SUMMARY
Hypothalamic neurons expressing Agouti-related peptide (AgRP) are critical for initiating food intake. But druggable biochemical pathways that control this response remain elusive. Thus, genetic ablation of anorexigenic signaling by insulin or leptin in AgRP neurons fails to affect food intake. FoxO1 is a shared mediator of both pathways, and its inhibition is required for their anorexigenic effects. We postulated that FoxO1 effectors include pathways regulating food intake. Accordingly, FoxO1 ablation in AgRP neurons of mice results in reduced food intake, leanness, improved glucose homeostasis, and increased sensitivity to insulin and leptin. Expression profiling of flow-sorted FoxO1-deficient AgRP neurons identifies G protein-coupled receptor Gpr17 as a FoxO1 target whose expression is regulated by nutritional status. Intracerebroventricular injection of Gpr17 agonists induces food intake, while Gpr17 antagonist cangrelor curtails it. These effects are absent in Agrp-Foxo1 knockouts, suggesting that pharmacological modulation of this pathway has therapeutic potential to treat obesity.
INTRODUCTION
The prevalence of obesity has grown at alarming rates, reaching pandemic proportions. One third of U.S. adults are obese, leading to increased numbers of patients affected by the comorbidities of obesity, such as cardiovascular disease, type 2 diabetes, respiratory disorders and cancer (Wang et al., 2011). Considering the staggering cost and serious challenges to public health associated with this condition, identification of biochemical pathways that can be effectively and safely targeted for obesity therapy has acquired new urgency.
Hypothalamic neurons expressing AgRP have been directly implicated in promoting feeding. AgRP neuron activation by either light-gated Channelrhodopsin-2 or ligand-activated G-proteins rapidly increases food intake (Aponte et al., 2011; Krashes et al., 2011). Conversely, acute ablation of AgRP neurons in adulthood causes cessation of feeding and results in starvation (Luquet et al., 2005). Despite the established functional importance of AgRP neurons in feeding behavior, genetic analyses of specific hormonal pathways that impinge on this process have yielded limited and occasionally conflicting results. For example, transgenic overexpression of Agrp results in obesity, and acute intracerebroventricular delivery of Agrp or Neuropeptide Y (Npy), another peptide produced in AgRP neurons, elicits hyperphagia (Levine et al., 2004; Ollmann et al., 1997; Rossi et al., 1998). However, mice with loss of Agrp, Npy, or both exhibit no feeding or body weight phenotypes and maintain a normal response to starvation (Qian et al., 2002).
The key anorexigenic hormones, insulin and leptin, inhibit AgRP neurons, raising their activation threshold (Konner et al., 2007; Takahashi and Cone, 2005). Surprisingly though, genetic ablation of insulin receptor in AgRP neurons has no effect on energy homeostasis, even as it impairs insulin-mediated suppression of hepatic glucose production (Konner et al., 2007). Similarly, inactivation of leptin receptors in AgRP neurons results in a mild increase of body weight, without affecting feeding behavior and energy balance (van de Wall et al., 2008). Thus, neither pathway appears to exert obligate control over AgRP neuron-dependent feeding and energy homeostasis.
The goal of this study was twofold: to solve the apparent paradox of the absence of food intake abnormalities following ablation of leptin or insulin receptors, and to identify alternative pathways that regulate AgRP neuron-dependent food intake. To this end, we adopted a two-pronged strategy. As transcription factor FoxO1 integrates both leptin and insulin signaling (Kim et al., 2006; Kitamura et al., 2006), it’s plausible that greater effects in AgRP neurons might be engendered by FoxO1 ablation. Therefore, we knocked out FoxO1 in AgRP neurons, with the intent to bring about a metabolic and energy balance phenotype that recapitulated constitutive insulin and leptin signaling in this cell type. We found that mice lacking FoxO1 in AgRP neurons are lean, eat less, and show improved glucose homeostasis as well as increased insulin/leptin sensitivity. Having achieved this goal, we sought to identify FoxO1-dependent pathways that modulate energy homeostasis and can be enlisted to develop anti-obesity medications. Using transcriptional profiling of flow-sorted AgRP neurons lacking FoxO1 and functional in vivo and ex vivo testing, we identified Gpr17, a purinergic G protein-coupled receptor, as a prominent Foxo1 target whose pharmacological inhibition reduces food intake and increases brain sensitivity to hormones and nutrients.
RESULTS
Generation and analysis of Agrp-Specific FoxO1 knockout mice
To ablate FoxO1 in AgRP neurons, we crossed Foxo1loxP/loxP and Agrp-Ires-cre mice (Tong et al., 2008). Cre-mediated deletion of the loxP-flanked Foxo1 DNA resulted in null Foxo1 alleles in AgRP neurons (hereafter Agrp-Foxo1−/−). To assess the extent of Foxo1 ablation, we introduced a reporter allele encoding red fluorescent protein upon Cre-mediated recombination (Rosa-tomato) into Agrp-Foxo1−/− animals. The resulting Agrp-Foxo1−/−; Rosa-tomato mice displayed a red fluorescence pattern in the arcuate nucleus (ARH) consistent with AgRP neuron localization (Figure 1A). We didn’t detect differences in the relative number or size of ARH AgRP neurons between wild-type and Agrp-Foxo1−/− mice. Immunohistochemistry indicated that FoxO1 was highly expressed in ARH neurons, including AgRP neurons, of wild-type mice, but was selectively undetectable in AgRP neurons of Agrp-Foxo1−/− mice (Figure 1A).
Figure 1. Generation and analysis of Agrp-Foxo1−/− mice.
(A) Immunohistochemistry of ARH in wild-type (WT) and Agrp-Foxo1−/− mice (KO) using Rfp as a reporter of Cre-mediated recombination (red) and FoxO1 (green) to determine co-localization and DAPI to counterstain nuclei (blue). Scale bar 50μm.
(B) Growth curves of female and male WT and KO on chow diet.
(C) Body fat and lean mass content in female (n=12–13 for each genotype) and male (n=6 for each genotype) mice analyzed by MRI.
(D) Locomotor activity in light and dark phase (n=5–8 for each genotype).
(E–F) Cumulative 24-h (E) and dark phase food intake (F) (g of food per mouse) (n=5–8 for each genotype).
(G) Locomotor activity during overnight fasting (n=5 for each genotype).
(H) Rebound food intake (g/g lean body mass) after an overnight fast (n=9 for each genotype).
(I) Hypothalamic neuropeptide (αMSH and AGRP) levels after overnight fast (n=56 for each genotype). Agrp and Pomc mRNA in ARH after overnight fast (n=6 for each genotype).
(J) Percentage of body weight gain of 10wk-old mice after switching to HFD (n=8–9).
We present data as means ± SEM. * = P <0.05, ** = P <0.01.
Improved energy homeostasis in Agrp-Foxo1−/− mice
Body weight of female and male Agrp-Foxo1−/− mice was comparable to wild-type controls or trended lower without reaching statistical significance (Figure 1B). Both female and male Agrp-Foxo1−/− mice showed altered body composition, with a remarkable 18–30% decrease of fat mass and a significant 3–5% increase of lean mass (Figure 1C).
We selected male mice of matched body weight and composition for calorimetry studies. We measured respiratory exchanges, energy expenditure and food intake. When fed ad libitum, Agrp-Foxo1−/− mice were comparable to wild-type controls with regard to oxygen consumption (VO2), respiratory quotient (RQ), and energy expenditure (Figure S1A–C), but showed reduced locomotor activity during light and dark phases of the light cycle (Figure 1D), as well as reduced cumulative food intake during the 24-h period (Figure 1E) that was accounted for by reduced intake during the dark phase of the light cycle (Figure 1F).
