Arabidopsis heterotrimeric G proteins function as a converging point of plant defense signaling by mediating responses initiated by multiple receptor-like kinases, which may fulfill equivalent roles to GPCRs in fungi and animals.
Abstract
In fungi and metazoans, extracellular signals are often perceived by G-protein-coupled receptors (GPCRs) and transduced through heterotrimeric G-protein complexes to downstream targets. Plant heterotrimeric G proteins are also involved in diverse biological processes, but little is known about their upstream receptors. Moreover, the presence of bona fide GPCRs in plants is yet to be established. In Arabidopsis (Arabidopsis thaliana), heterotrimeric G protein consists of one Gα subunit (G PROTEIN α-SUBUNIT1), one Gβ subunit (ARABIDOPSIS G PROTEIN β-SUBUNIT1 [AGB1]), and three Gγs subunits (ARABIDOPSIS G PROTEIN γ-SUBUNIT1 [AGG1], AGG2, and AGG3). We identified AGB1 from a suppressor screen of BAK1-interacting receptor-like kinase1-1 (bir1-1), a mutant that activates cell death and defense responses mediated by the receptor-like kinase (RLK) SUPPRESSOR OF BIR1-1. Mutations in AGB1 suppress the cell death and defense responses in bir1-1 and transgenic plants overexpressing SUPPRESSOR OF BIR1-1. In addition, agb1 mutant plants were severely compromised in immunity mediated by three other RLKs, FLAGELLIN-SENSITIVE2 (FLS2), Elongation Factor-TU RECEPTOR (EFR), and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1), respectively. By contrast, G PROTEIN α-SUBUNIT1 is not required for either cell death in bir1-1 or pathogen-associated molecular pattern-triggered immunity mediated by FLS2, EFR, and CERK1. Further analysis of agg1 and agg2 mutant plants indicates that AGG1 and AGG2 are also required for pathogen-associated molecular pattern-triggered immune responses mediated by FLS2, EFR, and CERK1, as well as cell death and defense responses in bir1-1. We hypothesize that the Arabidopsis heterotrimeric G proteins function as a converging point of plant defense signaling by mediating responses initiated by multiple RLKs, which may fulfill equivalent roles to GPCRs in fungi and animals.
Receptor-like kinases (RLKs) represent one of the largest protein families in plants and play diverse roles in plant development and stress signaling (Morillo and Tax, 2006). In Arabidopsis (Arabidopsis thaliana), there are over 600 RLKs (Shiu and Bleecker, 2001). Most RLKs contain an extracellular domain, a single transmembrane motif, and a cytoplasmic kinase domain. It is believed that the extracellular domains are involved in ligand recognition that subsequently leads to activation of the cytoplasmic kinase domain. Pathogen-associated molecular pattern (PAMP) receptors FLAGELLIN-SENSITIVE2 (FLS2), Elongation Factor (EF)-TU RECEPTOR (EFR), and CHITIN ELICITOR RECEPTOR KINASE1 (CERK1) all belong to the RLK family. FLS2 and EFR function as receptors for bacterial flagellin and EF-Tu, respectively (Gómez-Gómez and Boller, 2000; Zipfel et al., 2006), while CERK1 is involved in the perception of chitin, a common component of the fungal cell wall (Miya et al., 2007; Wan et al., 2008). CERK1 was also shown to play an important role in defense against bacterial pathogens (Gimenez-Ibanez et al., 2009). Another RLK, Brassinosteroid Insensitive1-associated receptor kinase1 (BAK1), functions as a coreceptor for FLS2 and EFR (Chinchilla et al., 2007; Heese et al., 2007). In addition, a rice (Oryza sativa) RLK, Xa21, functions as the receptor for a peptide derived from AvrXa21 (Song et al., 1995; Lee et al., 2009).
Activation of different PAMP receptors often leads to rapid downstream responses, such as oxidative burst, calcium influx, mitogen-activated protein kinase (MAPK) activations, and the up-regulation of defense gene expression (Boller and Felix, 2009). However, our knowledge of how defense responses are regulated downstream of the RLKs remains limited. Genetic analysis of mutants defective in EFR-mediated PAMP responses showed that endoplasmic reticulum (ER) quality control plays an important role in the accumulation of EFR (Li et al., 2009; Lu et al., 2009; Nekrasov et al., 2009; Saijo et al., 2009). ER-resident chaperones are also required for the accumulation of the tobacco (Nicotiana tabacum) INDUCED RECEPTOR-LIKE KINASE (Caplan et al., 2009). BOTRYTIS-INDUCED KINASE1 (BIK1) encodes a cytoplasmic RLK that directly interacts with FLS2 and likely EFR and CERK1 (Lu et al., 2010; Zhang et al., 2010). Knocking out BIK1 leads to modest reductions of PAMP-induced callose deposition, H2O2 accumulation, and pathogen resistance. Recently, a group of redundant calcium-dependent protein kinases (CDPKs) were identified as critical regulators of MAPK-independent defense pathways downstream of FLS2 (Boudsocq et al., 2010).
In yeast (Saccharomyces cerevisiae) and metazoans, heterotrimeric G proteins, composed of α-, β-, and γ-subunits, serve as essential signaling intermediates between cell surface G-protein-coupled receptors (GPCRs) and their downstream targets (Temple and Jones, 2007). Binding of ligands to GPCRs leads to the exchange of GDP for GTP in the α-subunit, resulting in the activation of the G protein. In Arabidopsis, there is one Gα subunit (G PROTEIN α-SUBUNIT1 [GPA1]), one Gβ subunit (ARABIDOPSIS G PROTEIN β-SUBUNIT1 [AGB1]), and three Gγs subunits (ARABIDOPSIS G PROTEIN γ-SUBUNIT1 [AGG1], AGG2, and AGG3; Temple and Jones, 2007; Chakravorty et al., 2011). Whereas AGG1 and AGG2 are closely related, AGG3 only shares very limited homology to AGG1 and AGG2. GPCR-like proteins have been identified in plants, and their interactions with Gα have been experimentally proven, although their status as bona fide GPCRs remains controversial (Liu et al., 2007; Gookin et al., 2008; Pandey et al., 2009).
Heterotrimeric G proteins have been shown to be involved in a wide range of biological processes, including plant immunity (Perfus-Barbeoch et al., 2004). Analysis of rice dwarf1 mutants with defects in the Gα subunit indicates that the rice Gα subunit plays an important role in resistance against rice blast (Suharsono et al., 2002). In Arabidopsis, loss of function of the Gβ or Gγ subunits leads to compromised resistance against the necrotrophic pathogen Plectosphaerella cucumerina (Llorente et al., 2005; Delgado-Cerezo et al., 2012). By contrast, loss of function of the Gα subunit GPA1 results in enhanced resistance against the pathogen (Llorente et al., 2005). Resistance against other necrotrophic pathogens, such as Fusarium oxysporum, Alternaria brassicicola, and Botrytis cinerea, was also found to be compromised in AGB1- and AGG1/AGG2-deficient mutants (Trusov et al., 2006, 2007, 2009). In addition, loss of function of the Gβ subunit leads to reduced elf18-induced resistance against Agrobacterium tumefaciens as well as reduced reactive oxygen species (ROS) production triggered by flagellin22 (flg22) and elf18 (an 18-amino acid peptide that represents the N terminus of bacterial EF-Tu; Ishikawa, 2009). Multiple surface residues of AGB1 were recently shown to play important roles in resistance against necrotrophic pathogens and flg22-induced ROS production (Jiang et al., 2012).
Arabidopsis GPA1 was also found to play an important role in stomatal defense. In gpa1 mutants, flg22-induced inhibition of stomatal opening and inward K+ channels in guard cells are blocked (Zhang et al., 2008b). Growth of the coronatine-deficient Pseudomonas syringae pv tomato DC3118 is dramatically increased in gpa1 mutant plants (Zeng and He, 2010). In Nicotiana benthamiana, silencing of the Gα and Gβ subunits also leads to reduced elicitor-induced stomatal closure as well as hypersensitive responses induced by harpin (Zhang et al., 2012a).
Arabidopsis BAK1-INTERACTING RECEPTOR-LIKE KINASE1 (BIR1) encodes a BAK1-associated RLK (Gao et al., 2009). Knocking out BIR1 results in constitutive activation of cell death and defense responses in a manner that is partially dependent on PHYTOALEXIN DEFICIENT4 (PAD4), a positive regulator of resistance mediated by the Toll-Interleukin-1 Receptor-like-Nucleotide-Binding-Leu-Rich Repeat domain class of resistance proteins. To identify signaling components downstream of BIR1, a suppressor screen was performed in the bir1-1 pad4-1 double-mutant background. A number of suppressor of bir1-1 (sobir) mutants suppressing the seedling lethality phenotype of bir1-1 were identified. SOBIR1 encodes another RLK whose overexpression is sufficient to activate cell death and defense responses (Gao et al., 2009). Combining the sobir1-1 and pad4-1 mutations results in complete suppression of cell death and enhanced pathogen resistance in bir1-1, suggesting that SOBIR1 and PAD4 function in parallel to regulate cell death and defense responses. Here, we report the discovery and characterization of SOBIR2, which encodes the Arabidopsis heterotrimeric G-protein β-subunit AGB1 that functions downstream of SOBIR1 to regulate cell death and defense responses.
