Abstract
Background
The newly developed Brief–Balance Evaluation System Test (Brief-BESTest) may be useful for measuring balance and predicting falls in individuals with Parkinson disease (PD).
Objectives
The purposes of this study were: (1) to describe the balance performance of those with PD using the Brief-BESTest, (2) to determine the relationships among the scores derived from the 3 versions of the BESTest (ie, full BESTest, Mini-BESTest, and Brief-BESTest), and (3) to compare the accuracy of the Brief-BESTest with that of the Mini-BESTest and BESTest in identifying recurrent fallers among people with PD.
Design
This was a prospective cohort study.
Methods
Eighty participants with PD completed a baseline balance assessment. All participants reported a fall history during the previous 6 months. Fall history was again collected 6 months (n=51) and 12 months (n=40) later.
Results
At baseline, participants had varying levels of balance impairment, and Brief-BESTest scores were significantly correlated with Mini-BESTest (r=.94, P<.001) and BESTest (r=.95, P<.001) scores. Six-month retrospective fall prediction accuracy of the Brief-BESTest was moderately high (area under the curve [AUC]=0.82, sensitivity=0.76, and specificity=0.84). Prospective fall prediction accuracy over 6 months was similarly accurate (AUC=0.88, sensitivity=0.71, and specificity=0.87), but was less sensitive over 12 months (AUC=0.76, sensitivity=0.53, and specificity=0.93).
Limitations
The sample included primarily individuals with mild to moderate PD. Also, there was a moderate dropout rate at 6 and 12 months.
Conclusions
All versions of the BESTest were reasonably accurate in identifying future recurrent fallers, especially during the 6 months following assessment. Clinicians can reasonably rely on the Brief-BESTest for predicting falls, particularly when time and equipment constraints are of concern.
Falls are common among people with Parkinson disease (PD). A retrospective study demonstrated that 38.3% of individuals with PD fell since being diagnosed and 67% of fallers fell more than once since diagnosis.1 One devastating complication of falling is hip fracture, which is associated with high mortality in people with PD.2,3 Other consequences include: immobility, reduced quality of life, and fear of falling.4 Postural instability and impaired gait are independently associated with increased falls in PD.5,6 In addition, rehabilitation intervention trials suggest that programs targeted at fall prevention are successful at improving postural competence and reducing falls in PD.7,8 As such, accurate and time-efficient measures are critically needed to direct appropriate interventions for those at risk. Measures to predict falls in people with PD should be: (1) theoretically grounded in examining the systems controlling balance and gait, (2) accurate in their ability to predict falls, and (3) feasible and practical for clinical use.
The Balance Evaluation Systems Test (BESTest) was developed from a theoretical understanding of balance control systems. It includes 36 items that evaluate performance of 6 balance systems: biomechanical constraints, stability limits/verticality, anticipatory postural adjustments, postural responses, sensory orientation, and stability in gait.9 The BESTest was effective in determining which individuals with PD fell in the previous 6 months and accurate in prospectively predicting falls 6 months from original assessment, but was less useful for predicting falls 12 months from original assessment.10,11 One concern regarding the BESTest is that it can take at least 40 minutes to complete for someone with mild PD and even longer than 40 minutes with greater disease severity. In addition, equipment such as a ramp, a foam block, a meter stick, a table, and a 2.27-kg (5-lb) weight are necessary for the BESTest and may not be readily available for clinicians. These concerns suggest that the BESTest, although measuring balance control systems and possessing reasonable accuracy in predicting falls, may not be practical for regular use in all clinical settings.
Through psychometric analysis, a condensed version of the BESTest, the Mini-BESTest, was developed to enhance clinical usefulness. The period of time to complete the Mini-BESTest is substantially shorter when compared with the period of time to complete the full BESTest.12 The Mini-BESTest is useful in identifying individuals who will fall in the next 6 months, but its accuracy over 12 months is severely diminished.11 Although the Mini-BESTest reduces the time needed to evaluate balance, the items included are theoretically inconsistent with the full BESTest. The Mini-BESTest examines only 4 of the 6 balance systems assessed in the full BESTest. Because of this omission, deficits in the 2 untested systems (biomechanical constraints and stability limits/verticality) may go undetected and unaddressed.
In response to the limitations of the BESTest and Mini-BESTest, the Brief-BESTest was recently developed.13 The Brief-BESTest is a shortened version of the full BESTest that, in contrast to the Mini-BESTest, contains items that assess all 6 balance systems originally outlined by the original BESTest, using the original scale for scoring items. Despite evaluating 2 additional balance systems, the Brief-BESTest requires less administration time and less equipment than the Mini-BESTest, which could make the Brief-BESTest more feasible for clinical use. The accuracy of the Brief-BESTest in people with PD is unknown.
The objectives of this study were: (1) to describe balance performance in PD using the Brief-BESTest, (2) to determine relationships among the 3 versions of the BESTest (ie, full BESTest, Mini-BESTest, and Brief-BESTest) and relationships between the individual items of the Brief-BESTest and their related sections of the full BESTest, and (3) to determine the accuracy of the Brief-BESTest compared with the Mini-BESTest and full BESTest in retrospectively and prospectively identifying recurrent fallers among people with PD. We hypothesized that Brief-BESTest scores would correlate with Mini-BESTest and BESTest scores and that the Brief-BESTest would be equally or more accurate than the Mini-BESTest in identifying recurrent fallers.
