Abstract
The human epidermal receptor-2/neu (HER-2/neu) oncogene encodes a transmembrane tyrosine kinase receptor. This molecule could have a diagnostic value since the extracellular domain of c-erbB-2 (HER-2) transmembrane is shed into the blood as a circulating antigen. The diagnostic value of serum HER-2/neu was calculated along with the conventional marker carbohydrate antigen 15-3 (CA15-3) and carcinoembryonic antigen (CEA) at 85th percentiles. Serum levels of breast carcinoma antigens HER-2/neu, CEA and CA15-3 were determined in 175 normal individuals and 268 malignant patients. The soluble form of serum HER-2/neu, CEA and CA15-3 was assayed by enzyme linked immunosorbent assay in control and breast cancer patients prior to treatment. Serum levels of the tested tumor markers HER-2/neu and CA15-3 and CEA were significantly higher in cancer patients compared to controls. At 85th percentile the sensitivity of HER-2/neu was 51.12 %; the specificity was 86.29 % and the overall accuracy was 64.56 %. The sensitivity of CA15-3 was 73.13 %; the specificity was 85.14 % and the overall accuracy was 77.88 %. The sensitivity of the combined testing was 82.84 %; the specificity was 73.71 % and the overall accuracy was 80.01 %. The sensitivity and the overall accuracy of combined testing were higher than those of HER-2/neu and CA15-3 testing single. The combined testing of HER-2/neu and CA15-3 can increase the sensitivity and overall accuracy of breast cancer diagnosis. The results of this study suggest that the use of multiple tumor markers may be employed as combination and at 85th percentiles to assess the prognosis.
Keywords: Diagnostic value, CA15-3, HER-2/neu, Breast cancer
Introduction
Breast cancer is one of the most common cancers worldwide and causes more deaths per year than other cancers. Several tumor markers have been evaluated in breast cancer. Most of the markers are of prognostic value. The diagnostic statistics can be calculated for various tumor markers. The diagnostic values of each marker separately or in combinations were evaluated. The optimal cut-off values of each marker were defined. Diagnostic value of markers can be increased by combination of multiple marker values. Further, the diagnostic values can be improved at 85th percentiles. The receiver operating characteristic (ROC) curve is a simple and meaningful measure to assess the usefulness of diagnostic markers. Serum carcinoembryonic antigen (CEA) and carbohydrate antigen 15-3 (CA15-3) are established prognostic markers in breast cancer patients.
The human epidermal receptor-2 (HER-2) oncogene encodes a transmembrane tyrosine kinase receptor [1]. This molecule could be a new marker for prognosis and response to therapy in patients with advanced breast cancer. The HER-2 oncogene (also named erbB-2 and HER-2/neu) codifies for the HER-2 oncoprotein (also called p185erbB-2), which has a structure of growth factor receptor [2]. The HER-2 extracellular domain (ECD) can be found in the circulation [3, 4]. The ECD of c-erbB-2 (HER-2) transmembrane receptor undergoes proteolytic cleavage from the fulllength protein by metalloproteases, and is shed into the blood as a circulating antigen [5]. To determine the clinical utility of this oncoprotein, the soluble form of HER-2 was assayed in the serum of breast cancer patients.
In Health care industry, diagnosis of any disease plays major role, any reduction in false negative is given utmost importance as the patients do not miss the treatment. In this connection, cost/kit or any other matter does not prevail. Therefore one can take the support of how best one can reduce the false negatives in diagnostic performance of markers. A single marker alone may not be effective in reducing the false negative. Combinations of markers help in increasing the sensitivity as well as reducing the false negative.
The aim of this study is to determine the diagnostic value of serum HER-2 and to compare it with the conventional tumor markers: CA15-3, CEA and to evaluate whether a combination of two or three biomarkers might give better prognostic information.
Materials and Methods
Patients registered in the Breast service unit of Kidwai Memorial Institute of Oncology, Bangalore, were included in the study. In this case control study, we have taken 443 subjects with 175 normal individuals and 268 malignant cases of breast cancer. Impact and effect of disease on serum CA15-3, HER-2/neu, and CEA were studied.
