Abstract
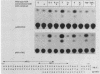
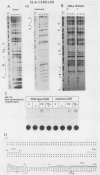
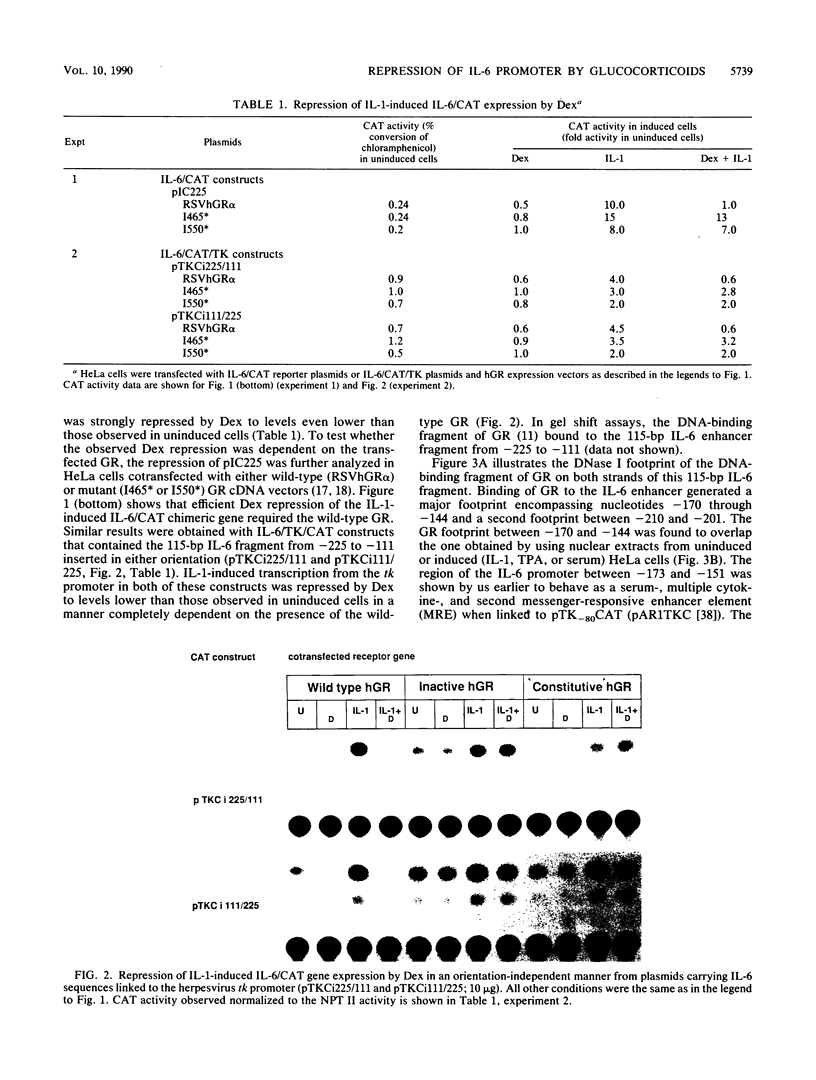
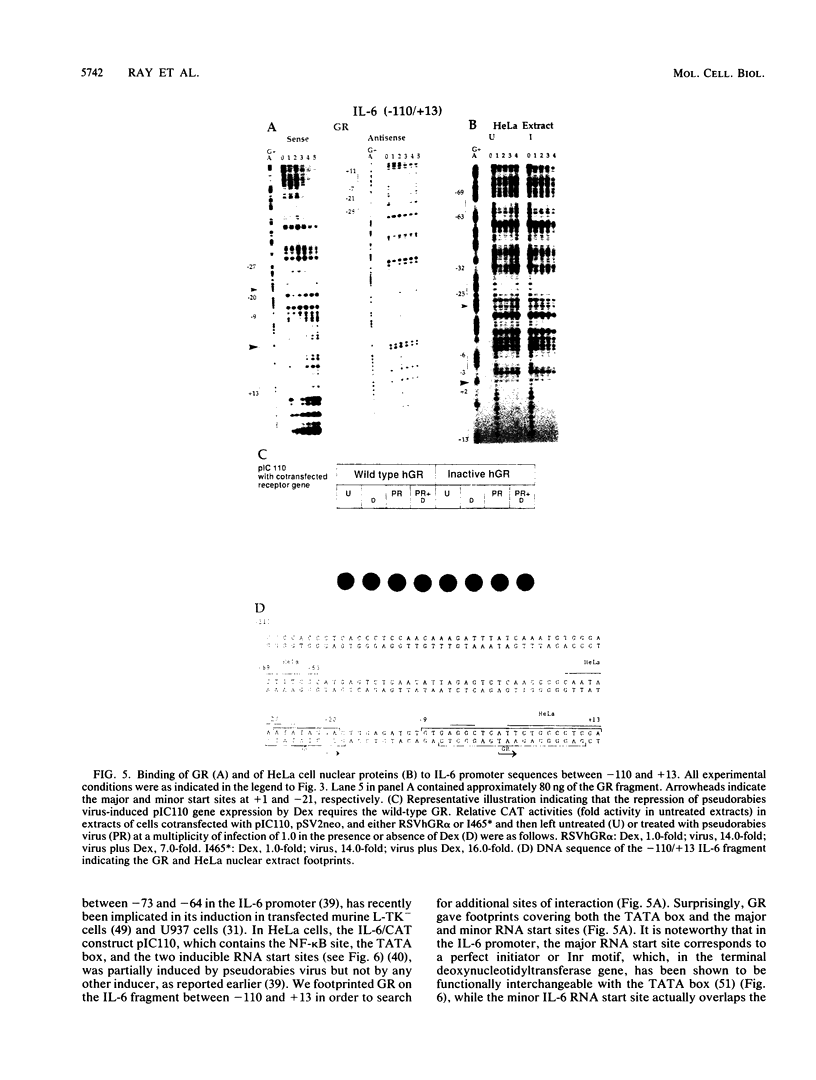
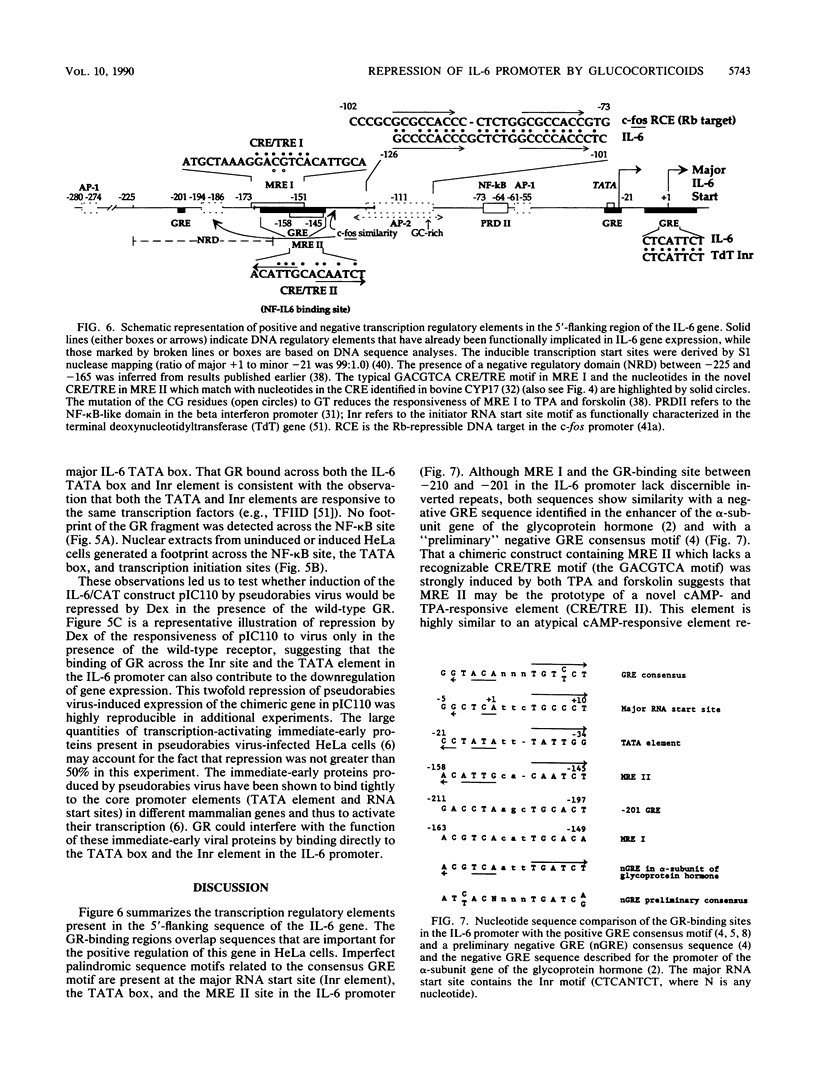
The feedback inhibition of interleukin-6 (IL-6) gene expression by glucocorticoids represents a regulatory link between the endocrine and immune systems. The mechanism of the efficient repression of the IL-6 promoter by dexamethasone (Dex) was investigated in HeLa cells transiently transfected with plasmid constructs containing different IL-6 promoter elements linked to the herpesvirus thymidine kinase gene (tk) promoter and the bacterial chloramphenicol acetyltransferase gene (cat) and cotransfected with cDNA vectors constitutively expressing either the active wild-type or inactive mutant human glucocorticoid receptor (GR). The induction by interleukin-1, tumor necrosis factor, phorbol ester, or forskolin of IL-6-tk-cat chimeric constructs containing a single copy of the IL-6 DNA segment from -173 to -151 (MRE I) or from -158 to -145 (MRE II), which derive from within the multiple cytokine- and second-messenger-responsive enhancer (MRE) region, was strongly repressed by Dex in a wild-type GR-dependent fashion irrespective of the inducer used. The induction by pseudorabies virus of an IL-6 construct containing the IL-6 TATA box and the RNA start site ("initiator" or Inr element) but not the MRE region was also repressed by Dex in the presence of wild-type GR. DNase I footprinting showed that the purified DNA-binding fragment of GR bound across the MRE, the TATA box, and the Inr site in the IL-6 promoter; this footprint overlapped that produced by proteins present in nuclear extracts from uninduced or induced HeLa cells. Imperfect palindromic nucleotide sequence motifs moderately related to the consensus GR-responsive element (GRE) motif were present at the Inr, the TATA box, and the MRE II site in the IL-6 promoter; although MRE I and a GR-binding site between -201 and -210 in IL-6 both lacked a discernible inverted repeat motif, their sequences showed considerable similarity with negative GRE sequences in other Dex-repressed genes. Surprisingly, chimeric genes containing MRE II, which lacks a recognizable GACGTCA cyclic AMP- and phorbol ester-responsive motif, were strongly induced by both phorbol ester and forskolin, suggesting that MRE II (ACATTGCACAATCT) may be the prototype of a novel cyclic AMP- and phorbol ester-responsive element. Taken together, these observations suggest that ligand-activated GR represses the IL-6 gene by occlusion not only of the inducible IL-6 MRE enhancer region but also of the basal IL-6 promoter elements.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler S., Waterman M. L., He X., Rosenfeld M. G. Steroid receptor-mediated inhibition of rat prolactin gene expression does not require the receptor DNA-binding domain. Cell. 1988 Mar 11;52(5):685–695. doi: 10.1016/0092-8674(88)90406-0. [DOI] [PubMed] [Google Scholar]