Method
Participants
Participants were recruited from Washington University's Movement Disorders Center and the Volunteers for Health database for participation in a multicenter longitudinal study.14 Individuals were eligible for participation if diagnosed with definite idiopathic PD (Hoehn and Yahr [H&Y] stages I–IV).15 Potential participants were excluded if they had: a history or presence of a neurological disorder other than PD, musculoskeletal injury limiting ability to walk, or any other serious medical condition. Participants agreed to complete assessments at 3 time points: baseline, 6 months, and 12 months. All participants provided informed consent according to the policies and procedures of the Human Research Protection Office at Washington University.
Outcome Measures
The full BESTest contains 36 items scored from 0 to 3, with 3 representing no impairment of balance and 0 representing severely impaired balance or inability to perform a task without falling.9 The maximum score is measured as a percentage of the points scored out of 108 total points possible. In addition, 6 subsection scores are generated, each representing a specific balance system (biomechanical constraints, stability limits/verticality, anticipatory postural adjustments, postural responses, sensory orientation, and stability in gait). The BESTest has high interrater and test-retest reliability in PD.16 The BESTest requires the following equipment: a table for sitting, a meter stick, a step stool, a 2.27-kg (5-lb) weight, a 1.36-kg (3-lb) weight, a foam block, a ramp, an obstacle, a stopwatch, and a relatively large walkway to complete. In our experience, the BESTest takes at least 40 minutes to complete when assessing an individual with mild to moderate PD, and more time is necessary for those with more severe PD.
The Mini-BESTest contains 14 items from the original BESTest.17 The items collectively represent only 4 of the 6 balance systems identified by the full test. In contrast to the original BESTest, each item is scored 0 to 2, with 2 representing no impairment in balance and 0 representing severe impairment of balance. Two items have right and left components, and the maximum total score is 32. The Mini-BESTest has high interrater and test-retest reliability in PD.10 The equipment needed to complete the Mini-BESTest includes: a foam block, a ramp, an obstacle, a stopwatch, and a relatively large walkway to complete. In our experience, the Mini-BESTest takes approximately 15 minutes to conduct for most ambulatory individuals with PD.
The Brief-BESTest is a 6-item balance assessment containing 1 item from each of the 6 subsections of the full BESTest (for a copy, see Padgett et al13). Items were chosen based on correlational analysis. The 1 item from each specific section of the BESTest with the strongest correlation to that total section score was included in the Brief-BESTest.13 Each item is administered and scored the same as in the original test (ie, performance is rated 0 to 3, with 3 representing no balance impairment and 0 representing severe balance impairment or inability to perform a task without falling).13 Because 2 of the items in the Brief-BESTest have left and right components, the maximum possible score for the Brief-BESTest is 24. The Brief-BESTest requires only a foam block, a stopwatch, a meter stick, and enough space to complete the Timed “Up & Go” Test. In our experience, the Brief-BESTest requires approximately 10 minutes to complete with most ambulatory individuals with PD. The interrater reliability of the Brief-BESTest was evaluated and noted to be high in a mixed group that included individuals without neurological diagnoses and individuals with varied neurological diagnoses of PD, multiple sclerosis, stroke, neuropathy, and essential tremor.13
Procedure
From July 2009 to December 2009, baseline assessments were conducted with participants on antiparkinson medication approximately 1 to 1.5 hours after medication administration. Age and sex data were collected using a custom-designed form, which was completed by each participant. Motor symptom severity was determined using section III of The Movement Disorder Society–Unified Parkinson's Disease Rating Scale (MDS-UPDRS III).18 Hoehn & Yahr stage was determined as a part of the MDS-UPDRS III assessment. Balance performance was evaluated by a trained physical therapist using the full, original BESTest. A custom-designed worksheet allowed the examiner to simultaneously record BESTest item scores and Mini-BESTest item scores, each of which have distinct scoring scales. Following assessment, Brief-BESTest scores were extracted from the relevant subset of BESTest items.
Hoehn & Yahr stage and MDS-UPDRS III scores were collected at each time point (baseline, 6 months, and 12 months), as was self-reported 6-month fall history, using a custom-designed form with a forced choice response paradigm (ie, zero falls, 1 fall, 2–10 falls, weekly falls, or daily falls). This form, along with the disease severity ratings, was administered by the same physical therapist who conducted baseline assessments; however, to maintain blinding with respect to fall history, the form was completed following the administration of all other outcome measures. Prior to completing the form, each participant was informed that a fall was defined as an unintentional event in which any part of the body comes into contact with the ground. This definition has been used previously by investigators studying fall prediction in people with and without PD.19,20
Data Analysis
Descriptive statistics were used to describe mean sample characteristics for age, sex, MDS-UPDRS III score, H&Y stage, and balance performance. These values also were determined separately for those who dropped out of the study. Individuals reporting 2 or more falls during the analysis period of interest were considered recurrent fallers.11 For the retrospective analysis of the 6 months prior to baseline, we used baseline fall history data. For the prospective analysis of the 6 months following baseline, we used the 6-month follow-up fall history data. For the prospective analysis of the 12 months following baseline, we determined total fall count with 6-month and 12-month fall history data. Participants who did not report fall history at 6 or 12 months were not included in statistical analyses at those respective time points.
