Abstract
Several pathways and pathologies have been suggested as connections between obesity and diabetes, including inflammation of adipose and other tissues, toxic lipids, endoplasmic reticulum stress, and fatty liver. One specific proposal is that insulin resistance induces a vicious cycle in which hyperinsulinemia increases hepatic lipogenesis and exacerbates fatty liver, in turn further increasing insulin resistance. Here I suggest that reversing this cycle via suppression of the lipogenic transcription factor SREBP-1c is a common thread that connects the antidiabetic effects of a surprising number of nuclear hormone receptors, including CAR, LRH-1, TRβ, ERα and FXR/SHP.
The diverse members of the nuclear receptor superfamily exert a wide range of metabolic regulatory effects. Within the context of insulin resistance and type 2 diabetes, the best known may be the classic hyperglycemic and diabetogenic effects of glucocorticoids (Ingle, 1941; Ingle et al., 1945; Long et al., 1940; Munck, 1971), and GR has been found more recently to directly downregulate key components of the insulin signaling cascade (Rose et al., 2010). Glucorticoids can be produced outside the adrenal, and local production by visceral adipose tissue is thought to be a contributor to insulin resistance in obesity (Gathercole and Stewart, 2010). In the liver, glucocorticoid excess promotes steatosis (Rose et al., 2010), and glucocorticoids have emerged more recently as modulators of additional hepatic metabolic pathways, including bile acid homeostasis (Rose et al., 2011).
Activation of the oxysterol receptors LXRα and LXRβ may also have negative effects, since they are well known inducers of hepatic lipogenesis and fatty liver, and double knockouts do not become insulin resistant when fed a high fat diet (Kalaany et al., 2005; Kalaany and Mangelsdorf, 2005). They are essential for the activation of SREBP-1c expression by insulin (Horton et al., 2002; Repa et al., 2000; Yoshikawa et al., 2001), and the elevated expression of LXRα observed in human subjects with non-alcoholic fatty liver disease (NAFLD) is correlated with increased expression of SREBP-1c and its downstream lipogenic targets (Higuchi et al., 2008). However, synthetic LXR agonists can have antidiabetic effects in some cases (Cao et al., 2003; Commerford et al., 2007; Grefhorst et al., 2005; Herzog et al., 2007; Laffitte et al., 2003; Liu et al., 2006). Such effects have been attributed to promotion of peripheral glucose uptake, particularly by inducing Glut4 expression in adipose tissues, and suppression of hepatic gluconeogenesis via repression of hepatic GR and gluconeogenic gene expression.
The bile acid receptor FXR may also have conflicting effects in different contexts. Loss of FXR results in steatosis (Sinal et al., 2000) and insulin resistance (Ma et al., 2006; Zhang et al., 2006) in mice fed normal chow, and there are reports of beneficial effects of both bile acids and synthetic FXR ligands on insulin sensitivity (Cipriani et al., 2010; Sanyal et al., 2009; Watanabe et al., 2006; Zhang et al., 2006). However, FXR deficiency reportedly improves glucose homeostasis in mouse models of obesity (Prawitt et al., 2011), and long term FXR activation via a synthetic agonist induces obesity and insulin resistance, perhaps via suppression of endogenous bile acid pools (Watanabe et al., 2011).
In contrast to these diabetogenic effects, activation of surprisingly large number of other nuclear receptors has beneficial effects on insulin sensitivity (Table 1). Some, such as the antidiabetic actions of agonist ligands for PPARγ and PPARα, are very well recognized (Lalloyer and Staels, 2010; Lehrke and Lazar, 2005). Others are less so, including the clear beneficial impact of estrogen receptor activation (Mauvais-Jarvis, 2011), and the well documented insulin sensitizing effects of phenobarbital in human type 2 diabetes (Lahtela et al., 1985; Sotaniemi and Karvonen, 1989). The beneficial impact of HNF-4α is revealed by the genetic consequences of its mutation in human patients with Mature Onset Diabetes of the Young type 1 (MODY-1) (Vaxillaire and Froguel, 2008), and also in mice with β-cell specific deletion of HNF-4α (Gupta et al., 2005; Miura et al., 2006). The potential antidiabetic effects of vitamin D in human patients remain controversial (Takiishi et al., 2010). Finally, there are reports of antidiabetic effects of specific RXR agonists (Pinaire and Reifel-Miller, 2007), although it remains unclear whether these effects are due to activation of RXR alone or a heterodimer. All-trans retinoic acid treatment can also have positive metabolic effects in mice, including weight loss and improved glucose tolerance (Amengual et al., 2010; Berry and Noy, 2009; Manolescu et al., 2010), suggesting that one or more of the retinoic acid receptor (RAR) isoforms could exert antidiabetic effects. More broadly, however, retinoids are associated with negative effects in humans (Gerber and Erdman, 1982), and the association of distinct components of the vitamin A – retinol - retinoic acid axis with insulin sensitivity is both complex and contradictory. Thus, while specific antidiabetic effects may exist, none of the 6 retinoid receptors are included in the table.
Table 1.
Diverse impact of nuclear receptors on insulin resistance.
| Impacts of Nuclear Receptors on Insulin Resistance
| ||
|---|---|---|
| Mouse models | Human patients | |
| GR | − | − |
| LXRα/β | +/− | ? |
| FXR | +/− | +? |
| PPARα/γ/δ | +/+/+ | +/+/? |
| ERα/β | + | + |
| CAR | + | + |
| HNF-4α | +* | +* |
| VDR | + | ? |
| TRβ | + | ? |
| LRH-1 | + | ? |
| NR4A1–3 | + | ? |
+ indicates significant evidence for antidiabetic effects, −, evidence for deleterious effects, ?, no evidence, or suggestive but inconclusive evidence. PPARs (Barish et al., 2006; Lalloyer and Staels, 2010; Lehrke and Lazar, 2005); ERα (Bryzgalova et al., 2008b; Mauvais-Jarvis, 2011); CAR (Dong et al., 2009; Lahtela et al., 1985; Sotaniemi and Karvonen, 1989); FXR (Cipriani et al., 2010; Sanyal et al., 2009; Watanabe et al., 2006; Zhang et al., 2006); HNF-4α (* revealed by impact of MODY mutations in humans (Vaxillaire and Froguel, 2008) and pancreatic knockouts in mice (Gupta et al., 2005; Miura et al., 2006)), VDR (Takiishi et al., 2010); TRβ (Amorim et al., 2009; Bryzgalova et al., 2008a); LRH-1 (Lee et al., 2011); LXRα/β (Cao et al., 2003; Commerford et al., 2007; Grefhorst et al., 2005; Herzog et al., 2007; Laffitte et al., 2003; Liu et al., 2006); NR4A (Chao et al., 2009; Pols et al., 2008) (note – NR4A effects are based on induction or loss of receptor expression rather than ligand responses).
Overall, however, it is clear that activation of a remarkably large number of nuclear receptors improves insulin sensitivity. This striking functional convergence raises a simple question: despite their very diverse physiologic roles, does a common mechanistic thread connect their antidiabetic effects?