- Akerblom I. E., Slater E. P., Beato M., Baxter J. D., Mellon P. L. Negative regulation by glucocorticoids through interference with a cAMP responsive enhancer. Science. 1988 Jul 15;241(4863):350–353. doi: 10.1126/science.2838908. [DOI] [PubMed] [Google Scholar]
- Akira S., Isshiki H., Sugita T., Tanabe O., Kinoshita S., Nishio Y., Nakajima T., Hirano T., Kishimoto T. A nuclear factor for IL-6 expression (NF-IL6) is a member of a C/EBP family. EMBO J. 1990 Jun;9(6):1897–1906. doi: 10.1002/j.1460-2075.1990.tb08316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M., Arnemann J., Chalepakis G., Slater E., Willmann T. Gene regulation by steroid hormones. J Steroid Biochem. 1987;27(1-3):9–14. doi: 10.1016/0022-4731(87)90288-3. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Cromlish W. A., Abmayr S. M., Workman J. L., Horikoshi M., Roeder R. G. Transcriptionally active immediate-early protein of pseudorabies virus binds to specific sites on class II gene promoters. J Virol. 1989 May;63(5):1869–1876. doi: 10.1128/jvi.63.5.1869-1876.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin J., Trifiro M. A., Plante R. K., Nemer M., Eriksson P., Wrange O. Glucocorticoid receptor binding to a specific DNA sequence is required for hormone-dependent repression of pro-opiomelanocortin gene transcription. Mol Cell Biol. 1989 Dec;9(12):5305–5314. doi: 10.1128/mcb.9.12.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feyen J. H., Elford P., Di Padova F. E., Trechsel U. Interleukin-6 is produced by bone and modulated by parathyroid hormone. J Bone Miner Res. 1989 Aug;4(4):633–638. doi: 10.1002/jbmr.5650040422. [DOI] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Freedman L. P., Luisi B. F., Korszun Z. R., Basavappa R., Sigler P. B., Yamamoto K. R. The function and structure of the metal coordination sites within the glucocorticoid receptor DNA binding domain. Nature. 1988 Aug 11;334(6182):543–546. doi: 10.1038/334543a0. [DOI] [PubMed] [Google Scholar]