Among 390 selected patients, 21 cases had benign breast disease, 4 were male patients and 97 patients were treated cases and hence excluded from the study. Remaining 268 female patients aged 26–75 year were histologically confirmed breast cancer cases and included in the study.
For control samples 175 female individuals were selected from among volunteers without any history of major illness and matched with cases for age (±2 years). Blood samples were collected in plain tubes, centrifuged to separate the serum. The serum samples (0.3 ml) were stored at −20 °C until analysis (within a month).
The study protocol was approved by the Ethics committee of Kidwai Memorial Institute of Oncology, Bangalore. A written informed consent was obtained from each patient. The markers (HER-2/neu, CA15-3 and CEA) were analysed by sandwich enzyme linked immunosorbent assay (ELISA) method.
HER-2/neu levels
The levels of serum HER-2/neu were measured by modified sandwich enzyme immunoassay [6]. An anti-sp185HER-2 human monoclonal coating antibody was adsorbed on to micro wells. Sp185HER-2 present in the sample or standards bound to antibodies adsorbed to the micro wells; a horse radish peroxidase (HRP) conjugated monoclonal anti-sp185HER-2 antibody was then added to the wells. Following incubation, unbound enzyme conjugated anti-sp185HER-2 was removed by washing and substrate tetramethylbenzidine (TMB) solution reactive with HRP was added to the wells. A blue colored product was formed in proportion to the amount of soluble sp185HER-2 present in the sample. The reaction was terminated by addition of 2/3 N sulphuric acid and resulted yellow color was measured at 450 nm. A standard curve was prepared from stock standard with assay buffer and sample concentration was determined.
Carcinoembryonic Antigen
The levels of serum CEA were measured in the microwells coated with monoclonal antibody (MAb). CEA in the patients’ serum bound to anti CEA MAb adsorbed on to microwells; a HRP conjugated goat anti CEA was then added to the wells. Unbound protein and HRP conjugate was washed off by wash buffer phosphate buffer saline (PBS). Upon the addition of the TMB substrate a blue colored product was formed. The intensity of color was proportional to the concentration of CEA in the samples. The color development was stopped with the addition of stop solution (1 N HCl). The resulted yellow color was read at 450 nm in the ELISA reader.
Carbohydrate Antigen 15-3
The primary MAb directed against an antigenic determinant on the intact CA15-3 molecule was adsorbed on to the micro wells. The CA15-3 present in serum sample was bound to MAb. The secondary antibody solution contained rabbit anti CA15-3 antibody conjugated to HRP was added. Following incubation unbound anti CA15-3 was removed by washing with PBS. A solution of TMB reagent was added and incubated, resulting in the development of a blue color. The color development was stopped with the addition of stop solution (1 N HCl). The resulted yellow colored product was measured at 450 nm.
A standard curve was prepared relating color intensity to the concentration of the markers.
Statistical Analysis
Area under curve had been computed to find the diagnostic value of each study parameters at different cut-off points. Bayesian Statistics namely sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), positive and negative likelihood ratio (LR+), (LR−) were used to find the diagnostic value of study parameters. Effect size due to Cohen (Bias corrected) had been used to find the effect on each study parameters in cases when compared to controls. Student’s ‘t’ test was used to compare serum HER-2/neu levels between patients and controls.
Results
The ROC curves are calculated for each marker (Figs. 1, 2, 3) and the diagnostic cut-off point is selected as the value that offered a specificity of 100 %. Table 1 shows diagnostic value of study parameters as single marker and also with combination based on standard cut off. CA15-3 shows highest sensitivity (51.12) and CEA the least (21.27). HER-2/neu levels have low sensitivity (27.24), with high specificity (100). When utility of two markers (HER-2/neu and CA15-3) are combined, sensitivity increased to 54.85 %with the classification accuracy of 72.69.
