Abstract
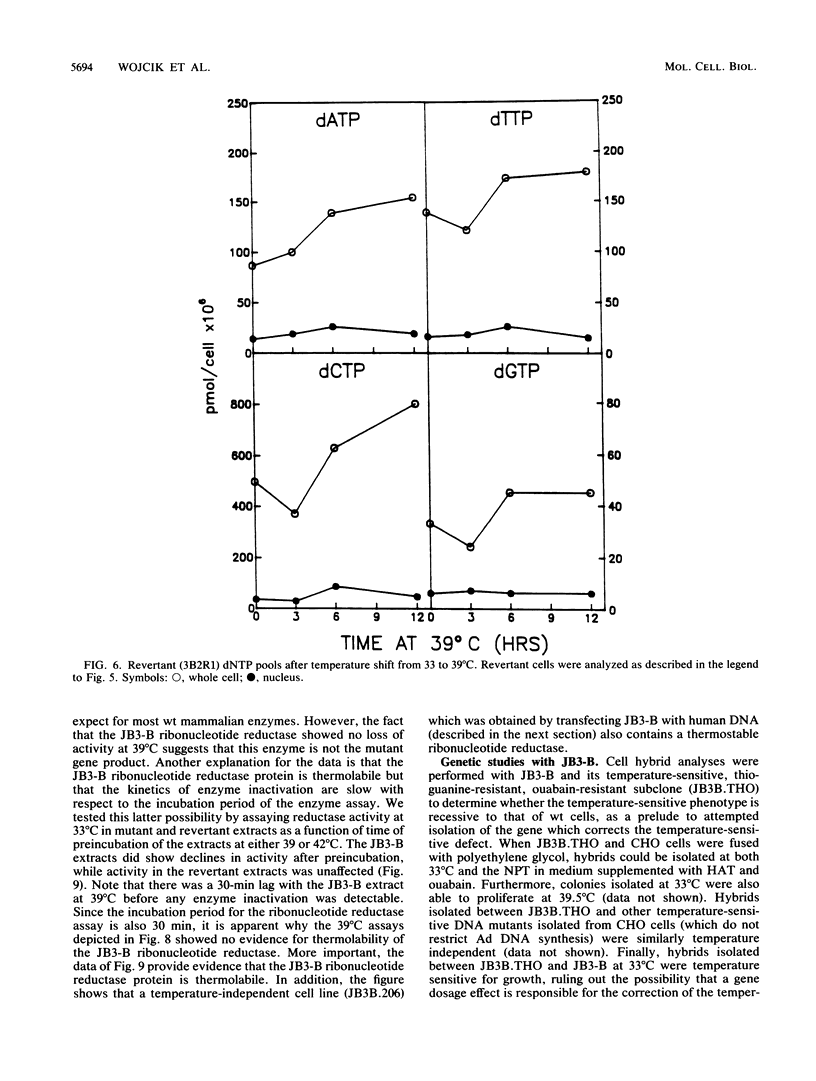
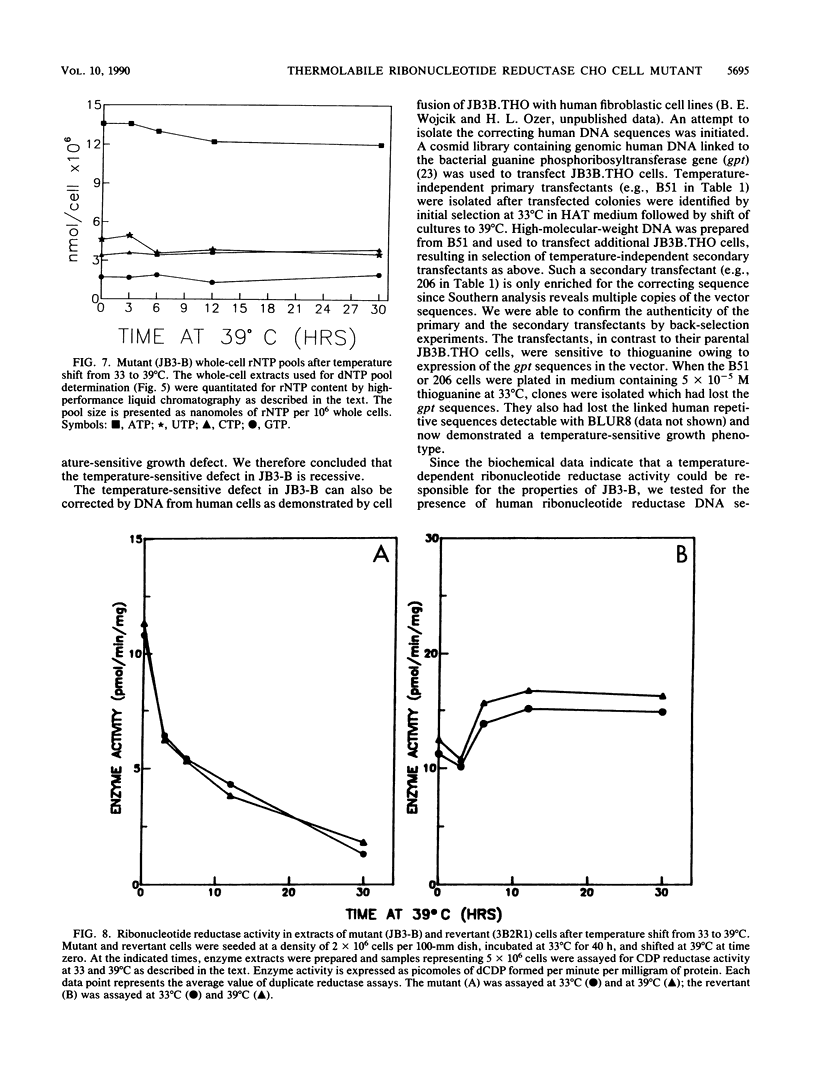
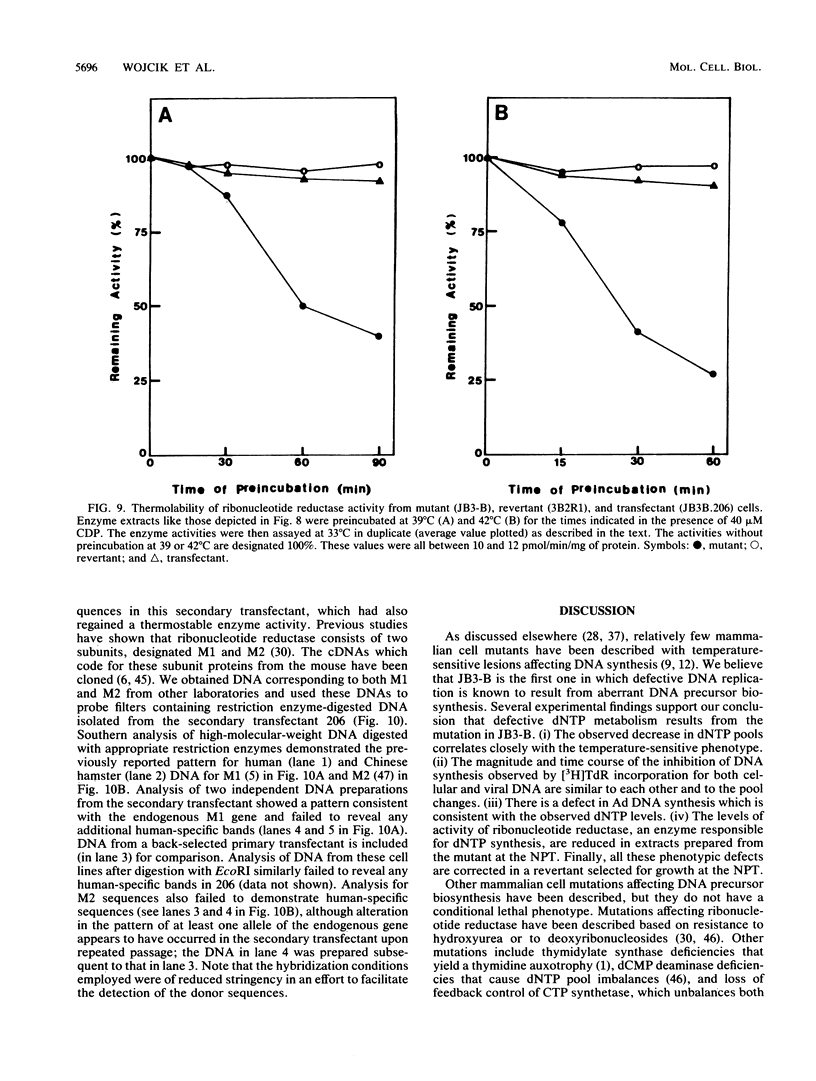
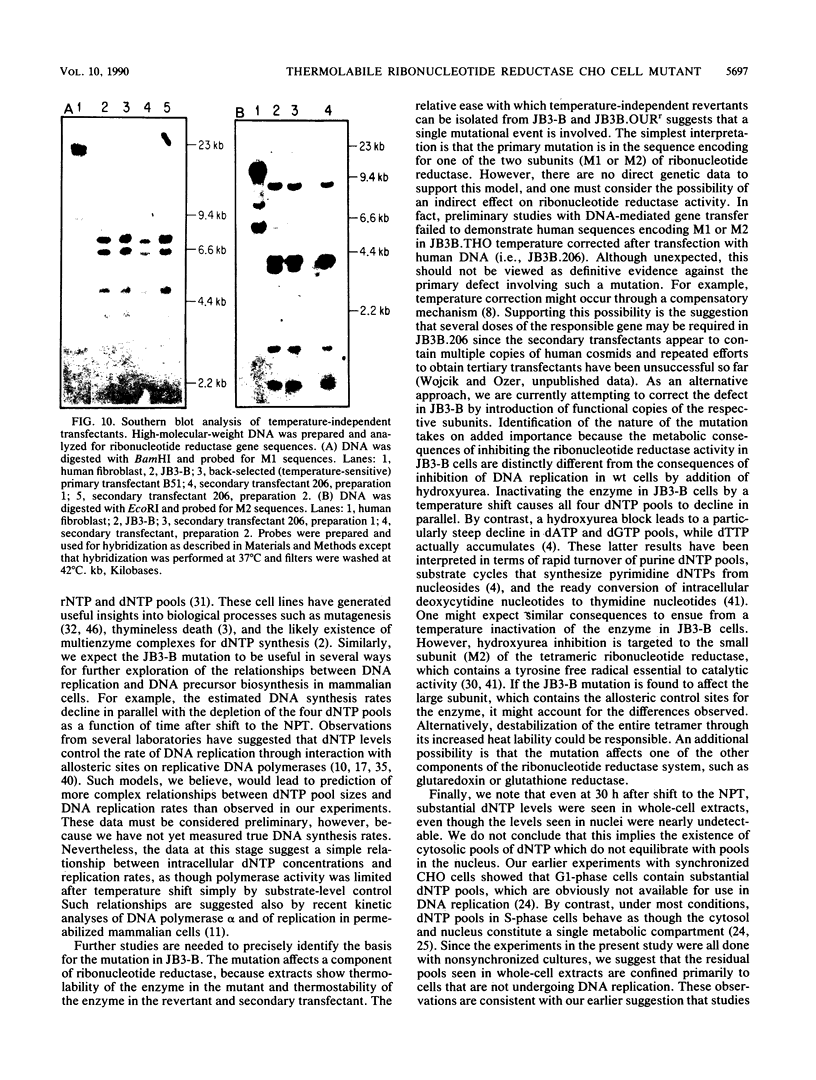
JB3-B is a Chinese hamster ovary cell mutant previously shown to be temperature sensitive for DNA replication (J. J. Dermody, B. E. Wojcik, H. Du, and H. L. Ozer, Mol. Cell. Biol. 6:4594-4601, 1986). It was chosen for detailed study because of its novel property of inhibiting both polyomavirus and adenovirus DNA synthesis in a temperature-dependent manner. Pulse-labeling studies demonstrated a defect in the rate of adenovirus DNA synthesis. Measurement of deoxyribonucleoside triphosphate (dNTP) pools as a function of time after shift of uninfected cultures from 33 to 39 degrees C revealed that all four dNTP pools declined at similar rates in extracts prepared either from whole cells or from rapidly isolated nuclei. Ribonucleoside triphosphate pools were unaffected by a temperature shift, ruling out the possibility that the mutation affects nucleoside diphosphokinase. However, ribonucleotide reductase activity, as measured in extracts, declined after cell cultures underwent a temperature shift, in parallel with the decline in dNTP pool sizes. Moreover, the activity of cell extracts was thermolabile in vitro, consistent with the model that the JB3-B mutation affects the structural gene for one of the ribonucleotide reductase subunits. The kinetics of dNTP pool size changes after temperature shift are quite distinct from those reported after inhibition of ribonucleotide reductase with hydroxyurea. An indirect effect on ribonucleotide reductase activity in JB3-B has not been excluded since human sequences other than those encoding the enzyme subunits can correct the temperature-sensitive growth defect in the mutant.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Koyama H., Iwata K., Seno T. Single-step selection of mouse FM3A cell mutants defective in thymidylate synthetase. Somatic Cell Genet. 