To determine whether reduced food intake was due to increased satiety, we examined mice during fasting and refeeding. Male Agrp-Foxo1−/− mice showed significantly reduced locomotor activity during an overnight fast, suggesting reduced food foraging behavior (Figure 1G). Moreover, they showed significantly reduced rebound food intake after an overnight fast (Figure 1H). In light of the role of FoxO1 in orexigenic and anorexigenic neuropeptide expression (Kitamura et al., 2006; Plum et al., 2009), we examined AGRP and αMSH levels. After fasting, AGRP was significantly reduced in the mediobasal hypothalamus (MBH) of male Agrp-Foxo1−/− mice, while αMSH was comparable to wild-type controls (Figure 1I). Agrp is a transcriptional target of FoxO1 (Kitamura et al., 2006). Accordingly, we found decreased Agrp mRNA in ARH punch biopsies from overnight–fasted Agrp-Foxo1−/− mice (Figure 1I). In contrast, transcript levels of the aMSH precursor, Pomc, were unaltered (Figure 1I). Moreover, Agrp-Foxo1−/− mice were largely protected from high fat diet-induced weight gain (Figure 1J). These data suggest that FoxO1 in AgRP neurons is important to regulate satiety and diet-induced obesity.
Altered hepatic glucose production and gene expression in Agrp-Foxo1−/− mice
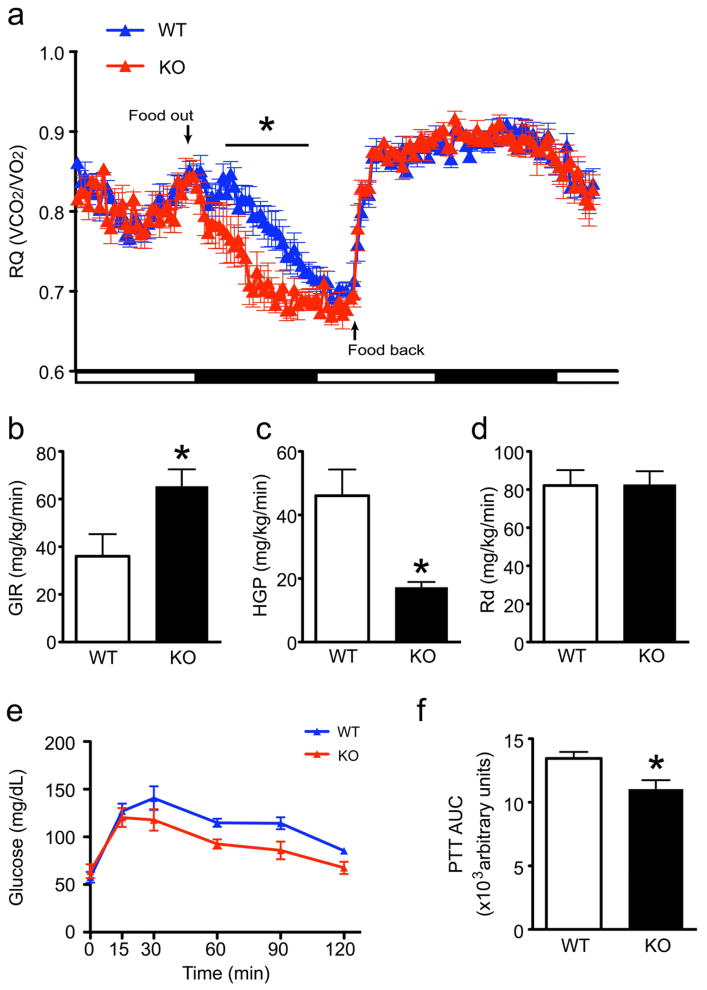
Given the key role of FoxO1 in hormone-dependent hepatic glucose production (HGP) (Matsumoto et al., 2007), and the effect of insulin receptor signaling in AgRP neurons on this process (Konner et al., 2007), we investigated the liver response to FoxO1 ablation in AgRP neurons. After acclimating mice to the calorimetry cage for >72 hrs, we subjected them to an overnight fast; shortly upon starting the fast, Agrp-Foxo1−/− mice showed a more rapid drop of RQ than controls, but they had rebound RQ similar to controls once refed (Figure 2A). This finding indicates that fasted Agrp-Foxo1−/− mice adapt more rapidly from carbohydrates to fatty acids as energy source.
Figure 2. Respiratory quotient and glucose production in Agrp-Foxo1−/− mice.
(A) RQ measured during fasting and refeeding in Agrp-Foxo1−/− (KO) and wild-type (WT) mice (n=5–7 for each genotype).
(B–D) Hyperinsulinemic euglycemic glucose clamps showing glucose infusion rates (GIR) (B), glucose disposal (Rd) (C), and hepatic glucose production (HGP) (D) with insulin (3.6 μU/ml) (n=6 for each genotype).
(E,F) Time course (E) and area under curve (AUC) (F) of pyruvate tolerance tests (PTT) (n=6 for each genotype). We present data as means ± SEM. * = P <0.05.
We investigated whether this adaptation to fast was due to inability to mobilize hepatic glycogen. To avoid confounders due to differences in fat content (Butler and Kozak, 2010), we performed hyperinsulinemic euglycemic clamps in mice that were closely matched for body weight and composition. Agrp-Foxo1−/− mice had a 50% increase in glucose infusion rates (GIR) (Figure 2B) and a 70% reduction of HGP (Figure 2C), but comparable disposal rates (Rd) (Figure 2D). Furthermore, when mice were challenged with pyruvate after an overnight fast, Agrp-Foxo1−/− mice had a significantly reduced rise in glucose levels, consistent with decreased hepatic gluconeogenesis (Figure 2E,F). Therefore, Agrp-Foxo1−/− mice show altered energy partitioning during fasting and increased metabolic flexibility.
To examine mechanisms of decreased HGP, we studied Agrp-Foxo1−/− mice after fasting and refeeding. During fasting, mRNA levels of the gluconeogenic gene G6pc were decreased (Figure S2A), as was glucokinase (Gck) (Figure S2B). Phosphoenolpyruvate carboxykinase–1 (Pck1) and pyruvate dehydrogenase kinase–4 (Pdk4) remained unchanged (Figure S3). During fasting, hepatic glycogenolysis is the main source of glucose (Lin and Accili, 2011). Consistent with decreased HGP, Agrp-Foxo1−/− mice had significantly increased hepatic glycogen and lipid after prolonged fasting (Figure S2C,D). To test whether the latter was due to increased de novo hepatic lipogenesis, we examined the expression of genes involved in fatty acid synthesis, oxidation, and mobilization (Figure S2E–G and Figure S3). mRNAs encoding key lipogenic enzymes were either comparable to wild-type levels (Figure S2E and Figure S3) or slightly decreased in Agrp-Foxo1−/− mice (Figure S2F and Figure S3). In contrast, hepatic Mcad was expressed at lower levels–consistent with the possibility that fatty acid oxidation is decreased (Figure S2G). Other genes involved in liver FFA metabolism were unchanged (Figure S3). These data raised the possibility that the increased lipid accumulation in the liver of Agrp-Foxo1−/− mice was due to increased flux of FFA from adipose tissue.