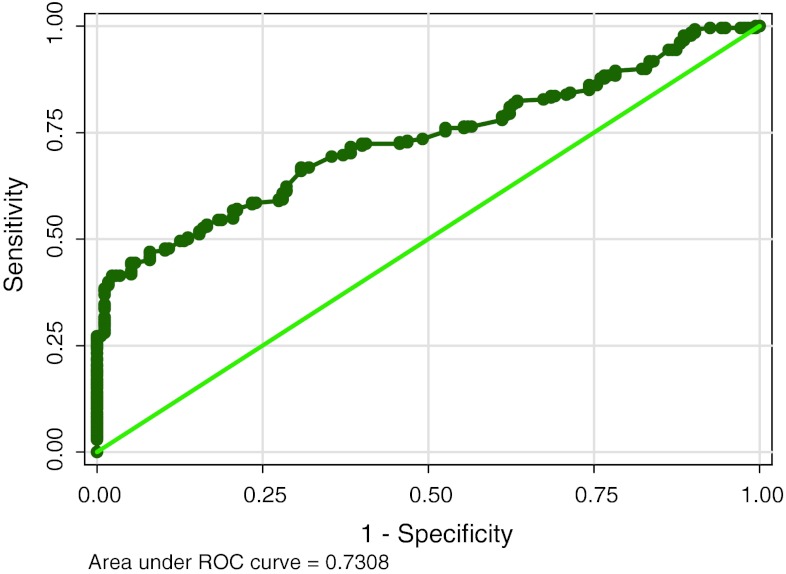
Fig. 1.
Showing the ROC and area under curve for HER-2/neu
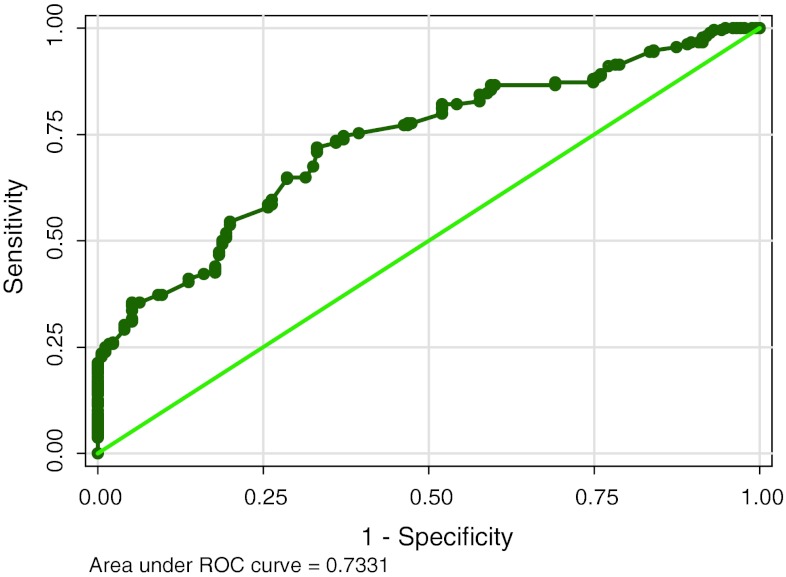
Fig. 2.
Showing the ROC and area under curve for CEA
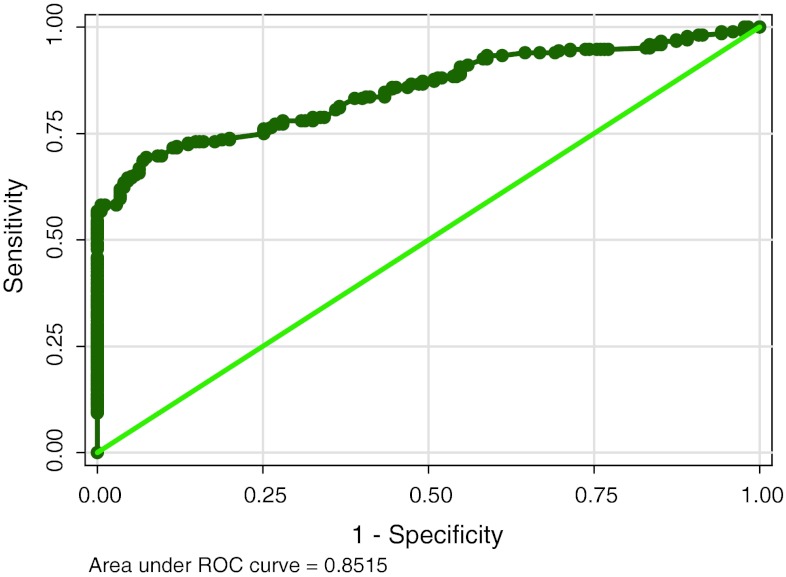
Fig. 3.
