Abstract
Tumor necrosis factor (TNF-α) is a cytokine involved in systemic inflammation during acute phase reactions. The current study was designed to investigate the levels of pro-inflammatory cytokine (TNF-α) along with the anti-inflammatory cytokine (IL-10) during progression of non-alcoholic fatty liver disease (NAFLD) from simple steatosis to non-alcoholic steatohepatitis (NASH) and fibrosis in diabetic patients, and correlate the levels of cytokines with the progression of NAFLD. Fifty-two diabetic patients compared to 18 healthy controls were participated in this study. Based on clinical diagnosis, patients were divided into three groups: simple steatosis, NASH and fibrosis. Serum liver function tests, fasting blood glucose, bilirubin, ALT, AST, TNF-α, IL-10 and lipid profile were measured. TNF-α levels were significantly higher in NAFLD patients compared to control subjects with a significant positive correlation with body mass index and fasting blood glucose (FBG) but with negative correlation with IL-10. Serum IL-10 levels were significantly lower in NAFLD patients compared with controls. A positive correlation between IL-10 and HDL-C with concomitant negative correlation between IL-10 and FBG and triacylglycerides was found. Cytokine analyses showed that there was a prominent imbalance between TNF-α and IL-10 in patients with NAFLD, and this imbalance increase by increasing the progression of NAFLD especially in obese diabetic patients. TNF-α and IL-10 could be used in diagnosis and follow-up of NAFLD stages in a way to avoid liver biopsies in greater proportion of patients.
Keywords: Non-alcoholic fatty liver disease, Tumor necrosis factor-alpha, Interleukin-10, Steatohepatitis, Diagnosis, Progression
Introduction
Non-alcoholic fatty liver disease (NAFLD) is one of the most common liver diseases worldwide nowadays affecting 20–30 % of adults in developed countries and up to 80 % of obese people [1]. NAFLD is defined as an excessive accumulation of fat in the liver leads to chronic liver injury in which at least 5 % of hepatocytes display lipid droplets that exceed 5–10 % of liver weight in patients who do not consume significant amounts of alcohol [2, 3]. It is a heterogeneous disease that may range from a relatively mild and subtle disease (hepatic steatosis) to a much more active and progressive disease, in which significant necroinflammatory fatty infiltration of hepatocytes are involved, designated non-alcoholic steatohepatitis (NASH) [1, 4]. The first step of the pathophysiology of NASH is the lipid accumulation in the liver causing steatosis. This increases the sensitivity of the liver to injury and inflammation [5]. The second step involves cytokines which causing oxidative stress and lipid peroxidation, in time leading to steatohepatitis [6, 7] which eventually might develop cirrhosis and hepatocellular carcinoma [8]. Obesity, type 2 (non-insulin dependent) diabetes mellitus, and hyperlipidemia are coexisting conditions frequently associated with NAFLD [9, 10]. NASH progression is mediated by an inflammatory process in the liver with concomitant tissue damage and fibrosis [7]. The gene expression of proinflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and TNF receptors is increased in the liver of patients with NASH compared with both normal liver and fatty liver, and the expression is higher in those patients with more severe NASH [11].
Cytokines are classified as T helper 1 (Th1) and T helper 2 (Th2) subtypes. The main proinflammatory Th1 cytokines are TNF-α, interleukin (IL)-1, IL-6 and interferon; whereas the main Th2 anti-inflammatory cytokines are IL-4 and IL-10. In general, Th1 cytokines inhibit Th2 cytokine production and vice versa [12]. Thus, normally there is a balance between proinflammatory and anti-inflammatory cytokines [13, 14]. Therefore, the balance between pro- and anti-inflammatory acting cytokines appears to play a key role in hepatic and systemic insulin action, and they are supposed to have important functions in the development of NAFLD [15]. Neutralization of TNF-α activity improves insulin resistance and fatty liver disease in animals [11]. Therefore, the aim of the present study was to investigate the levels of pro-inflammatory cytokine (TNF-α) along with the anti-inflammatory cytokine (IL-10) in development and progression of NAFLD from simple steatosis to NASH and fibrosis in Egyptian diabetic patients, and to correlate the relationship between cytokine levels and progression of NAFLD.