Pearson correlation coefficients (r) (α=.05) were calculated to describe relationships between: (1) the Brief-BESTest and Mini-BESTest, (2) the Brief-BESTest and full BESTest total scores, and (3) the representative Brief-BESTest item scores and their respective BESTest subsection scores. To compare fall prediction accuracy of each outcome measure for each time interval (ie, 6 months prior to baseline, 0–6 months following baseline, and 0–12 months following baseline), we created receiver operating characteristic (ROC) curves and determined the area under the curve (AUC) for each.21 Secondary AUC analyses with just the 40 individuals who completed the full study also were conducted. Empirical tests of equivalence (2-tailed) were used to make pair-wise comparisons of AUCs (P<.05) in order to determine whether an AUC of 1 measure was different from that of another.22 From each ROC curve, we determined a cutoff score that maximized sensitivity and specificity values and calculated positive and negative likelihood ratios (LR+ and LR−) and posttest probabilities for predicting falls. All statistical analyses were conducted using Number Cruncher Statistical System (NCSS) software.23
A power analysis was conducted as a part of another study in which 81 participants were required to describe the ability of the BESTest and Functional Gait Assessment (FGA) to retrospectively predict falls in people with PD.16
Results
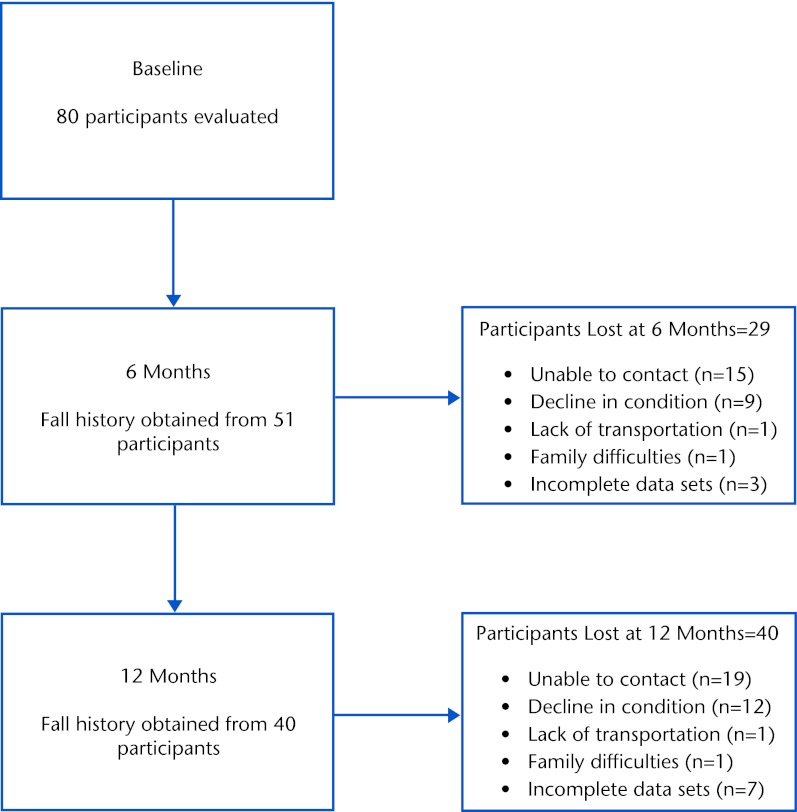
Eighty individuals (59% men and 41% women) with idiopathic PD were evaluated at baseline (Tab. 1). Of the original sample of 80 there were 25 (31% men and 69% women) with a retrospective history of 2 or more falls in the past 6 months. From that original sample, fall history data were collected from 51 individuals (14 recurrent fallers [27.5%]) at 6 months and from 40 individuals (13 recurrent fallers [32.5%]) at 12 months. Scores (mean [SD]) for each balance measure as well as disease severity are provided in Table 1. Figure 1 depicts the number of participants evaluated at baseline, 6 months, and 12 months, as well as the reasons for participant loss at each time point. Seven (3 men and 4 women) of the 11 individuals who were lost from 6 to 12 months were characterized as recurrent fallers.
Table 1.