- Garman R. D., Jacobs K. A., Clark S. C., Raulet D. H. B-cell-stimulatory factor 2 (beta 2 interferon) functions as a second signal for interleukin 2 production by mature murine T cells. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7629–7633. doi: 10.1073/pnas.84.21.7629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauldie J., Richards C., Harnish D., Lansdorp P., Baumann H. Interferon beta 2/B-cell stimulatory factor type 2 shares identity with monocyte-derived hepatocyte-stimulating factor and regulates the major acute phase protein response in liver cells. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7251–7255. doi: 10.1073/pnas.84.20.7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Helfgott D. C., May L. T., Sthoeger Z., Tamm I., Sehgal P. B. Bacterial lipopolysaccharide (endotoxin) enhances expression and secretion of beta 2 interferon by human fibroblasts. J Exp Med. 1987 Nov 1;166(5):1300–1309. doi: 10.1084/jem.166.5.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfgott D. C., Tatter S. B., Santhanam U., Clarick R. H., Bhardwaj N., May L. T., Sehgal P. B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989 Feb 1;142(3):948–953. [PubMed] [Google Scholar]
- Hollenberg S. M., Evans R. M. Multiple and cooperative trans-activation domains of the human glucocorticoid receptor. Cell. 1988 Dec 2;55(5):899–906. doi: 10.1016/0092-8674(88)90145-6. [DOI] [PubMed] [Google Scholar]
- Hollenberg S. M., Giguere V., Segui P., Evans R. M. Colocalization of DNA-binding and transcriptional activation functions in the human glucocorticoid receptor. Cell. 1987 Apr 10;49(1):39–46. doi: 10.1016/0092-8674(87)90753-7. [DOI] [PubMed] [Google Scholar]
- Isshiki H., Akira S., Tanabe O., Nakajima T., Shimamoto T., Hirano T., Kishimoto T. Constitutive and interleukin-1 (IL-1)-inducible factors interact with the IL-1-responsive element in the IL-6 gene. Mol Cell Biol. 1990 Jun;10(6):2757–2764. doi: 10.1128/mcb.10.6.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablons D. M., Mulé J. J., McIntosh J. K., Sehgal P. B., May L. T., Huang C. M., Rosenberg S. A., Lotze M. T. IL-6/IFN-beta-2 as a circulating hormone. Induction by cytokine administration in humans. J Immunol. 1989 Mar 1;142(5):1542–1547. [PubMed] [Google Scholar]
- Jensen E. V., Suzuki T., Kawashima T., Stumpf W. E., Jungblut P. W., DeSombre E. R. A two-step mechanism for the interaction of estradiol with rat uterus. Proc Natl Acad Sci U S A. 1968 Feb;59(2):632–638. doi: 10.1073/pnas.59.2.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P. F., McKnight S. L. Eukaryotic transcriptional regulatory proteins. Annu Rev Biochem. 1989;58:799–839. doi: 10.1146/annurev.bi.58.070189.004055. [DOI] [PubMed] [Google Scholar]