Showing the ROC and area under the curve for CA15-3
Table 1.
Diagnostic statistics of serum HER-2/neu, CEA and CA15-3 based on the standard cut-off values
| Diagnostic statistics | HER-2/neu (ng/ml) | CEA (ng/ml) | CA15-3 (IU/l) | HER-2/neu + CA15-3 | HER-2/neu + CEA | CEA + CA15-3 |
|---|---|---|---|---|---|---|
| Cut off | 15.0 | 5.0 | 35.0 | |||
| Sensitivity | 27.24 | 21.27 | 51.12 | 54.85 | 52.85 | 41.04 |
| Specificity | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| PPV | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| NPV | 47.30 | 45.34 | 57.19 | 59.12 | 59.12 | 52.55 |
| Classification accuracy | 55.98 | 52.37 | 70.43 | 72.69 | 71.49 | 64.33 |
| P value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
P value < 0.01 is significant
As shown in Table 2, ROC analysis for serum HER-2/neu is calculated at 85th percentile with LR+ 3.31 and LR− 0.578. Based on this correlation of serum markers between controls and cases are determined (Table 3). The cut off value for HER-2/neu is 11.0 ng/ml with odds ratio (OR) 6.39, 24 control and 135 cases have values above the cut off. The cut-off value for serum CA15-3 was 19.6 ng/ml. At this cut off 26 controls and 196 cases are included with OR 15.6. The cut-off value for CEA was 3.1 ng/ml at 85th percentile with OR 4.38 and 24 control and 110 cases have positive values.
Table 2.
Receiving operating characteristics (ROC) analysis of Her-2/neu
| Cut-off HER-2/neu (ng/ml) | Sensitivity (%) | Specificity (%) | % of Correct classification | LR+ | LR− |
|---|---|---|---|---|---|
| 10.4 | 56.72 | 79.43 | 65.69 | 2.757 | 0.5449 |
| 10.42 | 55.22 | 79.43 | 64.79 | 2.6845 | 0.5637 |
| 10.5 | 54.85 | 79.43 | 64.56 | 2.6664 | 0.5684 |
| 10.56 | 54.48 | 81.14 | 65.01 | 2.889 | 0.561 |
| 10.6 | 54.48 | 81.71 | 65.24 | 2.9792 | 0.5571 |
| 10.64 | 53.36 | 83.43 | 65.24 | 3.2199 | 0.5591 |
| 10.65 | 52.99 | 83.43 | 65.01 | 3.1974 | 0.5635 |
| 10.8 | 52.61 | 84.00 | 65.01 | 3.2882 | 0.5641 |
| 10.88 | 51.87 | 84.57 | 64.79 | 3.3617 | 0.5692 |
| 10.98 | 51.49 | 84.57 | 64.56 | 3.3375 | 0.5736 |
| 11 | 51.12 | 86.29 | 64.56 | 3.3133 | 0.578 |
| 11.12 | 50.37 | 86.29 | 64.56 | 3.673 | 0.5751 |
| 11.2 | 50.00 | 86.29 | 64.33 | 3.6458 | 0.5795 |
| 11.25 | 49.63 | 86.86 | 64.33 | 3.776 | 0.58 |
| 11.3 | 49.63 | 87.43 | 64.56 | 3.9476 | 0.5762 |
| 11.32 | 47.76 | 89.14 | 64.11 | 4.3991 | 0.586 |
| 11.34 | 47.76 | 89.71 | 64.33 | 4.6434 | 0.5823 |
Italicized row is related to 85th percentile
Table 3.
