Abstract
Background
Although periacetabular osteotomy (PAO) for developmental dysplasia of the hip (DDH) provides conceptual advantages compared with other osteotomies and reportedly is associated with joint survivorship of 60% at 20 years, the beneficial effect of proper acetabular reorientation with concomitant arthrotomy and creation of femoral head-neck offset on 10-year hip survivorship remains unclear.
Questions/purposes
We asked the following questions: (1) Does the 10-year survivorship of the hip after PAO improve with proper acetabular reorientation and a spherical femoral head; (2) does the Merle d’Aubigné-Postel score improve; (3) can the progression of osteoarthritis (OA) be slowed; and (4) what factors predict conversion to THA, progression of OA, or a Merle d’Aubigné-Postel score less than 15 points?
Methods
We retrospectively reviewed 147 patients who underwent 165 PAOs for DDH with two matched groups: Group I (proper reorientation and spherical femoral head) and Group II (improper reorientation and aspherical femoral head). We compared the Kaplan-Meier survivorship, Merle d’Aubigné-Postel scores, and progression of OA in both groups. A Cox regression analysis (end points: THA, OA progression, or Merle d’Aubigné-Postel score less than 15) was performed to detect factors predicting failure. The minimum followup was 10 years (median, 11 years; range, 10–14 years).
Results
An increased survivorship was found in Group I. The Merle d’Aubigné-Postel score did not differ. Progression of OA in Group I was slower than in Group II. Factors predicting failure included greater age, lower preoperative Merle d’Aubigné-Postel score, and the presence of a Trendelenburg sign, aspherical head, OA, subluxation, postoperative acetabular retroversion, excessive acetabular anteversion, and undercoverage.
Conclusions
Proper acetabular reorientation and the creation of a spherical femoral head improve long-term survivorship and decelerate OA progression in patients with DDH.
Level of Evidence
Level III, therapeutic study. See Guidelines for Authors for a complete description of levels of evidence.
Introduction
The Bernese periacetabular osteotomy (PAO) has become an established method for treating developmental dysplasia of the hip (DDH) in adolescents and adults. Since the first PAO in 1984 [7], the surgical technique has evolved with increasing experience and better understanding of hip pathomechanics. In particular, femoroacetabular impingement (FAI) has been recognized as a major pathomechanism, leading to hip pain and osteoarthritis (OA) [8]. This concept is important when correcting dysplastic hips because acetabular malpositioning may lead to an iatrogenic pincer-type impingement, especially in cases with lateral overcoverage or retroversion [23, 50]. The typically aspherical femoral head in DDH [34] will increase the risk of secondary cam-type FAI that is reportedly symptomatic in 48% of all cases after reorientation [50].
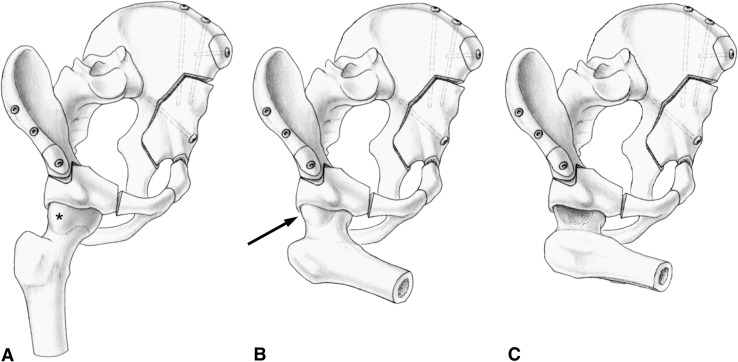
Based on these observations, in 1996 we modified our surgical technique. We took care to avoid retroverting the acetabulum. Nonspherical heads were treated by correcting the femoral head-neck offset through an anterior arthrotomy. Osteochondroplasty was performed with chisels or a high-speed burr and was indicated if internal rotation in 90° flexion was compromised as a result of an impingement between the aspherical head-neck portion and the reoriented acetabulum. As a result of the decreased lunate surface area in DDH [12], it is impossible to restore normal femoral coverage by reorienting the dysplastic acetabulum. We therefore introduced “optimal acetabular orientation”, which was intended to provide the best reasonable orientation given the anatomic constraints. Acetabular orientation was assessed by the following six radiographic parameters: (1) total femoral coverage; (2) anterior coverage; (3) posterior coverage; (4) lateral center-edge (LCE) angle; (5) acetabular index; and (6) extrusion index [37]. We considered an acetabular reorientation as optimal if at least four of six of these radiographic parameters were within a previously determined normal range [36]. Although the PAO provides conceptual advantages and reportedly is associated with a preservation rate of the native joint of 60% at 20 years [33], the beneficial effect of an optimal acetabular reorientation with concomitant arthrotomy and creation of the femoral head-neck offset (Fig. 1) remains unclear.
Fig. 1A–C.
The current technique of periacetabular osteotomy is shown. (A) Typically, dysplastic hips present with an aspherical femoral head (asterisk). Before PAO, this decreased head-neck offset is compensated by the diminished anterior acetabular coverage. (B) After proper reorientation, femoroacetabular impingement may become apparent in deep flexion and internal rotation (arrow). Iatrogenic acetabular overcoverage can increase this conflict. (C) Through an intraoperative arthrotomy, the FAI conflict can be assessed. If necessary, a concomitant osteochondroplasty of the femoral neck can be performed.
We, therefore, raised the following questions: (1) Does the cumulative 10-year survivorship of the hip after PAO improve with an optimal acetabular reorientation and a spherical femoral head compared with hips after PAO with suboptimal acetabular reorientation and/or an aspherical femoral head; (2) are the clinical outcomes (Merle d’Aubigné-Postel score, impingement and apprehension tests, limp, Trendelenburg sign, and ROM) in these hips superior at 10 years; (3) can the progression of radiographic OA be slowed in these hips; and (4) what factors predict conversion to THA, progression of OA, or a Merle d’Aubigné-Postel score less than 15 points?
Materials and Methods
We retrospectively reviewed 147 patients who underwent 165 PAOs between 1984 and 2010. During that time, we performed more than 1300 PAOs for symptomatic DDH in adolescents and adults. For this study two series of PAOs were included: the first series consisted of all 75 PAOs in 63 patients (12 bilateral) performed from April 1984 to December 1987 [7, 30, 33]. The second series consisted of all 90 PAOs in 83 patients (seven bilateral) from January 1997 to January 2000. The indication for PAO was painful DDH in both series [45]. The contraindications were: (1) OA Grade 3 according to Tönnis [40] in patients older than 30 years; (2) skeletally immature patients with an open triradiate cartilage; and (3) high dislocation with the femoral head articulating with a pseudoacetabulum [45]. Beginning with the first case of the second series, arthrotomy was routinely performed for observation and as a potential treatment of FAI. We allocated each of the 165 hips to two groups: Group I consisted of 43 hips (42 patients) with an optimal acetabular reorientation and an intraoperatively corrected or a priori present spherical femoral head. Group II consisted of 122 hips (105 patients) with a suboptimal acetabular reorientation and/or a persistent aspherical femoral head. All patients from the two series were allocated to one of the groups and no patient was excluded. The allocation was based on radiographic parameters for subsequently found morphologic features of the acetabular and femoral head. Group I included eight hips (19%) from the first series and 35 hips (81%) from the second series. Group II consisted of 67 hips (55%) from the first series and 55 hips (45%) from the second series. Three patients (four hips [2%]) were lost to followup. One patient (two hips [2.5%]) died 6 years postoperatively from a cause unrelated to the PAO. Two patients (two hips [2.5%]) were lost to followup 1.2 and 1.8 years after surgery without undergoing conversion to THA. The average followup of the remaining patients was 11 ± 1 year (range, 10–13 years) for Group I and 11 ± 1 year (range, 10–14 years) for Group II. Our institutional review board approved the study.