Altered adipocyte metabolism in Agrp-Foxo1−/− mice
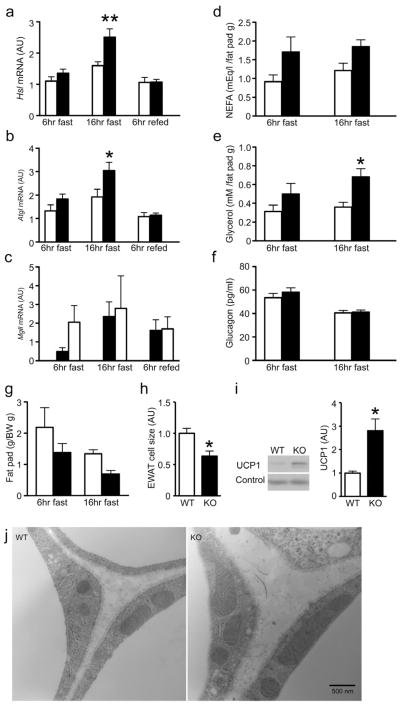
To test this hypothesis, we examined expression of lipolytic genes. Both Hsl and Atgl showed significantly increased expression after an overnight fast (Figure 3A,B), while Mgll showed a non-significant trend toward increase only at the 6-hr time point (Figure 3C) in Agrp-Foxo1−/− mice. Consistently, fasting plasma FFA and glycerol were elevated (Figure 3D,E). Glucagon levels were unchanged between control and Agrp-Foxo1−/− mice (Figure 3F). Fat pad weight decreased in Agrp-Foxo1−/− mice (Figure 3G), consistent with increased fasting-induced lipolysis. Histomorphometry of epididymal white adipose tissue (EWAT) demonstrated significantly smaller cells in Agrp-Foxo1−/− mice (Figure 3H). Moreover, Agrp-Foxo1−/− mice showed increased UCP1 in EWAT (Figure 3I). Electron microscopy revealed that EWAT from Agrp-Foxo1−/− mice contained larger mitochondria of normal morphology (Figure 3J).
Figure 3. Altered white adipose tissue metabolism in Agrp-Foxo1−/− mice.
(A–C) Expression of lipolytic genes Hsl (A), Atgl (B), and Mgl1 (C) under the following conditions: 6-hr fast (WT n=7, KO n=10), 16-hr fast (WT n=7, KO n=9), 6-hr refed (WT n=8, KO n=7).
(D–E) Fasting plasma NEFA and glycerol (n=7–11 for each genotype). Values are normalized by epididymal fat pad weight.
(F) Fasting plasma glucagon (n=6–11 for each genotype).
(G) Epididymal fat pad weight after 6-hr or 16-hr fasting (n=7–11 for each genotype).
(H) Histomorphometry of epididymal adipose cells size (n=9–11 for each genotype).
(I) Representative western blot and quantification of UCP1 expression in epididymal white adipose tissue (n=10 for each genotype).
(J) Representative electron microscopic image illustrating larger mitochondria in epididymal adipocytes of Agrp-Foxo1−/− mice. We present data as means ± SEM. * = P <0.05, ** = P <0.01. AU: arbitrary units.
Increased nutrient signaling in CNS of Agrp-Foxo1−/− mice
The common thread in the sub-phenotypes observed in Agrp-Foxo1−/− mice is the dissociation between decreased food intake and reduced fasting response. These two aspects could be reconciled by increased sensitivity to hormonal and nutrient signals conveyed through AgRP neurons. To test this possibility, we looked at hormonal and nutritional signaling events.
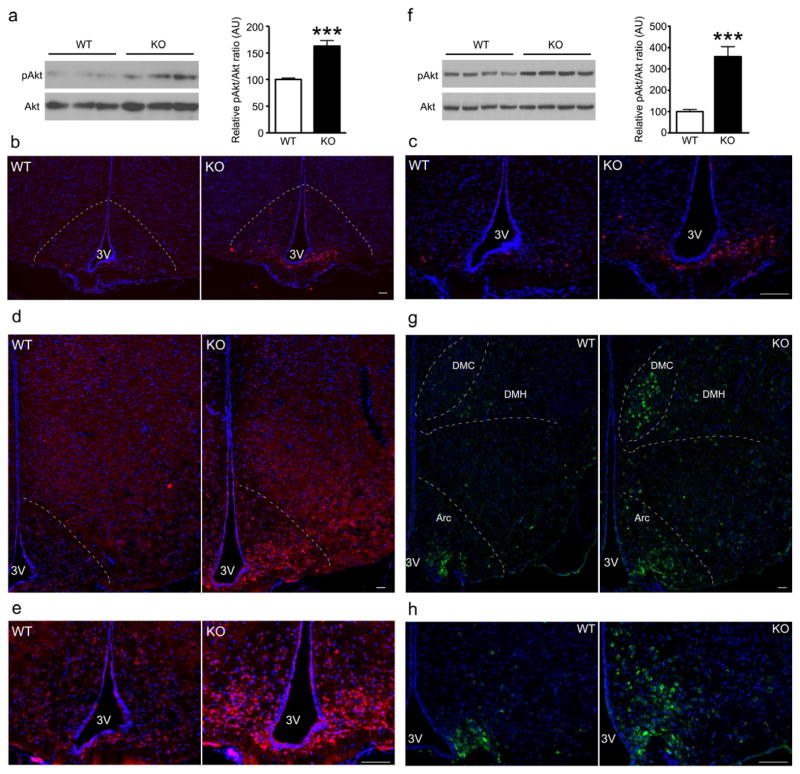
Insulin and leptin inhibit food intake (Halaas et al., 1995; Woods et al., 1979). We used immunohistochemistry with phospho (p)Akt and pStat3, respectively, to examine these signaling pathways. Fasted Agrp-Foxo1−/− mice had increased pAkt staining in MBH that wasn’t limited to AgRP neurons (red cells) (Figure S4A). Western blots confirmed the increase of pAkt in MBH (Figure 4A). After refeeding, despite decreased serum leptin (Table S1), Agrp-Foxo1−/− mice showed increased pStat3 staining in ARH (Figure 4B,C) and increased hypothalamic pAkt (Figure 4D), especially in ARH (Figure 4E). Western blots confirmed the increase of pAkt in ARH (Figure 4F). Therefore, knocking out Foxo1 in AgRP neurons renders mice more sensitive to leptin and insulin signaling in the hypothalamus.
Figure 4. Increased hypothalamic signaling by hormones and nutrients in mutant mice.
(A,F) Representative western blotting and quantitative analysis on ARH protein extracts in 6-hr-fasted Agrp-Foxo1−/− (KO) and wild-type (WT) (A) or 4-hr-refed Agrp-Foxo1−/− (KO) and wild-type (WT) (F). We present data as means ± SEM. *** = P<0.001.
(B–E, G–H) Representative immunohistochemistry with pStat3 (B,C), pAkt (D,E) and pS6 (G,H) in mice fasted overnight and refed for 3–4 hrs (n=6 for each genotype). Scale bar 100μm.
Amino acid sensing by the mTOR pathway promotes satiety (Cota et al., 2006). We monitored mTOR activity by immunohistochemistry with antibodies against its substrate ribosomal protein S6 in refed mice. ARH AgRP neurons project to DMH (Broberger et al., 1998). Agrp-Foxo1−/− mice showed increased pS6 staining in ARH and DMH (Figure 4G,H). Thus, hormone and nutrient signaling is uniformly increased in Agrp-Foxo1−/− mice.