- Karin M., Haslinger A., Holtgreve H., Richards R. I., Krauter P., Westphal H. M., Beato M. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature. 1984 Apr 5;308(5959):513–519. doi: 10.1038/308513a0. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-Destefano D., Sehgal P. B., Vilcek J. Dexamethasone inhibits feedback regulation of the mitogenic activity of tumor necrosis factor, interleukin-1, and epidermal growth factor in human fibroblasts. J Cell Physiol. 1987 Aug;132(2):271–278. doi: 10.1002/jcp.1041320211. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Green S., Stack G., Berry M., Jin J. R., Chambon P. Functional domains of the human estrogen receptor. Cell. 1987 Dec 24;51(6):941–951. doi: 10.1016/0092-8674(87)90581-2. [DOI] [PubMed] [Google Scholar]
- LeMay L. G., Vander A. J., Kluger M. J. Role of interleukin 6 in fever in rats. Am J Physiol. 1990 Mar;258(3 Pt 2):R798–R803. doi: 10.1152/ajpregu.1990.258.3.R798. [DOI] [PubMed] [Google Scholar]
- Levine M., Manley J. L. Transcriptional repression of eukaryotic promoters. Cell. 1989 Nov 3;59(3):405–408. doi: 10.1016/0092-8674(89)90024-x. [DOI] [PubMed] [Google Scholar]
- Libermann T. A., Baltimore D. Activation of interleukin-6 gene expression through the NF-kappa B transcription factor. Mol Cell Biol. 1990 May;10(5):2327–2334. doi: 10.1128/mcb.10.5.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J., Ahlgren R., Wu D. H., Kagimoto M., Simpson E. R., Waterman M. R. Transcriptional regulation of the bovine CYP17 (P-450(17)alpha) gene. Identification of two cAMP regulatory regions lacking the consensus cAMP-responsive element (CRE). J Biol Chem. 1990 Feb 25;265(6):3304–3312. [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Mordacq J. C., Linzer D. I. Co-localization of elements required for phorbol ester stimulation and glucocorticoid repression of proliferin gene expression. Genes Dev. 1989 Jun;3(6):760–769. doi: 10.1101/gad.3.6.760. [DOI] [PubMed] [Google Scholar]
- Morrison N. A., Shine J., Fragonas J. C., Verkest V., McMenemy M. L., Eisman J. A. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989 Dec 1;246(4934):1158–1161. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- Naitoh Y., Fukata J., Tominaga T., Nakai Y., Tamai S., Mori K., Imura H. Interleukin-6 stimulates the secretion of adrenocorticotropic hormone in conscious, freely-moving rats. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1459–1463. doi: 10.1016/s0006-291x(88)81305-6. [DOI] [PubMed] [Google Scholar]
- Nishida T., Nakai S., Kawakami T., Aihara K., Nishino N., Hirai Y. Dexamethasone regulation of the expression of cytokine mRNAs induced by interleukin-1 in the astrocytoma cell line U373MG. FEBS Lett. 1989 Jan 16;243(1):25–29. doi: 10.1016/0014-5793(89)81210-4. [DOI] [PubMed] [Google Scholar]