We performed a power analysis for the primary research question regarding survivorship at 10 years followup with a two-sided level of significance of 5%, beta error of 20%, known survivorship of 87.6% [30, 33], SD of 3.85% [30, 33], and minimal detectable difference of 2% resulting in a minimal sample size of 30 hips per group.
To analyze whether a specific morphologic feature of the hip influences survivorship after PAO, the two groups should be comparable for confounding variables such as known predictive factors or other demographic factors. Known factors predicting the survival of the native hip after PAO reported in the literature are preoperative age, OA, Merle d’Aubigné-Postel score, anterior impingement test, limp, and postoperative extrusion index [33]. The two study groups did not differ for any of these known predictive factors or any other demographic (Table 1), preoperative clinical (Table 2), or preoperative radiographic parameters (Table 3). In terms of demographic factors (Table 1), Group II had a higher prevalence of previous surgery (25% versus 14%; p = 0.106). We found no difference for age at operation, operation time, or blood loss (Table 1). The prevalence of positive impingement or apprehension tests was increased in Group I (p = 0.061 and p = 0.995, respectively), whereas the prevalence for limp was increased in Group II (p = 0.247). We observed no clinically important difference (p = 0.395) in the mean Merle d’Aubigné-Postel score between the two groups. In Group II the prevalence of hips with advanced OA (Tönnis Grades 2 and 3) was 7% compared with 18% in Group I (p = 0.421). There were no differences for acetabular morphologic features (Table 3).
Table 1.
Demographic data of the patients
| Parameter | Group I | Group II | p value |
|---|---|---|---|
| Number of patients (hips) | 42 (43) | 105 (122) | |
| Age at surgery* (years) | 29 ± 11 (12–55) [26–31] | 28 ± 9 (13–44) [27–31] | – |
| Sex (% of all male hips) | 31 | 22 | 0.721 |
| Side (% of all right hips) | 60 | 48 | 0.124 |
| Weight* (kg) | 64 ± 14 (47–113) [59–70] | 63 ± 14 (31–108) [60–66] | – |
| Height* (cm) | 169 ± 9 (153–187) [165–172] | 166 ± 10 (144–193) [164–168] | – |
| BMI* (kg/m2) | 23 ± 4 (19–33) [21–24] | 23 ± 4 (15–33) [22–23] | – |
| Comorbidity (%) | 5 | 7 | 0.491 |
| Previous surgery (%) | 14 | 25 | 0.106 |
| Operation time* (hours) | 2.9 ± 0.5 (2–5) [2.8–3.1] | 3.2 ± 0.8 (2–5) [3.1–3.3] | – |
| Blood loss* (L) | 1.9 ± 0.9 (0.4–4.6) [1.5–2.1] | 1.8 ± 0.8 (0.7–4.4) [1.7–2.1] | – |
| Concomitant intertrochanteric osteotomy (%) | 10 | 23 | 0.166 |
* Values of continuous parameters are expressed as mean ± SD with range in parentheses and confidence intervals in brackets.
Table 2.
Clinical data for Group I and Group II
| Parameter | Group I | Group II | ||
|---|---|---|---|---|
| Preoperative | 10-year followup | Preoperative | 10-year followup | |
| Merle d’Aubigné-Postel score* [3] | 15 ± 1.7 (8–18) [14–15] | 16 ± 2.1 (9–18)‡ [15–17] | 15 ± 2.1 (7–18) [14–15] | 16 ± 1.9 (10–18)‡ [16–16] |
| Impingement test [38] (% of all hips) | 79 | 10†,‡ | 62 | 26‡ |
| Apprehension test [38] (% of all hips) | 48 | 11‡ | 29 | 20 |
| Limp (% of all hips) | 28 | 10†,‡ | 35 | 26 |
| Trendelenburg sign [9] (% of all hips) | 28 | 11 | 20 | 17 |
| ROM | ||||
| Flexion* (°) | 112 ± 15 (90–140) [107–117] | 99 ± 11 (70–120)‡ [95–103] | 112 ± 18 (80–140) [109–115] | 101 ± 12 (80–130)‡ [98–103] |
| Extension* (°) | 2 ± 6 (−20 to 15) [0–4] | 5 ± 5 (0–15) [3–7] | 1 ± 6 (−10 to 20) [0–2] | 6 ± 8 (0–30)‡ [4–7] |
| Internal rotation* (°) | 28 ± 19 (−50 to 70) [22–34] | 22 ± 15 (0–45) [17–27] | 26 ± 16 (−10 to 70) [23–29] | 19 ± 14 (0–60)‡ [16–21] |
| External rotation* (°) | 38 ± 11 (10–60) [34–41] | 33 ± 12 (0–50) [28–37] | 39 ± 15 (10–80) [36–42] | 30 ± 17 (0–80)‡ [27–33] |
| Abduction* (°) | 39 ± 12 (10–80) [36–43] | 37 ± 12 (10–60) [32–42] | 38 ± 11 (10–70) [36–40] | 34 ± 12 (0–60) [31–36] |
| Adduction* (°) | 29 ± 10 (10–60) [26–33] | 28 ± 11 (10–50) [23–33] | 28 ± 9 (1–50) [26–30] | 26 ± 8 (10–50) [24–27] |
* Values of continuous parameters are expressed as mean ± SD with range in parentheses and confidence intervals in brackets; †significant difference compared with Group II; ‡significant difference compared with preoperative status.
Table 3.
Radiographic data for Group I and Group II
| Parameter | Normal values [36] | Group I | Group II | ||
|---|---|---|---|---|---|
| Preoperative | Postoperative | Preoperative | Postoperative | ||
| Osteoarthritis score [40] (%)† | ‡ | ||||
| Grade 0 | 47 | 47 | 44 | 41 | |
| Grade 1 | 47 | 44 | 38 | 35 | |
| Grade 2 | 5 | 0 | 18 | 11§ | |
| Grade 3 | 2 | 9 | 0 | 13§ | |
| Total femoral coverage* (%) | 70–83 | 59 ± 9 (34–76) [57–62] | 81 ± 7 (68–100)§ [78–83] | 59 ± 15 (12–100) [56–62] | 84 ± 14 (10–100)§ [81–86] |
| Anterior coverage* (%) | 15–26 | 14 ± 7 (1–31) [12–16] | 16 ± 6 (4–33) [14–17] | 14 ± 7 (0–31) [12–15] | 16 ± 10 (0–47)§ [15–18] |
| Posterior coverage* (%) | 36–47 | 35 ± 8 (17–50) [32–36] | 38 ± 8 (3–48)§ [36–40] | 34 ± 12 (0–63) [31–36] | 40 ± 15 (1–82)§ [37–42] |
| Lateral center-edge angle* [46] (°) | 23–33 | 9 ± 8 (−17 to 24) [6–11] | 29 ± 6 (21–48)‡,§ [27–31] | 7 ± 9 (−20 to 30) [5–10] | 33 ± 12 (2–67)§ [31–36] |
| Acetabular index* [41] (°) | 3–13 | 22 ± 8 (3–50) [19–24] | 3 ± 5 (−9 to 20)§ [2–5] | 24 ± 10 (0–60) [21–25] | 2 ± 9 (−20 to 30)§ [1–4] |
| Extrusion index* [22] (%) | 17–27 | 40 ± 7 (26–63) [37–42] | 20 ± 7 (−5 to 32)‡,§ [18–22] | 40 ± 11 (7–81) [38–42] | 15 ± 11 (−13 to 39)§ [13–17] |
| Crossover sign [28] (% positive) | Negative | 36 | 15§ | 42 | 21§ |
| Shenton’s line intact (% intact) | Intact | 67 | 91‡,§ | 73 | 72 |
* Values of continuous parameters are expressed as mean ± SD with range in parentheses and confidence intervals in brackets; †osteoarthritis values were obtained at the most recent followup; hips with conversion to THA were included and graded as Tönnis Grade 3; ‡significant difference compared with Group II; §significant difference compared with preoperative status.