Baseline Demographic Characteristics of Participantsa
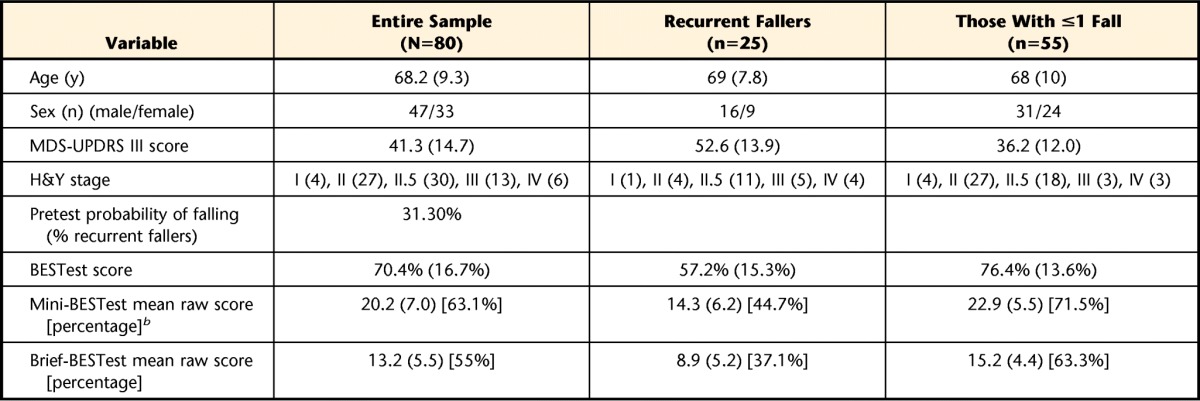
Values are mean (SD). MDS-UPDRS III=Movement Disorder Society–Unified Parkinson's Disease Rating Scale motor examination section, H&Y=Hoehn and Yahr scale.
b Mini-BESTest and Brief-BESTest percentage scores are included in brackets to allow comparison with BESTest scores.
Figure 1.
Flow diagram describing number of participants evaluated and reasons for loss at designated time points.
Demographic characteristics of those who dropped out compared to those who completed the full study are provided in Table 2. On average, participants who dropped out were no different in terms of age or gender, but did have a higher percentage of recurrent fallers as defined at baseline evaluation and had greater disease severity (H&Y and MDS-UPDRS III) when compared with those who completed the full 12 months of the study. When comparing the available sample at each time point across the study, however, there were no significant changes in disease severity or percentage of recurrent fallers from baseline to 6 to 12 months.
Table 2.
Comparison of Baseline Characteristics Between Participants Who Did or Did Not Drop Out at 12 Monthsa
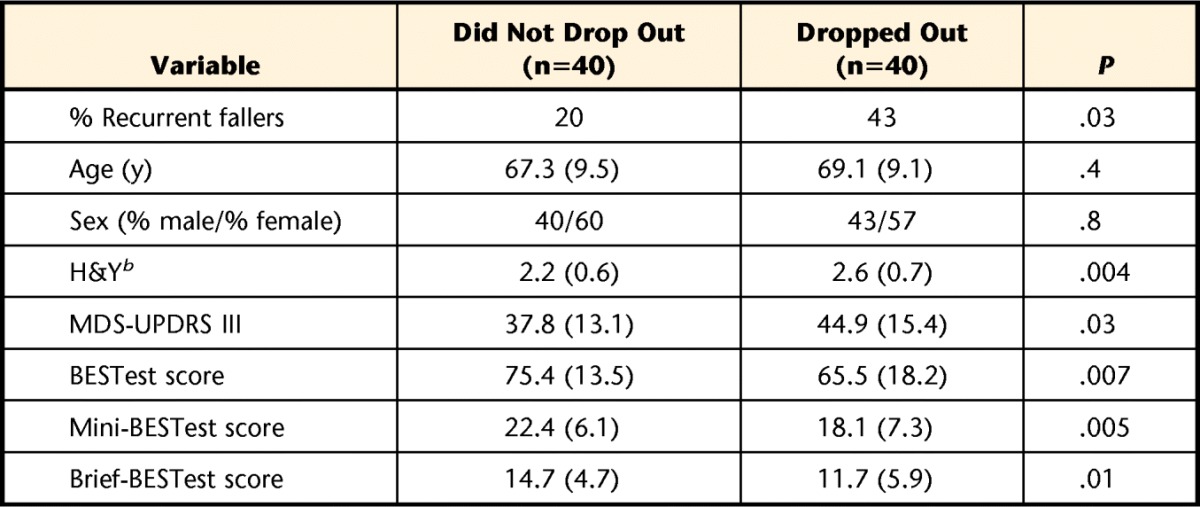
Values are mean (SD). Independent samples t tests were conducted unless otherwise indicated. MDS-UPDRS III=Movement Disorder Society–Unified Parkinson's Disease Rating Scale motor examination section, H&Y=Hoehn and Yahr scale.
b Mann-Whitney U test was used for differences.
Brief-BESTest Relationships
Brief-BESTest scores were significantly correlated with scores on the Mini-BESTest and BESTest (r=.94, P<.001, and r=.95, P<.001, respectively). Each item score on the Brief-BESTest correlated with its respective section score on the full BESTest (all P<.0001). The biomechanical constraints (r=.61) and stability limits/verticality (r=.69) sections of the Brief-BESTest demonstrated the lowest correlations with their respective sections of the BESTest. The highest correlations between the Brief-BESTest and BESTest sections were for anticipatory postural adjustments (r=.89) and postural responses (r=.91), while sensory orientation (r=.78) and stability in gait (r=.78) were slightly less correlated.