1980 Mar;6(2):261–270. doi: 10.1007/BF01538800. [DOI] [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Takeishi K., Seno T. Accumulation of DNA strand breaks during thymineless death in thymidylate synthase-negative mutants of mouse FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12448–12454. [PubMed] [Google Scholar]
- Ayusawa D., Shimizu K., Koyama H., Takeishi K., Seno T. Unusual aspects of human thymidylate synthase in mouse cells introduced by DNA-mediated gene transfer. J Biol Chem. 1983 Jan 10;258(1):48–53. [PubMed] [Google Scholar]
- Bianchi V., Pontis E., Reichard P. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J Biol Chem. 1986 Dec 5;261(34):16037–16042. [PubMed] [Google Scholar]
- Brissenden J. E., Caras I., Thelander L., Francke U. The structural gene for the M1 subunit of ribonucleotide reductase maps to chromosome 11, band p15, in human and to chromosome 7 in mouse. Exp Cell Res. 1988 Jan;174(1):302–308. doi: 10.1016/0014-4827(88)90165-6. [DOI] [PubMed] [Google Scholar]
- Caras I. W., Martin D. W., Jr Molecular cloning of the cDNA for a mutant mouse ribonucleotide reductase M1 that produces a dominant mutator phenotype in mammalian cells. Mol Cell Biol. 1988 Jul;8(7):2698–2704. doi: 10.1128/mcb.8.7.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Choi W. J., Campbell J. L., Kuo C. L., Jong A. Y. The Saccharomyces cerevisiae SOC8-1 gene and its relationship to a nucleotide kinase. J Biol Chem. 1989 Sep 15;264(26):15593–15599. [PubMed] [Google Scholar]
- Dermody J. J., Wojcik B. E., Du H., Ozer H. L. Identification of temperature-sensitive DNA- mutants of Chinese hamster cells affected in cellular and viral DNA synthesis. Mol Cell Biol. 1986 Dec;6(12):4594–4601. doi: 10.1128/mcb.6.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley J. F. Affinity labeling the DNA polymerase alpha complex. Identification of subunits containing the DNA polymerase active site and an important regulatory nucleotide-binding site. J Biol Chem. 1988 Dec 15;263(35):19126–19131. [PubMed] [Google Scholar]