The original operative technique was described previously in detail [7, 29]. In brief, a modified Smith-Petersen approach was performed followed by four osteotomies and a controlled fracture to completely mobilize the acetabulum. This technique allows for substantial acetabular reorientation potential. The posterior column of the pelvis remains intact, maintaining stability of the pelvic ring and adding protection for the sciatic nerve. After recognition of the FAI concept, the most relevant technical changes included (1) correct three-dimensional (3-D) reorientation of the acetabulum; and (2) routine anterior capsulotomy and, if necessary, correction of the femoral head-neck offset. In all cases, one or more intraoperative radiographs were acquired to verify the orientation of the acetabular fragment with special emphasis on the version of the acetabular fragment. A routine anterior T-shaped capsulotomy was performed after temporary acetabular reorientation with assessment of ROM. Often, dysplastic hips present with an elliptical femoral head [34]. Before acetabular correction, this lack of head-neck offset is compensated by the diminished anterior acetabular coverage. After proper reorientation, this can lead to iatrogenic FAI with the acetabular rim (Fig. 1). If necessary, creation of the anterolateral femoral head-neck offset is accomplished by resecting the aspherical head-neck portion with chisels or a high-speed burr to achieve the desired minimum ROM of 90° flexion and 30° internal rotation. In the second series including only PAOs with a concomitant arthrotomy, offset creation was performed in 51 hips (57%). Surgical treatment of the labrum was performed in 21 hips (49%), including one hip (2%) with labrum refixation, 10 hips (23%) with labrum resection, and 10 hips (23%) with resection of labral cysts.
As a result of the continuity of the pelvic ring, early crutch mobilization with partial weightbearing was possible. Weightbearing was restricted to 15 kg for 8 weeks. During their hospital stay (range, 5–12 days), patients were maintained on continuous passive motion to prevent capsular adhesions. Patients were advanced to full weightbearing after 8 weeks if beginning of bone healing was visible on radiographs. At 8 weeks postoperatively, physiotherapy was started to improve ROM and muscle strength, typically for a duration of 2 to 3 months.
Patients were routinely evaluated clinically and radiographically in our outpatient clinic at 8 weeks, 3 months, 1 year, 2 years, and every 5 years after surgery. Clinical examinations included anterior impingement and apprehension test, limp, Trendelenburg sign, and full ROM. Radiographic evaluation consisted of a supine AP pelvic radiograph and a lateral crosstable radiograph [6] of the proximal femur before surgery and at followup. An additional standing false profile view [18] and an AP pelvic view radiograph with the hip in abduction were performed preoperatively for the first series. At 10 years we were able to examine 126 of the 147 patients. Using our records and contacting patients by phone, we identified all remaining 18 patients who had known conversions to THAs and recorded the dates of conversions. There were no patients without clinical examination or telephone followup. Clinical evaluations of the patients in both study groups were performed preoperatively and at the 10-year followups. The clinical grading system of Merle d’Aubigné-Postel was used [3]. Additionally, the clinical evaluation included the anterior impingement and apprehension test [38], limp, the Trendelenburg sign [9], and ROM determined by a goniometer.
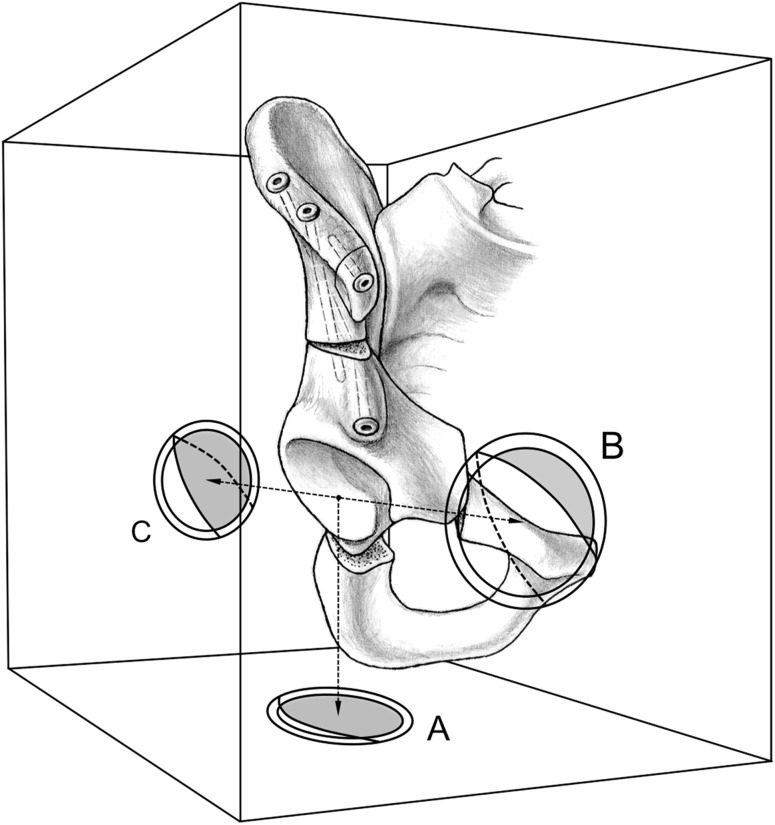
Two of us (CEA, SDS, neither a treating surgeon) graded the preoperative and postoperative OA according to the Tönnis classification [40]. For evaluation of OA progression, hips with conversion to THA were graded Tönnis Grade 3. Evaluation of interobserver and intraobserver agreement for the Tönnis classification in hips with DDH and PAO was performed in a previous study [33]. Interobserver agreement showed a kappa of 0.74 for both measurements and intraobserver agreement had a kappa of 0.73 for Observer 1 and a kappa of 0.76 for Observer 2. To describe the morphologic features of the acetabulum and femoral head, a series of eight standard radiographic parameters (Table 3) was assessed and computerized by two observers (CEA, SDS; no treating surgeons) using previously described and validated computer software (Hip2Norm software; University of Bern, Bern, Switzerland) [37, 39, 49]. The acetabular orientation was assessed by the following six radiographic parameters: (1) total femoral coverage; (2) anterior coverage; (3) posterior coverage (Fig. 2); (4) LCE angle; (5) acetabular index; and (6) extrusion index (Table 3). Because the morphologic features of a dysplastic acetabulum differ from those of a normal acetabulum [12], the acetabular reorientation was defined as optimal if at least four of these six radiographic parameters were within a previously determined normal range [36]. Because the original radiographs were not calibrated, we did not adjust the radiographic values for variances of pelvic tilt but for pelvic rotation using Hip2Norm [37, 39, 49]. In addition, head sphericity was assessed by the following four parameters: (1) head sphericity index [34]; (2) pistol grip deformity [35]; (3) sagging rope sign [1]; and (4) alpha angle [25]. A head was defined as aspherical if one of the four parameters was not within normal range: (1) sphericity index of 0.87 or less (n = 91 [82%]); (2) pistol grip deformity [35] (n = 14 [13%]); (3) sagging rope sign [1] (n = 20 [18%]); and/or (4) an alpha angle of 50° or greater [25] (n = 35 [32%]).