Twenty years ago, the late Denis McGarry provocatively suggested that dysregulation of fatty acid metabolism is more important than altered glucose homeostasis in the development of type 2 diabetes (McGarry, 1992). The crux of his argument was that the elevation of insulin levels in the early stages of insulin resistance results in increased lipogenesis and lipid deposition. This results in increased insulin resistance that drives insulin levels yet higher, resulting overall in a self-reinforcing vicious cycle (Fig. 1, counterclockwise, red). Although he also focused on skeletal muscle and other peripheral tissues, the tight correlation of hepatic steatosis with insulin resistance in humans (Fabbrini et al., 2010; Korenblat et al., 2008; Petersen et al., 2005; Samuel et al., 2010) suggests a central role for the liver in his basic proposal. In accord with this, increased lipogenesis contributes significantly to the elevated liver triglycerides in insulin resistant human subjects with nonalcoholic fatty liver disease (Donnelly et al., 2005).
Figure 1. Proposed model for McGarry’s lipogenic vicious cycle, and its reversal.
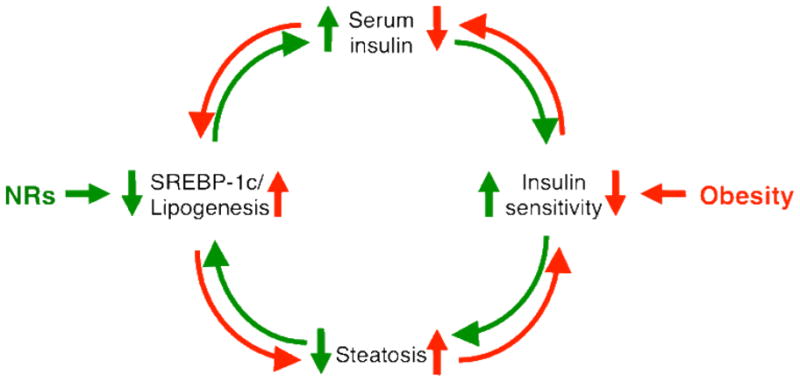
In the red, counterclockwise cycle, insulin resistance generates a self-reinforcing negative regulatory loop in which elevated insulin levels increase SREBP-1c expression and steatosis. This further decreases insulin sensitivity and increases in serum insulin levels to continue the negative cycle. Several NRs, including ERα CAR, LRH-1 TRβ and FXR/SHP act to decrease SREBP-1c expression, lowering steatosis and setting up a positive, anti-lipogenic cycle to improved insulin sensitivity.
McGarry was very aware of the lipogenic impact of insulin, but his mechanistic views were based on acute allosteric effects on metabolic pathways and the Randle cycle. Only a year after his commentary appeared, however, his University of Texas Southwestern Medical Center colleagues Michael Brown and Joe Goldstein (Briggs et al., 1993), and also Bruce Spiegelman ((Tontonoz et al., 1993) as ADD1), first described the transcription factor SREBP-1c, which we now know drives the lipogenic response to insulin in the liver (Horton et al., 2002). Much more recently, Brown and Goldstein described a mechanism that could explain the seemingly paradoxical observations that the insulin resistant liver loses the ability to shut down gluconeogenesis in response to elevated insulin levels, while at the same time maintaining elevated SREBP-1c expression (Brown and Goldstein, 2008; Li et al., 2010). They identified a bifurcation in the insulin signaling pathway in which the induction of lipogenesis, but not the gluconeogenic response, is under the control of the mTOR pathway. Thus, the mTOR inhibitor rapamycin blocked SREBP-1c induction in response to insulin, but did not prevent insulin suppression of PEPCK expression. Both responses were blocked by the PI3kinase/AKT inhibitor wortmannin, placing this early target in the insulin signaling cascade upstream of the divergent mTORC1 - SREBP-1c and FoxO1 - PEPCK branches.
Despite the insulin resistance of the gluconeogenic arm, persistently elevated SREBP-1c expression in response to hyperinsulinemia, or potentially other signals (Ferre and Foufelle, 2010), could neatly account for the induction of lipogenesis and increased hepatic steatosis in insulin resistant liver. Consistent with this, elevated expression of SREBP-1c and its downstream lipogenic targets is positively correlated with insulin resistance in subjects with steatosis (Pettinelli et al., 2009). Expression of SREBP-1c and its targets was also markedly elevated in the livers of morbidly obese, hyperinsulinemic human patients prior to gastric bypass, relative to the patients who experienced massive weight loss and reversal of insulin resistance after bypass (Elam et al., 2010).
The involvement of mTOR in SREBP-1c activation and steatosis in response to western diet feeding in mice was confirmed in liver specific knockouts of raptor, the defining mTORC1 component (Peterson et al., 2011). Increases in both hepatic triglycerides and the SREBP-1c target fatty acid synthase (FAS) in response to the diet were completely absent in these Li-RapKO mice. However, mTORC1 activation is not sufficient to drive SREBP-1c activation and steatosis. Thus, recent results demonstrate that the loss of Akt2 in the liver of refed mice prevents the induction of lipogenesis by refeeding, despite activation of the mTORC1 - SREBP-1c pathway (Wan et al., 2011). Moreover, the constitutive, insulin independent activation of mTORC1 in liver specific knockouts of the inhibitor Tsc1 blocks, rather than promotes SREBP-1c activation and steatosis (Yecies et al., 2011). In accord with the Akt2 knockout results, this block was reversed by expression of a constitutively active form of Akt2. These results suggest that Akt has additional downstream lipogenic targets, and raise interesting questions about the relationship of the mTOR and Akt pathways to the LXRs, which are required for insulin dependent activation of SREBP-1c (Repa et al., 2000), and also directly target downstream lipogenic genes (Kalaany and Mangelsdorf, 2005).
In McGarry’s vicious cycle, inappropriate activation of SREBP-1c in the context of insulin resistance drives lipogenesis and fatty liver, which is then linked to further increases in insulin resistance. Fatty liver is now widely recognized as a consequence of insulin resistance (Abdelmalek and Diehl, 2007; Malhi and Gores, 2008). Nonetheless, it is also quite clear from a number of rodent models (Anstee and Goldin, 2006; Lan et al., 2003; Monetti et al., 2007), as well as some human examples (Amaro et al., 2010; Romeo et al., 2008) that simply elevating liver triglycerides is not sufficient to produce insulin resistance. The intriguing phenomenon of obese but metabolically normal individuals (Pataky et al., 2010) is consistent with this, although there is evidence that such individuals have markedly lower liver fat than those who are obese and insulin resistant (Stefan et al., 2008).
Nonetheless, many of the cases in which liver triglycerides are dissociated from insulin resistance represent unusual genetic variations, or situations that may not reflect more normal physiology or pathophysiology, and could be considered exceptions to the general pattern. Thus, there is no doubt that human population studies demonstrate a strong correlation of increased steatosis with insulin resistance (Fabbrini et al., 2010; Korenblat et al., 2008; Samuel et al., 2010), which is independent of body weight (Seppala-Lindroos et al., 2002), and various high fat or hypercaloric diets reproducibly induce both steatosis and insulin resistance in rodent models, which is associated with increased SREBP-1c expression (Biddinger et al., 2005; Shimomura et al., 1999). In the opposite direction, fasting serum glucose was normalized in human subjects with type 2 diabetes after the loss of only a small amount of body weight, and this was correlated with a marked decrease in intrahepatic lipid content but no effect on peripheral glucose uptake (Petersen et al., 2005). Improved insulin sensitivity in response to decreased steatosis is also observed in numerous mouse models (Tilg and Moschen, 2008), including those described below.