Functional and gene expression analysis of FoxO1-deficient AgRP neurons
To identify AgRP and Pomc neurons, we generated Agrp-Foxo1−/− mice carrying Npy-Gfp (Agrp-Foxo1−/−; Rosa-tomato; Npy-Gfp) or Pomc-Gfp transgenes (Agrp-Foxo1−/−; Rosa-tomato; Pomc-Gfp). Foxo1–ablated AgRP neurons expressed Npy-gfp, but not Pomc-gfp (Figure 5A). We conclude that FoxO1-deficient AgRP neurons retain their neuronal identity, thereby excluding developmental defects due to trans-differentiation (Padilla et al., 2010). Next, we used Agrp-Foxo1−/−; Rosa-tomato;Npy-Gfp mice to flow-sort green Npy neurons and red AgRP neurons. FACS analysis demonstrated that 100% of AgRP neurons (labeled by tomato in red) (Figure 5B) are also Gfp–positive (i.e., they express Npy-Gfp). Having thus validated the FACS approach, we isolated mRNA from AgRP neurons of wild-type (Foxo1loxP/+; Agrp-Cre; Rosa-tomato) and Agrp-Foxo1−/−; Rosa-tomato mice (Figure 5C) and performed transcriptome profiling. Gene set enrichment analysis of the microarray data demonstrated increased expression of genes encoding components of the electron transport chain, translation, generation of precursor metabolites and energy, mitochondrial genome maintenance, and cellular component biogenesis in FoxO1-deficient AgRP neurons (Table S2 and Figure S4B). This anabolic gene expression profile of the mutant neurons is consistent with a state of increased energy availability, as one would predict based on increased insulin, leptin, and mTOR signaling (Figure 4). In other words, FoxO1-deficient AgRP neurons display gene expression patterns consistent with a state of energy sufficiency.
Figure 5. FACS, RNA profiling and electrophysiology of AgRP neurons.
(A) Fluorescence microscopy of ARH from Agrp-Foxo1−/−; Rosa-tomato mice carrying a Npy-Gfp or Pomc-Gfp transgene. FoxO1–deficient AgRP neurons (red) retain Npy-Gfp expression (green, upper panel), giving rise to merged yellow color (upper panel), whereas they fail to co-localize with Pomc-Gfp (green, lower panel). Scale bar 100μm.
(B) FACS analysis of dissociated hypothalamic neurons of Agrp-Foxo1−/− mice carrying a Npy-Gfp transgene (Agrp-Foxo1−/−; Rosa-tomato; Npy-Gfp). Npy neurons were sorted by green fluorescence (FITC, horizontal axis) and AgRP neurons were identified by red fluorescence (Tomato, vertical axis).
(C) Representative FACS analysis of dissociated hypothalamic neurons of Agrp-Foxo1−/−; Rosa-tomato mice and Agrp-Foxo1−/loxP; Rosa-tomato mice.
(D–F) Representative traces recorded in AgRP neurons from WT and Agrp-Foxo1−/− mice (D). Quantified results: firing frequency normalized to WT without insulin treatment (E), membrane potential (F). WT − insulin n=6, WT + insulin n=10, KO − insulin n=11, KO + insulin n=4.
(G,H) mEPSC (G) and mIPSC (H) recorded in AgRP neurons from WT and Agrp-Foxo1−/− mice illustrating representative traces (left), peak amplitude (middle) and size distribution (right) (mEPSC n=30, and mIPSC n=27–29 for each genotype). We present data as means ± SEM. * = P <0.05, ** = P <0.01, *** = P <0.001.
To explore this possibility, we examined the electrophysiological properties of AgRP neurons identified using fluorescent reporters in brain slices from wild-type and Agrp-Foxo1−/− mice (Figure 5D–F). Under basal conditions, FoxO1-deficient AgRP neurons had significantly lower membrane potential and firing frequency. Furthermore, insulin suppressed the firing frequency of wild-type cells to ~ 35% of basal level, a nearly three-fold reduction. Despite the lower basal firing frequency, insulin further suppressed the firing frequency of knockout neurons, with a 100-fold reduction. Insulin caused a similar reduction in membrane potential in wild-type and knockout cells (~ −20mV). Therefore, the electrophysiological data are consistent with the physiologic and signaling findings in Agrp-Foxo1−/− mice, indicating that knockout nerurons are more insulin-sensitive.
Next, we examined expression of ion channels and neurotransmitter receptors (Table S3 and Figure S4C). Of note, most GABAergic receptors showed increased expression, suggesting that GABAergic activity is increased, whereas most glutamatergic receptors were expressed at lower levels in FoxO1-deficient AgRP neurons, suggesting that glutamatergic signaling is decreased. To provide a functional validation of the mRNA measurements, we probed electrophysiological responses of AgRP neurons identified in brain slices from control and Agrp-Foxo1−/− mice. Mini excitatory post-synaptic currents (mEPSC) are typically mediated by glutamatergic neurotransmitters, while mini inhibitory post-synaptic currents (mIPSC) are elicited by GABAergic neurotransmitters (Pinto et al., 2004). FoxO1-ablated AgRP neurons showed a significant reduction of mEPSC peak amplitude (Figure 5G), but no change in frequency (data not shown). Conversely, they showed a significant increase of peak amplitude and size distribution of mIPSC (Figure 5H), without significantly altered frequency (data not shown). Therefore, the electrophysiological findings corroborate the results from gene expression profiling and indicate that Foxo1 ablation in AgRP neurons leads to increased GABAergic and decreased glutamatergic responses, as might be expected from altered neurotransmitter receptor expression at the post-synaptic level. Pre-synaptic plasticity of Npy neurons in ob/ob mice is modulated by acute exposure to leptin (Pinto et al., 2004). The changes at the post-synaptic level could partly contribute to or reflect adaptive consequences of the anorexigenic phenotype in Agrp-Foxo1−/− mice.
G protein-coupled receptor Gpr17 is a FoxO1 target in AgRP neurons
Given the favorable metabolic profile of Agrp-Foxo1−/− mice, we sought to identify FoxO1 targets that mediate its effects and are readily targetable by chemical means. Our microarray analysis indicated that mRNA encoding the G protein-coupled receptor, Gpr17, was profoundly decreased in flow-sorted FoxO1-deficient AgRP neurons. Gpr17 has been deorphanized as a dual receptor for uracil nucleotides and cysteinyl-leukotrienes (Ciana et al., 2006). Inhibition of Gpr17 in vivo can reduce ischemic damage in rodents, presumably by lowering neuronal excitability (Ciana et al., 2006). In addition, purinergic receptors, including Gpr17, have been widely reported to modulate Ca2+ and K+ channels and neurotransmitter receptors (Fischer and Krugel, 2007; Pugliese et al., 2009). But neither the role of Gpr17 in satiety and glucose homeostasis, nor its CNS distribution is known (Chen et al., 2009; Lecca et al., 2008). To examine the latter point, we labeled neurons with Rfp by intercrossing Synapsin-Cre and Rosa-tomato mice, then flow-sorted neuron and non-neuron populations and measured Gpr17 levels. qPCR analysis showed that Gpr17 is overwhelmingly enriched in neurons (Figure 6A). Next, we examined Gpr17 regulation in ARH punch biopsies from fasted and refed mice. Gpr17 was expressed at significantly higher levels during fasting (Figure 6B). To investigate the function of Gpr17 in AgRP neurons, we tested the electrophysiological properties of fluorescently labeled AgRP neurons in response to the Gpr17 antagonist, cangrelor. Upon treatment with cangrelor, AgRP neurons showed significantly reduced firing frequency and lower membrane potential (Figure 6C–E). Therefore, inhibition of Gpr17 can affect the electrical activity of AgRP neurons in an insulin-mimetic fashion, supporting our hypothesis.