RESULTS
Identification and Characterization of sobir2-1 bir1-1 pad4-1
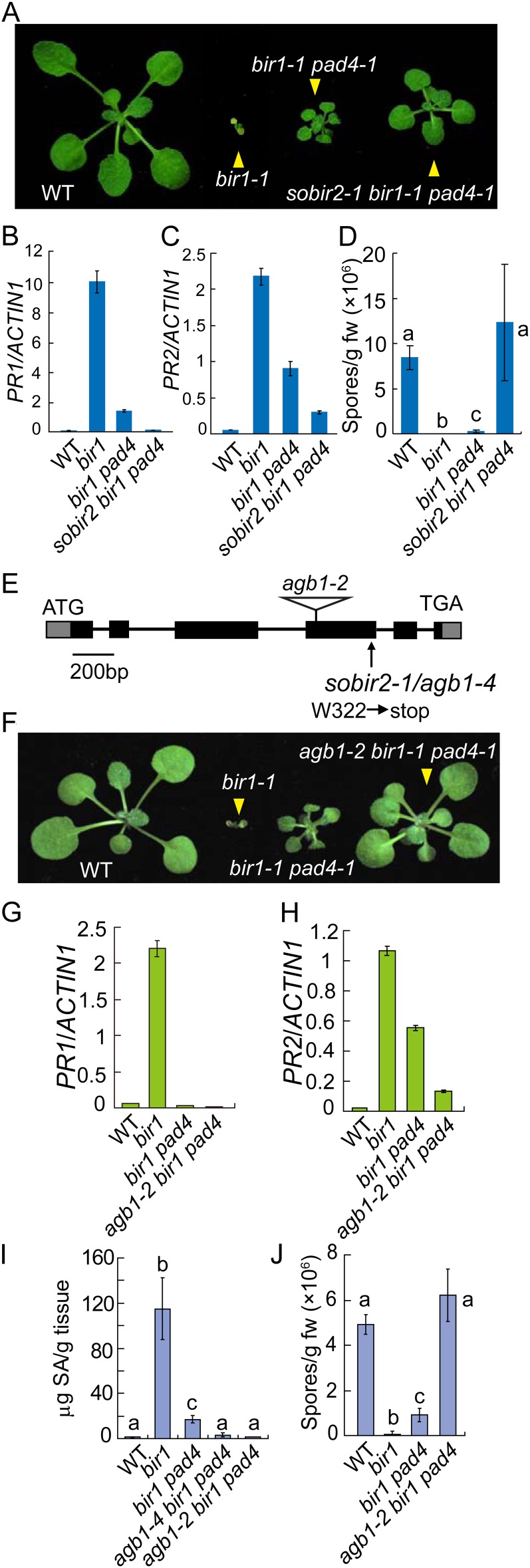
The sobir2-1 mutant was identified from a suppressor screen in the bir1-1 pad4-1 background as previously described (Gao et al., 2009). The sobir2-1 bir1-1 pad4-1 triple mutant is significantly larger than bir1-1 pad4-1 (Fig. 1A) and can easily grow to maturity and set seeds at 23°C. The expression levels of defense marker genes PATHOGENESIS-RELATED1 (PR1; Fig. 1B) and PR2 (Fig. 1C) in sobir2-1 bir1-1 pad4-1 are considerably lower than those in bir1-1 pad4-1. In addition, enhanced resistance to Hyaloperonospora arabidopsidis Noco2 in bir1-1 pad4-1 is completely abolished in the sobir2-1 bir1-1 pad4-1 triple mutant (Fig. 1D). Taken together, our data show that sobir2-1 suppresses the constitutive defense responses observed in bir1-1 pad4-1.
Figure 1.
Characterization and cloning of sobir2-1. A, Morphology of wild-type, bir1-1, bir1-1 pad4-1, and sobir2-1 bir1-1 pad4-1 plants. Plants were grown on soil at 23°C and photographed about 3 weeks after planting. B and C, PR1 (B) and PR2 (C) expression in wild-type, bir1-1, bir1-1 pad4-1, and sobir2-1 bir1-1 pad4-1 seedlings. Values were normalized to the expression of ACTIN1. Error bars represent sds from means of three measurements. D, Growth of H. arabidopsidis Noco2 on the wild type, bir1-1, bir1-1 pad4-1, and sobir2-1 bir1-1 pad4-1. E, Predicted gene structure of SOBIR2/AGB1. Boxes are exons, and lines indicate introns. The ATG start and TGA stop codons are indicated. F, Morphology of the wild type, bir1-1, bir1-1 pad4-1, and agb1-2 bir1-1 pad4-1. G and H, PR1 (G) and PR2 (H) expression in the wild type, bir1-1, bir1-1 pad4-1, and agb1-2 bir1-1 pad4-1. Values were normalized to the expression of ACTIN1. Error bars represent sds from means of three measurements. I, Total SA levels in the wild type, bir1-1, bir1-1 pad4-1, agb1-4 bir1-1 pad4-1, and agb1-2 bir1-1 pad4-1. Statistical differences among different genotypes are labeled with different letters (P < 0.01). J, Growth of H. arabidopsidis Noco2 on the wild type, bir1-1, bir1-1 pad4-1, and agb1-2 bir1-1 pad4-1. Statistical differences among different genotypes are labeled with different letters (P < 0.001). WT, Wild type. [See online article for color version of this figure.]
SOBIR2 Encodes the Heterotrimeric G-Protein β-Subunit
To map the sobir2-1 mutation, sobir2-1 bir1-1 pad4-1 (in the Columbia ecotype background) was crossed with the Landsberg erecta ecotype to generate a segregating mapping population. Crude mapping using the F2 progeny showed that the sobir2-1 mutation is located between marker T16L1 and F8D20 on chromosome 4. Further fine mapping narrowed the sobir2-1 mutation to a 40-kb region between markers F10M10 and T4L20 (Supplemental Fig. S1). Sequence analysis of genes in this region in the sobir2-1 mutant identified a single G-to-A mutation in At4g34460, which encodes the heterotrimeric G-protein β-subunit. The mutation created an early stop codon in the gene (Fig. 1E); thus, sobir2-1 was renamed agb1-4. Semiquantitative reverse transcription (RT)-PCR analysis showed that AGB1 is expressed at a slightly lower level in agb1-4 compared with the wild type (Supplemental Fig. S2), suggesting that a truncated AGB1 protein may still be expressed in the mutant. Whether the truncated protein retains part of the function of AGB1 is unclear.
To confirm that the mutation in At4g34460 causes suppression of the bir1-1 phenotypes, we crossed agb1-2 into bir1-1 pad4-1. agb1-2 contains a transfer DNA insertion that disrupts the expression of AGB1 (Supplemental Fig. S2). The agb1-2 bir1-1 pad4-1 triple mutant restored the size and appearance of bir1-1 pad4-1 to almost wild-type levels (Fig. 1F), suggesting that SOBIR2 encodes AGB1. Further analysis showed that expression of both PR1 and PR2 was dramatically reduced in the triple mutant (Fig. 1, G and H). In both agb1-4 bir1-1 pad4-1 and agb1-2 bir1-1 pad4-1, the levels of salicylic acid (SA) were much lower than those in bir1-1 and bir1-1 pad4-1 (Fig. 1I). In addition, enhanced resistance to H. arabidopsidis Noco2 in bir1-1 pad4-1 was completely lost in agb1-2 bir1-1 pad4-1 (Fig. 1J).
Mutations in AGB1 Suppress Cell Death and Defense Responses in bir1-1
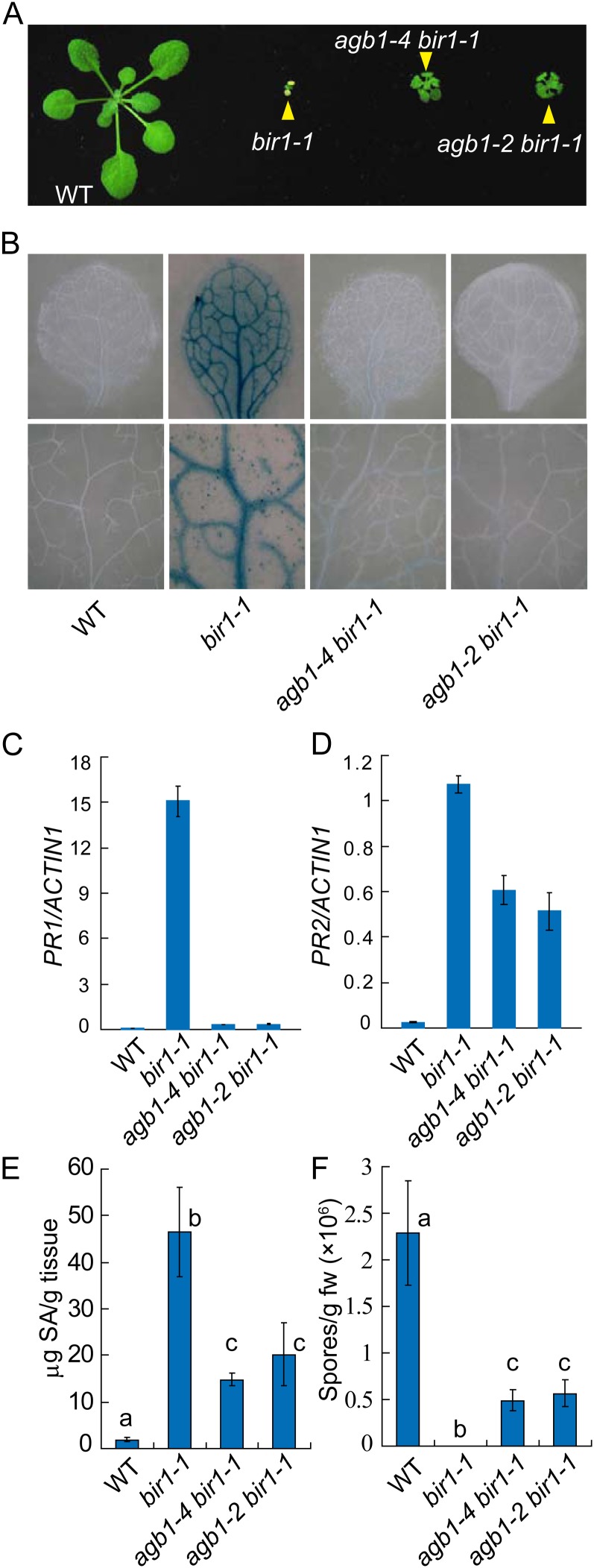
To determine whether mutations in AGB1 can suppress the cell death and defense responses in bir1-1, we obtained the agb1-4 bir1-1 double mutant by crossing agb1-4 bir1-1 pad4-1 with wild-type Columbia and agb1-2 bir1-1 by crossing agb1-2 and bir1-1. Unlike bir1-1 mutant plants, agb1-4 bir1-1 and agb1-2 bir1-1 can grow to maturity and set seeds at 23°C. agb1-4 bir1-1 and agb1-2 bir1-1 are much larger than bir1-1, though smaller than the wild type (Fig. 2A). To determine whether cell death was blocked in the double mutants, trypan blue staining was performed on seedlings. As shown in Figure 2B, cell death in bir1-1 was inhibited by the agb1-4 and agb1-2 mutations. Accumulation of H2O2 in bir1-1 was also partially blocked in agb1-4 bir1-1 and agb1-2 bir1-1 (Supplemental Fig. S3). In addition, constitutive expression of PR1 in bir1-1 was dramatically reduced in the double mutants (Fig. 2C), whereas the expression of PR2 was only modestly reduced (Fig. 2D), suggesting that cell death is a major contributor to the activation of PR1 expression and the expression of PR2 is largely independent of cell death in bir1-1. Both agb1-4 bir1-1 and agb1-2 bir1-1 accumulated less SA than bir1-1, but SA levels in the double mutants were still much higher than that in the wild type (Fig. 2E). Consistent with the reduced SA levels, agb1-4 bir1-1 and agb1-2 bir1-1 supported much higher growth of H. arabidopsidis Noco2 than bir1-1, but supported less growth of the pathogen than the wild type (Fig. 2F).