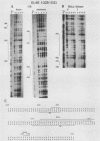
- Ray A., Sassone-Corsi P., Sehgal P. B. A multiple cytokine- and second messenger-responsive element in the enhancer of the human interleukin-6 gene: similarities with c-fos gene regulation. Mol Cell Biol. 1989 Dec;9(12):5537–5547. doi: 10.1128/mcb.9.12.5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Tatter S. B., May L. T., Sehgal P. B. Activation of the human "beta 2-interferon/hepatocyte-stimulating factor/interleukin 6" promoter by cytokines, viruses, and second messenger agonists. Proc Natl Acad Sci U S A. 1988 Sep;85(18):6701–6705. doi: 10.1073/pnas.85.18.6701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Tatter S. B., Santhanam U., Helfgott D. C., May L. T., Sehgal P. B. Regulation of expression of interleukin-6. Molecular and clinical studies. Ann N Y Acad Sci. 1989;557:353–362. [PubMed] [Google Scholar]
- Ringold G. M. Steroid hormone regulation of gene expression. Annu Rev Pharmacol Toxicol. 1985;25:529–566. doi: 10.1146/annurev.pa.25.040185.002525. [DOI] [PubMed] [Google Scholar]
- Robbins P. D., Horowitz J. M., Mulligan R. C. Negative regulation of human c-fos expression by the retinoblastoma gene product. Nature. 1990 Aug 16;346(6285):668–671. doi: 10.1038/346668a0. [DOI] [PubMed] [Google Scholar]
- Sakai D. D., Helms S., Carlstedt-Duke J., Gustafsson J. A., Rottman F. M., Yamamoto K. R. Hormone-mediated repression: a negative glucocorticoid response element from the bovine prolactin gene. Genes Dev. 1988 Sep;2(9):1144–1154. doi: 10.1101/gad.2.9.1144. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Ransone L. J., Verma I. M. Cross-talk in signal transduction: TPA-inducible factor jun/AP-1 activates cAMP-responsive enhancer elements. Oncogene. 1990 Mar;5(3):427–431. [PubMed] [Google Scholar]
- Scheidereit C., Beato M. Contacts between hormone receptor and DNA double helix within a glucocorticoid regulatory element of mouse mammary tumor virus. Proc Natl Acad Sci U S A. 1984 May;81(10):3029–3033. doi: 10.1073/pnas.81.10.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B., Helfgott D. C., Santhanam U., Tatter S. B., Clarick R. H., Ghrayeb J., May L. T. Regulation of the acute phase and immune responses in viral disease. Enhanced expression of the beta 2-interferon/hepatocyte-stimulating factor/interleukin 6 gene in virus-infected human fibroblasts. J Exp Med. 1988 Jun 1;167(6):1951–1956. doi: 10.1084/jem.167.6.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B. Interleukin-6: a regulator of plasma protein gene expression in hepatic and non-hepatic tissues. Mol Biol Med. 1990 Apr;7(2):117–130. [PubMed] [Google Scholar]