One relatively simple resolution of this apparent paradox is that triglyceride accumulation is not by itself the cause of the metabolic imbalance initiated by insulin resistance, but is rather a symptom or an associated comorbidity. In this scenario, lipid metabolism could somehow be balanced in the exceptional cases, perhaps by detoxification of free fatty acids via incorporation into triglycerides (Choi and Diehl, 2008) or sequestration of lipids into stable droplets (Greenberg et al., 2011), while other lipids or signaling molecules would drive metabolic imbalance in the context of insulin resistance. The simplest “culprit” might be free fatty acids, which are elevated via increased lipolysis in insulin resistance and are, of course, also the immediate products of lipogenesis. Elevated levels of free fatty acids are associated with insulin resistance in the liver and also in peripheral tissues (Boden, 2011). In accord with this, plasma free fatty acids were higher in individuals with type 2 diabetes and fatty liver than in diabetics who did not show evidence for fatty liver, and this elevation was associated with increased insulin resistance (Kelley et al., 2003). A more recent study of both normal subjects and those with type 2 diabetes found correlations of elevated liver fat and free fatty acids with the worsening of many metabolic parameters, including fasting plasma glucose and insulin levels, hepatic insulin resistance index, and, in the euglycemic clamp, decreased glucose clearance (Gastaldelli et al., 2007). There are a number of other candidates in addition to free fatty acids, including diacylglycerol (Samuel et al., 2010), ceramides (Haus et al., 2009; Holland and Summers, 2008; Yang et al., 2009) and hepatic cytokines (Cai et al., 2005; Solinas et al., 2007), all of which are known to be increased in the context of steatosis. Inappropriately elevated hepatic production and serum accumulation of any of these known insulin signaling inhibitors could contribute to whole body insulin resistance.
I suggest that inhibition of SREBP-1c is a common mechanistic thread that connects at least a subset of the antidiabetic effects in Table 1. This thread is particularly evident in our recent studies on the insulin sensitizing effects of agonist ligands for the nuclear receptors CAR (Dong et al., 2009) and LRH-1 (Lee et al., 2011). We demonstrated that CAR activation can account for the insulin sensitizing effects of phenobarbital first described by Sotaniemi and colleagues in human patients (Lahtela et al., 1985; Sotaniemi and Karvonen, 1989), and also in ob/ob mice (Karvonen et al., 1989). A similar impact of CAR activation was observed independently by Xie and colleagues (Gao et al., 2009). We also found that a novel LRH-1 agonist, dilauroyl phosphatidylcholine, exerts antidiabetic effects in mice that are strikingly similar to those of phenobarbital. In particular, activation of either nuclear receptor repressed expression of SREBP-1 mRNA, as well as that of its downstream lipogenic targets including fatty acid synthase, stearoyl CoA desaturase-1, and acetyl CoA carboxylase. In addition to the decrease in lipogenesis expected from these responses, we showed that CAR activation also increases fatty acid β-oxidation (Dong et al., 2009). This is likely due to decreased malonyl-CoA, the product of acetyl CoA carboxylase, which McGarry showed is a potent inhibitor of fatty acid uptake into mitochondria via the carnitine dependent shuttle (McGarry and Foster, 1980). The net result in both cases is a striking decrease in hepatic steatosis, but not overall body weight, which is associated with improved whole body insulin sensitivity. The lack of significant expression of either CAR or LRH-1 in other key metabolic target tissues, particularly skeletal muscle and adipose (Bookout et al., 2006), and the loss of the LRH-1 response in liver specific knockouts (Lee et al., 2011) highlight the central role of the liver in both antidiabetic effects.
TRβ is the best studied of at least 3 other antidiabetic nuclear receptors that also repress SREBP-1c. Activation of both thyroid hormone receptor isoforms in hyperthyroidsm decreases insulin sensitivity in humans (Dimitriadis et al., 1985). However, selective liver/TRβ activation decreases serum triglycerides and cholesterol in both rodent models and humans (Angelin and Rudling, 2010). A number of studies show that SREBP-1c expression is repressed by activation of TRβ via T3, or by the selective agonists GC-1, KB-141, MB07344 and MB07811 (Angelin and Rudling, 2010; Bryzgalova et al., 2008a; Erion et al., 2007). This is thought to be due to direct repression of SREBP-1c promoter activity (Hashimoto et al., 2006; Johansson et al., 2005). Although studies with these compounds have primarily focused on their effects on serum lipids and body weight, KB-141 decreased hepatic triglycerides and improved insulin sensitivity in ob/ob mice (Bryzgalova et al., 2008a), and both KB-141 and MB07811 repressed SREBP-1c expression and decreased hepatic triglycerides and serum glucose in the diet induced obesity mouse model of insulin resistance (Erion et al., 2007). In both of these studies, the antidiabetic effects were also correlated with decreased body weight.
The impact of FXR on insulin resistance appears complex, as noted above, but FXR activation can have positive effects (Cipriani et al., 2010; Sanyal et al., 2009; Watanabe et al., 2006; Zhang et al., 2006). Auwerx and collaborators attributed the beneficial effects of modestly elevated bile acids to suppression of SREBP-1c expression via induction of the nuclear receptor corepressor SHP (Watanabe et al., 2004). In human subjects with non-alcoholic fatty liver disease, increased expression of SREBP-1c and its downstream lipogenic targets was recently correlated with decreased FXR expression (Yang et al., 2010).
Estrogen also has well established antidiabetic effects in women (Mauvais-Jarvis, 2011). Studies in rodent models indicate that estradiol suppresses hepatic expression of SREBP-1c or its downstream targets (Bryzgalova et al., 2008b; Paquette et al., 2008). This could be linked to its ability to induce SHP (Gao et al., 2008), or the reported ability of ERβ selective ligands to suppress expression of the site 1 protease required for SREBP activation in the endoplasmic reticulum (Shin et al., 2007).
Finally, a report suggesting that hepatic Nur77 overexpression suppresses SREBP-1c activity (Pols et al., 2008), and another indicating that a general Nur77 knockout increases steatosis and insulin resistance in high fat fed mice (Chao et al., 2009) raise the possibility that this common thread may extend to other nuclear receptors.
I propose that the antidiabetic effects of these diverse nuclear receptor ligands reverse McGarry’s vicious cycle (Fig. 1, clockwise, green). In this “reverse McGarry effect,” the inversion of that pathologic response generates a positive cycle that is also self-reinforcing. This starts with direct suppression of SREBP-1c expression, which decreases steatosis and improves insulin sensitivity. This results in decreased insulin levels and a further decrement in SREBP-1c expression, followed by further improvements in steatosis and insulin resistance that reinforce the decrease in SREBP-1c (Fig. 1, clockwise, green). From this perspective, the loss of metabolic balance that is initiated by insulin resistance in McGarry’s vicious cycle is restored by its equally self-reinforcing reversal.