Patients and Methods
Patients
Patients were selected from inpatient and outpatient clinics of Internal Medicine Department enrolled Italian Hospital in Cairo who was referred for the assessment of abnormal liver function tests. NAFLD patients were identified on the basis of questionnaire, laboratory investigations, clinical findings and ultrasound scan imaging. All regular alcohol consumers were excluded from this study. A complete clinical history, including anthropometric measurements of the subjects was obtained. Anthropometric evaluation included measures of body weight, height, and body mass index (BMI). BMI was calculated as weight (kg) divided by height (m) squared.
Ultrasonographic abdominal examination was performed for all the enrolled patients by a single sonographer following not less than 8 h fasting using FFsonic UF-4100 (Japan). Liver echopattern was graded according to Mottin et al. [16]. Degree of NAFLD (steatosis) was determined throughout the ultrasonography scan imaging of liver echogenicity and liver biopsy when appropriate.
Disorders such as drug induced liver disease, alcoholic liver disease, viral hepatitis, Schistosomiasis, autoimmune hepatitis, primary biliary cirrhosis, sclerosing cholangitis, α1-antitrypsin deficiency, hemochromatosis, Wilson’s disease and biliary obstruction were excluded. Other exclusion criteria were recent gastrointestinal surgery, pregnancy, suffering from malignancy or under any kind of medication.
Study protocol was approved by National Ethics Committee according to ethics guidelines of the 1975 Declaration of Helsinki, and all patients gave their written informed consent to the study.
Subjects who had visited the internal medicine department at Italian hospital (Cairo) for routine health check-up programmes without any specific problem and with no abnormality in the clinical and laboratory findings were considered ‘normal controls’. Eighteen healthy individuals (12 males and 6 females) of 30 up to 51 years old are served as controls. None of them had any evidence of fatty liver or previous liver disease.
NAFLD patients represented by 52 diabetics previously diagnosed with diabetes mellitus type 2 (35 males and 17 females). Based on clinical diagnosis and investigations patients were divided into the following groups:
Simple Steatosis group: This group included 26 patients having simple steatosis (17 males & 9 females) of 39 up to 59 years old.
Steatohepatitis group: This group included 16 patients (11 males & 5 females) having NASH of 45 up to 60 years old.
Fibrosis group: This group included 10 patients having hepatic fibrosis (7 males & 3 females) of 54 up to 67 years old.
Laboratory Investigations
Blood sampling were performed in the morning, following a not less than 12 h fasting period.
Serum triglycerides, total cholesterol (TC), and high-density lipoprotein cholesterol (HDL-C), fasting blood glucose (FBG), total proteins, albumin, total bilirubin, direct bilirubin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were estimated using commercial kits on the synchron CX5 analyzer from Beckman Instruments Inc., California (USA). Low-density cholesterol (LDL-C) and very low density lipoprotein cholesterol (VLDL-C) were calculated using Friedewald’s formulae [17].
Interleukin-10 and TNF-α levels in serum were estimated according to Stepaniak et al. [18] throughout ELISA technique following the manufacturer’s instructions.
Statistical Analyses
Results are expressed as mean ± standard deviation (SD). Correlation coefficient between quantitative data was determined using Pearson’s test at 95 % confidence. The Statistical Package for Social Science (SPSS) version 11.5.0 (SPSS® Chicago, IL, USA) program was used. Significance (P) less than 0.01 (two-tailed) was considered statistically significant.
Results
NAFLD diabetic patients showed significantly higher age values in NASH and fibrosis groups when compared to control groups (Table 1). Significant increased levels of BMI and FBG were observed in NASH and fibrosis groups when compared with simple steatosis along with control subjects (Table 1). No significant changes were observed with severity of liver disease in any group for T. cholesterol and LDL-C among NAFLD subgroups (Table 1). Comparison between NAFLD subgroups showed that patients with fibrosis had higher significant levels for TG along with VLDL-C and lower levels of HDL-C than patients with simple steatosis and NASH (Table 1).
Table 1.