- Dresler S. L., Frattini M. G., Robinson-Hill R. M. In situ enzymology of DNA replication and ultraviolet-induced DNA repair synthesis in permeable human cells. Biochemistry. 1988 Sep 20;27(19):7247–7254. doi: 10.1021/bi00419a011. [DOI] [PubMed] [Google Scholar]
- Eki T., Enomoto T., Miyajima A., Miyazawa H., Murakami Y., Hanaoka F., Yamada M., Ui M. Isolation of temperature-sensitive cell cycle mutants from mouse FM3A cells. Characterization of mutants with special reference to DNA replication. J Biol Chem. 1990 Jan 5;265(1):26–33. [PubMed] [Google Scholar]
- Eriksson S., Skog S., Tribukait B., Wallström B. Deoxyribonucleoside triphosphate metabolism and the mammalian cell cycle. Effects of hydroxyurea on mutant and wild-type mouse S49 T-lymphoma cells. Exp Cell Res. 1987 Jan;168(1):79–88. doi: 10.1016/0014-4827(87)90417-4. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Garrett C., Santi D. V. A rapid and sensitive high pressure liquid chromatography assay for deoxyribonucleoside triphosphates in cell extracts. Anal Biochem. 1979 Nov 1;99(2):268–273. doi: 10.1016/s0003-2697(79)80005-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grindey G. B., Winkler M., Otten M., Steinberg J. A. Inhibition of calf thymus DNA polymerase-alpha by deoxyribonucleoside triphosphates. Mol Pharmacol. 1980 Mar;17(2):256–261. [PubMed] [Google Scholar]
- Jelinek W. R., Toomey T. P., Leinwand L., Duncan C. H., Biro P. A., Choudary P. V., Weissman S. M., Rubin C. M., Houck C. M., Deininger P. L. Ubiquitous, interspersed repeated sequences in mammalian genomes. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1398–1402. doi: 10.1073/pnas.77.3.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha K. K., Siniscalco M., Ozer H. L. Temperature-sensitive mutants of BALB/3T3 cells. III. Hybrids between ts2 and other mouse mutant cells affected in DNA synthesis and correction of ts2 defect by human X chromosome. Somatic Cell Genet. 1980 Sep;6(5):603–614. doi: 10.1007/BF01538640. [DOI] [PubMed] [Google Scholar]