Figure 6. G protein-coupled receptor Gpr17 is a FoxO1 target in AgRP neurons.

(A) Gpr17 mRNA levels in flow-sorted neurons and non-neurons (identified using Synapsin-Cre; Rosa-tomato mice) (n=2 for each genotype).
(B) Gpr17 mRNA in mediobasal hypothalamus from overnight-fasted and refed mice (n=7 for each genotype).
(C–E) Representative traces of electrophysiological recordings from the same AgRP neurons before and after cangrelor treatment (90nM) (C). Quantified results: firing frequency normalized to the values before cangrelor treatment (D) and membrane potential (E). (N=9)
(F,G) Foxo1 (F) and Gpr17 (G) expression in flow-sorted AgRP neurons from WT and KO mice (n=3 for each genotype).
(H) ChIP assay of the Gpr17 promoter in Neuro2A cells transfected with constitutively nuclear FoxO1.
(I) Endogenous Gpr17 mRNA in Neuro2A cells transfected with GFP-tagged wild-type Foxo1 (Foxo1-gfp) or constitutively nuclear Foxo1 (ADA) (n=3 for each genotype).
We present data as means ± SEM. * = P <0.05, ** = P <0.01, *** = P <0.001.
Consistent with the microarray data, Gpr17 expression in FoxO1-deficient AgRP neurons (Figure 6F) was dramatically reduced (18-fold over control) (Figure 6G). Gpr17 levels are extremely high in AgRP neurons, being comparable to β-actin (qPCR Ct = 18.44 ± 0.02 vs. 20.61 ± 0.06 for β-actin and Gpr17, respectively). Foxo1, in contrast, is considerably less abundant (Ct =23.43 ± 0.03).
We investigated the possibility that Gpr17 is a direct FoxO1 target. We found potential FoxO1 binding sites by analyzing the mouse Gpr17 promoter. Accordingly, chromatin immunoprecipitation (ChIP) assays showed that FoxO1 binds to the Gpr17 promoter (Figure 6H). Furthermore, quantitative analysis of the ChIP data indicated a 40-fold enrichment of the FoxO1 ChIP compared with IgG controls, indicating that binding is extremely robust.
Next, we used cultured Neuro2A cells to test the role of FoxO1 in Gpr17 regulation. Endogenous Foxo1 and Gpr17 are expressed at low levels in this cell line. Transfection of either wild-type FoxO1-Gfp or constitutively active FoxO1 (FoxO1-ADA, in which the key phosphorylation sites have been mutated by prevent FoxO1 inactivation) (Nakae et al., 2003) significantly increased Gpr17 levels (Figure 6I).
Opposing actions of GPR17 agonists and antagonists on AgRP neuron signaling and food intake in vivo
To test whether Gpr17 function affects ARH hormonal signaling in vivo, we delivered cangrelor directly into the 3rd ventricle of mice after an overnight fast and determined the co-localization of pSTAT3, pS6, and pAkt with either POMC or AgRP neurons, identified by fluorescent reporter, after refeeding. Cangrelor treatment specifically and dramatically increased pSTAT3 content in AgRP neurons, but not in POMC neurons (Figure 7A, compare yellow fluorescence in the third row, and lack thereof in the second row). Cangrelor also increased pAkt (Figure 7B, third row) and pS6 (Figure 7C, third row) in a subset of AgRP neurons, without effecting detectable changes in POMC neurons, as indicated by the dearth of yellow merged fluorescence in the POMC neuron panels (Figure 7B,C, second row panels). This striking result indicates that Gpr17 antagonism can directly and specifically increase pSTAT3 content in AgRP neurons in vivo, with lesser increases in pS6 and pAkt. The specificity of the effect of cangrelor in AgRP neurons may not be unrelated to the fact that Gpr17 mRNA is exceedingly abundant in this cell type.
Figure 7. Gpr17 modulation affects pSTAT3 content of AgRP neurons and food intake.
(A–C) ARH immunohistochemistry of refed AgRP or POMC reporter mice with pStat3 (A), pS6 (B), and pAkt (C) following administration of cangrelor into the 3V after an overnight fast. Scale bar 100μm.
(D) Food intake during refeeding in overnight-fasted WT and Agrp-Foxo1−/− mice following ICV administration of saline or cangrelor (n=6). We present data as means ± SEM. * = P <0.05.
(E) ARH immunohistochemistry of refed WT and Agrp-Foxo1−/− AgRP reporter mice with pStat3 following administration of LTD4 into the 3V after an overnight fast. Scale bar 100μm.
(F) Food intake in satiated WT and Agrp-Foxo1−/− mice after ICV injection of saline or Gpr17 agonist (n=5–9). We present data as means ± SEM. *** = P <0.001.
(G) Under fasting conditions (nuclear FoxO1, indicated by the green color), Gpr17 is induced and modulates ion channel activity, leading to increased orexigenic neuropeptide secretion as well as glutamatergic activity (+ labeled synapse), and contributing to food intake and HGP. Under fed conditions (phenocopied by the FoxO1 knockout) insulin and leptin signaling concur to inactivate Foxo1 and reduce Gpr17 expression. Together with increased GABAergic activity (− labeled synapse), this leads to decreased food intake and HGP.
To interrogate the consequences of these biochemical changes, we assessed the effect of delivering cangrelor into the 3rd ventricle of mice on food intake. In wild-type mice, we detected a significant decrease of food intake starting 1 hr after re-feeding (Figure 7D). Consistent with our previous data (Figure 2), Agrp-Foxo1−/− mice had a significantly decreased re-feeding intake during the same period of time that was completely unaffected by cangrelor administration.
Next, we examined the consequences of activating Gpr17 in vivo. First, we delivered LTD4 directly into the 3rd ventricle of mice after an overnight fast and determined the co-localization of pSTAT3 with AgRP neurons after refeeding. LTD4 treatment decreased pSTAT3 content in AgRP neurons (Figure 7E, compare yellow fluorescence in the first and second row, and lack thereof in the third row). Second, based on data indicating a synergistic effect of the two classes of Gpr17 ligands–purines and leukotrienes–on Gpr17 signaling (Daniele et al., 2011) we infused UDP-Glucose and LTD4 into the 3rd ventricle of satiated mice and measured food intake. Activating Gpr17 by this approach rapidly induced food intake in wild type, but not in Agrp-Foxo1−/− mice (Figure 7F). Thus, functional data support the hypothesis that Gpr17 is a direct FoxO1 target, and that its decreased expression mediates–at least partly–the anorexigenic phenotype of Agrp-Foxo1−/− mice.
DISCUSSION
In this work, we present three important findings (Figure 7G): (i) transcription factor FoxO1 exerts integrative control of leptin, insulin and amino acids in AgRP neurons, effectively coordinating food intake with peripheral metabolism; (ii) the identification of a heretofore unrecognized FoxO1 pathway that regulates food intake through the G protein-coupled receptor, Gpr17; and (iii) the profound decrease of HGP following FoxO1 ablation in AgRP neurons, effectively unifying direct and indirect regulation of HGP control under a FoxO1-dependent mechanism. The availability of Gpr17 inhibitors in the clinic lends potential relevance to our findings, providing impetus to explore this pathway for the pharmacological treatment of obesity.