A prediction of this model is that inhibiting SREBP-1c expression via other avenues should suppress steatosis and improve insulin sensitivity. Total (Yahagi et al., 2002) or selective SREBP-1c knockout (Moon et al., 2012) in ob/ob mice resulted in decreased steatosis, but did not improve insulin sensitivity. However, interpretation of the phenotypic consequences of these knockouts is complicated by a compensatory increase in hepatic SREBP-2 expression (Liang et al., 2002; Shimano et al., 1997). Consistent with this, inactivation of both SREBP-1a/c and SREBP-2 by liver specific knockout of SCAP, which is required for processing and activation of all 3 isoforms, decreased liver triglycerides further (Moon et al., 2012). This more extreme double SREBP knockout also did not improve insulin sensitivity, perhaps due to increased lipogenesis in adipose and other tissues in response to the near total loss of hepatic lipogenesis in the SCAP deficient mice (Moon et al., 2012).
In contrast, a number of other studies have linked beneficial effects on both steatosis and glucose homeostasis to more modest inhibition of SREBP-1c. For example, hepatic overexpression of the suppressors of cytokine signaling SOCS-1 and SOCS-3 in normal chow fed mice is sufficient to induce both whole body insulin resistance and steatosis, which is linked to increasing SREBP-1c expression (Ueki et al., 2004). In contrast, antisense knockdown of the elevated expression of SOCS-3 in db/db mice normalized the increased expression of SREBP-1c, and dramatically improved hepatic steatosis and insulin sensitivity (Ueki et al., 2004). PGC-1 reportedly coactivates the ability of SREBP-1c to induce its own expression (Lin et al., 2005), and the improvements in both steatosis and whole body insulin sensitivity that resulted from knockdown of hepatic PGC-1α expression were attributed to decreased SREBP-1c expression (Nagai et al., 2009). In a very different model, based on the fact that SREBP-1c activation depends on the same regulated intramembrane proteolytic pathway that is activated by endoplasmic reticulum stress, overexpression of the chaperone GRP78 in ob/ob mouse liver decreased SREBP-1c expression and steatosis, and improved overall insulin sensitivity (Kammoun et al., 2009).
In addition to the effects of nuclear receptor ligands, a number of other pharmacologic interventions have been described in which improved metabolic outcomes are linked with decreased SREBP-1c expression (eg. (Del Bas et al., 2008; Kim et al., 1999; Li et al., 2011; Park et al., 2008; Ponugoti et al., 2010). Many are natural products, raising issues regarding specificity and mechanism of action. Others, such as metformin, are much better characterized. However, while metformin and the more specific AMP kinase activator AICAR clearly lower the expression of SREBP-1c (Zhou et al., 2001), they exert many other effects that presumably also contribute to their well known metabolic effects. Finally, and in accord with the proposed role of the mTOR pathway in lipogenesis, rapamycin inhibits the acute activation of SREBP-1c expression in response to insulin and refeeding (Li et al., 2010; Yecies et al., 2011). In one study of high fat fed mice, chronic rapamycin treatment decreased steatosis and lowered serum insulin levels (Chang et al., 2009). However, mTOR has additional and complex effects on insulin signaling and other pathways, and other rapamycin studies have indicated deleterious results (Fraenkel et al., 2008; Houde et al., 2010). Rapamycin treatment for immunosuppression in human patients are associated with hyperlipidemia and glucose intolerance (Stallone et al., 2009).
More broadly, the focus here on this hepatocentric mechanism is not meant to discount the fundamental impact of adipose tissue inflammation or other mechanisms in the development of insulin resistance and diabetes (Hotamisligil, 2006; Shoelson et al., 2006). In addition, it is apparent that this mechanism cannot account for all of the antidiabetic effects of nuclear receptor agonists. PPARγ agonists primarily target adipocytes, and potentially macrophages, although this may result in a less direct impact on steatosis that could contribute to their insulin sensitizing effects (Lehrke and Lazar, 2005; Olefsky and Glass, 2010; Tontonoz and Spiegelman, 2008). PPARα and PPARδ/β agonists directly induce fatty acid β-oxidation in multiple tissues (Barish et al., 2006), although this could also decrease steatosis and contribute indirectly to insulin sensitivity. Potential effects of vitamin D may be due to its anti-inflammatory actions (Takiishi et al., 2010), and the NR4A receptors may directly regulate expression of glucose homeostatic genes in skeletal muscle and other tissues (Pearen and Muscat, 2010). The most obvious exception, as noted above, is the antidiabetic effects of LXR agonists (Cao et al., 2003; Commerford et al., 2007; Grefhorst et al., 2005; Herzog et al., 2007; Laffitte et al., 2003; Liu et al., 2006), which are based on other pathways that somehow overcome their well-known ability to induce SREBP-1c. Finally, based on the very different physiologic functions of the antidiabetic nuclear receptors that exhibit the reverse McGarry effect, it is apparent that each must have additional targets and functions, notably the effects of TRβ selective agonists on body weight, and the ability of Nur77 and the other NR4A members to increase hepatic glucose output (Pearen and Muscat, 2010). Thus, other pleiotropic activities of these receptors may be just as important - or in some cases even more important – than the inhibition of lipogenesis in mediating their anti-diabetic effects.
Overall, however, it is apparent that inhibition of SREBP-1c expression is a common thread that connects the antidiabetic effects of a surprising number of nuclear hormone receptors. As McGarry said: “According to this formulation, hyperinsulinemia is an early event and serves to drive hepatic lipogenesis and VLDL synthesis. … The progress of these events leads from simple insulin resistance to glucose intolerance with elevated glucose levels causing even greater postprandial hyperinsulinemia, which sets up a vicious cycle.” Repressing SREBP-1c to run McGarry’s vicious cycle backwards, via nuclear receptor activation or other strategies, is an attractive approach to treating steatosis and type 2 diabetes.