Correlation of serum markers between controls and cases on 85th percentiles
| Markers | Cut-off | Control (n = 175) | Cases (n = 268) | P value | OR | 95 % CI |
|---|---|---|---|---|---|---|
| CA15-3 (IU/l) | 19.6 | 26 (14.9 %) | 196 (73.1 %) | <0.001 | 15.60 | 9.49–25.63 |
| HER-2/neu (ng/ml) | 11.0 | 24 (13.7 %) | 135 (50.4 %) | <0.001 | 6.39 | 3.90–10.45 |
| CEA (ng/ml) | 3.10 | 24 (13.7 %) | 110 (41.0 %) | <0.001 | 4.38 | 2.67–7.18 |
P value < 0.01 is significant
85th percentile of the present data supports for the diagnostic cut-off for HER-2/neu and CA15-3 and validated by good Bayesian statistics and area under ROC (Table 4). Serum HER-2/neu has an added value over the CA15-3 in terms of reducing the large number of false negative patients. The false negative rate for HER-2/neu was 49.63 %, 26.87 % for CA15-3 and significantly reduced to 17.16 % (Z = 4.379, P < 0.001) when both HER-2/neu was combined with CA15-3 as biomarkers for carcinoma cases. Therefore, combined use of CA15-3 and HER-2/neu might increase the diagnostic value as well as it could reduce the false negative rate at significant level when compared to CA15-3 alone.
Table 4.
Diagnostic value of HER-2/neu and CA15-3 at 85th percentile
| Bayesian statistics | HER-2/neu (ng/ml) | CA15-3 (IU/l) | HER-2/neu + CA15-3 |
|---|---|---|---|
| 85th percentile | 11.00 | 19.6 | – |
| AUROC (95 % CI) | 0.73 (0.68–0.78) | 0.85 (0.82–0.89) | – |
| Pick-up rate in cases (%) | 84.91 | 88.29 | 82.84 |
| Sensitivity (%) | 51.12 | 73.13 | 82.84 |
| Specificity (%) | 86.29 | 85.14 | 73.71 |
| % of Correct classification | 64.56 | 77.88 | 80.01 |
| False negative rate (%) | 49.63 | 26.87 | 17.16 |
Discussion
Serum HER-2/neu, CA15-3 and CEA are not diagnostic markers. Still the markers have diagnostic value to increase the pickup rate of diseases. Combined values of two markers have increased the diagnostic values. Similarly cut off point at 85th percentile could reduce false negative rate. CA15-3 measurements served as “gold standard” to which HER-2 diagnostic and/or prognostic value was compared.
By calculating ROC curves we observed the highest area under curve for CA15-3, which points to the usefulness of this tumor marker in diagnosis.
The sensitivity, specificity, accuracy, PPV and NPV of the tumor markers were also determined both individually and in combination. The PPV = TP/[TP + FP] for the test sample is 100 % and the NPV = TN/[FN + TN] is 59.12 %.
The best combination of tumor markers which revealed 100 % specificity and 100 % PPV with 51.12 % sensitivity could be obtained by measurement of CA15-3 in serum. A higher sensitivity (54.85 %) with the same level of specificity (100 %) could be obtained by the addition of HER-2/neu measurement.
Studies from Kobayashi et al. [7] showed the usefulness of eight tumor markers in ovarian cancer at an early stage and was evaluated from the point of view of their diagnostic value. According to Visintin et al. [8] six markers provided a significant improvement over CA-125 alone for ovarian cancer detection. Also Rossi et al. [9] proved the combination of two marker HBME-1 and galectin-3 in tissue samples increased the sensitivity and specificity for thyroid cancer detection.
There are two ways to define the cut-off values viz; standard cut-off (conventional) and study defined (or calculated) cut off. Standard cut-off is already established by the manufacturers using large population, whereas study defined cut off is calculated at 85th percentile and the diagnostic value is measured in this study. When the percentile of cut off is decreased from 100 to 85 more number of patients and few control individuals show the positive values for markers. So when the diagnostic values of two markers are combined at this point, false negative rate is decreased. Careful monitoring for these groups of patient may help in the management of disease.
When diagnostic utility of two markers were combined (HER-2/neu + CA15-3), sensitivity was increased and false negative rate was decreased to 17.16 %. HER-2/neu had an added value over the CA15-3 in terms of reducing the large number patients of false negative values indicating that combined markers had an added value while predicting markers’ diagnostic statistics. HER-2/neu can be able to pick up an additional number (10.82 %) of new patients when it is combined with CA15-3.