Fall Prediction Using the Brief-BESTest
The ROC curves for the 3 measures are presented in Figure 2. Details of the predictive abilities of the Brief-BEST at all 3 time points are presented in Table 3. These same values for the Mini-BESTest and BESTest have been reported previously for this sample.10,11 Retrospectively, the Brief-BESTest had the highest posttest probability of falling with a score less than or equal to the cutoff when compared with the BESTest and Mini-BESTest. Also at this time point, the LR+ for the Brief-BESTest exceeded those of the other measures. At 6 months, the highest LR+ and lowest LR− were derived from the BESTest (Tab. 3). The LR+ for the Brief-BESTest was 5.29, and the LR− was the highest of the 3 measures. Pretest probability of falling at 6 months was 27.5%, and after administration of the 3 measures, the posttest probability of falling was 60% or higher for each test at 6 months, with the BESTest highest at 69%. At 12 months, predictive values for most measures were lower than at 6 months (Tab. 3).
Figure 2.
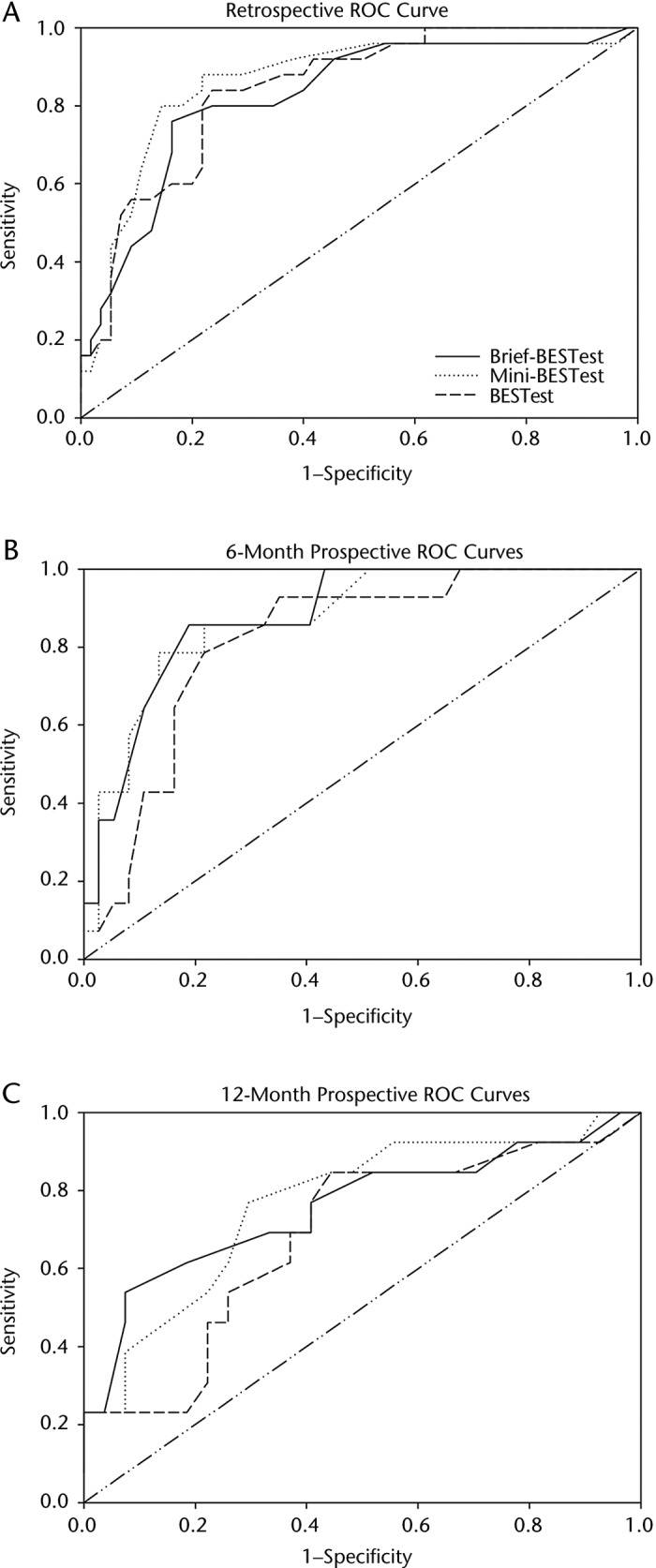
Receiver operating characteristic (ROC) curves for the Brief-BESTest, Mini-BESTest, and BESTest for retrospective fall prediction over 6 months (A) and prospective fall prediction over 6 months (B) and 12 months (C).
Table 3.