Figure 2.
Suppression of cell death and defense responses in bir1-1 by agb1-4 and agb1-2. A, Morphology of wild-type, bir1-1, agb1-4 bir1-1, and agb1-2 bir1-1 plants. Plants were grown on soil at 23°C and photographed about 3 weeks after planting. B, Trypan blue staining of the wild-type, bir1-1, agb1-4 bir1-1, and agb1-2 bir1-1 mutant seedlings. Plants were grown at 23°C for 2 weeks on one-half-strength MS plates. C and D, PR1 (C) and PR2 (D) expression in the wild type, bir1-1, agb1-4 bir1-1, and agb1-2 bir1-1. Values were normalized to the expression of ACTIN1. Error bars represent sds from means of three measurements. E, Total SA levels in the wild type, bir1-1, agb1-4 bir1-1, and agb1-2 bir1-1. Statistical differences among different genotypes are labeled with different letters (P < 0.01). F, Growth of H. arabidopsidis Noco2 on the wild type, bir1-1, agb1-4 bir1-1, and agb1-2 bir1-1. Statistical differences among different genotypes are labeled with different letters (P < 0.001). WT, Wild type. [See online article for color version of this figure.]
AGB1 Is Required for Cell Death in Transgenic Plants Overexpressing SOBIR1
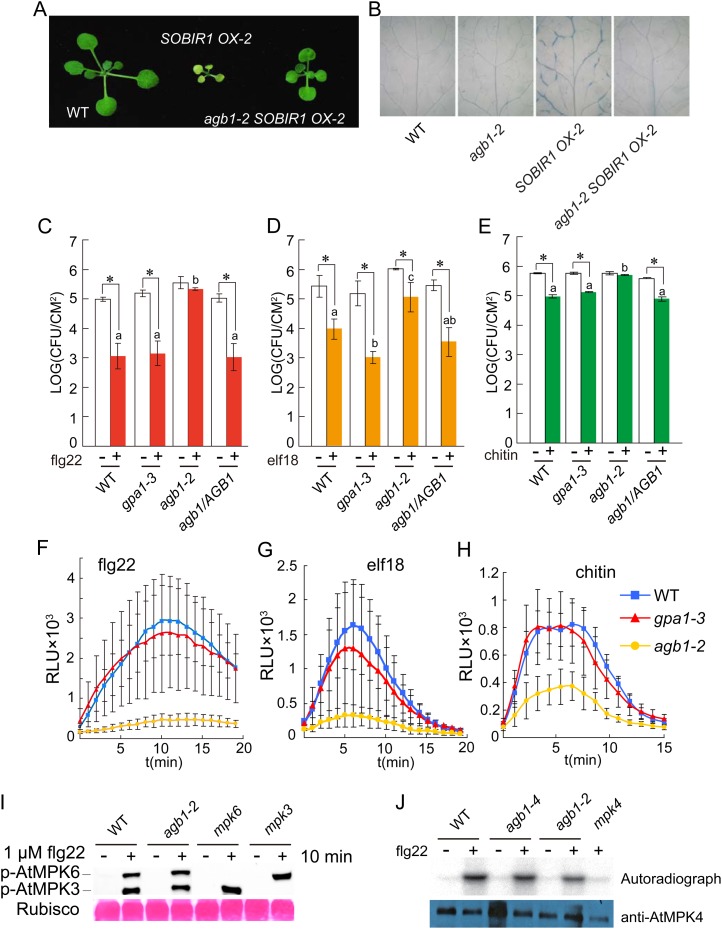
It was previously shown that bir1-1 activates SOBIR1-dependent cell death (Gao et al., 2009). To test whether AGB1 functions downstream of SOBIR1, we introduced the agb1-2 mutation into SOBIR1 overexpression (OX-2), a transgenic line overexpressing SOBIR1 that exhibits spontaneous cell death (Gao et al., 2009). Semiquantitative RT-PCR analysis indicates that SOBIR1 is expressed at similar levels in the wild type and agb1-2 carrying the 35S-SOBIR1 transgene (Supplemental Fig. S4). As shown in Figure 3A, agb1-2 partially suppresses the dwarf phenotype of the SOBIR1 overexpression line. Trypan blue staining showed that cell death in the transgenic line was suppressed by agb1-2 (Fig. 3B), suggesting that AGB1 functions downstream of the RLK SOBIR1 to regulate cell death.
Figure 3.
agb1-2 suppresses cell death in transgenic plants overexpressing SOBIR1 and compromised PAMP-triggered immunity. A, Morphology of the wild type, 35S-SOBIR1 transgenic line number 2 (SOBIR1 OX-2; Gao et al., 2009), and agb1-2 carrying the 35S-SOBIR1 transgene from line number 2 (agb1-2 SOBIR1 OX-2). Plants were grown on soil at 23°C and photographed approximately 3 weeks after planting. B, Trypan blue staining of the indicated genotypes. Plants were grown at 23°C for 2 weeks on one-half-strength MS plates. C to E, flg22-induced (C), elf18-induced (D), or chitin-induced (E) resistance to P. syringae pv tomato DC3000 in the wild type, gpa1-3 (SALK_066823), agb1-2, and a transgenic line expressing AGB1 under its own promoter in agb1-2 background (agb1/AGB1). Plants were pretreated with the indicated PAMPs (+) or water (−) 1 d before infiltration with P. syringae pv tomato DC3000 (OD600 = 0.001). Bacterial titers on day 3 are shown. Error bars represent sds from means of six measurements. Asterisks above the bars indicate significant difference between samples treated with or without the indicated elicitors (*P < 0.01). Statistical differences among the elicitor-treated samples are labeled with different letters (P < 0.01). F to H, Oxidative burst triggered by flg22 (F), elf18 (G), or chitin (H) in the indicated genotypes. Leaf slices of 4-week-old plants were treated with 1 μm flg22, 1 μm elf18, or 200 μg mL–1 chitin, and ROS was subsequently measured. Error bars represent sds from means of eight samples. I, Activation of MPK3 and MPK6 in the wild type and agb1-2 by flg22. Two-week-old seedlings grown on one-half-strength MS medium were treated with or without 1 μm flg22. Phosphorylated MPK3 and MPK6 were detected by western blot using an anti-Erk1/2 antibody specific for the phosphorylated MAPKs. mpk3 and mpk6 knockout mutants were included as controls. J, Activation of MPK4 in the wild type and agb1 mutants by flg22. Two-week-old seedlings grown on one-half-strength MS medium were treated with or without 1 μm flg22. MPK4 was immunoprecipitated from total protein extracts using anti-MPK4 antibodies (Sigma) and assayed for its kinase activity (autoradiograph) using MBP as the substrate. WT, Wild type. [See online article for color version of this figure.]
AGB1 Is Required for PAMP-Mediated Immunity
To determine whether AGB1 is also required for resistance responses mediated by three other RLKs, FLS2, EFR, and CERK1, we first analyzed bacterial growth in wild-type and agb1-2 plants pretreated with flg22, a peptide derived from bacterial flagellin that is recognized by FLS2 (Gómez-Gómez and Boller, 2000). As shown in Figure 3C, flg22-induced resistance to P. syringae pv tomato DC3000 was blocked in agb1-2 compared with wild-type plants. Analysis of bacterial growth in the wild type and agb1-2 treated with elf18, a peptide derived from bacterial EF-Tu, showed that elf18-induced resistance to P. syringae pv tomato DC3000 was also reduced in agb1-2 (Fig. 3D). Furthermore, chitin-induced resistance to P. syringae pv tomato DC3000 was lost in agb1-2 as well (Fig. 3E). By contrast, flg22-, elf18-, and chitin-induced resistance to P. syringae pv tomato DC3000 was not impaired in the Gα mutant gpa1-3 (Fig. 3, C–E). The reduction in PAMP-induced resistance observed in agb1-2 can be complemented by expressing the wild-type AGB1 with a C-terminal FLAG tag. These results suggest that AGB1 is required for PAMP-triggered immunity mediated by the RLKs FLS2, EFR, and CERK1.
