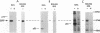Abstract
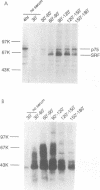
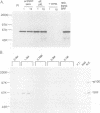
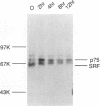
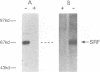
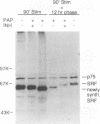
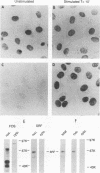
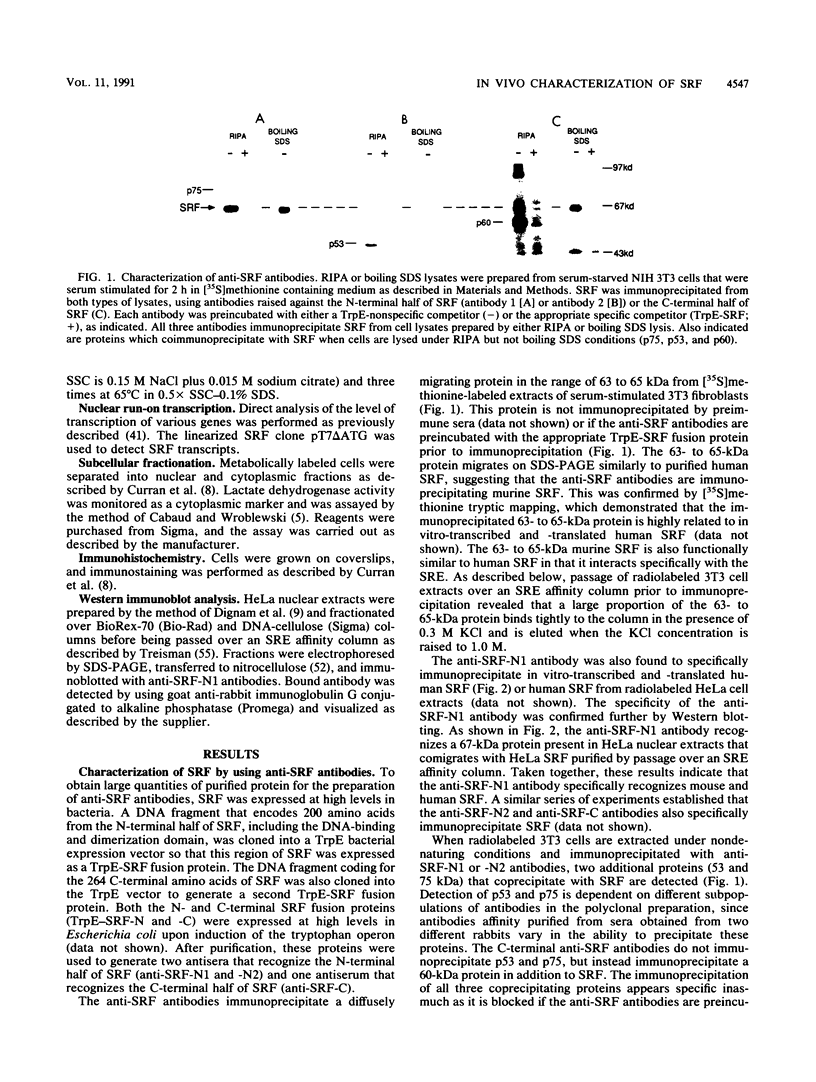
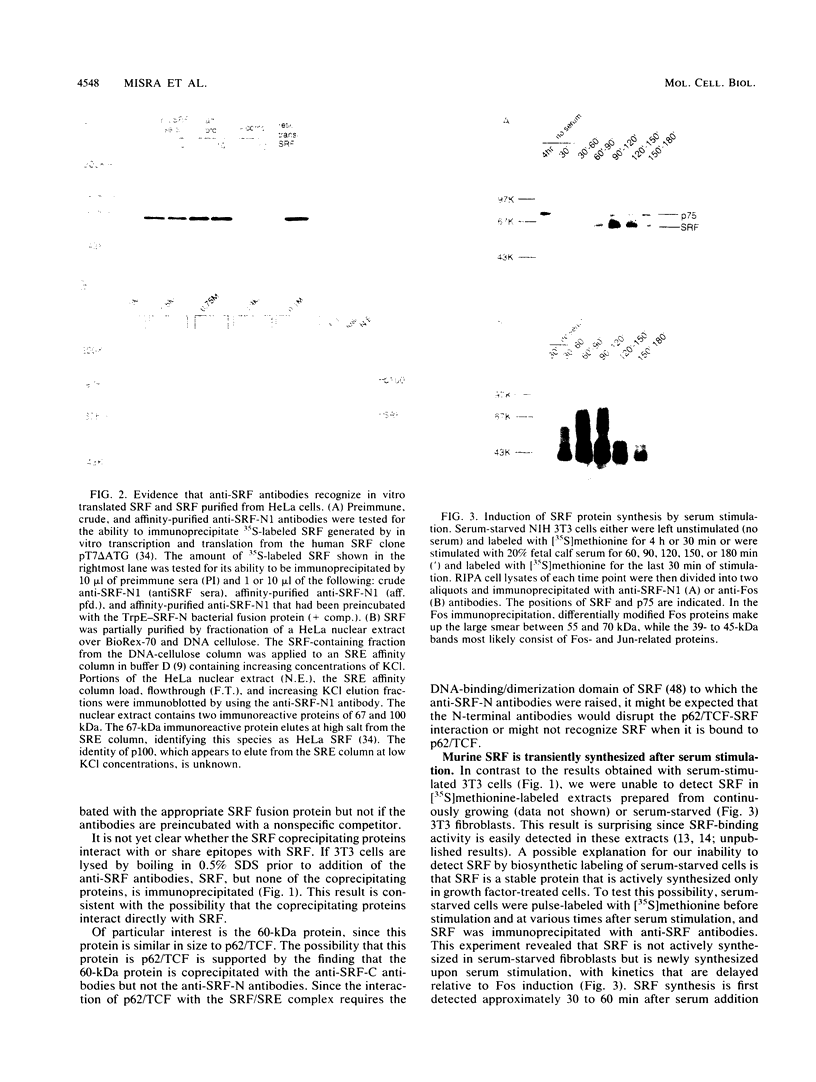
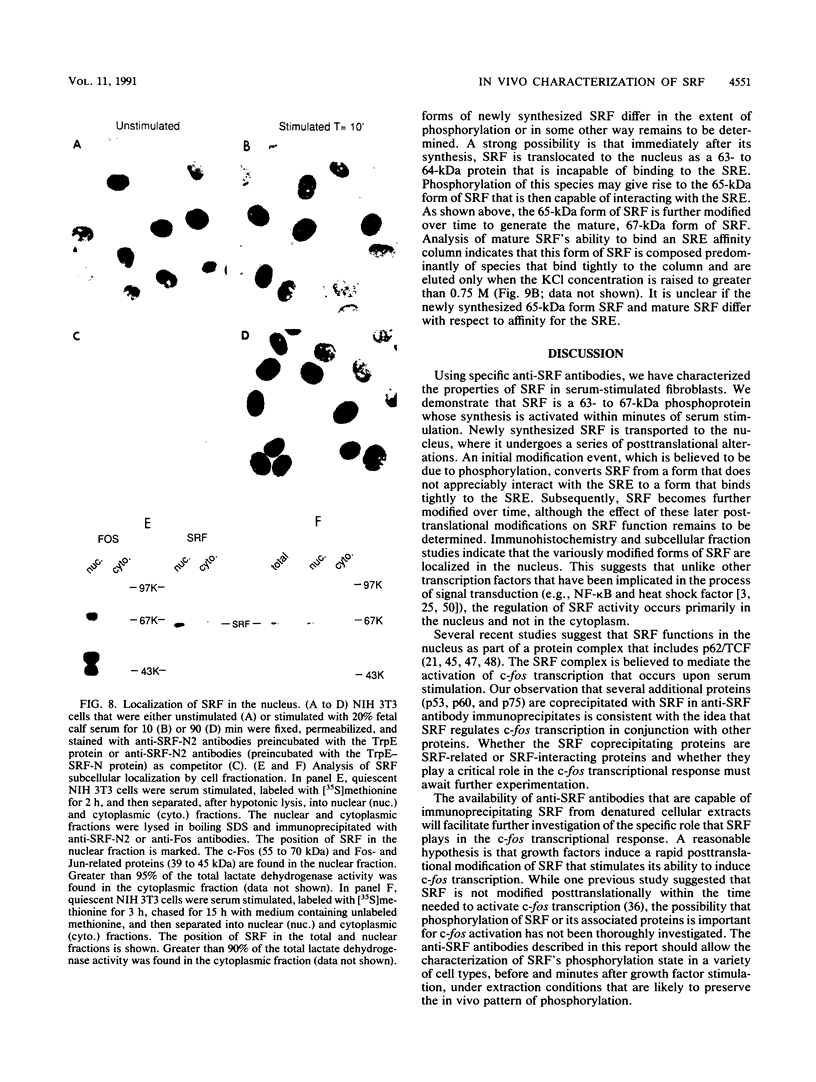
Growth factor regulation of c-fos proto-oncogene transcription is mediated by a 20-bp region of dyad symmetry, termed the serum response element. The inner core of this element binds a 67-kDa phosphoprotein, the serum response factor (SRF), that is thought to play a pivotal role in the c-fos transcriptional response. To investigate the mechanism by which SRF regulates c-fos expression, we generated polyclonal anti-SRF antibodies and used these antibodies to analyze the biochemical properties of SRF. These studies indicate that the synthesis of SRF is transient, occurring within 30 min to 4 h after serum stimulation of quiescent fibroblasts. Newly synthesized SRF is transported to the nucleus, where it is increasingly modified by phosphorylation during progression through the cell cycle. Within 2 h of serum stimulation, differentially modified forms of SRF can be distinguished on the basis of the ability to bind a synthetic serum response element. SRF protein exhibits a half-life of greater than 12 h and is predominantly nuclear, with no change occurring in its localization upon serum stimulation. We find that the induction of SRF synthesis is regulated at the transcriptional level and that cytoplasmic SRF mRNA is transiently expressed with somewhat delayed kinetics compared with c-fos mRNA expression. These features of SRF expression suggest a model whereby newly synthesized SRF functions in the shutoff of c-fos transcription.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Lenardo M., Pierce J. W., Baltimore D. Phorbol-ester-induced activation of the NF-kappa B transcription factor involves dissociation of an apparently cytoplasmic NF-kappa B/inhibitor complex. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):789–798. doi: 10.1101/sqb.1988.053.01.089. [DOI] [PubMed] [Google Scholar]