Predictive Values for the Brief-BESTest, Mini-BESTest, and BESTest at Each Time Pointa
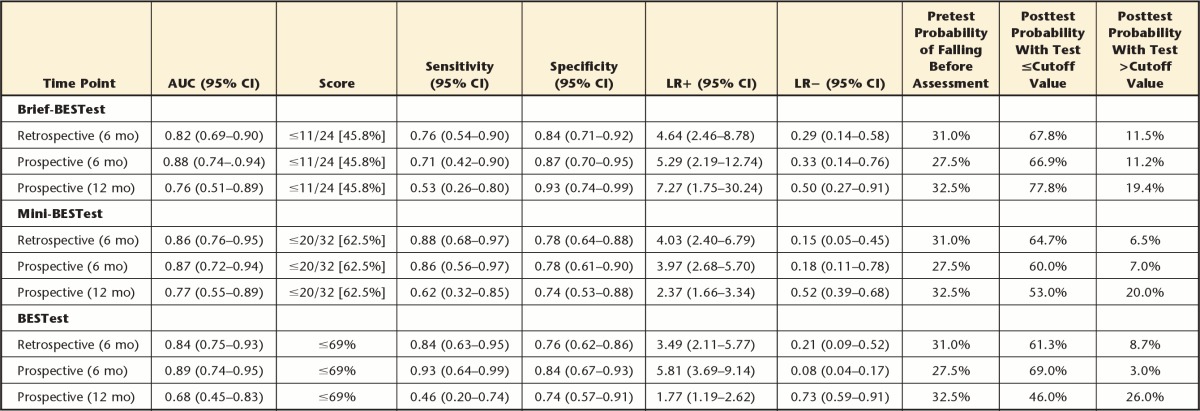
Values are mean (SD). Values inside brackets indicate percentage score determined from raw score to ease comparison to the BESTest. AUC=area under the curve, 95% CI=95% confidence interval, LR+=positive likelihood ratio, LR−=negative likelihood ratio.
Regarding comparisons between the Brief-BESTest, Mini-BESTest, and BESTest when used to retrospectively predict falls, equivalence tests of the AUCs revealed no significant differences between the 3 balance tests for retrospective or prospective fall prediction across 6 or 12 months.
Discussion
To our knowledge, this is the first study to describe balance performance as assessed by the Brief-BESTest in people with PD. This newly derived balance assessment includes items from each of the 6 systems examined using the original full BESTest, as opposed to the Mini-BESTest, which only includes items from 4 of the 6 systems.13
Total scores from each test were strongly related to one another, supporting our hypothesis. Perhaps the most interesting relationship was that between the Brief-BESTest and Mini-BESTest. The strength of the correlation suggests that overall the Brief-BESTest and Mini-BESTest result in similar outcomes despite the fact that items are included in the Brief-BESTest from the 2 systems not examined with the Mini-BESTest. Interestingly, the 2 systems tested in the Brief-BESTest that are not included in the Mini-BESTest had lower correlations with scores from those systems in the full BESTest. There are some potential explanations for the lower correlations. First, it is possible that these 2 systems are either inadequately tested with the items in the full BESTest or that these systems have less impact on the overall balance score on the Brief-BESTest among people with PD. Second, it is important to note the heterogeneity between items within both of these BESTest sections, such as body alignment and postural transition items contained in the same section as ankle and hip strength items. Third, both of these sections are the least reliable in the BESTest examination; however, it is important to note that the Brief-BESTest items representing these sections have higher reliability than other items in those sections.9 Finally, it is possible that the items in the Brief-BESTest representing these 2 balance systems may not be the best items to detect impairments in their respective domains in people with PD.
To determine whether the Brief-BESTest would be an appropriate and equally valuable examination tool in detecting fall risk in people with PD, we evaluated its accuracy in retrospectively and prospectively predicting who would fall in a given time period. The Brief-BESTest (AUC=0.82) compared well with the Mini-BESTest and BESTest (AUC=0.86 and 0.84, respectively) for identifying recurrent fallers based on retrospective fall reports over the previous 6 months10 and for prospective identification of recurrent fallers over 6 and 12 months. Accuracy of all 3 measures was less than ideal over the 12-month prospective period. Sensitivity for all 3 tests ranged from 0.46 (BESTest) to 0.62 (Mini-BESTest).10 At 12 months, the posttest probability of falling with a score greater than the proposed cutoff was between 19.4% (Brief-BESTest) and 26% (BESTest), suggesting that approximately 1 of 5 individuals identified as not at risk for falls would fall in the next 12 months.11 The negative posttest probability at 6 months ranged from 3% (BESTest) to 11.2% (Brief-BESTest), indicating that any of the balance assessments at 6-month intervals are more likely to accurately identify individuals with PD at risk for falls as compared with assessments performed on a yearly basis.10
Other investigators have studied outcome measures and their ability to prospectively predict falls in people with PD. A meta-analysis of 6 studies revealed that a prior history of 2 or more falls in the previous year, not MDS-UPDRS score, was the best predictor of falls over the next 3 months.24 This finding may lead to skepticism regarding why a clinician would take time to complete balance assessments instead of simply asking for fall history if both are equally accurate predictors of future falls. We think that, although it is a useful predictor of falls, fall history can be unreliable and does not provide information to clinicians concerning the cause of the falls.25 As such, clinicians would have no information on which to base their rehabilitation treatment in an attempt to reduce the likelihood of future falls.