- Klein C. E., Ozer H. L., Traganos F., Atzpodien J., Oettgen H. F., Old L. J. A transformation-associated 130-kD cell surface glycoprotein is growth controlled in normal human cells. J Exp Med. 1988 May 1;167(5):1684–1696. doi: 10.1084/jem.167.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bella F., Ozer H. L. Differential replication of SV40 and polyoma DNAs in Chinese hamster ovary cells. Virus Res. 1985 Jun;2(4):329–344. doi: 10.1016/0168-1702(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Versatile cosmid vectors for the isolation, expression, and rescue of gene sequences: studies with the human alpha-globin gene cluster. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5225–5229. doi: 10.1073/pnas.80.17.5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds J. M., Mathews C. K. Cell cycle-dependent effects on deoxyribonucleotide and DNA labeling by nucleoside precursors in mammalian cells. Mol Cell Biol. 1987 Jan;7(1):532–534. doi: 10.1128/mcb.7.1.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeds J. M., Slabaugh M. B., Mathews C. K. DNA precursor pools and ribonucleotide reductase activity: distribution between the nucleus and cytoplasm of mammalian cells. Mol Cell Biol. 1985 Dec;5(12):3443–3450. doi: 10.1128/mcb.5.12.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longiaru M., Horwitz M. S. Chinese hamster ovary cells replicate adenovirus deoxyribonucleic acid. Mol Cell Biol. 1981 Mar;1(3):208–215. doi: 10.1128/mcb.1.3.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus M., Fainsod A., Diamond G. The genetic analysis of mammalian cell-cycle mutants. Annu Rev Genet. 1985;19:389–421. doi: 10.1146/annurev.ge.19.120185.002133. [DOI] [PubMed] [Google Scholar]