Fig. 2.
The definition of differing degrees of acetabular coverage is shown. The total femoral coverage is defined as the craniocaudal coverage of the femoral head by the acetabular rim (A). The anterior acetabular coverage is defined as the amount of coverage of the femoral head by the anterior acetabular wall in the AP direction (B). The posterior acetabular coverage is defined as the amount of coverage of the femoral head by the posterior acetabular wall in the posteroanterior direction (C).
Survivorship analysis was performed according to Kaplan and Meier [15]. The end points were defined as (1) conversion to THA; (2) radiographic progression of OA; or (3) a Merle d’Aubigné-Postel score less than 15 points. The differences between two survivorship curves of the two groups were calculated by using the log-rank test. Differences in clinical and radiographic outcomes between the two groups preoperatively and at 10 years followup were calculated using the Wilcoxon signed-rank test for continuous data and Fisher’s exact test for binominal data. Preoperative and postoperative differences in each group were calculated using the Mann-Whitney U test for continuous data and Fisher’s exact test for binominal data. Differences in the Tönnis classifications were analyzed with the Kruskal-Wallis test between Groups I and II and the Friedman test for changes within the groups. The univariate Cox proportional hazards model was used to detect possible predictive factors to reach one of the previously defined end points [2]. The resulting total of 16 factors from the univariate analysis (Table 4) then was adjusted using the multivariate Cox proportional hazard model and corresponding hazard ratios were calculated.
Table 4.
Predictive factors for conversion to THA, radiographic progression of OA, or Merle d’Aubigné-Postel score less than 15 points with corresponding hazard ratios
| Category | Parameter | Crude hazard ratio | p value (hazard ratio) | Adjusted hazard ratio | p value (adjusted hazard ratio) |
|---|---|---|---|---|---|
| Demographic factors | Age > 30 years | 3.3 (2.5–4.1) | < 0.001 | 4.1 (3.3–4.9) | < 0.001 |
| Preoperative Merle d’Aubigne-Postel score < 15 | 3.3 (2.5–4.1) | < 0.001 | 4.7 (3.9–5.6) | < 0.001 | |
| Preoperative positive Trendelenburg sign | 2.0 (1.5–2.6) | 0.017 | 2.2 (1.6–2.9) | 0.015 | |
| Preoperative radiographic factors | Nonspherical head | 2.0 (1.4–2.5) | 0.013 | 2.0 (1.5–2.6) | 0.010 |
| Preoperative osteoarthritis ≥ Grade 1 | 5.8 (4.9–6.7) | < 0.001 | 3.7 (2.4–5.0) | 0.042 | |
| Severin > Grade 3 | 1.9 (1.3–2.6) | 0.05 | 2.4 (1.6–3.3) | 0.047 | |
| Postoperative factors related to surgical accuracy | Total femoral coverage of < 70% (undercoverage) | 2.6 (1.8–3.4) | 0.017 | NA | – |
| Total femoral coverage of > 83% (overcoverage) | 2.5 (1.7–3.4) | 0.031 | NA | – | |
| Anterior femoral coverage of > 27% (overcoverage) | 1.9 (1.3–2.5) | 0.045 | NA | – | |
| Posterior femoral coverage of < 36% (undercoverage) | 1.9 (1.2–2.5) | 0.045 | NA | – | |
| Excessive acetabular anteversion* | 2.2 (1.4–2.9) | 0.043 | 6.0 (4.5–7.5) | 0.019 | |
| Acetabular retroversion† | 3.3 (2.8–3.8) | 0.013 | 3.7 (2.9–4.4) | < 0.001 | |
| Extrusion index > 27% (undercoverage) | 2.2 (1.6–2.8) | 0.011 | NA | – | |
| Acetabular index > 14° (undercoverage) | 2.5 (1.7–3.3) | 0.023 | NA | – | |
| LCE < 22° (undercoverage) | 2.2 (1.6–2.9) | 0.013 | 2.2 (1.5–2.9) | 0.022 | |
| No offset correction in a nonspherical femoral head | 2.4 (2.1–2.7) | 0.003 | 2.8 (2.2–3.4) | < 0.001 |
Values expressed as mean with 95% confidence interval in parentheses; * defined as deficient anterior acetabular coverage (< 15%) and excessive acetabular posterior coverage (> 47%); †defined as excessive anterior acetabular coverage (> 27%) and deficient posterior acetabular coverage (< 36%) [30]; LCE = lateral center-edge angle; NA = not applicable.
Results
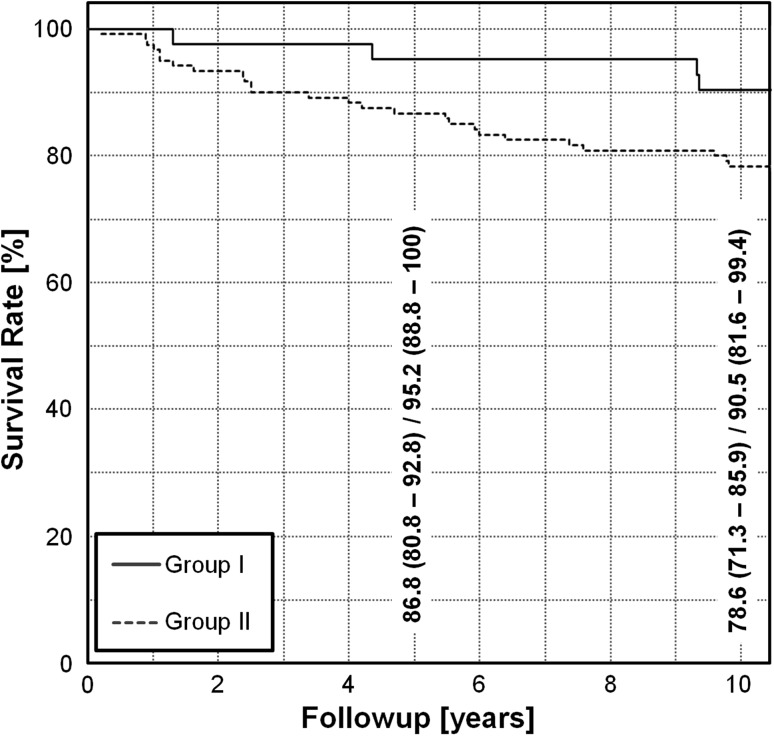
The cumulative 10-year survivorship of Group I was higher (p = 0.039) than that of Group II: 90% (95% CI, 82%–99%) versus 78% (95% CI, 71%–86%), respectively (Fig. 3). In Group I, three hips (7%) were converted to a THA, and one patient (2%) had a Merle d’Aubigné-Postel score less than 15 points. In Group II, 16 hips (13%) were converted to a THA, three hips (2%) had evidence of radiographic progression of OA, and seven hips (6%) had a Merle d’Aubigné-Postel score less than 15 points.
Fig. 3.
The Kaplan-Meier survivor analysis is shown for both groups with the end points defined as a conversion to THA, progression of OA, or a Merle d’Aubigné-Postel score of less than 15. Group I was comprised of all the hips with optimal acetabular reorientations and corrected or a priori spherical femoral heads. Group II was comprised of the hips with suboptimal acetabular reorientations and/or aspherical heads.