Anthropometric and biochemical parameters of NAFLD patients and normal subjects*
| Parameter | Groups | |||
|---|---|---|---|---|
| Controls (n = 18) | NAFLD patients (n = 52) | |||
| Simple steatosis (n = 26) | NASH (n = 16) | Fibrosis (n = 10) | ||
| Age (years) | 42.67 ± 6.63 | 48.58 ± 6.22 | 53 ± 4.65a | 59 ± 3.94a, b |
| BMI (kg/m2) | 25.6 ± 2.85 | 27.9 ± 4.26 | 31.0 ± 6.18a | 33.2 ± 5.32a, b |
| ALT (IU/L) | 26.9 ± 7.34 | 58.4 ± 8.31a | 81 ± 7.31a, b | 95.8 ± 8.62a, b, c |
| AST (IU/L) | 20.8 ± 6.86 | 41.7 ± 6.17a | 52.3 ± 5.95a | 67.6 ± 6.48a, b |
| FBG (mg/dL) | 85.6 ± 10.28 | 114.4 ± 20.18a | 132.3 ± 18.63a | 156 ± 26.31a, b |
| T. chol. (mg/dL) | 172.6 ± 28.05 | 191.8 ± 37.16 | 184.8 ± 28.72 | 173 ± 19.24 |
| TG (mg/dL) | 110.6 ± 23.89 | 146.4 ± 25.70a | 187.3 ± 31.40a | 203 ± 27.26a, b |
| HDL-C (mg/dL) | 45.3 ± 5.10 | 41.6 ± 5.75 | 34.8 ± 5.03a, b | 30.9 ± 4.56a, b |
| LDL-C (mg/dL) | 105.2 ± 25.44 | 120.9 ± 33.16 | 112.6 ± 26.70 | 101.5 ± 17.29 |
| VLDL-C (mg/dL) | 22.1 ± 4.78 | 29.3 ± 9.14a | 37.5 ± 7.08a | 40.6 ± 5.45a, b |
| T. protein (g/dL) | 7.81 ± 0.17 | 7.76 ± 0.18 | 7.60 ± 0.19 | 7.39 ± 0.19 |
| Albumin (g/dL) | 4.85 ± 0.14 | 4.79 ± 0.19 | 4.49 ± 0.31 | 4.10 ± 0.24 |
| T. bilirubin (mg/dL) | 0.48 ± 0.16 | 0.51 ± 0.20 | 0.70 ± 0.23a | 1.30 ± 0.24a, b |
| D. bilirubin (mg/dL) | 0.18 ± 0.08 | 0.19 ± 0.09 | 0.30 ± 0.13a | 0.49 ± 0.17a, b |
*Values are mean ± SD of number of observation (n)
aP < 0.01 Significant compared to control group
bP < 0.01 Significant compared to simple steatosis group
cP < 0.01 Significant compared to steatohepatitis group
The levels of serum ALT and AST were significantly elevated in NAFLD subgroups compared to control group while levels of serum bilirubin were significantly elevated in fibrosis patients (Table 1).
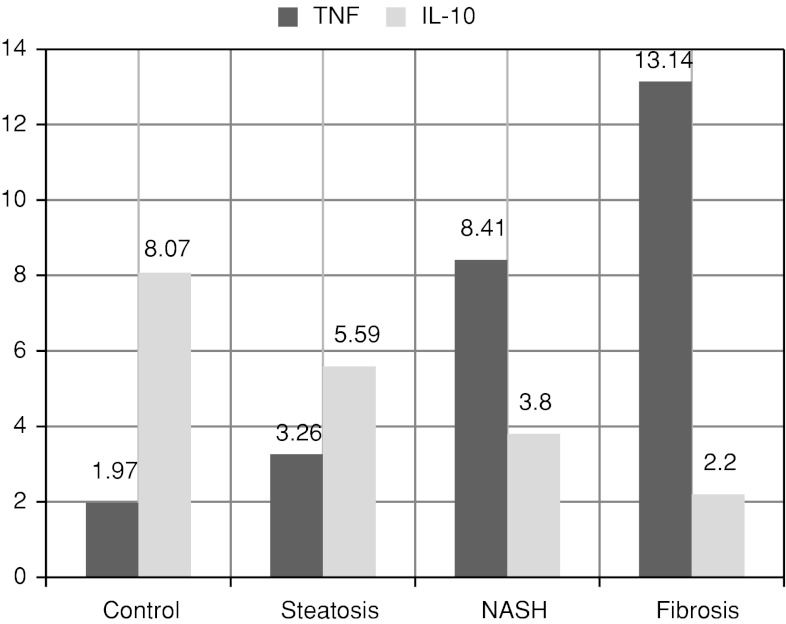
In this study, TNF-α significantly increased by progression of NAFLD from simple steatosis to fibrosis while IL-10 significantly decreased (Table 2 and Fig. 1). TNF-α and IL-10 showed statistically higher significant differences in fibrosis and NASH groups when compared to simple steatosis and control groups.