- Mathews C. K., Moen L. K., Wang Y., Sargent R. G. Intracellular organization of DNA precursor biosynthetic enzymes. Trends Biochem Sci. 1988 Oct;13(10):394–397. doi: 10.1016/0968-0004(88)90182-x. [DOI] [PubMed] [Google Scholar]
- McClarty G. A., Chan A. K., Engstrom Y., Wright J. A., Thelander L. Elevated expression of M1 and M2 components and drug-induced posttranscriptional modulation of ribonucleotide reductase in a hydroxyurea-resistant mouse cell line. Biochemistry. 1987 Dec 1;26(24):8004–8011. doi: 10.1021/bi00398a068. [DOI] [PubMed] [Google Scholar]
- Meuth M. Sensitivity of a mutator gene in Chinese hamster ovary cell to deoxynucleoside triphosphate pool alterations. Mol Cell Biol. 1981 Jul;1(7):652–660. doi: 10.1128/mcb.1.7.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuth M. The molecular basis of mutations induced by deoxyribonucleoside triphosphate pool imbalances in mammalian cells. Exp Cell Res. 1989 Apr;181(2):305–316. doi: 10.1016/0014-4827(89)90090-6. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld D. S., Ripley S., Henderson A., Ozer H. L. Immortalization of human fibroblasts transformed by origin-defective simian virus 40. Mol Cell Biol. 1987 Aug;7(8):2794–2802. doi: 10.1128/mcb.7.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicander B., Reichard P. Aphidicolin sensitivity of variant 3T6 cells selected for changes in ribonucleotide reductase. Biochem Biophys Res Commun. 1981 Nov 16;103(1):148–155. doi: 10.1016/0006-291x(81)91672-7. [DOI] [PubMed] [Google Scholar]
- North T. W., Bestwick R. K., Mathews C. K. Detection of activities that interfere with the enzymatic assay of deoxyribonucleoside 5'-triphosphates. J Biol Chem. 1980 Jul 25;255(14):6640–6645. [PubMed] [Google Scholar]
- Radna R. L., Foellmer B., Feldman L. A., Francke U., Ozer H. L. Restriction of human adenovirus replication in Chinese hamster cell lines and their hybrids with human cells. Virus Res. 1987 Nov;8(4):277–299. doi: 10.1016/0168-1702(87)90001-3. [DOI] [PubMed] [Google Scholar]