For both groups the Merle d’Aubigné-Postel scores improved (p < 0.001 for both) for surviving hips by the 10-year followup (Table 2). We found no difference (p = 0.984) in the Merle d’Aubigné-Postel scores at 10 years between the two groups. The prevalence of the anterior impingement test decreased (p < 0.001 for both) in both groups at 10 years. At followup, the prevalence of a positive impingement test was lower (p = 0.014) for Group I in comparison to Group II. The prevalence of a positive apprehension test decreased (p < 0.001) for Group I. The prevalence of limp decreased in Group I from preoperatively to postoperatively (p < 0.001) and was lower than in Group II postoperatively (p < 0.001). There were no differences in the prevalence of a Trendelenburg sign or ROM between groups at 10 years followup.
Radiographic OA did not progress (p = 0.647) in Group I in comparison to Group II in which the Tönnis grade increased (p = 0.014). Including the hips with conversion to THA and counting them as Tönnis Grade 3, there was an increase of OA in three hips (7%) in Group I and 19 hips (16%) in Group II (p = 0.119).
We found 10 factors predicting failure after PAO (Table 4). Of those 10 factors, three were demographic, three were preoperative radiographic factors, and four were related to surgical accuracy. These included correct 3-D orientation of the acetabular fragment and adequate treatment of an additional cam deformity.
Discussion
During the last decade, PAO has become a state-of-the-art treatment for adolescent and adult patients with DDH. The recognition of FAI, improved imaging modalities, and technical advancements have contributed to improved understanding of the 3-D anatomy of the hip. Because of this, more emphasis was put on correct acetabular orientation and surgical technique was adapted including an arthrotomy of the joint with offset creation if necessary. Other than case reports [23], however, the benefit from those modifications has not been documented in a large patient cohort. We, therefore, raised the following questions: (1) Does the cumulative 10-year survivorship of the hip after PAO improve with optimal acetabular reorientation and a spherical femoral head compared with hips after PAO with suboptimal acetabular reorientation and/or an aspherical femoral head; (2) are the clinical outcomes (Merle d’Aubigné-Postel score, impingement and apprehension test, limp, Trendelenburg sign, and ROM) in these hips superior at 10 years; (3) can the progression of radiographic OA be slowed in these hips; and (4) what factors predict conversion to THA, progression of OA, or a Merle d’Aubigné-Postel score less than 15 points?
This study has several limitations. First, we pooled two patient series from two different decades. The goal of the study was to test whether hips with optimal femoral and acetabular morphologic features have superior survivorship after PAO compared with hips with a potential impingement conflict. Therefore, all other factors predicting survivorship should be comparable in these two groups. We allocated the hips from the two series to the two study groups based on radiographic parameters describing acetabular and femoral morphologic features. No hip was excluded and no selection bias existed. However, the prevalence of possibly confounding demographic, clinical, and morphologic factors (Tables 1–3) is not always perfectly matched. The two groups are still considered comparable because discrepancies were evenly distributed between the two groups. Although the Tönnis grade of preoperative OA, prevalence of limp, number of previous surgeries, and comorbidities were higher in Group II, we found a lower preoperative Merle d’Aubigné-Postel score, higher mean age, higher prevalence of the preoperative anterior impingement test, apprehension test, and Trendelenburg sign in Group I. In addition, the p values for all factors between the groups never fell below 0.11 indicating that none of the confounding factors relevantly influence the survivorship. Therefore, we believe the resulting difference of 12% in survivorship after PAO at 10 years followup is the result of the different acetabular and femoral morphologic features between the two groups. Second, all clinical parameters were assessed by different observers at each followup. This is an unavoidable potential source of error for a retrospective study spanning more than a decade. Nevertheless, because the assessed clinical parameters could be proven to have substantial interobserver and intraobserver agreement [13, 16], we do not believe this would jeopardize our observations or conclusions. Third, the radiographs in the first series were not taken in a standardized manner. Therefore, the radiographic measurements are prone to error as a result of pelvic malpositioning during radiograph acquisition. To minimize this source of error, the radiographs were corrected for malrotation using the software Hip2Norm [37, 39, 49].
We found higher survivorship of hips after PAO at the 10-year followup if the acetabulum was properly oriented and a spherical femoral head was present preoperatively or after femoral osteochondroplasty through an arthrotomy (Fig. 4). Compared with the literature, Group I in the current study showed a superior survivorship rate of 90% at 10 years followup (Table 5) [5, 11, 17, 21, 26, 33, 44, 48]. Reported survivorship rates for PAO ranged from 54% to 85% [11, 17, 21, 33]. For triple and Chiari osteotomies, the reported survival rates at 10 years followup were 73% and 84%, respectively (Table 5) [5, 26]. The survivorship rate in our Group II, including hips with morphologic features predisposing to FAI, was 78% and was at the lower range of the reported rates in the literature (Table 5). Survival rates depend on the definition of end points, which could explain differences when comparing our results with those in the literature. Some studies [5, 17, 21] used a combination of clinical and radiographic end points and therefore are comparable to our study. There are two clinical implications of these findings. First, accuracy of acetabular reorientation is crucial and difficult. Before the acetabular fragment is fixed definitively, the temporary acetabular orientation should be assessed carefully by using intraoperative radiographs. It is important to recognize a dysplastic acetabulum differs from a normal acetabulum. Hipp et al. [12] showed that the lunate surface in normal hips was 26% larger when compared with dysplastic hips. A dysplastic acetabulum, therefore, can be reoriented only as close as possible to a normal acetabulum. The deficient contact area between the femoral head and acetabulum remains unchanged. Reconstitution of completely normal femoral coverage is rare after PAO. Only 10 hips (6%) of all patients had a postoperative acetabular configuration with all six predefined radiographic parameters lying in the normal range. Second, we believe it is important to correct the head-neck offset if an aspherical femoral head is present. This should be addressed because it reportedly improves survivorship [8, 27, 31]. The assessment of the asphericity can be made preoperatively by conventional radiographs, MRI arthrography with radial sequencing, or intraoperatively by arthrotomy. We found no negative effect on survivorship if an arthrotomy was performed to check for potential impingement without creating a femoral head-neck offset. Of the 114 hips (69%) without an offset correction, 39 hips (24%) had an arthrotomy and 75 hips (45%) did not have an arthrotomy. The survivorship of hips with an arthrotomy was 87% (95% CI, 77%–98%) versus 77% (95% CI, 67%–87%) without an arthrotomy after 10 years followup. The dynamic intraoperative assessment, however, is only possible through a capsulotomy with a (modified) Smith-Petersen approach [32]. Other approaches such as the classic ilioinguinal [20] or the more recently described transsartorial approach [42] do not offer access for this visual evaluation. In case of normal intraoperative ROM after acetabular reorientation, a capsulotomy might not be necessary.