ARH hormone signaling integrates satiety and energy homeostasis
CNS resistance to the two anorexient hormones, insulin and leptin, is likely to be an early contributor to the progressive weight gain that is associated with a “western” lifestyle (Schwartz et al., 2003). In addition to a plethora of genetic studies indicating that various sub-populations of ARH neurons control body weight, decreased leptin signaling through Jak-Stat has been demonstrated early on during the progression of diet-induced obesity (Gautron and Elmquist, 2011). We have been attracted to studying FoxO1 by its brisk regulation in response to hormones and nutrients, but also because it straddles leptin and insulin CNS signaling by virtue of its opposing actions on Agrp and Pomc expression (Kim et al., 2006; Kitamura et al., 2006). Similar to FoxO1 ablation, transgenic overexpression of constitutive activated Stat3 in AgRP neurons leads to leanness, but not as a result of decreased food intake (Mesaros et al., 2008). Moreover, constitutively active Stat3 causes feedback inhibition of leptin and insulin signaling (Ernst et al., 2009). These findings suggest that it is necessary to simultaneously sensitize both signaling pathways to engender an anorexient response. This contention is borne out from studies in Pomc neurons, in which ablation of either insulin or leptin receptor had modest consequences on metabolism and energy balance (Balthasar et al., 2004; Shi et al., 2008), while combined ablation caused systemic insulin resistance and female infertility (Hill et al., 2010). Our studies demonstrates phenotypes in Agrp-Foxo−/− mice that are not readily detectable by manipulating either insulin or leptin pathways alone.
Reconciling direct and indirect mechanisms of HGP control
The substantial decrease of HGP in Agrp-Foxo1−/− mice provides an unexpected answer to the longstanding question on the relative roles of direct and indirect mechanisms of insulin control over HGP. We have shown that ablation of FoxO in the liver (Matsumoto et al., 2007) reduced glucose production to the point of causing fasting hypoglycemia (Haeusler et al., 2010). The present data are noteworthy in several respects: they show that the indirect control of insulin on HGP through the CNS is mediated through the same signaling module utilized in liver; and that such control trumps the role of FFA and glycerol (Lewis et al., 1995), since Agrp-Foxo1−/− mice show decreased HGP in the face of elevated levels of these metabolites. Our data expand the conclusions reached in mice lacking insulin receptors in AgRP neurons, indicating that the failure of insulin inhibition of HGP in that model likely results from unchecked FoxO1 activity (Konner et al., 2007). We should point out that we endeavored to select mice with closely matched body weight and composition for the clamps. Thus, the decrease in HGP appears to occur independently in changes to energy balance, and provides–admittedly circumstantial–evidence that insulin control of liver metabolism through the CNS occurs independently of its effects on energy balance. Future studies are warranted to test how this regulation is achieved and to identify effector pathways.
The accelerated transition from carbohydrate-based to lipid-based metabolism in Agrp-Foxo1−/− mice is consistent with increased metabolic flexibility. It might be compensatory of their inability to mobilize hepatic glycogen, or it might reflect increased sympathetic tone of adipose tissue, as suggested by increased lipase expression, Ucp1 levels, and mitochondrial content. The latter interpretation would be consistent with the phenotype of mice lacking Pten in leptin receptor neurons (Plum et al., 2007) – although the present phenotype is considerably milder.
Anabolic profile of FoxO1-deficient AgRP neurons
The combination of immunohistochemical and gene profiling studies in Agrp-Foxo1−/− mice provides unprecedented insight into the mechanics of hypothalamic FoxO1 signaling. The data show increased basal and hormone-dependent signaling, consistent with a state of chronic inhibition of the electrical activity of these neurons. This conclusion is supported by the unusual and remarkable gene expression profile of FoxO1-deficient AgRP neurons, with increased anabolic pathways related to electron transport chain, translation, generation of precursor metabolites and energy, mitochondrial genome maintenance, and cellular component biogenesis. Thus, we suggest that the decreased food intake could be secondary to this increased anabolic activity. Consistently, mTOR pathway signaling in neurons is linked to feeding behavior (Cota et al., 2006). Combined expression patterns of the relevant receptors, as well as electrophysiological properties of FoxO1-deficient AgRP neurons are likely to increase GABAergic and diminish glutamatergic tone, a condition that would be expected to decrease food intake and increase metabolic flexibility. This is conceptually consistent with recent findings indicating that changes in energy storage and metabolic status in AgRP neurons are associated with altered neurotransmitter release and feeding behavior (Kaushik et al., 2011; Liu et al., 2012; Yang et al., 2011). We postulate that this could stem from adaptation to increased sensitivity to hormonal and nutrient cues.
Therapeutic prospects
Desirable as it might be to inhibit FoxO1 to treat metabolic diseases, this transcription factor is a poor drug target (Pajvani et al., 2011). Hence, we sought to identify a vicarious pathway with better therapeutic prospects. Gpr17 meets several criteria in this respect: we show it to be abundant in AgRP neurons, regulated by nutritional status, and a FoxO1 target. Moreover, Gpr17 antagonists mimic the effects of FoxO1 ablation on hypothalamic hormone signaling. Importantly, pharmacological inhibition of this receptor inhibits food intake, providing a ready alternative to FoxO1 inhibition. About one-third of all approved drugs target G-protein-coupled receptors (Erlinge, 2011). Gpr17 antagonists are currently in clinical use for the treatment of asthma by inhibiting leukotriene release (e.g., montelukast and pranlukast), and cangrelor has been used as short-acting anti-platelet aggregation agent (Kastrati and Ndrepepa, 2009). Therefore, the hypothesis emerging from our studies should be easily testable.
The broad distribution of Gpr17 in the CNS raises obvious concerns regarding its suitability as a target for the treatment of obesity. These concerns are partly allayed by the demonstration that direct ICV infusion of the Gpr17 antagonist cangrelor resulted in a remarkably specific activation of STAT3 phosphorylation in AgRP neurons (Figure 7A), while the agonist LTD4 caused a decrease of phospho-STAT3 (Figure 7E). This is consistent with our observation that Gpr17 levels are extraordinarily high in this cell type (only 2 cycles less abundant than actin by qPCR), and thus AgRP neurons might be singularly sensitive to manipulations of Gpr17, despite the latter’s broad distribution. There are currently no reported metabolic effects of these treatments, but this may simply reflect pharmacokinetics, CNS bioavailability, or confounders from the multiple comorbidities associated with each therapeutic indication. Further work is necessary before Gpr17 can be declared a bona fide target for intervention in obesity, but our observations provide impetus to pursue this research.
EXPERIMENTAL PROCEDURES
Mice
C57BL/6 and Gt(Rosa)26Sortm9(CAG-tdTomato)Hze mice were from The Jackson Laboratories. The Columbia University Animal Care and Utilization Committee approved all procedures. Normal chow diet (NCD) had 62.1% calories from carbohydrates, 24.6% from protein and 13.2% from fat (PicoLab rodent diet 20, 5053; Purina Mills). We measured weight and length to calculate body mass index and estimated body composition by nuclear magnetic resonance (Bruker Optics). We generated Agrp-specific FoxO1 knockouts by mating Agrp-Ires-Cre–transgenic mice (Tong et al., 2008) with Foxo1loxP/loxP mice (Paik et al., 2007) and genotyped them as previously described. We excluded from analyses Agrp-Foxo1−/− mice that showed somatic recombination owing to stochastic embryonic expression of Agrp-Ires-Cre.