Mak and Pang26 noted that the Activities-specific Balance Confidence Scale (ABC) had an AUC of 0.82, a sensitivity of 0.93, and a specificity of 0.67 when attempting to prospectively predict falls over the next 12 months in people with PD. Perhaps the biggest limitation in this outcome measure is that it requires subjective responses, which could be unreliable in the PD population. Second, if used alone, the therapist would be provided no physically objective data from the ABC and would not gain insight into a potential mechanism for falls that could be used to guide treatment. Kerr and colleagues27 prospectively studied fall predictors over 6 months in people with PD and noted that commonly used outcome measures were not good predictors of falls when used alone. Notably, the Tinetti total score (AUC=0.72, sensitivity=0.67, and specificity=0.59), the Berg Balance Scale (BBS) (AUC=0.61, sensitivity=0.65, and specificity=0.51), and the Timed “Up & Go” Test (AUC=0.65, sensitivity=0.69, and specificity=0.62) all demonstrated worse predictive ability than all 3 versions of the BESTest noted in the present study at 6 months.27 Finally, the FGA did not perform as well as the BESTest and Mini-BESTest when used to determine prospective fall risk in people with PD.11 We speculate that the BESTest, Mini-BESTest, and Brief-BESTest may be more accurate for fall prediction than these measures due to the fact that they are essentially batteries of tests measuring more than 1 factor related to falling. Because falls are multifactorial in nature, it might be best to measure as many constructs related to falling rather than focusing on 1 construct.
Identification of the BESTest, Mini-BESTest, and Brief-BESTest as being accurate in predicting future falls in PD is vital so that, with these measures, clinicians can detect fall risk before a fall occurs and implement effective rehabilitation programs for people with PD, with the goal of preventing falls.7,8 Because the Brief-BESTest and Mini-BESTest are used to assess the validity of constructs associated with fall risk, are accurate in predicting future falls, and can be completed in a clinically reasonable amount of time, we suggest their use so that clinicians can determine which constructs should be targeted in physical rehabilitation. Although the BESTest outperformed the Mini-BESTest and Brief-BESTest at 6 months and optimally would be used for fall risk assessment in PD, time constraints in daily practice may not permit its use. As such, we think sacrificing a small amount of accuracy by using the Mini-BESTest or Brief-BESTest is reasonable when time does not permit administration of the full BESTest.
Study Limitations
The interpretation of results from this study should be tempered by the following limitations. The cutoff score for the Brief-BESTest is meant only to assist clinical decision making. Because there are false positives and false negatives with any of the 3 balance tests, cutoff scores should not be considered definitive points to classify individuals as likely recurrent fallers. The sample included primarily individuals with mild to moderate PD, which limits generalizability to the overall population with PD. As these findings are specific to PD, investigators should compare these outcome measures across other populations and study their usefulness with respect to other variables of interest other than falls. Assessments took place only with participants on antiparkinson medication. It is unclear whether the accuracy of fall prediction using the 3 versions of the test would have changed if participants were assessed off antiparkinson medication. If falls are more common during times when medications are not working effectively, one might expect off-medication testing to yield better predictive results. This is an important area for future research. Future studies also should track falls on shorter time intervals (eg, daily or weekly) through a falls diary or phone interviews. This approach would likely enhance fall reporting and be superior to the retrospective reporting method used in the present study. Finally, the dropout rates from baseline to 6 and 12 months were moderate at 36% and 49% of the original sample, respectively, resulting in sample sizes at 6 and 12 months that did not meet the requirements of our power analysis. As many of the individuals dropping out were considered recurrent fallers, it is unclear how this factor might have affected our data had they remained in the study. However, our data suggest that disease severity of the samples was consistent across time points. We also conducted secondary ROC curve analyses for baseline and 6 months of only the 40 individuals who completed the full 12 months of the study, and our results (not reported) did not change, further suggesting that dropout of individuals may not have substantially affected our results.
Conclusion
The BESTest, Mini-BESTest, and Brief-BESTest are valuable measures to assess fall risk in PD. If equipment or time is limited, clinicians may prefer the Brief-BESTest. Given the limited ability to prospectively predict falls over a 1-year time period, we recommend that balance testing should be conducted every 6 months for people with PD.
Footnotes
All authors provided concept/idea/research design. Dr Duncan and Dr Earhart provided writing and data analysis. Dr Duncan, Dr Leddy, and Dr Earhart provided data collection. Dr Dibble, Dr Foreman, and Dr Earhart provided project management, study participants, and facilities/equipment. Dr Leddy, Dr Dibble, Dr Ellis, Dr Ford, Dr Foreman, and Dr Earhart provided fund procurement. Dr Foreman provided institutional liaisons. Dr Leddy, Dr Dibble, Dr Ellis, Dr Ford, Dr Foreman, and Dr Earhart provided consultation (including review of manuscript before submission).
This study was approved by the Human Research Protection Office at Washington University.
A poster presentation of this work was given at the Combined Sections Meeting of the American Physical Therapy Association; January 21–24, 2013; San Diego, California.
This work was funded by the Davis Phinney Foundation, Parkinson's Disease Foundation, and NIH UL1 TR000448. The funding source had no impact or input on the design, conduct, or reporting of this study. Thanks to Vanessa Heil-Chapdelaine, Samantha Herriott, and Brian Morrell for data entry.