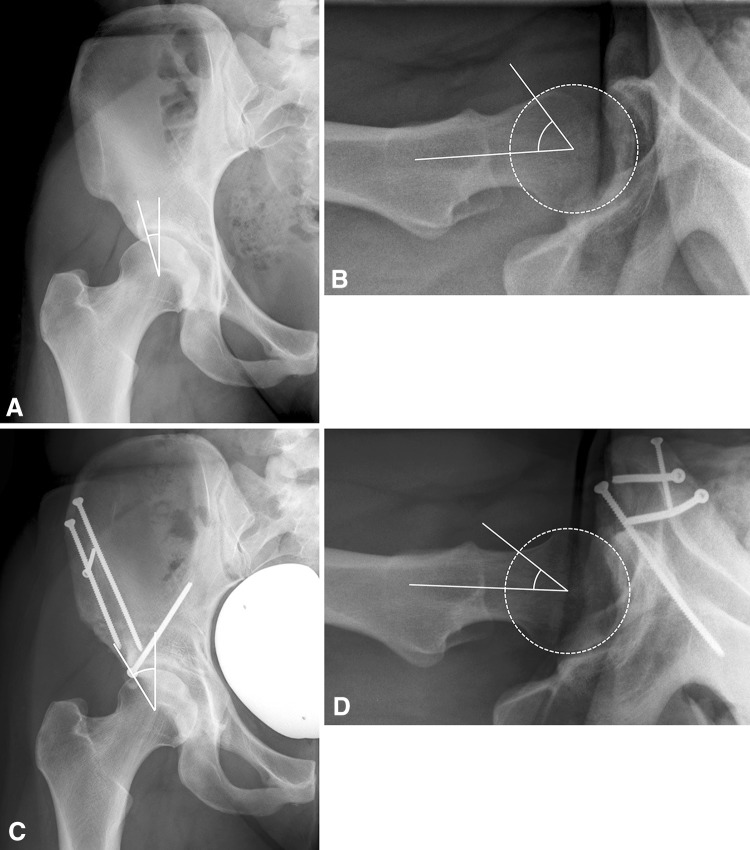
Fig. 4A–D.
The radiographs of a 24-year-old woman from Group I (optimal acetabular retroversion and spherical femoral head) are shown. (A) The preoperative AP radiograph showed deficient lateral coverage. (B) Femoral head-neck offset was decreased in the preoperative crosstable view. At the 10-year followup the patient presented with a Merle d’Aubigné-Postel score of 17 points. (C) The AP radiograph showed sufficient lateral coverage and corrected version of the acetabulum without signs of progression of OA. (D) The decreased head-neck offset was corrected by performing an osteochondroplasty of the femoral head-neck junction, leading to impingement-free ROM.
Table 5.
Studies reporting survival rate and predictors of failure
| Study | Number of hips | Age at surgery (years) (range) | Type of osteotomy | Followup (years) | End points | Survival | Predictive factors |
|---|---|---|---|---|---|---|---|
| Hartig-Andreasen et al. [11] | 401 | 34 (13–61) | PAO | 8 (4–12) | THA | 10 years: 75% | Age, OA, suboptimal achieved center-edge angle, reduced postoperative joint space width incongruence |
| Troelsen et al. [43] | 116 | 30 (14–57) | PAO | 7 (5–9) | THA | 5 years: 91% 9 years: 82% |
Severe dysplasia, presence of os acetabuli, OA, excessive lateral and proximal dislocation |
| Matheney et al. [21] | 135 | 27 (10–45) | PAO | 9 | WOMAC™ score ≥ 10 points, THA | 5 years: 96% 10 years: 84% |
Age > 35 years, poor congruency |
| Steppacher et al. [33] |
75 | 29 (13–56) | PAO | 20 (19–23) | THA | 10 years: 85% 20 years: 61% |
Age, preoperative Merle d’Aubigné-Postel score, positive impingement test, limb, OA grade, insufficient acetabular coverage |
| Kralj et al. [17] | 26 | 34 | PAO | 12 (7–15) | THA, OA Tönnis Grade 3, WOMAC™ > 20 points | 10 years: 54% | Postoperative normalized peak contact stress, preoperative WOMACTM score, preoperative Tönnis score |
| Hsieh et al. [14] | 46 | 31 (18–58) | Modified PAO | 4 (2–5) | Nonunion of osteotomy | 4 years: 100% | – |
| Naito et al. [24] | 128 | 35 (16–59) | Modified PAO | 4 (2–8) | Pubic nonunion | 4 years: 98% | – |
| van Hellemondt et al. [44] | 51 | 28 (15–46) | Triple | 15 (13–20) | THA, Merle d’Aubigné-Postel score | 15 years: 64% | Preoperative OA, preoperatively low Merle d’Aubigné-Postel score |
| de Kleuver et al. [4, 5] |
51 | 28 (14–46) | Triple | 10 (8–15) | THA, progression of OA, walking ability | 10 years: 73% | Reduced coverage of the posterolateral quadrant of the femoral head |
| Yanagimoto et al. [48] | 74 | 32 (6–64) | Chiari | 13 (10–20) | Decreased Japanese Orthopaedic Association hip score, THA | 13 years: 91% | Advanced DDH, spherical femoral head |
| Ohashi et al. [26] | 86 | 18 (6–48) | Chiari | 17 (4–37) | Progression of OA | 10 years: 84% | Severity of the preoperative OA, shape of the femoral head, the level of osteotomy |
| Current study | Group I: 43 | 28 (13–44) | PAO | 11 (10–14) | THA, progression of OA, Merle d’Aubigné-Postel score < 15 points | Group I: 10 years: 91% | Age > 30 years, preoperative Merle d’Aubigne-Postel score < 15, preoperative positive Trendelenburg sign, nonspherical head, preoperative OA ≥ Grade 1, Severin > Grade 3, excessive acetabular anteversion, acetabular retroversion, LCE < 22° (undercoverage), no offset correction in a nonspherical femoral head |
| Group II: 122 | 29 (12–55) | Group II: 10 years: 80% |
PAO = periacetabular osteotomy; OA = osteoarthritis; DDH = developmental dysplasia of the hip; LCE = lateral center-edge angle.
Both groups of patients had improved mean Merle d’Aubigné-Postel scores at followup with no differences between Group I and Group II. In Group I there was a decrease of 69% of the positive impingement test, whereas in Group II, the decrease was only 36%. This result is consistent with the different morphologic features of the hip in these two groups: Group I with optimal acetabular and femoral morphologic features had a higher decrease than Group II with morphologic features predisposing for FAI. The anterior impingement test is positive for labral lesions and reportedly has a high positive predictive value [10]. Ten percent of the hips in Group I still have a positive impingement test at followup despite the radiographic exclusion of morphologic features of FAI. An explanation for this might be that hips with DDH frequently have labral lesions [20], which persist after PAO without labral treatment. Flexion decreased in both groups, whereas extension and internal and external rotation only decreased in Group II. This could be explained by the morphologic features of the hip in Group II leading to FAI.
In Group I the progression of OA was less than that in Group II. The percentages of hips with progression of OA in Groups I and II were 7% and 16%, respectively. These results are lower than the reported values in the literature ranging from 21% to 35% after PAO and other pelvic osteotomies [5, 17, 21, 26, 44].
Some of the predictive factors for survivorship after PAO or other pelvic osteotomies found in our study (Table 4) have been reported previously: age [11, 21, 33], preoperative OA [11, 17, 33, 43], preoperative clinical scoring systems such as Merle d’Aubigné-Postel [33, 44] or WOMACTM score [17], nonspherical femoral head [26], degree of DDH [43, 48], and postoperatively decreased acetabular coverage [4, 11, 33]. Novel factors predicting the survivorship after PAO were related to acetabular reorientation and included postoperative excessive acetabular anteversion and acetabular retroversion (Fig. 5). Predictive factors related to acetabular reorientation after PAO are rarely reported in the literature. Xie et al. [47] found iatrogenic acetabular retroversion after curved PAO predicted failure. Similarly, a reduced acetabular anteversion angle less than 10° on postoperative CT increased the risk for conversion to THA by a factor of 4.3 [43]. Unfortunately, this measurement is not available intraoperatively. The computerized radiographic evaluation, as used in our study, allows the evaluation with potential correction of the acetabular fragment using intraoperative radiographs.