- Bartel D. P., Sheng M., Lau L. F., Greenberg M. E. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989 Mar;3(3):304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- CABAUD P. G., WROBLEWSKI F. Colorimetric measurement of lactic dehydrogenase activity of body fluids. Am J Clin Pathol. 1958 Sep;30(3):234–236. doi: 10.1093/ajcp/30.3.234. [DOI] [PubMed] [Google Scholar]
- Carroll D., Marshak D. R. Serum-stimulated cell growth causes oscillations in casein kinase II activity. J Biol Chem. 1989 May 5;264(13):7345–7348. [PubMed] [Google Scholar]
- Cochran B. H., Zullo J., Verma I. M., Stiles C. D. Expression of the c-fos gene and of an fos-related gene is stimulated by platelet-derived growth factor. Science. 1984 Nov 30;226(4678):1080–1082. doi: 10.1126/science.6093261. [DOI] [PubMed] [Google Scholar]
- Curran T., Miller A. D., Zokas L., Verma I. M. Viral and cellular fos proteins: a comparative analysis. Cell. 1984 Feb;36(2):259–268. doi: 10.1016/0092-8674(84)90219-8. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrner T. J., Carroll S. L., Milbrandt J. The NGFI-B protein, an inducible member of the thyroid/steroid receptor family, is rapidly modified posttranslationally. Mol Cell Biol. 1990 Dec;10(12):6454–6459. doi: 10.1128/mcb.10.12.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel M. P., Biskis B. O., Jinkins P. B. Virus induction of osteosarcomas in mice. Science. 1966 Feb 11;151(3711):698–701. doi: 10.1126/science.151.3711.698. [DOI] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman M. Z. The c-fos serum response element responds to protein kinase C-dependent and -independent signals but not to cyclic AMP. Genes Dev. 1988 Apr;2(4):394–402. doi: 10.1101/gad.2.4.394. [DOI] [PubMed] [Google Scholar]
- Gilman M. Z., Wilson R. N., Weinberg R. A. Multiple protein-binding sites in the 5'-flanking region regulate c-fos expression. Mol Cell Biol. 1986 Dec;6(12):4305–4316. doi: 10.1128/mcb.6.12.4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gius D., Cao X. M., Rauscher F. J., 3rd, Cohen D. R., Curran T., Sukhatme V. P. Transcriptional activation and repression by Fos are independent functions: the C terminus represses immediate-early gene expression via CArG elements. Mol Cell Biol. 1990 Aug;10(8):4243–4255. doi: 10.1128/mcb.10.8.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham R., Gilman M. Distinct protein targets for signals acting at the c-fos serum response element. Science. 1991 Jan 11;251(4990):189–192. doi: 10.1126/science.1898992. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Siegfried Z., Ziff E. B. Mutation of the c-fos gene dyad symmetry element inhibits serum inducibility of transcription in vivo and the nuclear regulatory factor binding in vitro. Mol Cell Biol. 1987 Mar;7(3):1217–1225. doi: 10.1128/mcb.7.3.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Hazel T. G., Misra R., Davis I. J., Greenberg M. E., Lau L. F. Nur77 is differentially modified in PC12 cells upon membrane depolarization and growth factor treatment. Mol Cell Biol. 1991 Jun;11(6):3239–3246. doi: 10.1128/mcb.11.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf U., Büscher M., Schönthal A., Rahmsdorf H. J., Herrlich P. Autoregulation of fos: the dyad symmetry element as the major target of repression. EMBO J. 1989 Sep;8(9):2559–2566. doi: 10.1002/j.1460-2075.1989.tb08394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J. S., Schuetz T. J., Kingston R. E. Activation in vitro of sequence-specific DNA binding by a human regulatory factor. Nature. 1988 Sep 22;335(6188):372–375. doi: 10.1038/335372a0. [DOI] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung S., Miyamoto N. G. Point mutational analysis of the human c-fos serum response factor binding site. Nucleic Acids Res. 1989 Feb 11;17(3):1177–1195. doi: 10.1093/nar/17.3.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. Growth-related changes in specific mRNAs of cultured mouse cells. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4271–4275. doi: 10.1073/pnas.80.14.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucibello F. C., Lowag C., Neuberg M., Müller R. trans-repression of the mouse c-fos promoter: a novel mechanism of Fos-mediated trans-regulation. Cell. 1989 Dec 22;59(6):999–1007. doi: 10.1016/0092-8674(89)90756-3. [DOI] [PubMed] [Google Scholar]
- Manak J. R., de Bisschop N., Kris R. M., Prywes R. Casein kinase II enhances the DNA binding activity of serum response factor. Genes Dev. 1990 Jun;4(6):955–967. doi: 10.1101/gad.4.6.955. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Curran T., Verma I. M. c-fos protein can induce cellular transformation: a novel mechanism of activation of a cellular oncogene. Cell. 1984 Jan;36(1):51–60. doi: 10.1016/0092-8674(84)90073-4. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Norman C., Runswick M., Pollock R., Treisman R. Isolation and properties of cDNA clones encoding SRF, a transcription factor that binds to the c-fos serum response element. Cell. 1988 Dec 23;55(6):989–1003. doi: 10.1016/0092-8674(88)90244-9. [DOI] [PubMed] [Google Scholar]