References
- 1. Contreras A, Grandas F. Risk of falls in Parkinson's disease: a cross-sectional study of 160 patients. Parkinsons Dis. 2012;2012:362572 2012 Jan 15 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Johnell O, Melton LJ, III, Atkinson EJ, et al. Fracture risk in patients with parkinsonism: a population-based study in Olmsted County, Minnesota. Age Ageing. 1992;21:32–38 [DOI] [PubMed] [Google Scholar]
- 3. Coughlin L, Templeton J. Hip fractures in patients with Parkinson's disease. Clin Orthop Relat Res. 1980;148:192–195 [PubMed] [Google Scholar]
- 4. Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884 [DOI] [PubMed] [Google Scholar]
- 5. Rudzińska M, Bukowczan S, Banaszkiewicz K, et al. Causes and risk factors of falls in patients with Parkinson's disease. Neurol Neurochir Pol. 2008;42:216–222 [PubMed] [Google Scholar]
- 6. Plotnik M, Giladi N, Dagan Y, Hausdorff JM. Postural instability and fall risk in Parkinson's disease: impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Exp Brain Res. 2011;210:529–538 [DOI] [PubMed] [Google Scholar]
- 7. Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson's disease. N Engl J Med. 2012;366:511–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Goodwin VA, Richards SH, Henley W, et al. An exercise intervention to prevent falls in people with Parkinson's disease: a pragmatic randomised controlled trial. J Neurol Neurosurg Psychiatry. 2011;82:1232–1238 [DOI] [PubMed] [Google Scholar]
- 9. Horak FB, Wrisley DM, Frank J. The Balance Evaluation Systems Test (BESTest) to differentiate balance deficits. Phys Ther. 2009;89:484–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leddy AL, Crowner BE, Earhart GM. Utility of the Mini-BESTest, BESTest, and BESTest sections for balance assessments in individuals with Parkinson disease. J Neurol Phys Ther. 2011;35:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Duncan RP, Leddy AL, Cavanaugh JT, et al. Accuracy of fall prediction in Parkinson disease: six-month and 12-month prospective analyses. Parkinsons Dis. 2012;2012:237673 Epub 2011 Nov 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. King LA, Priest KC, Salarian A, et al. Comparing the Mini-BESTest with the Berg Balance Scale to evaluate balance disorders in Parkinson's disease. Parkinsons Dis. 2012;2012:375419 2011 Oct 24 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Padgett PK, Jacobs JV, Kasser SL. Is the BESTest at its best? A suggested brief version based on interrater reliability, validity, internal consistency, and theoretical construct. Phys Ther. 2012;92:1197–1207 [DOI] [PubMed] [Google Scholar]
- 14. Dibble LE, Cavanaugh JT, Earhart GM, et al. Charting the progression of disability in Parkinson disease: study protocol for a prospective longitudinal cohort study. BMC Neurol. 2010;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson's disease. Am J Med Genet. 1999;88:539–543 [PubMed] [Google Scholar]
- 16. Leddy AL, Crowner BE, Earhart GM. Functional gait assessment and balance evaluation system test: reliability, validity, sensitivity, and specificity for identifying individuals with Parkinson disease who fall. Phys Ther. 2011;91:102–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Franchignoni F, Horak F, Godi M, et al. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med. 2010;42:323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170 [DOI] [PubMed] [Google Scholar]
- 19. Ashburn A, Stack E, Pickering RM, Ward CD. Predicting fallers in a community-based sample of people with Parkinson's disease. Gerontology. 2001;47:277–281 [DOI] [PubMed] [Google Scholar]
- 20. Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the Timed Up & Go Test. Phys Ther. 2000;80:896–903 [PubMed] [Google Scholar]
- 21. Akobeng AK. Understanding diagnostic tests 3: Receiver operating characteristic curves. Acta Paediatr. 2007;96:644–647 [DOI] [PubMed] [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845 [PubMed] [Google Scholar]
- 23. Hintze JL. NCSS. Kaysville, UT: NCSS LLC; 2009. Available at: http://www.ncss.com [Google Scholar]
- 24. Pickering RM, Grimbergen YA, Rigney U, et al. A meta-analysis of six prospective studies of falling in Parkinson's disease. Mov Disord. 2007;22:1892–1900 [DOI] [PubMed] [Google Scholar]
- 25. Cummings SR, Nevitt MC, Kidd S. Forgetting falls. The limited accuracy of recall of falls in the elderly. J Am Geriatr Soc. 1988;36:613–616 [DOI] [PubMed] [Google Scholar]
- 26. Mak MK, Pang MY. Fear of falling is independently associated with recurrent falls in patients with Parkinson's disease: a 1-year prospective study. J Neurol. 2009;256:1689–1695 [DOI] [PubMed] [Google Scholar]
- 27. Kerr GK, Worringham CJ, Cole MH, et al. Predictors of future falls in Parkinson disease. Neurology. 2010;75:116–124 [DOI] [PubMed] [Google Scholar]