Fig. 5A–F.
(A) A 32-year-old woman from Group II presented with bilateral hip dysplasia. (B) The preoperative version of both acetabula is correct: the anterior wall (blue) does not cross the posterior wall (red). (C) The postoperative radiograph shows suboptimal acetabular reorientation on both sides (6 months postoperatively). (D) The right hip presents with excessive anteversion while the left hip presents with excessive retroversion. (E) Eight years after surgery, the right hip had severe progression of osteoarthritis. (F) In the left hip, a herniation pit formed as a result of iatrogenic pincer-type femoroacetabular impingement (arrow). The recurrent impingement resulted in joint space narrowing and subluxation of the joint. The patient required THA on the right side 9 years postoperatively and on the left side 12 years after surgery (not shown).
Our observations suggest a proper acetabular reorientation with correction of the femoral head-neck offset improved survival after PAO for DDH. The 3-D acetabular reorientation should be checked carefully intraoperatively. Preoperative radiographic evaluations should include assessment of a frequently present asphericity of the femoral head. Our findings suggest corrected hips with optimal acetabular reorientation and a spherical head have a lower progression rate of radiographic OA.
Acknowledgments
We thank Joseph M. Schwab MD for assistance with preparation of this article.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution has approved the human protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Apley AG, Wientroub S. The sagging rope sign in Perthes’ disease and allied disorders. J Bone Joint Surg Br. 1981;63:43–47. doi: 10.1302/0301-620X.63B1.7204473. [DOI] [PubMed] [Google Scholar]
- 2.Cox DR. Regression models and life-tables. J Roy Stat Soc B. 1972;34:187–191. [Google Scholar]
- 3.D’Aubigne RM, Postel M. Functional results of hip arthroplasty with acrylic prosthesis. J Bone Joint Surg Am. 1954;36:451–475. [PubMed] [Google Scholar]
- 4.de Kleuver M, Kapitein PJ, Kooijman MA, van Limbeek J, Pavlov PW, Veth RP. Acetabular coverage of the femoral head after triple pelvic osteotomy: no relation to outcome in 51 hips followed for 8–15 years. Acta Orthop Scand. 1999;70:583–588. doi: 10.3109/17453679908997846. [DOI] [PubMed] [Google Scholar]
- 5.de Kleuver M, Kooijman MA, Pavlov PW, Veth RP. Triple osteotomy of the pelvis for acetabular dysplasia: results at 8 to 15 years. J Bone Joint Surg Br. 1997;79:225–229. doi: 10.1302/0301-620X.79B2.7167. [DOI] [PubMed] [Google Scholar]
- 6.Eijer H, Leunig M, Mohamed N, Ganz R. Cross-table lateral radiographs for screening of anterior femoral head-neck offset in patients with femoro-acetabular impingement. Hip Int. 2001;11:37–41. [Google Scholar]
- 7.Ganz R, Klaue K, Vinh TS, Mast JW. A new periacetabular osteotomy for the treatment of hip dysplasias: technique and preliminary results. Clin Orthop Relat Res. 1988;232:26–36. [PubMed] [Google Scholar]
- 8.Ganz R, Parvizi J, Beck M, Leunig M, Notzli H, Siebenrock KA. Femoroacetabular impingement: a cause for osteoarthritis of the hip. Clin Orthop Relat Res. 2003;417:112–120. doi: 10.1097/01.blo.0000096804.78689.c2. [DOI] [PubMed] [Google Scholar]
- 9.Golub BS. The Duchenne-Trendelenburg sign. Bull Hosp Joint Dis. 1947;8:127–136. [PubMed] [Google Scholar]
- 10.Hananouchi T, Yasui Y, Yamamoto K, Toritsuka Y, Ohzono K. Anterior impingement test for labral lesions has high positive predictive value. Clin Orthop Relat Res. 2012;470:3524–3529. doi: 10.1007/s11999-012-2450-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hartig-Andreasen C, Troelsen A, Thillemann TM, Soballe K. What factors predict failure 4 to 12 years after periacetabular osteotomy? Clin Orthop Relat Res. 2012;470:2978–2987. doi: 10.1007/s11999-012-2386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hipp JA, Sugano N, Millis MB, Murphy SB. Planning acetabular redirection osteotomies based on joint contact pressures. Clin Orthop Relat Res. 1999;364:134–143. doi: 10.1097/00003086-199907000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Holm I, Bolstad B, Lutken T, Ervik A, Rokkum M, Steen H. Reliability of goniometric measurements and visual estimates of hip ROM in patients with osteoarthrosis. Physiother Res Int. 2000;5:241–248. doi: 10.1002/pri.204. [DOI] [PubMed] [Google Scholar]
- 14.Hsieh PH, Shih CH, Lee PC, Yang WE, Lee ZL. A modified periacetabular osteotomy with use of the transtrochanteric exposure. J Bone Joint Surg Am. 2003;85:244–250. doi: 10.1302/0301-620X.85B2.13074. [DOI] [PubMed] [Google Scholar]
- 15.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. doi: 10.1080/01621459.1958.10501452. [DOI] [Google Scholar]
- 16.Kirmit L, Karatosun V, Unver B, Bakirhan S, Sen A, Gocen Z. The reliability of hip scoring systems for total hip arthroplasty candidates: assessment by physical therapists. Clin Rehabil. 2005;19:659–661. doi: 10.1191/0269215505cr869oa. [DOI] [PubMed] [Google Scholar]
- 17.Kralj M, Mavcic B, Antolic V, Iglic A, Kralj-Iglic V. The Bernese periacetabular osteotomy: clinical, radiographic and mechanical 7–15-year follow-up of 26 hips. Acta Orthop. 2005;76:833–840. doi: 10.1080/17453670510045453. [DOI] [PubMed] [Google Scholar]
- 18.Lequesne M, de Seze S. [False profile of the pelvis: a new radiographic incidence for the study of the hip. Its use in dysplasias and different coxopathies][in French] Rev Rhum Mal Osteoartic. 1961;28:643–652. [PubMed] [Google Scholar]
- 19.Letournel E. The treatment of acetabular fractures through the ilioinguinal approach. Clin Orthop Relat Res. 1993;292:62–76. [PubMed] [Google Scholar]
- 20.Leunig M, Podeszwa D, Beck M, Werlen S, Ganz R. Magnetic resonance arthrography of labral disorders in hips with dysplasia and impingement. Clin Orthop Relat Res. 2004;418:74–80. doi: 10.1097/00003086-200401000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Matheney T, Kim YJ, Zurakowski D, Matero C, Millis M. Intermediate to long-term results following the Bernese periacetabular osteotomy and predictors of clinical outcome. J Bone Joint Surg Am. 2009;91:2113–2123. doi: 10.2106/JBJS.G.00143. [DOI] [PubMed] [Google Scholar]
