi: 10.1126/science.6267699. [DOI] [PubMed] [Google Scholar]
- Van Der Kooy K, Van Hout H, Marwijk H, Marten H, Stehouwer C, Beekman A. Depression and the risk for cardiovascular diseases: systematic review and meta analysisv. Int J Geriat Psychiatr. 2007;22:613–626. doi: 10.1002/gps.1723. [DOI] [PubMed] [Google Scholar]
- Von Frijtag JC, Reijmers LG, Van der Harst JE, Leus IE, Van den Bos R, Spruijt BM. Defeat followed by individual housing results in long-term impaired reward- and cognition-related behaviours in rats. Behav Brain Res. 2000;117:137–146. doi: 10.1016/s0166-4328(00)00300-4. [DOI] [PubMed] [Google Scholar]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hyper-noradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci USA. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Woods JH. Corticotropin-releasing factor receptor-1: a therapeutic target for cardiac autonomic disturbances. Expert Opin Ther Targets. 2007;11:1401–1413. doi: 10.1517/14728222.11.11.1401. [DOI] [PubMed] [Google Scholar]
- Wood SK, Verhoeven RE, Savit AZ, Rice KC, Fischbach PS, Woods JH. Facilitation of cardiac vagal activity by CRF-R1 antagonists during swim stress in rats. Neuropsychopharmacology. 2006;31:2580–2590. doi: 10.1038/sj.npp.1301085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Baez MA, Bhatnagar S, Valentino RJ. Social stress-induced bladder dysfunction: potential role of corticotropin-releasing factor. Am J Physiol Regul Integr Comp Physiol. 2009a;296:R1671–R1678. doi: 10.1152/ajpregu.91013.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Narasimhan D, Cooper Z, Sunahara RK, Woods JH. Prevention and reversal by cocaine esterase of cocaine-induced cardiovascular effects in rats. Drug Alcohol Depend. 2009b;106:219–229. doi: 10.1016/j.drugalcdep.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SK, Walker HE, Valentino RJ, Bhatnagar S. Individual differences in reactivity to social stress predict susceptibility and resilience to a depressive phenotype: role of corticotropin-releasing factor. Endocrinology. 2010;151:1795–1805. doi: 10.1210/en.2009-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla EP, Koob GF. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin Investig Drugs. 2004;13:799–828. doi: 10.1517/13543784.13.7.799. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Valdez GR, Nozulak J, Koob GF, Markou A. Effects of antalarmin, a CRF type 1 receptor antagonist, on anxiety-like behavior and motor activation in the rat. Brain Res. 2002;952:188–199. doi: 10.1016/s0006-8993(02)03189-x. [DOI] [PubMed] [Google Scholar]