- Sehgal P. B., May L. T., Tamm I., Vilcek J. Human beta 2 interferon and B-cell differentiation factor BSF-2 are identical. Science. 1987 Feb 13;235(4790):731–732. doi: 10.1126/science.3492764. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Mitomo K., Watanabe T., Okamoto S., Yamamoto K. Involvement of a NF-kappa B-like transcription factor in the activation of the interleukin-6 gene by inflammatory lymphokines. Mol Cell Biol. 1990 Feb;10(2):561–568. doi: 10.1128/mcb.10.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater E. P., Rabenau O., Karin M., Baxter J. D., Beato M. Glucocorticoid receptor binding and activation of a heterologous promoter by dexamethasone by the first intron of the human growth hormone gene. Mol Cell Biol. 1985 Nov;5(11):2984–2992. doi: 10.1128/mcb.5.11.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smale S. T., Schmidt M. C., Berk A. J., Baltimore D. Transcriptional activation by Sp1 as directed through TATA or initiator: specific requirement for mammalian transcription factor IID. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4509–4513. doi: 10.1073/pnas.87.12.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangelo B. L., Judd A. M., Isakson P. C., MacLeod R. M. Interleukin-6 stimulates anterior pituitary hormone release in vitro. Endocrinology. 1989 Jul;125(1):575–577. doi: 10.1210/endo-125-1-575. [DOI] [PubMed] [Google Scholar]
- Tabibzadeh S. S., Santhanam U., Sehgal P. B., May L. T. Cytokine-induced production of IFN-beta 2/IL-6 by freshly explanted human endometrial stromal cells. Modulation by estradiol-17 beta. J Immunol. 1989 May 1;142(9):3134–3139. [PubMed] [Google Scholar]
- Vankelecom H., Carmeliet P., Van Damme J., Billiau A., Denef C. Production of interleukin-6 by folliculo-stellate cells of the anterior pituitary gland in a histiotypic cell aggregate culture system. Neuroendocrinology. 1989 Jan;49(1):102–106. doi: 10.1159/000125097. [DOI] [PubMed] [Google Scholar]
- Walther Z., May L. T., Sehgal P. B. Transcriptional regulation of the interferon-beta 2/B cell differentiation factor BSF-2/hepatocyte-stimulating factor gene in human fibroblasts by other cytokines. J Immunol. 1988 Feb 1;140(3):974–977. [PubMed] [Google Scholar]
- Woloski B. M., Smith E. M., Meyer W. J., 3rd, Fuller G. M., Blalock J. E. Corticotropin-releasing activity of monokines. Science. 1985 Nov 29;230(4729):1035–1037. doi: 10.1126/science.2997929. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lin J. X., Vilcek J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem. 1988 May 5;263(13):6177–6182. [PubMed] [Google Scholar]