Table 2.
Cytokine levels among control and NAFLD patients*
| Parameter | Groups | |||
|---|---|---|---|---|
| Controls (n = 18) | NAFLD patients (n = 52) | |||
| Simple steatosis (n = 26) | NASH (n = 16) | Fibrosis (n = 10) | ||
| TNF-α (pg/mL) | 1.97 ± 0.41 | 3.26 ± 0.64a | 8.41 ± 1.10a, b | 13.14 ± 1.39a, b, c |
| IL-10 (pg/mL) | 8.07 ± 1.82 | 5.59 ± 1.03a | 3.80 ± 0.97a, b | 2.20 ± 0.42a, b |
*Values are mean ± SD of number of observation (n)
aP < 0.01 Significant compared to control group
bP < 0.01 Significant compared to simple steatosis group
cP < 0.01 Significant compared to steatohepatitis group
Fig. 1.
Comparative study of serum cytokine levels in-between NAFLD patients and controls (pg/ml)
Significant positive correlations were found between TNF-α along with BMI and ALT in NAFLD groups. A concomitant significant negative correlation between IL-10 and ALT in NASH and fibrosis groups observed while significant positive correlation between IL-10 and HDL-C recorded. There is highly significant negative correlation between TNF-α and IL-10 in both NASH and Fibrosis groups (Table 3).
Table 3.
Correlation Coefficients (95 % confidence) in-between selected variables along with TNF-α and IL-10 within NAFLD groups
| Parameter | Groups | |||||
|---|---|---|---|---|---|---|
| Simple steatosis | NASH | Fibrosis | ||||
| TNF-α | IL-10 | TNF-α | IL-10 | TNF-α | IL-10 | |
| BMI | 0.59* | −0.12 | 0.59* | −0.28 | 0.69* | 0.30 |
| ALT | 0.27 | −0.11 | 0.67* | −0.59* | 0.6* | −0.68* |
| TG | 0.35 | −0.59* | 0.46 | −0.71* | 0.44 | −0.64* |
| HDL-C | −0.48 | 0.62* | −0.49 | 0.63* | −0.5 | 0.64* |
| TNF-α | – | −0.42 | – | −0.59* | – | −0.64* |
| IL-10 | −0.42 | – | −0.59* | – | −0.64* | – |
*Correlation coefficient (r) is significant at 0.01 level (two-tailed)
Discussion
NAFLD is the most common growing public health problem worldwide presenting a wide spectrum of conditions ranging from fatty liver, that in general follows a benign nonprogressive clinical course, to nonalcoholic steatohepatitis (NASH), a serious form of NAFLD, that may progress to cirrhosis and end-stage liver disease with an increasing incidence in obesity, hyperlipidemia and insulin resistance (higher than normal insulin concentrations are needed to achieve normal metabolic responses) [1, 11, 19]. In this regard, fatty liver disease is a major contributor to cardiovascular and overall obesity-related morbidity and mortality [20]. In obese non-diabetic western adults, the prevalence of NAFLD ranges from 80 to 98 % [21]. Although the prevalence of NAFLD remains largely unknown in the Egyptian population, there is great concern about the impact of this fast-growing disease in this part of the world. Also, several efforts had been made to identify non-invasive markers of fibrosis in adults with NAFLD since most studies have focused on the use of clinical features and routine laboratory serum markers of liver fibrosis. Therefore, the objective of this study was to describe cytokine levels in patients with NAFLD to assess the possible involvement of candidate cytokines in disease progression and try to get simple non invasive parameters for NAFLD severity, thus sparing repeated liver biopsies especially in patients who are unfit for this procedure.