Next, we tested whether induction of ROS production by flg22 and elf18 was affected in agb1-2. As shown in Figure 3, F and G, flg22- and elf18-induced ROS production was clearly reduced in the mutant, consistent with the previous report that ROS production induced by flg22 and elf18 was reduced in agb1-2 (Ishikawa, 2009). We further tested whether induction of ROS production by chitin is affected in agb1-2. As shown in Figure 3H, chitin-induced ROS production was also reduced in agb1-2. These data suggest that AGB1 is required for PAMP-triggered induction of ROS. The gpa1-3 mutant lacking the Gα subunit has little or no effect on ROS induction by flg22, elf18, or chitin.
To test whether AGB1 functions as a general regulator of the stability of PAMP receptors, we analyzed the accumulation of FLS2 in agb1-2 and agb1-4 by western-blot analysis. As shown in Supplemental Figure S5, the levels of FLS2 protein in agb1-2 and agb1-4 were comparable in the wild type and the agb1 mutants, suggesting that AGB1 is not required for the accumulation of FLS2.
To test whether activation of the MAPKs MPK3 and MPK6 by flg22 is affected by agb1-2, we analyzed phosphorylated MPK3 and MPK6 in plants treated with flg22 by western blot using an anti-Erk1/2 (for Extracellular signal-regulated kinase) antibody specific for phosphorylated MAPKs. As shown in Figure 3I and Supplemental Figure S6, flg22-induced activation of MPK3 and MPK6 was not affected in the mutant, which is consistent with the previous report that activation of MPK3 and MPK6 by flg22 was not affected by agb1-2 (Ishikawa, 2009). We further tested whether agb1-2 affects flg22-induced expression of FLG22-INDUCED RECEPTOR-LIKE KINASE1 (FRK1) and WRKY DNA-BINDING PROTEIN29 (WRKY29), two marker genes for activation of the MPK3/MPK6-signaling pathway. As shown in Supplemental Figure S7, induction of these two genes was not affected in agb1-2 either, suggesting that AGB1 functions in a defense pathway independent of MPK3 and MPK6.
Next, we tested whether activation of MPK4 by flg22 is affected by mutations in AGB1. MPK4 was immunoprecipitated from the wild type and agb1 mutants using anti-MPK4 antibodies and assayed for its kinase activity using myelin basic protein (MBP) as a substrate. As shown in Figure 3J, activation of MPK4 was reduced in both agb1-2 and agb1-4, suggesting that AGB1 is required for full activation of MPK4.
Both MAPK-dependent and independent signaling pathways are activated during PAMP signaling. Induction of GLUTATHIONE S-TRANSFERASE1 (GST1) expression by flg22 was previously shown to be independent of MAPK signaling (Asai et al., 2002). To determine whether AGB1 is required for induction of GST1 by flg22, we compared the expression levels of GST1 in flg22-treated wild-type and agb1-2 plants. As shown in Supplemental Figure S8, induction of GST1 by flg22 was not affected in the agb1-2 mutant.
The agg1 agg2 Double Mutant Suppresses Cell Death in bir1-1
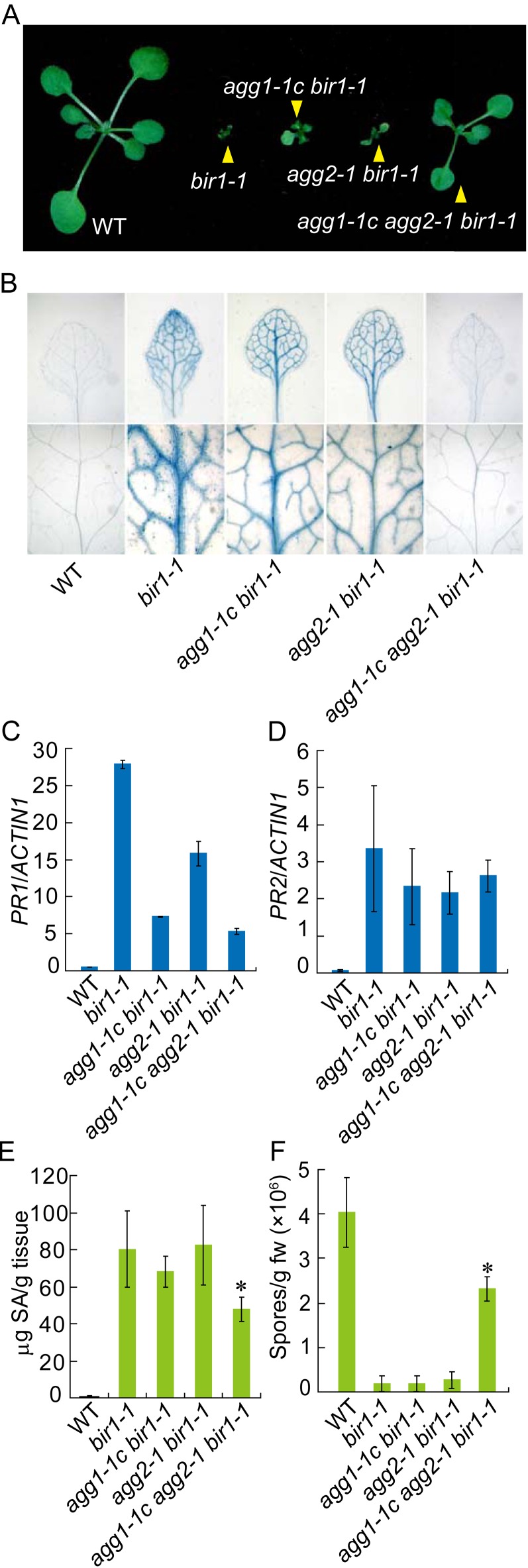
Next, we tested whether the G-protein subunits Gα and Gγ are also required for cell death and defense responses in bir1-1. The gpa1-3 bir1-1, gpa1-4 bir1-1, agg1-1c bir1-1, and agg2-1 bir1-1 double mutants and the agg1-1c agg2-1 bir1-1 triple mutant were obtained by crossing bir1-1 with gpa1-3, gpa1-4, and agg1-1c agg2-1, respectively. As shown in Supplemental Figure S9, gpa1-3 bir1-1 and gpa1-4 bir1-1 exhibited morphology similar to bir1-1 and were seedling lethal, suggesting that GPA1 is dispensable for the seedling lethality phenotype of bir1-1. Both agg1-1c bir1-1 and agg2-1 bir1-1 show slightly increased size compared with bir1-1 (Fig. 4A). By contrast, the agg1-1c agg2-1 bir1-1 triple mutant is much bigger than bir1-1 and is able to complete its life cycle at 23°C, suggesting that AGG1 and AGG2 function redundantly to regulate cell death in bir1-1. Suppression of cell death in bir1-1 by agg1-1c agg2-1 was further confirmed by trypan blue staining (Fig. 4B). Real-time RT-PCR analysis showed that the constitutive expression of PR1, but not PR2, was considerably reduced in agg1-1c bir1-1 and agg1-1c agg2-1 bir1-1 compared with bir1-1 (Fig. 4, C and D). The agg1-1c agg2-1 bir1-1 triple mutant also accumulated less SA (Fig. 4E) and supported much higher growth of H. arabidopsidis Noco2 (Fig. 4F) than bir1-1. Taken together, AGG1 and AGG2 are required for the cell death and part of the constitutive defense response phenotypes in bir1-1.
Figure 4.
Suppression of cell death and defense responses in bir1-1 by agg1-1c agg2-1. A, Morphology of wild-type, bir1-1, agg1-1c bir1-1, agg2-1 bir1-1, and agg1-1c agg2-1 bir1-1 plants. Plants were grown on soil at 23°C and photographed when they were about 3 weeks old. B, Trypan blue staining of the indicated genotypes. Plants were grown at 23°C for 2 weeks on one-half-strength MS plates. C to D, PR1 (C) and PR2 (D) expression in the indicated genotypes. Values were normalized to the expression of ACTIN1. Error bars represent sds from means of three measurements. E, SA levels in the indicated genotypes. Asterisks above the bars indicate significant difference from bir1-1 (*P < 0.01). F, Growth of H. arabidopsidis Noco2 on the indicated genotypes. Asterisks above the bars indicate significant difference from bir1-1 (*P < 0.001). WT, Wild type. [See online article for color version of this figure.]
AGG1 and AGG2 Are Involved in PAMP-Mediated Defense Responses
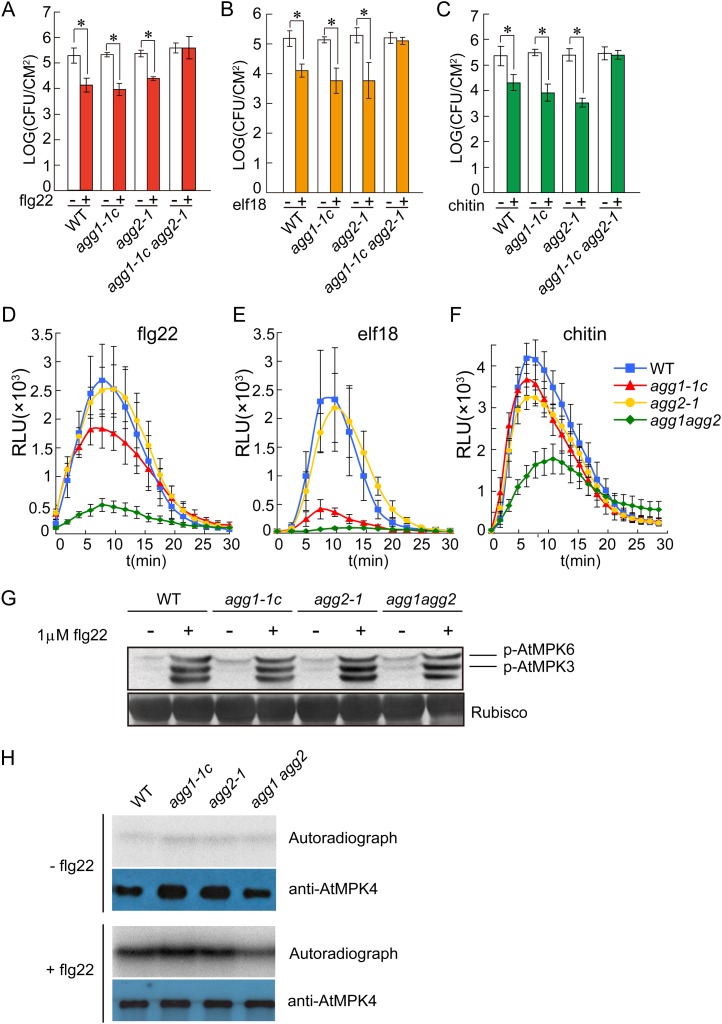
To determine whether AGG1 and/or AGG2 are also required for resistance responses mediated by the RLKs FLS2, EFR, and CERK1, we analyzed flg22-, elf18-, and chitin-induced resistance to P. syringae pv tomato DC3000 in the wild type, agg1, agg2, and the agg1 agg2 double mutant. Treatment with flg22 (Fig. 5A), elf18 (Fig. 5B), or chitin (Fig. 5C) resulted in reduction of bacterial growth in the wild type, agg1-1c, and agg2-1, but not in agg1-1c agg2-1, indicating that flg22-, elf18-, and chitin-induced resistance is blocked in the double mutant. Thus, AGG1 and AGG2 play redundant roles in the PAMP-mediated resistance induced by flg22, elf18, and chitin.