Metabolic analyses
We measured food intake and refeeding response as described (Plum et al., 2009), using a TSE Labmaster Platform (TSE Systems) for indirect calorimetry and activity measurements (Banks et al., 2008). We performed glucose clamps as described (Okamoto et al., 2005). We used ELISA for leptin, insulin, and glucagon measurements (Millipore), and colorimetric assays for plasma free fatty acids and glycerol (Wako).
Stereotactic injections
We performed stereotactic manipulations in 3–4 month-old C57BL/6 mice. We placed cannulae and allowed 1 week for recovery (−1.8 mm anterior and 0 mm lateral to bregma and 5.3 mm below the skull surface). We verified correct positioning of the cannulae by monitoring drinking activity after angiotensin injection. After an overnight fast and 20 min before refeeding, we injected cangrelor (0.455 nmol) into the 3rd ventricle and measured food intake at the times indicated. For immunohistochemistry studies, we perfused mice 4–5 hr after refeeding. We infused LTD4 0.02 nmol and UDP-glucose 0.45 nmol into the 3rd ventricle of satiated mice and measured food intake afterwards.
Immunostaining
We processed mouse brains and cut 10-μm-thick coronal sections for immunohistochemistry as described (Plum et al., 2009), using pAkt (Cell Signaling #4060), pSTAT3 (Cell Signaling #9131), and pS6 antibodies (Cell Signaling #4858); Foxo1 antibody (Santa Cruz, FKHR-H128, sc11350).
RNA procedures
We extracted RNA with Trizol (Invitrogen) and reverse transcribed with Superscript II reverse transcriptase. We performed quantitative PCR using primers spanning introns. Primer sequences are available upon request.
Western blotting
We used RIPA buffer to extract protein and loaded equal amount of protein for gel electrophoresis.
Chromatin immunoprecipitation assays
We isolated intact chromatin from cultured N2A cells using ChIP-IT Express Enzymatic kit (Active Motif). Putative Foxo1 binding site on the Gpr17 promoter was identified by genomatrix software. Multiple pairs of primers were used for the subsequent amplification of Gpr17 promoter and yielded the same result. The pair used for electrophoresis is (forward) 5′-CCACACAGCTTATGTAGCATTGAGG-3′ and (reverse) 5′-AGCAGGAAGGTCTCAGTAACTCCC-3′.
Hypothalamic neuropeptide assays
We prepared acid extracts from mediobasal hypothalamus, and measured AGRP and α-MSH as previously described (Plum et al., 2009). We used HPLC to characterize AGRP immunoreactivity. The elution profile of AGRP immunoactivity in the MBH of Agrp-Foxo1−/− and WT mice was similar and consisted primarily of C-terminal AGRP 83-132 , as reported (Breen et al., 2005).
Flow cytometry and gene profiling of AgRP neurons
We dissociated mediobasal hypothalami from Agrp-Foxo1loxP/− Rosa-tomato and Agrp-Foxo1−/− Rosa-tomato neonates (40–50 animals per genotype) with papain dissociation kit (Worthington Biochemical). We gated live neurons to collect Rfp–positive Foxo1+/loxP;Agrp-Ires-cre;Rfp and Foxo1loxP/loxP;Agrp-Ires-cre;Rfp AgRP neurons. We isolated RNA with Trizol LS reagent, amplified it (NuGEN WT-Ovation Pico RNA Amplification System) and processed it for hybridization with Affymetrix chips (GeneChip Mouse Exon 1.0 ST Array). We used GOrilla and R for pathway analysis and heatmap generation.
Electrophysiology and patch-clamp
We prepared 300–μm coronal ARH slices from 6- to 8-week-old mice using ice cold sucrose-based cutting solution (adjusted to pH 7.3) containing (in mM): 10 NaCl, 25 NaHCO3, 195 sucrose, 5 glucose, 2.5 KCl, 1.25 NaH2PO4, 2 Na pyruvate, 0.5 CaCl2, and 7 MgCl2 bubbled continuously with 95% O2 and 5% CO2. We allowed slices to recover at least 45 minutes at room temperature in recording solution (adjusted to pH 7.3) containing (in mM): 125 NaCl, 5 glucose, 25 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 2 CaCl2, and 1 MgCl2 bubbled continuously with 95% O2 and 5% CO2 before transferring them to a perfusion chamber for recording. We pulled patch pipettes to resistances of 7–9 MΩ and filled them with solution (adjusted to pH 7.3) containing 128 K gluconate, 10 KCl, 10 HEPES, 0.1 EGTA, 2 MgCl2, 0.3 Na-GTP, and 3 Mg-ATP. We selected cells using an upright microscope fitted with fluorescence optics (Nikon) and patched under IR-DIC optics. We assessed electrical access in the whole–cell recording mode using a small-voltage step-pulse before and at the end of the current clamp recordings. We discarded recordings in which the series resistance had been >15 MΩ. We acquired current clamp recordings with no current injection using a MultiClamp 700B amplifier and Clampex data acquisition software (Axon Instruments). We recorded at room temperature and analyzed action potential (AP) firing frequency, membrane potential, and AP amplitude using MiniAnalysis (Synaptosoft). Electrophysiological recording to measure mEPSC and mIPSC in brain slices was performed as previously described (Pinto et al., 2004).
Statistical analyses
We analyzed data with Student’s t test or one–way ANOVA using GraphPad Prism software. We used the customary threshold of P < 0.05 to declare statistical significance.