In our study, results of patients with fatty liver, NASH and cirrhosis showed that the progression of NAFLD increase with increasing the age, and older ages at higher risk than younger patients to develop fibrosis, these result confirm the finding of Collier [22] who reported that older ages often are predictive of more severe grading of fibrosis. Also, serum levels of T. bilirubin, ALT and AST were significantly highly increased in fibrosis patients compared to control group. These results indicate that ALT and AST liver function markers were increased according to the severity of the liver disease. This finding suggests that higher degree of steatosis results in an increase in inflammatory response, and subsequently elevated ALT levels which also being considered as a predictor of the progression of steatosis to steatohepatitis and fibrosis [23].
Nonalcoholic fatty liver disease is strongly associated with the metabolic syndrome, a cluster of related disorders that includes central obesity, insulin resistance and dyslipidemia [10, 24]. Angulo [9] and Riley et al. [25] reported that BMI was a positive risk factor for liver disease. The comparative study of the three groups showed that the severity of obesity is correlated with the progression of the NAFLD (Table 1). These data were consistent with Angulo [9] who reported that the prevalence of nonalcoholic fatty liver disease increases by a factor of 4.6 in obese people with a BMI of at least 30, and also with result of Riley et al. [25] who stated that BMI over 30 for more than 15 years increases the risk of advanced fibrosis in all chronic liver diseases with increased risk of alcohol ingestion.
In patients with NAFLD, hepatic glucose uptake was found to be less effectively stimulated by insulin, contributing to elevated plasma glucose concentrations [26]. In our study, all patients are previously diagnosed with diabetes mellitus type 2. The comparative study of the three groups showed that as FBG increase the progression of the NAFLD increase, which confirm that IR is very important risk factor in the development and progression of NAFLD. Similar results were reported by Angulo [9] who reported that the presence of type 2 diabetes mellitus significantly increases the risk and severity of nonalcoholic fatty liver disease. These observations due to insulin resistance affect hepatic fat accumulation by increasing fatty acids and triglycerides synthesis in the liver.
In studying the lipid profile in NAFLD patients, T. cholesterol and LDL-C hadn’t statistically significant differences in simple steatosis,NASH and fibrosis groups when compared with each other as well as with control group. These results confirm the finding of Uchil et.al [27] and Simonen et al. [28] who reported that there were no significant difference in-between T. cholesterol and LDL-C values of NAFLD patients and control subjects but hypertriglyceridemia rather than hypercholesterolemia can be considered to be a significant risk factor towards progression of NAFLD [27]. Nobili et.al [29] reported that hypertriglyceridemia may be considered an independent predictor of liver fibrosis in a study with obese children. The comparative study of NAFLD patients in the three groups showed that as TG increased, the progression of the NAFLD increased. These findings are in accordance with results of Uchil et.al. [27] and Mouralidarane et al. [30]. Also, HDL-C levels were significantly different in the three groups since fibrosis group showed the lowest values. These results are in agreement with Simonen et al. [28] who found a similar strong association between lipid metabolism abnormalities such as increase of serum triglyceride and decrease of HDL towards the development and progression of NASH.
In our correlation studies, there is significant positive correlation between TNF-α and BMI in NAFLD groups. This correlation indicates that adipose tissue is an important initiator for TNF-α and obesity is very important risk factor for NAFLD progression where TNF-α plays a central role in the state of insulin resistance associated with obesity [31] which considered being very important factor in the pathogeneses and progression of NAFLD. Also, there is significant positive correlation of TNF-α with ALT in NASH and fibrosis groups since the production of TNF-α is one of the earliest events of liver injury, triggering the production of other cytokines that together recruit inflammatory cells, kill hepatocytes [32]. TNF-α induced mitochondrial swelling followed by a bursting of the mitochondrial membrane which leading to a reduced content of respiratory proteins causing hepatic steatosis and liver injury [33]. These effects of TNF-α on the hepatocytes can explain the correlation between serum concentration of TNF-α and the elevation in the levels of ALT and AST during cellular injury. On the other side, our data showed that there is a significant positive correlation between IL-10 and HDL-C in steatosis, NASH and fibrosis groups with a concomitant significant negative correlation between IL-10 and ALT in NASH and fibrosis groups was found which being in agreement with Zhang and Wang [34] study which showed that liver injury induced by lipopolysaccharide complex in mice treated with IL-10 could markedly reduce serum transaminase activities.