Figure 5.
PAMP-trigged responses in the wild type, agg1-c, agg2-1, and agg1-1c agg2-1. A to C, flg22-induced (A), elf18-induced (B), or chitin-induced (C) resistance to P. syringae pv tomato DC3000 in the wild type, agg1-c, agg2-1, and agg1-1c agg2-1. Plants were pretreated with the indicated PAMP (+) or water (−) 1 d before infiltration with P. syringae pv tomato DC3000 (OD600 = 0.001). Bacterial titers on day 3 are shown. Error bars represent sds from means of six measurements. Asterisks above the bars indicate significant difference between samples treated with or without the indicated elicitors (*P < 0.01). D to F, Oxidative burst triggered by flg22 (A), elf18 (B), or chitin (C) in wild-type, agg1-c, agg2-1, and agg1-1c agg2-1 plants. Leaf slices of 4-week-old plants were treated with 1 μm flg22, 1 μm elf18, or 200 μg mL–1 chitin. ROS was measured using a luminol-dependent assay (Trujillo et al., 2008). Error bars represent sds from means of eight samples. G, Activation of MPK3 and MPK6 in the wild type, agg1-1c, agg2-1, and agg1-1c agg2-1 by flg22. Two-week-old seedlings grown on one-half-strength MS medium were treated with or without 1 μm flg22. Phosphorylated MPK3 and MPK6 were detected by western blot using an anti-Erk1/2 antibody specific for the phosphorylated MAPKs. H, Activation of MPK4 in the wild type, agg1-1c, agg2-1, and agg1-1c agg2-1 by flg22. Two-week-old seedlings were treated with or without 1 μm flg22. MPK4 was immunoprecipitated from total protein extracts using anti-MPK4 antibodies (Sigma) and assayed for its kinase activity (autoradiograph) using MBP as the substrate. WT, Wild type. [See online article for color version of this figure.]
Next, we tested whether mutations in AGG1 and AGG2 affect PAMP-induced oxidative burst. In wild-type plants, treatment with flg22, elf18, or chitin leads to a rapid oxidative burst. As shown in Figure 5, D and F, the oxidative bursts triggered by flg22 and chitin were not severely affected in either of the single Gγ mutants agg1-1c and agg2-1, but was dramatically reduced in the double agg1-1c agg2-1 mutant. The elf18-induced oxidative burst was markedly reduced in agg1-1c and almost completely blocked in agg1-1c agg2-1, while agg2-1 did not show any difference with the wild type (Fig. 5E). These data further support that AGG1 and AGG2 are critical signaling components in the response to flg22, elf18, and chitin.
We also analyzed flg22-induced activation of MPK3 and MPK6 in agg1-1c, agg2-1, and the agg1-1c agg2-1 double mutant by western blot. As shown in Figure 5G and Supplemental Figure S10, activation of MPK3 and MPK6 was not affected in these mutants. We further tested whether activation of MPK4 by flg22 is affected in agg1-1c, agg2-1, and agg1-1c agg2-1 mutant plants by assaying the kinase activity of MPK4 from the wild type and the mutant plants. As shown in Figure 5H, activation of MPK4 was reduced in the agg1-1c agg2-1 double mutant, suggesting that AGG1 and AGG2 are also required for full activation of MPK4.
Next, we tested whether flg22 induction of GST1, a marker gene activated independent of MAPK signaling, was affected in agg1-1c, agg2-1, and agg1-1c agg2-1 mutant plants. As shown in Supplemental Figure S11, induction of GST1 was comparable in the wild type and the mutant plants, suggesting that AGG1 and AGG2 are not required for the induction of GST1 by flg22.
The Gβ- and Gγ-Protein Subunits Are Required for Resistance against Nonpathogenic Bacteria
To further test whether AGB1 and GPA1 are required for PAMP-mediated resistance against nonpathogenic bacteria, we challenged agb1-2 and gpa1-4 with P. syringae pv tomato DC3000 hrcC (a nonpathogenic mutant defective in type III secretion). As shown in Figure 6A, growth of P. syringae pv tomato DC3000 hrcC is comparable in gpa1-4 and wild-type plants, but is significantly higher in agb1-2. We also challenged agg1, agg2, and agg1 agg2 double-mutant plants with P. syringae pv tomato DC3000 hrcC. As shown in Figure 6B, growth of P. syringae pv tomato DC3000 hrcC is comparable in wild-type, agg1-1c, and agg2-1 plants, but is about 3-fold higher in the agg1-1c agg2-1 double mutant. These data suggest that Gβ and Gγ, but not Gα, are required for PAMP-mediated resistance against nonpathogenic bacteria.
Figure 6.
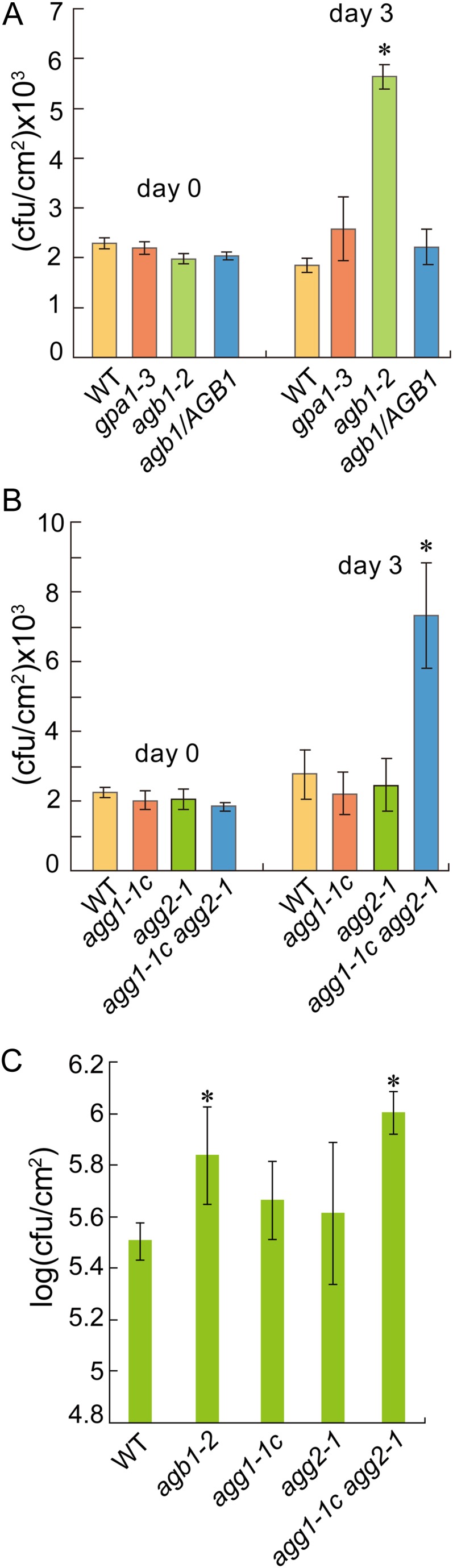
Growth of P. syringae pv tomato DC3000 hrcC and P. syringae pv tomato DC3118 in heterotrimeric G-protein mutants. A, Growth of P. syringae pv tomato DC3000 hrcC in the wild type, gpa1-3, agb1-2, and agb1-2 expressing a wild-type AGB1 transgene (agb1/AGB1). Asterisks above the bars indicate significant difference from the wild type (*P < 0.01). B, Growth of P. syringae pv tomato DC3000 hrcC in the wild type, agg1-1c, agg2-1, and agg1-1c agg2-1. Leaves of 6-week-old plants grown under short-day conditions (10-h day/14-h night cycles) were infiltrated with P. syringae pv tomato DC3000 hrcC (OD600 = 0.002). Bacterial titers at days 0 and 3 were measured by taking leaf discs within the inoculated area. Error bars represent sds from means of six samples. Asterisks above the bars indicate significant difference from the wild type (*P < 0.01). C, Growth of P. syringae pv tomato DC3118 in the wild type, agb1-2, agg1-1c, agg2-1, and agg1-1c agg2-1. Five-week-old plants were inoculated by spraying with a bacterial suspension at OD600 = 0.2. Samples were collected 3 d post inoculation to determine the bacterial titers. Asterisks above the bars indicate significant difference from the wild type (*P < 0.01). WT, Wild type. [See online article for color version of this figure.]