Acknowledgments
Supported by NIH R01 DK068804 and DK085372, USDA ARS 6250-52000-055, the BCM Diabetes Endocrine Research Center (supported by NIH DK-079638), the Alkek Foundation and the Robert R. P. Doherty Jr. Welch Chair in Science.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver disease as a complication of insulin resistance. Med Clin North Am. 2007;91:1125–1149. ix. doi: 10.1016/j.mcna.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Amaro A, Fabbrini E, Kars M, Yue P, Schechtman K, Schonfeld G, Klein S. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology. 2010;139:149–153. doi: 10.1053/j.gastro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amengual J, Ribot J, Bonet ML, Palou A. Retinoic acid treatment enhances lipid oxidation and inhibits lipid biosynthesis capacities in the liver of mice. Cell Physiol Biochem. 2010;25:657–666. doi: 10.1159/000315085. [DOI] [PubMed] [Google Scholar]
- Amorim BS, Ueta CB, Freitas BC, Nassif RJ, Gouveia CH, Christoffolete MA, Moriscot AS, Lancelloti CL, Llimona F, Barbeiro HV, et al. A TRbeta-selective agonist confers resistance to diet-induced obesity. J Endocrinol. 2009;203:291–299. doi: 10.1677/JOE-08-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelin B, Rudling M. Lipid lowering with thyroid hormone and thyromimetics. Curr Opin Lipidol. 2010;21:499–506. doi: 10.1097/MOL.0b013e3283402e9c. [DOI] [PubMed] [Google Scholar]
- Anstee QM, Goldin RD. Mouse models in non-alcoholic fatty liver disease and steatohepatitis research. Int J Exp Pathol. 2006;87:1–16. doi: 10.1111/j.0959-9673.2006.00465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barish GD, Narkar VA, Evans RM. PPAR delta: a dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry DC, Noy N. All-trans-retinoic acid represses obesity and insulin resistance by activating both peroxisome proliferation-activated receptor beta/delta and retinoic acid receptor. Mol Cell Biol. 2009;29:3286–3296. doi: 10.1128/MCB.01742-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddinger SB, Almind K, Miyazaki M, Kokkotou E, Ntambi JM, Kahn CR. Effects of diet and genetic background on sterol regulatory element-binding protein-1c, stearoyl-CoA desaturase 1, and the development of the metabolic syndrome. Diabetes. 2005;54:1314–1323. doi: 10.2337/diabetes.54.5.1314. [DOI] [PubMed] [Google Scholar]
- Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, Jeong Y, Downes M, Yu RT, Evans RM, Mangelsdorf DJ. Anatomical profiling of nuclear receptor expression reveals a hierarchical transcriptional network. Cell. 2006;126:789–799. doi: 10.1016/j.cell.2006.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MR, Yokoyama C, Wang X, Brown MS, Goldstein JL. Nuclear protein that binds sterol regulatory element of low density lipoprotein receptor promoter. I. Identification of the protein and delineation of its target nucleotide sequence. J Biol Chem. 1993;268:14490–14496. [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Selective versus total insulin resistance: a pathogenic paradox. Cell Metab. 2008;7:95–96. doi: 10.1016/j.cmet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Effendic S, Khan A, Rehnmark S, Barbounis P, Boulet J, Dong G, Singh R, Shapses S, Malm J, et al. Anti-obesity, anti-diabetic, and lipid lowering effects of the thyroid receptor beta subtype selective agonist KB-141. J Steroid Biochem Mol Biol. 2008a;111:262–267. doi: 10.1016/j.jsbmb.2008.06.010. [DOI] [PubMed] [Google Scholar]
- Bryzgalova G, Lundholm L, Portwood N, Gustafsson JA, Khan A, Efendic S, Dahlman-Wright K. Mechanisms of antidiabetogenic and body weight-lowering effects of estrogen in high-fat diet-fed mice. Am J Physiol Endocrinol Metab. 2008b;295:E904–912. doi: 10.1152/ajpendo.90248.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao G, Liang Y, Broderick CL, Oldham BA, Beyer TP, Schmidt RJ, Zhang Y, Stayrook KR, Suen C, Otto KA, et al. Antidiabetic action of a liver x receptor agonist mediated by inhibition of hepatic gluconeogenesis. J Biol Chem. 2003;278:1131–1136. doi: 10.1074/jbc.M210208200. [DOI] [PubMed] [Google Scholar]
- Chang GR, Chiu YS, Wu YY, Chen WY, Liao JW, Chao TH, Mao FC. Rapamycin protects against high fat diet-induced obesity in C57BL/6J mice. J Pharmacol Sci. 2009;109:496–503. doi: 10.1254/jphs.08215fp. [DOI] [PubMed] [Google Scholar]
- Chao LC, Wroblewski K, Zhang Z, Pei L, Vergnes L, Ilkayeva OR, Ding SY, Reue K, Watt MJ, Newgard CB, et al. Insulin resistance and altered systemic glucose metabolism in mice lacking Nur77. Diabetes. 2009;58:2788–2796. doi: 10.2337/db09-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SS, Diehl AM. Hepatic triglyceride synthesis and nonalcoholic fatty liver disease. Curr Opin Lipidol. 2008;19:295–300. doi: 10.1097/MOL.0b013e3282ff5e55. [DOI] [PubMed] [Google Scholar]
- Cipriani S, Mencarelli A, Palladino G, Fiorucci S. FXR activation reverses insulin resistance and lipid abnormalities and protects against liver steatosis in Zucker (fa/fa) obese rats. J Lipid Res. 2010;51:771–784. doi: 10.1194/jlr.M001602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commerford SR, Vargas L, Dorfman SE, Mitro N, Rocheford EC, Mak PA, Li X, Kennedy P, Mullarkey TL, Saez E. Dissection of the insulin-sensitizing effect of liver X receptor ligands. Mol Endocrinol. 2007;21:3002–3012. doi: 10.1210/me.2007-0156. [DOI] [PubMed] [Google Scholar]
- Del Bas JM, Ricketts ML, Baiges I, Quesada H, Ardevol A, Salvado MJ, Pujadas G, Blay M, Arola L, Blade C, et al. Dietary procyanidins lower triglyceride levels signaling through the nuclear receptor small heterodimer partner. Mol Nutr Food Res. 2008;52:1172–1181. doi: 10.1002/mnfr.200800054. [DOI] [PubMed] [Google Scholar]
- Dimitriadis G, Baker B, Marsh H, Mandarino L, Rizza R, Bergman R, Haymond M, Gerich J. Effect of thyroid hormone excess on action, secretion, and metabolism of insulin in humans. Am J Physiol. 1985;248:E593–601. doi: 10.1152/ajpendo.1985.248.5.E593. [DOI] [PubMed] [Google Scholar]
- Dong B, Saha PK, Huang W, Chen W, Abu-Elheiga LA, Wakil SJ, Stevens RD, Ilkayeva O, Newgard CB, Chan L, et al. Activation of nuclear receptor CAR ameliorates diabetes and fatty liver disease. Proc Natl Acad Sci U S A. 2009;106:18831–18836. doi: 10.1073/pnas.0909731106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elam MB, Yellaturu C, Howell GE, Deng X, Cowan GS, Kumar P, Park EA, Hiler ML, Wilcox HG, Hughes TA, et al. Dysregulation of sterol regulatory element binding protein-1c in livers of morbidly obese women is associated with altered suppressor of cytokine signaling-3 and signal transducer and activator of transcription-1 signaling. Metabolism. 2010;59:587–598. doi: 10.1016/j.metabol.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erion MD, Cable EE, Ito BR, Jiang H, Fujitaki JM, Finn PD, Zhang BH, Hou J, Boyer SH, van Poelje PD, et al. Targeting thyroid hormone receptor-beta agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc Natl Acad Sci U S A. 2007;104:15490–15495. doi: 10.1073/pnas.0702759104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology. 2010;51:679–689. doi: 10.1002/hep.23280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre P, Foufelle F. Hepatic steatosis: a role for de novo lipogenesis and the transcription factor SREBP-1c. Diabetes Obes Metab. 2010;12(Suppl 2):83–92. doi: 10.1111/j.1463-1326.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, Berthault MF, Magnan C, Cerasi E, Kaiser N, et al. mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes. 2008;57:945–957. doi: 10.2337/db07-0922. [DOI] [PubMed] [Google Scholar]