The balance between pro- and anti-inflammatory cytokines is very important for the function of the immune system. Overproduction of the pro-inflammatory cytokine TNF-α was connected with a defect in the production of anti-inflammatory cytokine IL-10 [35]. In our study, TNF-α is significantly increased by progression of the NAFLD from simple steatosis to fibrosis but on the other side IL-10 significantly decreased by progression of NAFLD. Thus, it evident that the increase in serum TNF-α and the decrease in serum IL-10 becomes more prominent as the case becomes complicated during progression of the NAFLD. In addition, TNF-α accelerates the hepatic synthesis of other cytokines, enhances neutrophils chemotaxis and leads to a severe inflammatory response, which results in hepatosteatosis and necrosis in the liver [11]. These results are confirmed with Uysal et al. [36] who suggest that liver iron and fat accumulation, oxidant stress, and inflammatory cytokines are closely related in which levels of serum ferritin, malonaldehyde, IL-6, TNF-α and IL-8 could represent the indices of activity and progression of NASH. Also, in our study a highly significant negative correlation between TNF-α and IL-10 in both NASH and fibrosis groups was found and the correlation increases by progression of liver disease where IL-10 secretion inhibited and increases in serum TNF-α concentrations were found. Treatment with IL-10 could significantly reduce TNF-α mRNA expression in the liver after administration of the toxins to sensitized mice [37–39]. These findings suggest that IL-10 is capable of regulating hepatic injury in vivo mediated by T cells and macrophages [34] thus it hypothesized that high IL-10 levels limiting the effects of the inflammatory response that is, by counter regulating the effects of pro-inflammatory cytokine so that the protective function of IL-10 in steatotic liver relies on suppression of TNF-α production [40].
Conclusion
TNF-α being an important determinant for the pathogenesis of NAFLD. Increased BMI, insulin resistance and hypertriglyceridaemia values considered risk factors involved in the development and progression of NAFLD just prior cirrhosis. TNF-α and IL-10 could be used in the follow up of simple steatosis versus NASH and fibrosis for sparing unnecessary liver biopsies in a greater proportion of patients within individualized risk management programs.
References
- 1.Zelber-Sagi S, Lotan R, Shlomai A, Webb M, Harrari G, Buch A, et al. Predictors for incidence and remission of NAFLD in the general population during a seven-year prospective follow-up. J Hepatol. 2012;56:1145–1151. doi: 10.1016/j.jhep.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 2.Marchesini G, Bugianesi E, Forlani G, Cerrelli F, Lenzi M, Manini R, et al. Nonalcoholic fatty liver, steatohepatitis, and the metabolic syndrome. Hepatology. 2003;37(4):917–923. doi: 10.1053/jhep.2003.50161. [DOI] [PubMed] [Google Scholar]
- 3.Cusi K. Role of obesity and lipotoxicity in the development of nonalcoholic steatohepatitis: pathophysiology and clinical implications. Gastroenterology. 2012;142(4):711–725. doi: 10.1053/j.gastro.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Charlton M. Nonalcoholic fatty liver disease: a review of current understanding and future impact. Clin Gastroenterol Hepatol. 2004;2:1048–1058. doi: 10.1016/S1542-3565(04)00440-9. [DOI] [PubMed] [Google Scholar]