- 22.Murphy SB, Ganz R, Muller ME. The prognosis in untreated dysplasia of the hip: a study of radiographic factors that predict the outcome. J Bone Joint Surg Am. 1995;77:985–989. doi: 10.2106/00004623-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Myers SR, Eijer H, Ganz R. Anterior femoroacetabular impingement after periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:93–99. doi: 10.1097/00003086-199906000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Naito M, Shiramizu K, Akiyoshi Y, Ezoe M, Nakamura Y. Curved periacetabular osteotomy for treatment of dysplastic hip. Clin Orthop Relat Res. 2005;433:129–135. doi: 10.1097/01.blo.0000153281.75265.1d. [DOI] [PubMed] [Google Scholar]
- 25.Notzli HP, Wyss TF, Stoecklin CH, Schmid MR, Treiber K, Hodler J. The contour of the femoral head-neck junction as a predictor for the rick of anterior impingement. J Bone Joint Surg Br. 2002;84:556–560. doi: 10.1302/0301-620X.84B4.12014. [DOI] [PubMed] [Google Scholar]
- 26.Ohashi H, Hirohashi K, Yamano Y. Factors influencing the outcome of Chiari pelvic osteotomy: a long-term follow-up. J Bone Joint Surg Br. 2000;82:517–525. doi: 10.1302/0301-620X.82B4.9583. [DOI] [PubMed] [Google Scholar]
- 27.Peters CL, Schabel K, Anderson L, Erickson J. Open treatment of femoroacetabular impingement is associated with clinical improvement and low complication rate at short-term followup. Clin Orthop Relat Res. 2010;468:504–510. doi: 10.1007/s11999-009-1152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds D, Lucas J, Klaue K. Retroversion of the acetabulum: a cause of hip pain. J Bone Joint Surg Br. 1999;81:281–288. doi: 10.1302/0301-620X.81B2.8291. [DOI] [PubMed] [Google Scholar]
- 29.Siebenrock KA, Leunig M, Ganz R. Periacetabular osteotomy: the Bernese experience. Instr Course Lect. 2001;50:239–245. [PubMed] [Google Scholar]
- 30.Siebenrock KA, Scholl E, Lottenbach M, Ganz R. Bernese periacetabular osteotomy. Clin Orthop Relat Res. 1999;363:9–20. doi: 10.1097/00003086-199906000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Siebenrock KA, Wahab KH, Werlen S, Kalhor M, Leunig M, Ganz R. Abnormal extension of the femoral head epiphysis as a cause of cam impingement. Clin Orthop Relat Res. 2004;418:54–60. doi: 10.1097/00003086-200401000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Smith-Petersen MN. Approach to and exposure of the hip joint for mold arthroplasty. J Bone Joint Surg Am. 1949;31:40–46. [PubMed] [Google Scholar]
- 33.Steppacher SD, Tannast M, Ganz R, Siebenrock KA. Mean 20-year followup of Bernese periacetabular osteotomy. Clin Orthop Relat Res. 2008;466:1633–1644. doi: 10.1007/s11999-008-0242-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steppacher SD, Tannast M, Werlen S, Siebenrock KA. Femoral morphology differs between deficient and excessive acetabular coverage. Clin Orthop Relat Res. 2008;466:782–790. doi: 10.1007/s11999-008-0141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stulberg SD, Cordell LD, Harris WH, Ramsey PL, MacEwen GD. Unrecognized childhood hip disease: a major cause of idiopathic osteoarthritis of the hip. In: Hip The., editor. Proceedings of the 3rd Meeting of The Hip Society. St Louis, MO, USA: CV Mosby Co; 1975. pp. 212–228. [Google Scholar]
- 36.Tannast M, Albers CE, Steppacher SD, Siebenrock KA. Hip pain in the young adult. In: Bentley G, editor. European Instructional Lectures. Berlin, Germany: Springer; 2011. pp. 141–154. [Google Scholar]
- 37.Tannast M, Mistry S, Steppacher SD, Reichenbach S, Langlotz F, Siebenrock KA, Zheng G. Radiographic analysis of femoroacetabular impingement with Hip2Norm: reliable and validated. J Orthop Res. 2008;26:1199–1205. doi: 10.1002/jor.20653. [DOI] [PubMed] [Google Scholar]
- 38.Tannast M, Siebenrock KA, Anderson SE. Femoroacetabular impingement: radiographic diagnosis–what the radiologist should know. AJR Am J Roentgenol. 2007;188:1540–1552. doi: 10.2214/AJR.06.0921. [DOI] [PubMed] [Google Scholar]
- 39.Tannast M, Zheng G, Anderegg C, Burckhardt K, Langlotz F, Ganz R, Siebenrock KA. Tilt and rotation correction of acetabular version on pelvic radiographs. Clin Orthop Relat Res. 2005;438:182–190. doi: 10.1097/01.blo.0000167669.26068.c5. [DOI] [PubMed] [Google Scholar]
- 40.Tönnis D. General radiography of the hip joint. In: Tönnis D, editor. Congenital Dysplasia, Dislocation of the Hip. New York, NY, USA: Springer; 1987. pp. 100–142. [Google Scholar]
- 41.Tonnis D, Heinecke A. Acetabular and femoral anteversion: relationship with osteoarthritis of the hip. J Bone Joint Surg Am. 1999;81:1747–1770. doi: 10.2106/00004623-199912000-00014. [DOI] [PubMed] [Google Scholar]
- 42.Troelsen A, Elmengaard B, Soballe K. A new minimally invasive transsartorial approach for periacetabular osteotomy. J Bone Joint Surg Am. 2008;90:493–498. doi: 10.2106/JBJS.F.01399. [DOI] [PubMed] [Google Scholar]
- 43.Troelsen A, Elmengaard B, Soballe K. Medium-term outcome of periacetabular osteotomy and predictors of conversion to total hip replacement. J Bone Joint Surg Am. 2009;91:2169–2179. doi: 10.2106/JBJS.H.00994. [DOI] [PubMed] [Google Scholar]
- 44.van Hellemondt GG, Sonneveld H, Schreuder MH, Kooijman MA, de Kleuver M. Triple osteotomy of the pelvis for acetabular dysplasia: results at a mean follow-up of 15 years. J Bone Joint Surg Br. 2005;87:911–915. doi: 10.1302/0301-620X.87B7.15307. [DOI] [PubMed] [Google Scholar]
- 45.Weber M, Ganz R. The Bernese periacetabular osteotomy. Operat Orthop Traumatol. 2002;2:99–121. doi: 10.1007/s00064-002-1040-9. [DOI] [Google Scholar]
- 46.Wiberg G. The anatomy and roentgenographic appearance of a normal hip joint. Acta Chir Scand. 1939;83:7–38. [Google Scholar]
- 47.Xie J, Naito M, Maeyama A. Evaluation of acetabular versions after a curved periacetabular osteotomy for dysplastic hips. Int Orthop. 2010;34:473–477. doi: 10.1007/s00264-009-0785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yanagimoto S, Hotta H, Izumida R, Sakamaki T. Long-term results of Chiari pelvic osteotomy in patients with developmental dysplasia of the hip: indications for Chiari pelvic osteotomy according to disease stage and femoral head shape. J Orthop Sci. 2005;10:557–563. doi: 10.1007/s00776-005-0942-4. [DOI] [PubMed] [Google Scholar]
- 49.Zheng G, Tannast M, Anderegg C, Siebenrock KA, Langlotz F. Hip2Norm: an object-oriented cross-platform program for 3D analysis of hip joint morphology using 2D pelvic radiographs. Comput Methods Programs Biomed. 2007;87:36–45. doi: 10.1016/j.cmpb.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 50.Ziebarth K, Balakumar J, Domayer S, Kim YJ, Millis MB. Bernese periacetabular osteotomy in males: is there an increased risk of femoroacetabular impingement (FAI) after Bernese periacetabular osteotomy? Clin Orthop Relat Res. 2011;469:447–453. doi: 10.1007/s11999-010-1544-9. [DOI] [PMC free article] [PubMed] [Google Scholar]