FLS2 plays a very important role in stomatal defense against P. syringae pv tomato DC3000 (Melotto et al., 2006; Zeng and He, 2010). To test whether the Gβ and Gγ subunits are required for stomatal defense, we sprayed agb1-2, agg1-1c, agg2-1, agg1-1c agg2-1, and wild-type plants with P. syringae pv tomato DC3118, a P. syringae pv tomato DC3000 mutant deficient in the phytotoxin coronatine. Compared with wild-type plants, a small but significant increase of P. syringae pv tomato DC3118 growth was observed in agb1-2 and agg1-1c agg2-1 (Fig. 6C).
PAMP-Triggered Defense Responses Are Not Affected in agg3 Single Mutants
To test whether the recently identified Gγ subunit AGG3 is required for PAMP-triggered defense responses, we analyzed induction of oxidative burst by flg22, elf18, and chitin in agg3-1 and agg3-2. As shown in Supplemental Figure S12, A to C, oxidative bursts triggered by flg22, elf18, and chitin were not affected in the agg3 mutants. Next, we tested flg22-induced resistance to P. syringae pv tomato DC3000 in agg3-1 and agg3-2. As shown in Supplemental Figure S12D, resistance to P. syringae pv tomato DC3000 induced by flg22 was not affected by the agg3 mutations. In addition, growth of P. syringae pv tomato DC3000 hrcC was comparable in wild-type and agg3 plants (Supplemental Fig. S12E). We also checked activation of MPK3 and MPK6 by flg22 in the agg3 mutants. As shown in Supplemental Figure S12F, activation of MPK3 and MPK6 by flg22 was not affected in the agg3 mutants either. These data suggest that PAMP-triggered defense responses are not affected in the agg3 single-mutant plants.
DISCUSSION
Arabidopsis BIR1 negatively regulates two parallel defense pathways, one dependent on PAD4 and the other dependent on the RLK SOBIR1 (Gao et al., 2009). From a suppressor screen of bir1-1, we found that mutations in AGB1 suppress cell death and defense responses in bir1-1. Combining mutations in AGB1 and PAD4 leads to more complete suppression of the mutant phenotypes and enhanced pathogen resistance in bir1-1, indicating that AGB1 functions in parallel with PAD4. AGB1 is also required for cell death in transgenic plants overexpressing SOBIR1, suggesting that the heterotrimeric G-protein β-subunit AGB1 functions downstream of the RLK SOBIR1 to regulate activation of cell death in bir1-1.
In agb1 null mutant plants, induction of PAMP-triggered defense responses mediated by three other RLKs, FLS2, EFR, and CERK1, is severely compromised, suggesting that AGB1 is a common signaling component for plant immunity mediated by different RLKs. In addition, we showed that cell death in bir1-1 and PAMP-triggered defense responses are severely attenuated in the agg1 agg2 double mutant, suggesting that the Gγ subunits AGG1 and AGG2 also play important roles in the regulation of cell death and PAMP-triggered immunity.
According to the classic paradigm, active G proteins dissociate into two functional signaling elements, the Gα subunit and the Gβγ dimer. Although it was initially believed that signaling only occurred via the Gα subunit, it is now clear that the Gβγ dimer is actively signaling in as many processes as the Gα subunit (Clapham and Neer, 1997). Plant Gβ and Gγ interact with each other in vitro and in vivo (Mason and Botella, 2000, 2001; Kato et al., 2004). Our results suggest that the Gβγ dimer acts alone in regulating PAMP-triggered defense responses and cell death in bir1-1 and that the Gα subunit does not play an active role in these processes. This kind of signaling mechanism by G proteins, in which only the Gβγ dimer is involved in the propagation of the signal, was named “classical route II” (Pandey et al., 2010). It can be used to explain the expression patterns of some abscisic acid-regulated genes in guard cells as well as roles of the Gβγ dimers in resistance against necrotrophic pathogens (Pandey et al., 2010; Delgado-Cerezo et al., 2012).
It will be important to determine the roles of the different γ-subunits in PAMP-mediated resistance and whether they confer specificity against different PAMP signals. Although our study has mostly shown redundant roles for AGG1 and AGG2, for some responses, such as elf18-induced oxidative burst, AGG1 seems to play the leading role. A recent report has described the third Gγ subunit in Arabidopsis, AGG3, with astonishing structural features never before seen in other plant or animal γ-subunits (Chakravorty et al., 2011). AGG3 is involved in stomatal ion channel regulation and development of reproductive organs and has no effect on resistance to F. oxysporum (Chakravorty et al., 2011). Our results suggest that AGG3 is probably not involved in PAMP-mediated defense either.
Plant heterotrimeric G proteins have been implicated in the regulation of a wide range of biological processes, yet the receptors that function upstream of G proteins in these processes are still poorly understood. Our study suggests that SOBIR1 and possibly other RLKs, such as FLS2, EFR, and CERK1, function upstream of AGB1 and AGG1/AGG2 to regulate cell death and plant immunity. It is very interesting that AGB1 and AGG1/AGG2 function not only as positive regulators of cell death in bir1-1, but also as essential components of PAMP-triggered immunity. One possibility is that there is a common signaling pathway downstream of SOBIR1 and the PAMP receptors FLS2, EFR, and CERK1 that is dependent on AGB1 and AGG1/AGG2. Constitutive activation of this signaling pathway in bir1-1 results in strong defense responses and activation of cell death, whereas treatment with PAMP signals induces weaker defense responses that do not cause cell death.
Another RLK that may function upstream of AGB1 is ER. agb1-1 was originally identified in a screen to look for mutants with an erecta (er) phenotype to find proteins that function together with the RLKs ER in the control of plant development (Lease et al., 2001). agb1 and er mutants share similar fruit phenotypes, and they all have round leaves and shorter stems. Similar to AGB1, ER is also required for resistance against P. cucumerina (Llorente et al., 2005). Recently, both er and agb1 mutants were found to have altered cell wall structure (Sánchez-Rodríguez et al., 2009; Klopffleisch et al., 2011; Delgado-Cerezo et al., 2012). The similarity of the mutant phenotypes in er and agb1 mutants suggests that AGB1 may be required for the functions of ER in the regulation of development, cell wall structure, and resistance against P. cucumerina.
agb1 mutant plants exhibit a number of developmental phenotypes, including rounder leaves. One question is whether the altered leaf morphology affects plant immune responses. The rounder leaf morphology is also present in agg3, but not in agg1, agg2, and agg1 agg2 mutants (Trusov et al., 2006; Chakravorty et al., 2011). Because attenuation of PAMP-triggered defense responses was observed in agb1 and agg1 agg2, but not in agg3 mutants, the rounder leaf morphology probably does not contribute to the loss of PAMP-triggered immunity in agb1 mutants.
The Gα subunit GPA1 was previously shown to play an important role in stomatal defense again bacterial pathogens. Growth of the coronatine-deficient P. syringae pv tomato DC3118 is dramatically increased in gpa1 mutant plants (Zeng and He, 2010). When agb1 and agg1 agg2 mutants were inoculated with P. syringae pv tomato DC3118 by spraying, they supported a small but significant increase of P. syringae pv tomato DC3118 growth compared with the wild type, suggesting that stomatal defense could be affected in these mutants as well. In agb1 mutants, stomatal density was shown to be higher than in the wild type (Zhang et al., 2008a; Klopffleisch et al., 2011). The increased stomatal density is probably also a contributing factor to higher P. syringae pv tomato DC3118 growth in the agb1 mutants.
AGB1 and AGG1 are colocalized to the plasma membrane (Adjobo-Hermans et al., 2006), suggesting that they may function together with RLKs such as SOBIR1, FLS2, EFR, and CERK1 at the plasma membrane. Preliminary experiments by our research team failed to detect any interaction between the Gα/Gβ/Gγ subunits and the kinase domains of SOBIR1, FLS2, EFR, CERK1, and BAK1 in yeast two-hybrid assays and bifluorescence complementation analysis, suggesting that additional components could be involved in transducing defense signals from the RLKs to Gβ/Gγ at the plasma membrane.
Recently, the MAPK/ERK KINASE KINASE1 (MEKK1)-MITOGEN ACTIVATED PROTEIN KINASE KINASE1 (MKK1)/MKK2-MPK4 kinase cascade was shown to positively regulate basal resistance against pathogens (Zhang et al., 2012b). We found that activation of MPK4, but not MPK3 and MPK6, was reduced in agb1 and agg1 agg2 mutants, suggesting that compromised activation of MPK4 is at least partly responsible for the loss of PAMP-triggered immunity in these mutants. Because blocking activation of MPK4 by mutations in its upstream kinases MEKK1 and MKK1/MKK2 does not affect flg22-induced oxidative burst (Zhang et al., 2012b), AGB1 and AGG1/AGG2 are most likely also involved in activation of defense pathways that are independent of MPK4. Multiple CDPKs functioning in a MAPK-independent pathway have been shown to be required for flg22-induced resistance against P. syringae pv tomato DC3000 (Boudsocq et al., 2010). It will be interesting to determine whether AGB1 and AGG1/AGG2 is required for activation of defense responses mediated by these CDPKs.
Our study suggests that heterotrimeric G-protein subunits AGB1 and AGG1/AGG2 function downstream of multiple RLKs, including SOBIR1, FLS2, EFR, and CERK1, to activate resistance responses against pathogens. We hypothesize that RLKs functioning upstream of the plant heterotrimeric G proteins may fulfill the roles of GPCRs in fungi and animals. How the Gβγ dimer is activated by the RLKs remains to be determined. It is also unclear how AGB1 and AGG1/AGG2 regulate downstream defense responses. Identification of downstream target proteins of AGB1 and AGG1/AGG2 should lead to a better understanding of the underlying mechanism of RLK-mediated immunity.