- Gao H, Falt S, Sandelin A, Gustafsson JA, Dahlman-Wright K. Genome-Wide Identification of Estrogen Receptor {alpha}-Binding Sites in Mouse Liver. Mol Endocrinol. 2008;22:10–22. doi: 10.1210/me.2007-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J, He J, Zhai Y, Wada T, Xie W. CAR is an anti-obesity nuclear receptor that improves insulin sensitivity. J Biol Chem. 2009 doi: 10.1074/jbc.M109.016808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A, Cusi K, Pettiti M, Hardies J, Miyazaki Y, Berria R, Buzzigoli E, Sironi AM, Cersosimo E, Ferrannini E, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- Gathercole LL, Stewart PM. Targeting the pre-receptor metabolism of cortisol as a novel therapy in obesity and diabetes. J Steroid Biochem Mol Biol. 2010;122:21–27. doi: 10.1016/j.jsbmb.2010.03.060. [DOI] [PubMed] [Google Scholar]
- Gerber LE, Erdman JW., Jr Changes in lipid metabolism during retinoid administration. J Am Acad Dermatol. 1982;6:664–674. doi: 10.1016/s0190-9622(82)80047-9. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, Coleman RA, Kraemer FB, McManaman JL, Obin MS, Puri V, Yan QW, Miyoshi H, Mashek DG. The role of lipid droplets in metabolic disease in rodents and humans. J Clin Invest. 2011;121:2102–2110. doi: 10.1172/JCI46069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefhorst A, van Dijk TH, Hammer A, van der Sluijs FH, Havinga R, Havekes LM, Romijn JA, Groot PH, Reijngoud DJ, Kuipers F. Differential effects of pharmacological liver X receptor activation on hepatic and peripheral insulin sensitivity in lean and ob/ob mice. Am J Physiol Endocrinol Metab. 2005;289:E829–838. doi: 10.1152/ajpendo.00165.2005. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, Matschinsky FM, Duncan SA, Kaestner KH. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115:1006–1015. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Yamada M, Matsumoto S, Monden T, Satoh T, Mori M. Mouse sterol response element binding protein-1c gene expression is negatively regulated by thyroid hormone. Endocrinology. 2006;147:4292–4302. doi: 10.1210/en.2006-0116. [DOI] [PubMed] [Google Scholar]
- Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B, Hallberg M, Seth A, Woods A, White R, Parker MG. The nuclear receptor cofactor, receptor-interacting protein 140, is required for the regulation of hepatic lipid and glucose metabolism by liver X receptor. Mol Endocrinol. 2007;21:2687–2697. doi: 10.1210/me.2007-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi N, Kato M, Shundo Y, Tajiri H, Tanaka M, Yamashita N, Kohjima M, Kotoh K, Nakamuta M, Takayanagi R, et al. Liver X receptor in cooperation with SREBP-1c is a major lipid synthesis regulator in nonalcoholic fatty liver disease. Hepatol Res. 2008;38:1122–1129. doi: 10.1111/j.1872-034X.2008.00382.x. [DOI] [PubMed] [Google Scholar]
- Holland WL, Summers SA. Sphingolipids, insulin resistance, and metabolic disease: new insights from in vivo manipulation of sphingolipid metabolism. Endocr Rev. 2008;29:381–402. doi: 10.1210/er.2007-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Houde VP, Brule S, Festuccia WT, Blanchard PG, Bellmann K, Deshaies Y, Marette A. Chronic rapamycin treatment causes glucose intolerance and hyperlipidemia by upregulating hepatic gluconeogenesis and impairing lipid deposition in adipose tissue. Diabetes. 2010;59:1338–1348. doi: 10.2337/db09-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle D. The production of glycosuria in the normal rat by means of 17-hydroxy-11-dehydrocorticosterone. Endocrinology. 1941;29:649–652. [Google Scholar]
- Ingle DJ, Sheppard R, et al. A comparison of adrenal steroid diabetes and pancreatic diabetes in the rat. Endocrinology. 1945;37:341–356. doi: 10.1210/endo-37-5-341. [DOI] [PubMed] [Google Scholar]
- Johansson L, Rudling M, Scanlan TS, Lundasen T, Webb P, Baxter J, Angelin B, Parini P. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci U S A. 2005;102:10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Gauthier KC, Zavacki AM, Mammen PP, Kitazume T, Peterson JA, Horton JD, Garry DJ, Bianco AC, Mangelsdorf DJ. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. LXRs and FXR: The Yin and Yang of Cholesterol and Fat Metabolism. Annu Rev Physiol. 2005 doi: 10.1146/annurev.physiol.68.033104.152158. [DOI] [PubMed]
- Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvonen I, Stengard JH, Huupponen R, Stenback FG, Sotaniemi EA. Effects of enzyme induction therapy on glucose and drug metabolism in obese mice model of non-insulin dependent diabetes mellitus. Diabetes Res. 1989;10:85–92. [PubMed] [Google Scholar]
- Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Takahashi M, Ezaki O. Fish oil feeding decreases mature sterol regulatory element-binding protein 1 (SREBP-1) by down-regulation of SREBP-1c mRNA in mouse liver. A possible mechanism for down-regulation of lipogenic enzyme mRNAs. J Biol Chem. 1999;274:25892–25898. doi: 10.1074/jbc.274.36.25892. [DOI] [PubMed] [Google Scholar]
- Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laffitte BA, Chao LC, Li J, Walczak R, Hummasti S, Joseph SB, Castrillo A, Wilpitz DC, Mangelsdorf DJ, Collins JL, et al. Activation of liver X receptor improves glucose tolerance through coordinate regulation of glucose metabolism in liver and adipose tissue. Proc Natl Acad Sci U S A. 2003;100:5419–5424. doi: 10.1073/pnas.0830671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahtela JT, Arranto AJ, Sotaniemi EA. Enzyme inducers improve insulin sensitivity in non-insulin-dependent diabetic subjects. Diabetes. 1985;34:911–916. doi: 10.2337/diab.34.9.911. [DOI] [PubMed] [Google Scholar]
- Lalloyer F, Staels B. Fibrates, glitazones, and peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2010;30:894–899. doi: 10.1161/ATVBAHA.108.179689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan H, Rabaglia ME, Stoehr JP, Nadler ST, Schueler KL, Zou F, Yandell BS, Attie AD. Gene expression profiles of nondiabetic and diabetic obese mice suggest a role of hepatic lipogenic capacity in diabetes susceptibility. Diabetes. 2003;52:688–700. doi: 10.2337/diabetes.52.3.688. [DOI] [PubMed] [Google Scholar]