- Rapaport E., Garcia-Blanco M. A., Zamecnik P. C. Regulation of DNA replication in S phase nuclei by ATP and ADP pools. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1643–1647. doi: 10.1073/pnas.76.4.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard P. From deoxynucleotides to DNA synthesis. Fed Proc. 1978 Jan;37(1):9–14. [PubMed] [Google Scholar]
- Reichard P. Interactions between deoxyribonucleotide and DNA synthesis. Annu Rev Biochem. 1988;57:349–374. doi: 10.1146/annurev.bi.57.070188.002025. [DOI] [PubMed] [Google Scholar]
- Sargent R. G., Mathews C. K. Imbalanced deoxyribonucleoside triphosphate pools and spontaneous mutation rates determined during dCMP deaminase-defective bacteriophage T4 infections. J Biol Chem. 1987 Apr 25;262(12):5546–5553. [PubMed] [Google Scholar]
- Slabaugh M. B., Johnson T. L., Mathews C. K. Vaccinia virus induces ribonucleotide reductase in primate cells. J Virol. 1984 Nov;52(2):507–514. doi: 10.1128/jvi.52.2.507-514.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Berg P. Isolation and characterization of expressible cDNA clones encoding the M1 and M2 subunits of mouse ribonucleotide reductase. Mol Cell Biol. 1986 Oct;6(10):3433–3442. doi: 10.1128/mcb.6.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg G., Ullman B., Martin D. W., Jr Mutator phenotypes in mammalian cell mutants with distinct biochemical defects and abnormal deoxyribonucleoside triphosphate pools. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2447–2451. doi: 10.1073/pnas.78.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang-Feng T. L., Barton D. E., Thelander L., Lewis W. H., Srinivasan P. R., Francke U. Ribonucleotide reductase M2 subunit sequences mapped to four different chromosomal sites in humans and mice: functional locus identified by its amplification in hydroxyurea-resistant cell lines. Genomics. 1987 Sep;1(1):77–86. doi: 10.1016/0888-7543(87)90108-x. [DOI] [PubMed] [Google Scholar]