MATERIALS AND METHODS
Plant Material
bir1-1, bir1-1 pad4-1, and the 35S-SOBIR1 transgenic line 2 were previously described (Gao et al., 2009). The sobir2-1 bir1-1 pad4-1 triple mutant was identified from an ethyl methanesulfonate-mutagenized bir1-1 pad4-1 population by looking for mutants that can grow to maturity and set seeds at 23°C. gpa1-3, gpa1-4, and agb1-2 were provided by Ligeng Ma at the National Institute of Biological Sciences. agg1-1c, agg2-1, agg1-1c agg2-1, agg3-1, and agg3-2 were described previously (Trusov et al., 2007; Chakravorty et al., 2011). agg1-1c was obtained by backcrossing agg1-1w with wild-type Columbia eight times. The agg1-1c and agg1-1c agg2-1 mutants were backcrossed one more time with Columbia plants to identify mutant plants with homozygous Columbia-FLS2. All other mutants used in this study are also in the Columbia background.
For transgene complementation analysis, a 2.9-kb genomic DNA fragment containing AGB1 lacking the stop codon was amplified by PCR and cloned into a modified pCAMBIA1305 vector with a 3xFLAG tag to obtain pCAMBIA1305-AGB1-3xFLAG for expressing the AGB1-3xFLAG fusion protein under its native promoter. The plasmid was transformed into Agrobacterium tumefaciens and subsequently into the agb1-2 by floral dipping (Clough and Bent, 1998).
Mutant Characterization
For trypan blue staining, 2-week-old seedlings grown on one-half-strength Murashige and Skoog (MS) plates were placed in microcentrifuge tubes containing 1 mL lactophenol trypan blue solution (10 mg trypan blue, 10 g phenol, 10 mL lactic acid, 10 mL glycerol, and 10 mL water) diluted 1:1 in ethanol and boiled for 2 min. After removing the staining solution, the samples were destained with 1.5 mL chloral hydrate solution (2.5 g mL–1 water) for 2 h and a second time overnight on an orbital shaker. The destained samples were kept in 70% (v/v) glycerol and examined by microscopy.
For 3,3′-diaminobenzidine staining, 2-week-old seedlings grown on one-half-strength MS plates were submerged in 2 mL 3,3′-diaminobenzidine solutions (1 mg mL–1, pH 3.8) in a 24-well tissue culture plate. The samples were vacuumed for 2 min before incubating on an orbital shaker for 1 h. After removing the staining solution, the samples were destained with 95% (v/v) ethanol and examined by microscopy.
For gene expression analysis, RNA was extracted from 2-week-old seedlings grown on one-half-strength MS plates and reverse transcribed to obtain total complementary DNA. Real-time PCR was subsequently performed using the complementary DNA as template to determine the expression levels of the target genes. Primers used for amplification of PR1, PR2, and Actin1 were described previously (Zhang et al., 2003).
Infection of Hyaloperonospora arabidopsidis Noco2 was carried out by spraying 2-week-old seedlings with spore suspensions at a concentration of 50,000 spores per mL water. The plants were kept at 18°C under 12-h day/12-h night cycles in a growth chamber with 95% humidity. Infection was scored 7 d later as previously described (Bi et al., 2010).
SA was extracted and quantified as previously described (Li et al., 1999). The FLS2 antibodies (Zhang et al., 2010) were provided by Jianmin Zhou. The anti-Erk1/2 antibody specific for the phosphorylated MAPKs was from Cell Signaling Technology (no. 4370). The anti-MPK4 antibody was from Sigma. MPK4 immunocomplex kinase assays were carried out as previously described (Gao et al., 2008).
Elicitor Protection Assays
For elicitor protection assays, 5- to 6-week-old plants grown at 23°C under short-day conditions (10-h day/14-h night cycles) were used. Two leaves from each plant were preinfiltrated with 1 μm flg22, 1 μm elf18, or 200 μg mL–1 chitin. Control plants were infiltrated with distilled, deionized water. The same leaves were subsequently infiltrated with Pseudomonas syringae pv tomato DC3000 (optical density at 600 nm [OD600] = 0.001) in 10 mm MgCl2 24 h later. One leaf disc was taken from each infiltrated leaf, and the two leaf discs from the same plant were mixed as one sample. The samples were ground, diluted in 10 mm MgCl2, and plated on King’s B medium. After incubation at 28°C for 2 d, bacterial colonies were counted, and colony-forming units were calculated.
Measurement of Oxidative Burst
Leaf strips with a size of approximately 3 × 5 mm from 5- to 6-week-old plants grown under short-day conditions were placed in a 96-well plate, with each well containing 200 μL water. After incubation at room temperature for about 12 h, the liquid was removed, and 180 μL elicitor solution containing 20 μm luminol, 10 μg mL–1 horseradish peroxidase, and 1 μm flg22, 1 μm elf18, or 200 mg mL–1 chitin was added to each sample. Luminescence was recorded using a GLOMAX 96 microplate luminometer (Promega).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Map-based cloning of sobir2-1.
Supplemental Figure S2. Semiquantitative RT-PCR analysis of AGB1 expression in the wild type, agb1-4, and agb1-2.
Supplemental Figure S3. DAB staining of wild-type, bir1-1, agb1-4 bir1-1, and agb1-2 bir1-1 mutant seedlings.
Supplemental Figure S4. Semiquantitative RT-PCR analysis of SOBIR1 expression in the wild type, 35S-SOBIR1 transgenic line #2 (SOBIR1 OX-2), and agb1-2 carrying the 35S-SOBIR1 transgene from line #2 (agb1-2 SOBIR1 OX-2).
Supplemental Figure S5. FLS2 protein levels in the wild type, agb1-4, and agb1-2.
Supplemental Figure S6. Activation of MPK3 and MPK6 in mature leaves of the wild type and agb1-2 by flg22.
Supplemental Figure S7. Induction of FRK1 and WRKY29 by flg22 in the wild type, agb1-2, and agb1-2 expressing a wild-type AGB1 transgene (agb1/AGB1).
Supplemental Figure S8. Induction of GST1 by flg22 is not affected in agb1-2.
Supplemental Figure S9. Morphology of wild-type, bir1-2, gpa1-3 bir1-1, and gpa1-4 bir1-1 plants.
Supplemental Figure S10. Activation of MPK3 and MPK6 in the wild type, agg1-1c, agg2-1, and agg1-1c agg2-1 by flg22.
Supplemental Figure S11. Induction of GST1 expression in wild-type, agg1-1c, agg2-1, and agg1-1c agg2-1 plants.
Supplemental Figure S12. AGG3 is not required for PAMP-triggered immunity.
Acknowledgments
We thank Ligeng Ma (National Institute of Biological Sciences, Beijing [NIBS]) and the Arabidopsis Biological Resource Center for mutant seeds, Jianmin Zhou (NIBS) for the FLS2 antibodies, Junrong Yang (NIBS) for technical assistance, and Kaeli Johnson (University of British Columbia) for careful reading and discussion of the manuscript.
Glossary
- GPCR
G-protein-coupled receptor
- RLK
receptor-like kinase
- MAPK
mitogen-activated protein kinase
- ER
endoplasmic reticulum
- CDPK
calcium-dependent protein kinase
- RT
reverse transcription
- SA
salicylic acid
- ROS
reactive oxygen species
- MS
Murashige and Skoog
- OD600
optical density at 600 nm
- MBP
myelin basic protein
- PAMP
pathogen-associated molecular pattern
References
- Adjobo-Hermans MJ, Goedhart J, Gadella TW., Jr (2006) Plant G protein heterotrimers require dual lipidation motifs of Galpha and Ggamma and do not dissociate upon activation. J Cell Sci 119: 5087–5097 [DOI] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Bi D, Cheng YT, Li X, Zhang Y. (2010) Activation of plant immune responses by a gain-of-function mutation in an atypical receptor-like kinase. Plant Physiol 153: 1771–1779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T, Felix G. (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Willmann MR, McCormack M, Lee H, Shan L, He P, Bush J, Cheng SH, Sheen J. (2010) Differential innate immune signalling via Ca(2+) sensor protein kinases. Nature 464: 418–422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan JL, Zhu X, Mamillapalli P, Marathe R, Anandalakshmi R, Dinesh-Kumar SP. (2009) Induced ER chaperones regulate a receptor-like kinase to mediate antiviral innate immune response in plants. Cell Host Microbe 6: 457–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravorty D, Trusov Y, Zhang W, Acharya BR, Sheahan MB, McCurdy DW, Assmann SM, Botella JR. (2011) An atypical heterotrimeric G-protein γ-subunit is involved in guard cell K⁺-channel regulation and morphological development in Arabidopsis thaliana. Plant J 67: 840–851 [DOI] [PubMed] [Google Scholar]
- Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, Felix G, Boller T. (2007) A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature 448: 497–500 [DOI] [PubMed] [Google Scholar]
- Clapham DE, Neer EJ. (1997) G protein beta gamma subunits. Annu Rev Pharmacol Toxicol 37: 167–203 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Delgado-Cerezo M, Sánchez-Rodríguez C, Escudero V, Miedes E, Fernández PV, Jordá L, Hernández-Blanco C, Sánchez-Vallet A, Bednarek P, Schulze-Lefert P, et al. (2012) Arabidopsis heterotrimeric G-protein regulates cell wall defense and resistance to necrotrophic fungi. Mol Plant 5: 98–114 [DOI] [PubMed] [Google Scholar]
- Gao M, Liu J, Bi D, Zhang Z, Cheng F, Chen S, Zhang Y. (2008) MEKK1, MKK1/MKK2 and MPK4 function together in a mitogen-activated protein kinase cascade to regulate innate immunity in plants. Cell Res 18: 1190–1198 [DOI] [PubMed] [Google Scholar]
- Gao M, Wang X, Wang D, Xu F, Ding X, Zhang Z, Bi D, Cheng YT, Chen S, Li X, et al. (2009) Regulation of cell death and innate immunity by two receptor-like kinases in Arabidopsis. Cell Host Microbe 6: 34–44 [DOI] [PubMed] [Google Scholar]
- Gimenez-Ibanez S, Hann DR, Ntoukakis V, Petutschnig E, Lipka V, Rathjen JP. (2009) AvrPtoB targets the LysM receptor kinase CERK1 to promote bacterial virulence on plants. Curr Biol 19: 423–429 [DOI] [PubMed] [Google Scholar]