- Lee JM, Lee YK, Mamrosh JL, Busby SA, Griffin PR, Pathak MC, Ortlund EA, Moore DD. A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474:506–510. doi: 10.1038/nature10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrke M, Lazar MA. The many faces of PPARgamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Li S, Brown MS, Goldstein JL. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc Natl Acad Sci U S A. 2010;107:3441–3446. doi: 10.1073/pnas.0914798107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. AMPK Phosphorylates and Inhibits SREBP Activity to Attenuate Hepatic Steatosis and Atherosclerosis in Diet-Induced Insulin-Resistant Mice. Cell Metab. 2011;13:376–388. doi: 10.1016/j.cmet.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang G, Yang J, Horton JD, Hammer RE, Goldstein JL, Brown MS. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J Biol Chem. 2002;277:9520–9528. doi: 10.1074/jbc.M111421200. [DOI] [PubMed] [Google Scholar]
- Lin J, Yang R, Tarr PT, Wu PH, Handschin C, Li S, Yang W, Pei L, Uldry M, Tontonoz P, et al. Hyperlipidemic effects of dietary saturated fats mediated through PGC-1beta coactivation of SREBP. Cell. 2005;120:261–273. doi: 10.1016/j.cell.2004.11.043. [DOI] [PubMed] [Google Scholar]
- Liu Y, Yan C, Wang Y, Nakagawa Y, Nerio N, Anghel A, Lutfy K, Friedman TC. Liver X receptor agonist T0901317 inhibition of glucocorticoid receptor expression in hepatocytes may contribute to the amelioration of diabetic syndrome in db/db mice. Endocrinology. 2006;147:5061–5068. doi: 10.1210/en.2006-0243. [DOI] [PubMed] [Google Scholar]
- Long C, Katzin B, Fry E. The adrenal cortex and carbohydrate metabolism. Endocrinology. 1940;26:309–344. doi: 10.1210/endo-61-6-724. [DOI] [PubMed] [Google Scholar]
- Ma K, Saha PK, Chan L, Moore DD. Farnesoid X receptor is essential for normal glucose homeostasis. J Clin Invest. 2006;116:1102–1109. doi: 10.1172/JCI25604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manolescu DC, Sima A, Bhat PV. All-trans retinoic acid lowers serum retinol-binding protein 4 concentrations and increases insulin sensitivity in diabetic mice. J Nutr. 2010;140:311–316. doi: 10.3945/jn.109.115147. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F. Estrogen and androgen receptors: regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends Endocrinol Metab. 2011;22:24–33. doi: 10.1016/j.tem.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry JD. What if Minkowski had been ageusic? An alternative angle on diabetes. Science. 1992;258:766–770. doi: 10.1126/science.1439783. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Miura A, Yamagata K, Kakei M, Hatakeyama H, Takahashi N, Fukui K, Nammo T, Yoneda K, Inoue Y, Sladek FM, et al. Hepatocyte nuclear factor-4alpha is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. J Biol Chem. 2006;281:5246–5257. doi: 10.1074/jbc.M507496200. [DOI] [PubMed] [Google Scholar]
- Monetti M, Levin MC, Watt MJ, Sajan MP, Marmor S, Hubbard BK, Stevens RD, Bain JR, Newgard CB, Farese RV, Sr, et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Moon YA, Liang G, Xie X, Frank-Kamenetsky M, Fitzgerald K, Koteliansky V, Brown MS, Goldstein JL, Horton JD. The Scap/SREBP Pathway Is Essential for Developing Diabetic Fatty Liver and Carbohydrate-Induced Hypertriglyceridemia in Animals. Cell Metab. 2012;15:240–246. doi: 10.1016/j.cmet.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munck A. Glucocorticoid inhibition of glucose uptake by peripheral tissues: old and new evidence, molecular mechanisms, and physiological significance. Perspect Biol Med. 1971;14:265–269. doi: 10.1353/pbm.1971.0002. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Yonemitsu S, Erion DM, Iwasaki T, Stark R, Weismann D, Dong J, Zhang D, Jurczak MJ, Loffler MG, et al. The role of peroxisome proliferator-activated receptor gamma coactivator-1 beta in the pathogenesis of fructose-induced insulin resistance. Cell Metab. 2009;9:252–264. doi: 10.1016/j.cmet.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- Paquette A, Wang D, Jankowski M, Gutkowska J, Lavoie JM. Effects of ovariectomy on PPAR alpha, SREBP-1c, and SCD-1 gene expression in the rat liver. Menopause. 2008;15:1169–1175. doi: 10.1097/gme.0b013e31817b8159. [DOI] [PubMed] [Google Scholar]
- Park KG, Min AK, Koh EH, Kim HS, Kim MO, Park HS, Kim YD, Yoon TS, Jang BK, Hwang JS, et al. Alpha-lipoic acid decreases hepatic lipogenesis through adenosine monophosphate-activated protein kinase (AMPK)-dependent and AMPK-independent pathways. Hepatology. 2008;48:1477–1486. doi: 10.1002/hep.22496. [DOI] [PubMed] [Google Scholar]
- Pataky Z, Bobbioni-Harsch E, Golay A. Open questions about metabolically normal obesity. Int J Obes (Lond) 2010;34(Suppl 2):S18–23. doi: 10.1038/ijo.2010.235. [DOI] [PubMed] [Google Scholar]
- Pearen MA, Muscat GE. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Mol Endocrinol. 2010;24:1891–1903. doi: 10.1210/me.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen KF, Dufour S, Befroy D, Lehrke M, Hendler RE, Shulman GI. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Sengupta SS, Harris TE, Carmack AE, Kang SA, Balderas E, Guertin DA, Madden KL, Carpenter AE, Finck BN, et al. mTOR Complex 1 Regulates Lipin 1 Localization to Control the SREBP Pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinelli P, Del Pozo T, Araya J, Rodrigo R, Araya AV, Smok G, Csendes A, Gutierrez L, Rojas J, Korn O, et al. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n-3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta. 2009;1792:1080–1086. doi: 10.1016/j.bbadis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Pinaire JA, Reifel-Miller A. Therapeutic potential of retinoid x receptor modulators for the treatment of the metabolic syndrome. PPAR Res. 2007;2007:94156. doi: 10.1155/2007/94156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pols TW, Ottenhoff R, Vos M, Levels JH, Quax PH, Meijers JC, Pannekoek H, Groen AK, de Vries CJ. Nur77 modulates hepatic lipid metabolism through suppression of SREBP1c activity. Biochem Biophys Res Commun. 2008;366:910–916. doi: 10.1016/j.bbrc.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Ponugoti B, Kim DH, Xiao Z, Smith Z, Miao J, Zang M, Wu SY, Chiang CM, Veenstra TD, Kemper JK. SIRT1 deacetylates and inhibits SREBP-1C activity in regulation of hepatic lipid metabolism. J Biol Chem. 2010;285:33959–33970. doi: 10.1074/jbc.M110.122978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawitt J, Abdelkarim M, Stroeve JH, Popescu I, Duez H, Velagapudi VR, Dumont J, Bouchaert E, van Dijk TH, Lucas A, et al. Farnesoid X receptor deficiency improves glucose homeostasis in mouse models of obesity. Diabetes. 2011;60:1861–1871. doi: 10.2337/db11-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repa JJ, Liang G, Ou J, Bashmakov Y, Lobaccaro JM, Shimomura I, Shan B, Brown MS, Goldstein JL, Mangelsdorf DJ. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000;14:2819–2830. doi: 10.1101/gad.844900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Diaz MB, Reimann A, Klement J, Walcher T, Krones-Herzig A, Strobel O, Werner J, Peters A, Kleyman A, et al. Molecular control of systemic bile acid homeostasis by the liver glucocorticoid receptor. Cell Metab. 2011;14:123–130. doi: 10.1016/j.cmet.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Rose AJ, Vegiopoulos A, Herzig S. Role of glucocorticoids and the glucocorticoid receptor in metabolism: insights from genetic manipulations. J Steroid Biochem Mol Biol. 2010;122:10–20. doi: 10.1016/j.jsbmb.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A, Mudaliar S, Henry R, Marchall H, Morrow L, Sciacca C, Dillon P, Clopton P, Kipnes M, Shapiro D. A new therapy for nonalcoholic fatty liver disease and diabetes? INT 747 — the first FXR hepatic therapeutic study. Hepatology. 2009;50:389a–390a. [Google Scholar]
- Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, Goto T, Westerbacka J, Sovijarvi A, Halavaara J, Yki-Jarvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- Shimano H, Shimomura I, Hammer RE, Herz J, Goldstein JL, Brown MS, Horton JD. Elevated levels of SREBP-2 and cholesterol synthesis in livers of mice homozygous for a targeted disruption of the SREBP-1 gene. J Clin Invest. 1997;100:2115–2124. doi: 10.1172/JCI119746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- Shin ES, Lee HH, Cho SY, Park HW, Lee SJ, Lee TR. Genistein downregulates SREBP-1 regulated gene expression by inhibiting site-1 protease expression in HepG2 cells. J Nutr. 2007;137:1127–1131. doi: 10.1093/jn/137.5.1127. [DOI] [PubMed] [Google Scholar]
- Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinal CJ, Tohkin M, Miyata M, Ward JM, Lambert G, Gonzalez FJ. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- Solinas G, Vilcu C, Neels JG, Bandyopadhyay GK, Luo JL, Naugler W, Grivennikov S, Wynshaw-Boris A, Scadeng M, Olefsky JM, et al. JNK1 in hematopoietically derived cells contributes to diet-induced inflammation and insulin resistance without affecting obesity. Cell Metab. 2007;6:386–397. doi: 10.1016/j.cmet.2007.09.011. [DOI] [PubMed] [Google Scholar]
- Sotaniemi EA, Karvonen I. Glucose tolerance and insulin response to glucose load before and after enzyme inducing therapy in subjects with glucose intolerance and patients with NIDDM having hyperinsulinemia or relative insulin deficiency. Diabetes Res. 1989;11:131–139. [PubMed] [Google Scholar]
- Stallone G, Infante B, Grandaliano G, Gesualdo L. Management of side effects of sirolimus therapy. Transplantation. 2009;87:S23–26. doi: 10.1097/TP.0b013e3181a05b7a. [DOI] [PubMed] [Google Scholar]
- Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, Balletshofer B, Machicao F, Fritsche A, Haring HU. Identification and characterization of metabolically benign obesity in humans. Arch Intern Med. 2008;168:1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- Takiishi T, Gysemans C, Bouillon R, Mathieu C. Vitamin D and diabetes. Endocrinol Metab Clin North Am. 2010;39:419–446. doi: 10.1016/j.ecl.2010.02.013. table of contents. [DOI] [PubMed] [Google Scholar]
- Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371–379. doi: 10.1016/j.tem.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Tontonoz P, Kim JB, Graves RA, Spiegelman BM. ADD1: a novel helix-loop-helix transcription factor associated with adipocyte determination and differentiation. Mol Cell Biol. 1993;13:4753–4759. doi: 10.1128/mcb.13.8.4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu Rev Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Ueki K, Kondo T, Tseng YH, Kahn CR. Central role of suppressors of cytokine signaling proteins in hepatic steatosis, insulin resistance, and the metabolic syndrome in the mouse. Proc Natl Acad Sci U S A. 2004;101:10422–10427. doi: 10.1073/pnas.0402511101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaxillaire M, Froguel P. Monogenic diabetes in the young, pharmacogenetics and relevance to multifactorial forms of type 2 diabetes. Endocr Rev. 2008;29:254–264. doi: 10.1210/er.2007-0024. [DOI] [PubMed] [Google Scholar]
- Wan M, Leavens KF, Saleh D, Easton RM, Guertin DA, Peterson TR, Kaestner KH, Sabatini DM, Birnbaum MJ. Postprandial hepatic lipid metabolism requires signaling through Akt2 independent of the transcription factors FoxA2, FoxO1, and SREBP1c. Cell Metab. 2011;14:516–527. doi: 10.1016/j.cmet.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Horai Y, Houten SM, Morimoto K, Sugizaki T, Arita E, Mataki C, Sato H, Tanigawara Y, Schoonjans K, et al. Lowering bile acid pool size with a synthetic farnesoid X receptor (FXR) agonist induces obesity and diabetes through reduced energy expenditure. J Biol Chem. 2011;286:26913–26920. doi: 10.1074/jbc.M111.248203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Mataki C, Christoffolete MA, Kim BW, Sato H, Messaddeq N, Harney JW, Ezaki O, Kodama T, et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature. 2006;439:484–489. doi: 10.1038/nature04330. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Houten SM, Wang L, Moschetta A, Mangelsdorf DJ, Heyman RA, Moore DD, Auwerx J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J Clin Invest. 2004;113:1408–1418. doi: 10.1172/JCI21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahagi N, Shimano H, Hasty AH, Matsuzaka T, Ide T, Yoshikawa T, Amemiya-Kudo M, Tomita S, Okazaki H, Tamura Y, et al. Absence of sterol regulatory element-binding protein-1 (SREBP-1) ameliorates fatty livers but not obesity or insulin resistance in Lep(ob)/Lep(ob) mice. J Biol Chem. 2002;277:19353–19357. doi: 10.1074/jbc.M201584200. [DOI] [PubMed] [Google Scholar]
- Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZX, Shen W, Sun H. Effects of nuclear receptor FXR on the regulation of liver lipid metabolism in patients with non-alcoholic fatty liver disease. Hepatol Int. 2010;4:741–748. doi: 10.1007/s12072-010-9202-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yecies JL, Zhang HH, Menon S, Liu S, Yecies D, Lipovsky AI, Gorgun C, Kwiatkowski DJ, Hotamisligil GS, Lee CH, et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 2011;14:21–32. doi: 10.1016/j.cmet.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa T, Shimano H, Amemiya-Kudo M, Yahagi N, Hasty AH, Matsuzaka T, Okazaki H, Tamura Y, Iizuka Y, Ohashi K, et al. Identification of liver X receptor-retinoid X receptor as an activator of the sterol regulatory element-binding protein 1c gene promoter. Mol Cell Biol. 2001;21:2991–3000. doi: 10.1128/MCB.21.9.2991-3000.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Lee FY, Barrera G, Lee H, Vales C, Gonzalez FJ, Willson TM, Edwards PA. Activation of the nuclear receptor FXR improves hyperglycemia and hyperlipidemia in diabetic mice. Proc Natl Acad Sci U S A. 2006;103:1006–1011. doi: 10.1073/pnas.0506982103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]