- Passmore S., Elble R., Tye B. K. A protein involved in minichromosome maintenance in yeast binds a transcriptional enhancer conserved in eukaryotes. Genes Dev. 1989 Jul;3(7):921–935. doi: 10.1101/gad.3.7.921. [DOI] [PubMed] [Google Scholar]
- Prywes R., Dutta A., Cromlish J. A., Roeder R. G. Phosphorylation of serum response factor, a factor that binds to the serum response element of the c-FOS enhancer. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7206–7210. doi: 10.1073/pnas.85.19.7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Fisch T. M., Roeder R. G. Transcriptional regulation of c-fos. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):739–748. doi: 10.1101/sqb.1988.053.01.084. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Inducible binding of a factor to the c-fos enhancer. Cell. 1986 Dec 5;47(5):777–784. doi: 10.1016/0092-8674(86)90520-9. [DOI] [PubMed] [Google Scholar]
- Prywes R., Roeder R. G. Purification of the c-fos enhancer-binding protein. Mol Cell Biol. 1987 Oct;7(10):3482–3489. doi: 10.1128/mcb.7.10.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera V. M., Greenberg M. E. Growth factor-induced gene expression: the ups and downs of c-fos regulation. New Biol. 1990 Sep;2(9):751–758. [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Ryan W. A., Jr, Franza B. R., Jr, Gilman M. Z. Two distinct cellular phosphoproteins bind to the c-fos serum response element. EMBO J. 1989 Jun;8(6):1785–1792. doi: 10.1002/j.1460-2075.1989.tb03572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter H., Mueller C. G., Meese K., Nordheim A. Synergism in ternary complex formation between the dimeric glycoprotein p67SRF, polypeptide p62TCF and the c-fos serum response element. EMBO J. 1990 Apr;9(4):1123–1130. doi: 10.1002/j.1460-2075.1990.tb08218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröter H., Shaw P. E., Nordheim A. Purification of intercalator-released p67, a polypeptide that interacts specifically with the c-fos serum response element. Nucleic Acids Res. 1987 Dec 23;15(24):10145–10158. doi: 10.1093/nar/15.24.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönthal A., Büscher M., Angel P., Rahmsdorf H. J., Ponta H., Hattori K., Chiu R., Karin M., Herrlich P. The Fos and Jun/AP-1 proteins are involved in the downregulation of Fos transcription. Oncogene. 1989 May;4(5):629–636. [PubMed] [Google Scholar]