- 5.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 6.Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver disease. Mol Aspects Med. 2008;29:9–16. doi: 10.1016/j.mam.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Koek GH, Liedorp PR, Bast A. The role of oxidative stress in non-alcoholic steatohepatitis. Clin Chim Acta. 2011;412:1297–1305. doi: 10.1016/j.cca.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Wong VW, Chan HL, Hui AY, Chan KF, Liew CT, Chan FK, Sung JJ. Clinical and histological features of non-alcoholic fatty liver disease in Hong Kong Chinese. Aliment Pharmacol Ther. 2004;20(1):45–49. doi: 10.1111/j.1365-2036.2004.02012.x. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. Non alcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 10.Vanni E, Bugianesia E, Kotronen A, De Minicis S, Yki-Järvinen H, Svegliati-Baroni G. From the metabolic syndrome to NAFLD or vice versa? Dig Liver Dis. 2010;42:320–330. doi: 10.1016/j.dld.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Rolo AP, Teodoro JS, Palmeira CM. Role of oxidative stress in the pathogenesis of nonalcoholic steatohepatitis. Free Radic Biol Med. 2012;52:59–69. doi: 10.1016/j.freeradbiomed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 12.Crews FT, Bechara R, Brown LA, Guidot DM, Mandrekar P, Oak S, Qin L, Szabo G, Wheeler M, Zou J. Cytokines and alcohol. Alcohol Clin Exp Res. 2006;30:720–730. doi: 10.1111/j.1530-0277.2006.00084.x. [DOI] [PubMed] [Google Scholar]
- 13.Cameron RG, Neuman MG. Novel morphologic findings in alcoholic liver disease. Clin Biochem. 1999;32(7):579–584. doi: 10.1016/S0009-9120(99)00058-2. [DOI] [PubMed] [Google Scholar]
- 14.Das SK, Balakrishnan V. Role of cytokines in the pathogenesis of non-alcoholic fatty liver disease. Ind J Clin Biochem. 2011;26(2):202–209. doi: 10.1007/s12291-011-0121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. 2010;28(1):179–185. doi: 10.1159/000282083. [DOI] [PubMed] [Google Scholar]
- 16.Mottin CC, Moretto M, Padoin AV. The role of ultrasound in the diagnosis of hepatic steatosis in morbidly obese patients. Obes Surg. 2004;14:635–637. doi: 10.1381/096089204323093408. [DOI] [PubMed] [Google Scholar]
- 17.Freidewald WT, Levy RJ, Fredrickson DS. Estimation of concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 18.Stepaniak J, Shuster J, Sundick R. Production and determination of recombinant human cytokines. J Cytokine Res. 1995;19:515–526. doi: 10.1089/107999099313965. [DOI] [PubMed] [Google Scholar]
- 19.Carter-Kent C, Zein NN, Feldstein AE. Cytokines in the pathogenesis of fatty liver and disease progression to steatohepatitis: implications for treatment cytokines in NAFLD. Am J Gastroenterol. 2008;103:1036–1042. doi: 10.1111/j.1572-0241.2007.01709.x. [DOI] [PubMed] [Google Scholar]
- 20.Lu H, Zeng L, Liang B, Shu X, Xie D. High prevalence of coronary heart disease in type 2 diabetic patients with non-alcoholic fatty liver disease. Arch Med Res. 2009;40:571–575. doi: 10.1016/j.arcmed.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Edens M, Kuipers F, Stolk R. Non-alcoholic fatty liver disease is associated with cardiovascular disease risk markers. Obes Rev. 2009;10:412–419. doi: 10.1111/j.1467-789X.2009.00594.x. [DOI] [PubMed] [Google Scholar]
- 22.Collier J. Non-alcoholic fatty liver disease. Medicine. 2007;35(2):86–88. doi: 10.1016/j.mpmed.2006.11.010. [DOI] [Google Scholar]
- 23.Giorgio R, Wolfgang K, Joseph S, Klaus P. Non-alcoholic fatty liver disease in obese children evaluated by magnetic resonance imaging. Acta Pediatr. 2006;95(7):833–837. doi: 10.1080/08035250500449890. [DOI] [PubMed] [Google Scholar]
- 24.Steve C, Anna M. Role of inflammation in nonalcoholic steatohepatitis. Curr Opin Gastroenterol. 2005;21(6):702–707. doi: 10.1097/01.mog.0000182863.96421.47. [DOI] [PubMed] [Google Scholar]
- 25.Riley TR, Taheri M, Schreibman IR. Does weight history affect fibrosis in the setting of chronic liver disease? J Gastrointest Liver Dis. 2009;18(3):299–302. [PubMed] [Google Scholar]