Supplementary Material
FoxO1 knockout in AgRP neurons mimics insulin and leptin action
FoxO1-deficient AgRP mice are lean and have reduced food intake
Gpr17 is a FoxO1 target with an unexpected role to promote food intake
Gpr17 antagonists curtail food intake
Acknowledgments
H.R. is the recipient of a Mentor-based post-doctoral fellowship from the American Diabetes Association. Supported by NIH grants DK58282 and DK57539 (D.A.), DK80003 (S.L.W.), DK45024 (R.G-J.), NS49442 (O.A.), and DK63608 (Columbia University Diabetes Research Center). We thank Ms. Taylor Y. Lu and Kana Meece for excellent technical support, and members of the Accili laboratory for critical discussion of the data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua SC, Jr, Elmquist JK, et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Banks AS, Kon N, Knight C, Matsumoto M, Gutierrez-Juarez R, Rossetti L, Gu W, Accili D. SirT1 gain of function increases energy efficiency and prevents diabetes in mice. Cell Metab. 2008;8:333–341. doi: 10.1016/j.cmet.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen TL, Conwell IM, Wardlaw SL. Effects of fasting, leptin, and insulin on AGRP and POMC peptide release in the hypothalamus. Brain Res. 2005;1032:141–148. doi: 10.1016/j.brainres.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nature neuroscience. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciana P, Fumagalli M, Trincavelli ML, Verderio C, Rosa P, Lecca D, Ferrario S, Parravicini C, Capra V, Gelosa P, et al. The orphan receptor GPR17 identified as a new dual uracil nucleotides/cysteinyl-leukotrienes receptor. Embo J. 2006;25:4615–4627. doi: 10.1038/sj.emboj.7601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science. 2006;312:927–930. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- Daniele S, Trincavelli ML, Gabelloni P, Lecca D, Rosa P, Abbracchio MP, Martini C. Agonist-induced desensitization/resensitization of human G protein-coupled receptor 17: a functional cross-talk between purinergic and cysteinyl-leukotriene ligands. The Journal of pharmacology and experimental therapeutics. 2011;338:559–567. doi: 10.1124/jpet.110.178715. [DOI] [PubMed] [Google Scholar]
- Erlinge D. P2Y receptors in health and disease. Adv Pharmacol. 2011;61:417–439. doi: 10.1016/B978-0-12-385526-8.00013-8. [DOI] [PubMed] [Google Scholar]
- Ernst MB, Wunderlich CM, Hess S, Paehler M, Mesaros A, Koralov SB, Kleinridders A, Husch A, Munzberg H, Hampel B, et al. Enhanced Stat3 activation in POMC neurons provokes negative feedback inhibition of leptin and insulin signaling in obesity. J Neurosci. 2009;29:11582–11593. doi: 10.1523/JNEUROSCI.5712-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer W, Krugel U. P2Y receptors: focus on structural, pharmacological and functional aspects in the brain. Curr Med Chem. 2007;14:2429–2455. doi: 10.2174/092986707782023695. [DOI] [PubMed] [Google Scholar]
- Gautron L, Elmquist JK. Sixteen years and counting: an update on leptin in energy balance. The Journal of clinical investigation. 2011;121:2087–2093. doi: 10.1172/JCI45888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeusler RA, Kaestner KH, Accili D. FoxOs function synergistically to promote glucose production. J Biol Chem. 2010;285:35245–35248. doi: 10.1074/jbc.C110.175851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, et al. Direct insulin and leptin action on pro- opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell metabolism. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastrati A, Ndrepepa G. Cangrelor - a champion lost in translation? The New England journal of medicine. 2009;361:2382–2384. doi: 10.1056/NEJMe0910677. [DOI] [PubMed] [Google Scholar]
- Kaushik S, Rodriguez-Navarro JA, Arias E, Kiffin R, Sahu S, Schwartz GJ, Cuervo AM, Singh R. Autophagy in hypothalamic AgRP neurons regulates food intake and energy balance. Cell metabolism. 2011;14:173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MS, Pak YK, Jang PG, Namkoong C, Choi YS, Won JC, Kim KS, Kim SW, Kim HS, Park JY, et al. Role of hypothalamic Foxo1 in the regulation of food intake and energy homeostasis. Nat Neurosci. 2006;9:901–906. doi: 10.1038/nn1731. [DOI] [PubMed] [Google Scholar]
- Kitamura T, Feng Y, Kitamura YI, Chua SC, Jr, Xu AW, Barsh GS, Rossetti L, Accili D. Forkhead protein FoxO1 mediates Agrp-dependent effects of leptin on food intake. Nat Med. 2006;12:534–540. doi: 10.1038/nm1392. [DOI] [PubMed] [Google Scholar]
- Konner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. Insulin Action in AgRP-Expressing Neurons Is Required for Suppression of Hepatic Glucose Production. Cell Metab. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. The Journal of clinical investigation. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecca D, Trincavelli ML, Gelosa P, Sironi L, Ciana P, Fumagalli M, Villa G, Verderio C, Grumelli C, Guerrini U, et al. The recently identified P2Y-like receptor GPR17 is a sensor of brain damage and a new target for brain repair. PLoS One. 2008;3:e3579. doi: 10.1371/journal.pone.0003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AS, Jewett DC, Cleary JP, Kotz CM, Billington CJ. Our journey with neuropeptide Y: effects on ingestive behaviors and energy expenditure. Peptides. 2004;25:505–510. doi: 10.1016/j.peptides.2004.01.005. [DOI] [PubMed] [Google Scholar]
- Lewis GF, Uffelman KD, Szeto LW, Weller B, Steiner G. Interaction between free fatty acids and insulin in the acute control of very low density lipoprotein production in humans. J Clin Invest. 1995;95:158–166. doi: 10.1172/JCI117633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HV, Accili D. Hormonal regulation of hepatic glucose production in health and disease. Cell metabolism. 2011;14:9–19. doi: 10.1016/j.cmet.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting Activation of AgRP Neurons Requires NMDA Receptors and Involves Spinogenesis and Increased Excitatory Tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Pocai A, Rossetti L, Depinho RA, Accili D. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6:208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Bruning JC. Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab. 2008;7:236–248. doi: 10.1016/j.cmet.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Nakae J, Kitamura T, Kitamura Y, Biggs WH, 3rd, Arden KC, Accili D. The forkhead transcription factor Foxo1 regulates adipocyte differentiation. Dev Cell. 2003;4:119–129. doi: 10.1016/s1534-5807(02)00401-x. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Obici S, Accili D, Rossetti L. Restoration of liver insulin signaling in Insr knockout mice fails to normalize hepatic insulin action. J Clin Invest. 2005;115:1314–1322. doi: 10.1172/JCI23096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nature medicine. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik JH, Kollipara R, Chu G, Ji H, Xiao Y, Ding Z, Miao L, Tothova Z, Horner JW, Carrasco DR, et al. FoxOs Are Lineage-Restricted Redundant Tumor Suppressors and Regulate Endothelial Cell Homeostasis. Cell. 2007;128:309–323. doi: 10.1016/j.cell.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, Kitajewski J, Accili D. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nature medicine. 2011;17:961–967. doi: 10.1038/nm.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, et al. The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med. 2009;15:1195–1201. doi: 10.1038/nm.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Rother E, Munzberg H, Wunderlich FT, Morgan DA, Hampel B, Shanabrough M, Janoschek R, Konner AC, Alber J, et al. Enhanced Leptin-Stimulated Pi3k Activation in the CNS Promotes White Adipose Tissue Transdifferentiation. Cell Metab. 2007;6:431–445. doi: 10.1016/j.cmet.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Trincavelli ML, Lecca D, Coppi E, Fumagalli M, Ferrario S, Failli P, Daniele S, Martini C, Pedata F, et al. Functional characterization of two isoforms of the P2Y-like receptor GPR17: [35S]GTPgammaS binding and electrophysiological studies in 1321N1 cells. American journal of physiology Cell physiology. 2009;297:C1028–1040. doi: 10.1152/ajpcell.00658.2008. [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, et al. Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Molecular and cellular biology. 2002;22:5027–5035. doi: 10.1128/MCB.22.14.5027-5035.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, et al. A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology. 1998;139:4428–4431. doi: 10.1210/endo.139.10.6332. [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL. Is the energy homeostasis system inherently biased toward weight gain? Diabetes. 2003;52:232–238. doi: 10.2337/diabetes.52.2.232. [DOI] [PubMed] [Google Scholar]
- Shi H, Strader AD, Sorrell JE, Chambers JB, Woods SC, Seeley RJ. Sexually different actions of leptin in proopiomelanocortin neurons to regulate glucose homeostasis. American journal of physiology Endocrinology and metabolism. 2008;294:E630–639. doi: 10.1152/ajpendo.00704.2007. [DOI] [PubMed] [Google Scholar]
- Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nature neuroscience. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, et al. Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology. 2008;149:1773–1785. doi: 10.1210/en.2007-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet. 2011;378:815–825. doi: 10.1016/S0140-6736(11)60814-3. [DOI] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, Porte D., Jr Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature. 1979;282:503–505. doi: 10.1038/282503a0. [DOI] [PubMed] [Google Scholar]
- Yang Y, Atasoy D, Su HH, Sternson SM. Hunger States Switch a Flip-Flop Memory Circuit via a Synaptic AMPK-Dependent Positive Feedback Loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.