- Shaw P. E., Frasch S., Nordheim A. Repression of c-fos transcription is mediated through p67SRF bound to the SRE. EMBO J. 1989 Sep;8(9):2567–2574. doi: 10.1002/j.1460-2075.1989.tb08395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P. E., Schröter H., Nordheim A. The ability of a ternary complex to form over the serum response element correlates with serum inducibility of the human c-fos promoter. Cell. 1989 Feb 24;56(4):563–572. doi: 10.1016/0092-8674(89)90579-5. [DOI] [PubMed] [Google Scholar]
- Sheng M., Dougan S. T., McFadden G., Greenberg M. E. Calcium and growth factor pathways of c-fos transcriptional activation require distinct upstream regulatory sequences. Mol Cell Biol. 1988 Jul;8(7):2787–2796. doi: 10.1128/mcb.8.7.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirakawa F., Mizel S. B. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989 Jun;9(6):2424–2430. doi: 10.1128/mcb.9.6.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhatme V. P., Kartha S., Toback F. G., Taub R., Hoover R. G., Tsai-Morris C. H. A novel early growth response gene rapidly induced by fibroblast, epithelial cell and lymphocyte mitogens. Oncogene Res. 1987 Sep-Oct;1(4):343–355. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification and purification of a polypeptide that binds to the c-fos serum response element. EMBO J. 1987 Sep;6(9):2711–2717. doi: 10.1002/j.1460-2075.1987.tb02564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treisman R. Identification of a protein-binding site that mediates transcriptional response of the c-fos gene to serum factors. Cell. 1986 Aug 15;46(4):567–574. doi: 10.1016/0092-8674(86)90882-2. [DOI] [PubMed] [Google Scholar]
- Treisman R. The SRE: a growth factor responsive transcriptional regulator. Semin Cancer Biol. 1990 Feb;1(1):47–58. [PubMed] [Google Scholar]
- Treisman R. Transient accumulation of c-fos RNA following serum stimulation requires a conserved 5' element and c-fos 3' sequences. Cell. 1985 Oct;42(3):889–902. doi: 10.1016/0092-8674(85)90285-5. [DOI] [PubMed] [Google Scholar]
- Vosatka R. J., Hermanowski-Vosatka A., Metz R., Ziff E. B. Dynamic interactions of c-fos protein in serum-stimulated 3T3 cells. J Cell Physiol. 1989 Mar;138(3):493–502. doi: 10.1002/jcp.1041380308. [DOI] [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]