- Gómez-Gómez L, Boller T. (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011 [DOI] [PubMed] [Google Scholar]
- Gookin TE, Kim J, Assmann SM. (2008) Whole proteome identification of plant candidate G-protein coupled receptors in Arabidopsis, rice, and poplar: computational prediction and in-vivo protein coupling. Genome Biol 9: R120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heese A, Hann DR, Gimenez-Ibanez S, Jones AM, He K, Li J, Schroeder JI, Peck SC, Rathjen JP. (2007) The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA 104: 12217–12222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa A. (2009) The Arabidopsis G-protein beta-subunit is required for defense response against Agrobacterium tumefaciens. Biosci Biotechnol Biochem 73: 47–52 [DOI] [PubMed] [Google Scholar]
- Jiang K, Frick-Cheng A, Trusov Y, Delgado-Cerezo M, Rosenthal DM, Lorek J, Panstruga R, Booker FL, Botella JR, Molina A, Ort DR, et al. (2012) Dissecting Arabidopsis Gβ signal transduction on the protein surface. Plant Physiol 159: 975–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato C, Mizutani T, Tamaki H, Kumagai H, Kamiya T, Hirobe A, Fujisawa Y, Kato H, Iwasaki Y. (2004) Characterization of heterotrimeric G protein complexes in rice plasma membrane. Plant J 38: 320–331 [DOI] [PubMed] [Google Scholar]
- Klopffleisch K, Phan N, Augustin K, Bayne RS, Booker KS, Botella JR, Carpita NC, Carr T, Chen JG, Cooke TR, et al. (2011) Arabidopsis G-protein interactome reveals connections to cell wall carbohydrates and morphogenesis. Mol Syst Biol 7: 532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease KA, Wen J, Li J, Doke JT, Liscum E, Walker JC. (2001) A mutant Arabidopsis heterotrimeric G-protein beta subunit affects leaf, flower, and fruit development. Plant Cell 13: 2631–2641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SW, Han SW, Sririyanum M, Park CJ, Seo YS, Ronald PC. (2009) A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 326: 850–853 [DOI] [PubMed] [Google Scholar]
- Li J, Zhao-Hui C, Batoux M, Nekrasov V, Roux M, Chinchilla D, Zipfel C, Jones JD. (2009) Specific ER quality control components required for biogenesis of the plant innate immune receptor EFR. Proc Natl Acad Sci USA 106: 15973–15978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Zhang Y, Clarke JD, Li Y, Dong X. (1999) Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1. Cell 98: 329–339 [DOI] [PubMed] [Google Scholar]
- Liu X, Yue Y, Li B, Nie Y, Li W, Wu WH, Ma L. (2007) A G protein-coupled receptor is a plasma membrane receptor for the plant hormone abscisic acid. Science 315: 1712–1716 [DOI] [PubMed] [Google Scholar]
- Llorente F, Alonso-Blanco C, Sánchez-Rodriguez C, Jorda L, Molina A. (2005) ERECTA receptor-like kinase and heterotrimeric G protein from Arabidopsis are required for resistance to the necrotrophic fungus Plectosphaerella cucumerina. Plant J 43: 165–180 [DOI] [PubMed] [Google Scholar]
- Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. (2010) A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA 107: 496–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Tintor N, Mentzel T, Kombrink E, Boller T, Robatzek S, Schulze-Lefert P, Saijo Y. (2009) Uncoupling of sustained MAMP receptor signaling from early outputs in an Arabidopsis endoplasmic reticulum glucosidase II allele. Proc Natl Acad Sci USA 106: 22522–22527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR. (2000) Completing the heterotrimer: isolation and characterization of an Arabidopsis thaliana G protein gamma-subunit cDNA. Proc Natl Acad Sci USA 97: 14784–14788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MG, Botella JR. (2001) Isolation of a novel G-protein gamma-subunit from Arabidopsis thaliana and its interaction with Gbeta. Biochim Biophys Acta 1520: 147–153 [DOI] [PubMed] [Google Scholar]
- Melotto M, Underwood W, Kocza`n J, Nomura K, He SY. (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980 [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N. (2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morillo SA, Tax FE. (2006) Functional analysis of receptor-like kinases in monocots and dicots. Curr Opin Plant Biol 9: 460–469 [DOI] [PubMed] [Google Scholar]
- Nekrasov V, Li J, Batoux M, Roux M, Chu ZH, Lacombe S, Rougon A, Bittel P, Kiss-Papp M, Chinchilla D, et al. (2009) Control of the pattern-recognition receptor EFR by an ER protein complex in plant immunity. EMBO J 28: 3428–3438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey S, Nelson DC, Assmann SM. (2009) Two novel GPCR-type G proteins are abscisic acid receptors in Arabidopsis. Cell 136: 136–148 [DOI] [PubMed] [Google Scholar]
- Pandey S, Wang RS, Wilson L, Li S, Zhao Z, Gookin TE, Assmann SM, Albert R. (2010) Boolean modeling of transcriptome data reveals novel modes of heterotrimeric G-protein action. Mol Syst Biol 6: 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perfus-Barbeoch L, Jones AM, Assmann SM. (2004) Plant heterotrimeric G protein function: insights from Arabidopsis and rice mutants. Curr Opin Plant Biol 7: 719–731 [DOI] [PubMed] [Google Scholar]
- Saijo Y, Tintor N, Lu X, Rauf P, Pajerowska-Mukhtar K, Häweker H, Dong X, Robatzek S, Schulze-Lefert P. (2009) Receptor quality control in the endoplasmic reticulum for plant innate immunity. EMBO J 28: 3439–3449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Rodríguez C, Estévez JM, Llorente F, Hernández-Blanco C, Jordá L, Pagán I, Berrocal M, Marco Y, Somerville S, Molina A. (2009) The ERECTA receptor-like kinase regulates cell wall-mediated resistance to pathogens in Arabidopsis thaliana. Mol Plant Microbe Interact 22: 953–963 [DOI] [PubMed] [Google Scholar]
- Shiu SH, Bleecker AB. (2001) Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA 98: 10763–10768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, et al. (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270: 1804–1806 [DOI] [PubMed] [Google Scholar]
- Suharsono U, Fujisawa Y, Kawasaki T, Iwasaki Y, Satoh H, Shimamoto K. (2002) The heterotrimeric G protein alpha subunit acts upstream of the small GTPase Rac in disease resistance of rice. Proc Natl Acad Sci USA 99: 13307–13312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple BR, Jones AM. (2007) The plant heterotrimeric G-protein complex. Annu Rev Plant Biol 58: 249–266 [DOI] [PubMed] [Google Scholar]
- Trujillo M, Ichimura K, Casais C, Shirasu K. (2008) Negative regulation of PAMP-triggered immunity by an E3 ubiquitin ligase triplet in Arabidopsis. Curr Biol 18: 1396–1401 [DOI] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Chakravorty D, Armour D, Schenk PM, Botella JR. (2006) Heterotrimeric G proteins facilitate Arabidopsis resistance to necrotrophic pathogens and are involved in jasmonate signaling. Plant Physiol 140: 210–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Rookes JE, Tilbrook K, Chakravorty D, Mason MG, Anderson D, Chen JG, Jones AM, Botella JR. (2007) Heterotrimeric G protein γ subunits provide functional selectivity in Gβγ dimer signaling in Arabidopsis. Plant Cell 19: 1235–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trusov Y, Sewelam N, Rookes JE, Kunkel M, Nowak E, Schenk PM, Botella JR. (2009) Heterotrimeric G proteins-mediated resistance to necrotrophic pathogens includes mechanisms independent of salicylic acid-, jasmonic acid/ethylene- and abscisic acid-mediated defense signaling. Plant J 58: 69–81 [DOI] [PubMed] [Google Scholar]
- Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, Stacey MG, Stacey G. (2008) A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell 20: 471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, He SY. (2010) A prominent role of the flagellin receptor FLAGELLIN-SENSING2 in mediating stomatal response to Pseudomonas syringae pv tomato DC3000 in Arabidopsis. Plant Physiol 153: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang M, Wang W, Li D, Huang Q, Wang Y, Zheng X, Zhang Z. (2012a) Silencing of G proteins uncovers diversified plant responses when challenged by three elicitors in Nicotiana benthamiana. Plant Cell Environ 35: 72–85 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, Zou Y, Gao M, Zhang X, Chen S, et al. (2010) Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7: 290–301 [DOI] [PubMed] [Google Scholar]
- Zhang L, Hu G, Cheng Y, Huang J. (2008a) Heterotrimeric G protein alpha and beta subunits antagonistically modulate stomatal density in Arabidopsis thaliana. Dev Biol 324: 68–75 [DOI] [PubMed] [Google Scholar]
- Zhang W, He SY, Assmann SM. (2008b) The plant innate immunity response in stomatal guard cells invokes G-protein-dependent ion channel regulation. Plant J 56: 984–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Tessaro MJ, Lassner M, Li X. (2003) Knockout analysis of Arabidopsis transcription factors TGA2, TGA5, and TGA6 reveals their redundant and essential roles in systemic acquired resistance. Plant Cell 15: 2647–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Wu Y, Gao M, Zhang J, Kong Q, Liu Y, Ba H, Zhou J, Zhang Y. (2012b) Disruption of PAMP-induced MAP kinase cascade by a Pseudomonas syringae effector activates plant immunity mediated by the NB-LRR protein SUMM2. Cell Host Microbe 11: 253–263 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, Felix G. (2006) Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell 125: 749–760 [DOI] [PubMed] [Google Scholar]