- 26.Bugianesi E, Zannoni C, Vanni E, Marzocchi R, Marchesini G. Non-alcoholic fatty liver and insulin resistance: a cause–effect relationship? Dig Liver Dis. 2004;36(3):165–173. doi: 10.1016/j.dld.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 27.Uchil D, Pipalia D, Chawla M, Patel R, Maniar S, Narayani, Juneja A. Non-alcoholic fatty liver disease (NAFLD)—The hepatic component of metabolic syndrome. J Assoc Physicians India. 2009;57:201–204. [PubMed] [Google Scholar]
- 28.Simonen P, Kotronen A, Hallikainen M, Sevastianova K, Makkonen J, Hakkarainen A, et al. Cholesterol synthesis is increased and absorption decreased in non-alcoholic fatty liver disease independent of obesity. J Hepatol. 2011;54:153–159. doi: 10.1016/j.jhep.2010.05.037. [DOI] [PubMed] [Google Scholar]
- 29.Nobili V, Alisi A, Vania A, Tiribelli C, Pietrobattista A, Bedogni G. The pediatric NAFLD fibrosis index: a predictor of liver fibrosis in children with non-alcoholic fatty liver disease. BMC Med. 2009;7(21). [DOI] [PMC free article] [PubMed]
- 30.Mouralidarane A, Oben JA, Soeda J. Pathophysiology and clinical management of nonalcoholic fatty liver disease. Medicine. 2011;39(10):592–596. doi: 10.1016/j.mpmed.2011.07.014. [DOI] [Google Scholar]
- 31.Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM. Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem. 1996;271(22):13018–13022. doi: 10.1074/jbc.271.22.13018. [DOI] [PubMed] [Google Scholar]
- 32.Tilg H, Diehl AM. Cytokines in alcoholic and nonalcoholic steatohepatitis. N Engl J Med. 2000;343:1467–1476. doi: 10.1056/NEJM200011163432007. [DOI] [PubMed] [Google Scholar]
- 33.Wei Y, Rector RS, Thyfault JP, Ibdah JA. Nonalcoholic fatty liver disease and mitochondrial dysfunction. World J Gastroenterol. 2008;14(2):193–199. doi: 10.3748/wjg.14.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang L, Wang XZ. Interleukin-10 and chronic liver disease. World J Gastroenterol. 2006;12(11):1681–1685. doi: 10.3748/wjg.v12.i11.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Straczkowski M, Kowalska I, Nikolajuk A, Krukowska A, Gorska M. Plasma interleukin-10 concentration is positively related to insulin sensitivity in young healthy individuals. Diabetes Care. 2005;28(8):2036–2037. doi: 10.2337/diacare.28.8.2036. [DOI] [PubMed] [Google Scholar]
- 36.Uysal S, Armutcu F, Aydogan T, Akin K, Ikizek M, Yigitoglu MR. Some inflammatory cytokine levels, iron metabolism and oxidan stress markers in subjects with nonalcoholic steatohepatitis. Clin Biochem. 2011;44(17–18):1375–1379. doi: 10.1016/j.clinbiochem.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 37.Angulo P. Treatment of non alcoholic fatty liver disease. Ann Hepatol. 2002;1(1):12–19. [PubMed] [Google Scholar]
- 38.Cintra DE, Pauli JR, Araújo EP, Moraes JC, De Souza CT, Milanski M, et al. Interleukin-10 is a protective factor against diet-induced insulin resistance in liver. J Hepatol. 2008;48(4):628–637. doi: 10.1016/j.jhep.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 39.Braunersreuther V, Viviani GL, Mach F, Montecucco F. Role of cytokines and chemokines in non-alcoholic fatty liver disease. World J Gastroenterol. 2012;18(8):727–735. doi: 10.3748/wjg.v18.i8.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashem RM, Mahmoud MF, El-Moselhy MA, Soliman HM. Interleukin-10 to tumor necrosis factor-alpha ratio is a predictive biomarker in nonalcoholic fatty liver disease: interleukin-10 to tumor necrosis factor-alpha ratio in steatohepatitis. Eur J Gastroenterol Hepatol. 2008;20(10):995–1001. doi: 10.1097/MEG.0b013e3282fdf65f. [DOI] [PubMed] [Google Scholar]