According to Baskic et al. [10] a combination of markers was more sensitive than using one marker alone. The combined measurement of CEA, CA15-3 and TAG 72 in pleural fluid is a useful complementary test in the differential diagnosis of pleural effusions of malignant origin [11]. Conversion to serum HER-2/neu positive status occurs in approximately 25 % of patients who receive first-line hormone therapy with either letrozole or tamoxifen [12]. Therefore, the utility of diagnostic values of serum markers may also be applicable in the follow up group of HER-2/neu negative patients. The results of our study suggest that the measurement of multiple tumor markers may be employed as combination and at 85th percentiles to assess the prognosis in breast cancer patients.
Acknowledgments
Authors thank Indian Council of Medical Research, Delhi, India for financial support (Grant No. 3|2|2|57|2004, NCD-III).
References
- 1.Akiyama T, Sudo C, Ogawara H, Toyoshima K, Yamamoto T. The product of the human c-erbB-2 gene: a 185-kilodalton glycoprotein with tyrosine kinase activity. Science. 1986;232:1644–1646. doi: 10.1126/science.3012781. [DOI] [PubMed] [Google Scholar]
- 2.Dougall WC, Qian X, Peterson NC, Miller MJ, Samanta A, Greene MI. The neu-oncogene: signal transduction pathways, transformation mechanisms and evolving therapies. Oncogene. 1994;9:2109–2123. [PubMed] [Google Scholar]
- 3.Mielke S, Meden H, Raab T, Wuttke W, Kuhn W. Effects of interfering and influencing factors on the analyses of p105 (c-erbB-2/HER-2) oncoprotein fragment in serum. Anticancer Res. 1997;17:3125–3227. [PubMed] [Google Scholar]
- 4.Wu JT, Zhang P, Astill ME, Lyons BW, Wu LH. Identification and characterization of c-erbB-2 proteins in serum, breast tumor tissue, and SK-BR-3 cell line. J Clin Lab Anal. 1995;9:141–150. doi: 10.1002/jcla.1860090302. [DOI] [PubMed] [Google Scholar]
- 5.Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER-2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999;59:1196–1201. [PubMed] [Google Scholar]
- 6.Brandt-Rauf PW. The c-erb B transmembrane growth factor receptors as serum biomarkers in human cancer studies. Mutat Res. 1995;333:203–208. doi: 10.1016/0027-5107(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H, Sumimoto K, Terao T, Kawashima Y. Diagnostic value of serological tumor marker tests in patients with ovarian cancer. Nippon Sanka Fujinka Gakkai Zasshi. 1989;41:1501–1506. [PubMed] [Google Scholar]
- 8.Visintin I, Feng Z, Longton G, Ward DC, Alvero AB, Lai Y, et al. Diagnostic markers for early detection of ovarian cancer. Clin Cancer Res. 2008;14:1065–1072. doi: 10.1158/1078-0432.CCR-07-1569. [DOI] [PubMed] [Google Scholar]
- 9.Rossi ED, Raffaelli M, Mule A, Miragilia A, Lombardi CP, Veccio FM, Fadda G. Simultaneous immunohistochemical expression of HBME-1 and galectin-3 differentiates papillary carcinomas from hyperfunctioning lesions of the thyroid. Histopathology. 2006;48:795–800. doi: 10.1111/j.1365-2559.2006.02428.x. [DOI] [PubMed] [Google Scholar]
- 10.Baskic D, Ristic P, Pavlovic S, Arsenijevic N. Serum HER-2 and CA15-3 in breast cancer patients. J BUON. 2004;9:289–294. [PubMed] [Google Scholar]
- 11.Gaspar MJ, Miguel JD, Garcia Diaz JD, Diez M. Clinical utility of a combination of tumour markers in the diagnosis of malignant pleural effusions. Anticancer Res. 2008;28:2947–2952. [PubMed] [Google Scholar]
- 12.Lipton A, Leitzel K, Ali SM, Demers L, Harvey HA, Chaudri-Ross HA, et al. Serum HER-2/neu conversion to positive at the time of disease progression in patients with breast carcinoma on hormone therapy. Cancer. 2005;104:257–263. doi: 10.1002/cncr.21202. [DOI] [PubMed] [Google Scholar]