Abstract
Background
A bulky compression dressing (Robert Jones bandage) is commonly used after TKA to reduce blood loss, pain, and swelling. However, it is unclear whether these dressings in fact reduce blood loss.
Questions/Purposes
We compared postoperative blood loss, pain, knee swelling, and postoperative complications in two types of postoperative dressings after TKA: a modified Robert Jones dressing (MRJB) and a conventional wound dressing.
Methods
We conducted a prospective, randomized, controlled trial of 60 patients who underwent a unilateral primary TKA at our institution between November 2010 and July 2011. After wound closure, the patients were allocated into two groups. Thirty patients had the MRJB applied for 24 hours (Group 1) and 30 patients had a conventional wound dressing applied (Group 2). Postoperative hemorrhages in the vacuum drain, units of transfused blood, postoperative pain, knee swelling, and complications were assessed at 24 and 48 hours postoperatively.
Results
We found no differences in the mean postoperative blood loss between the groups (Group 1, 418 mL versus Group 2, 467 mL). Blood transfusion amounts, postoperative pain, and knee swelling also were similar. Three patients in Group 1 experienced bruising and two patients in Group 2 also had bruising. One patient in Group 1 had a blister.
Conclusions
Although previous studies have shown reduced blood loss, pain, and knee swelling after application of a MRJB, we found no benefit of this bandage. Our data suggest a postoperative compression dressing is not necessary after primary TKAs.
Level of Evidence
Level I, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Major bleeding and blood transfusions after TKA occur in 3% to 5% [18] and 21% to 53% of patients [11, 15, 19], respectively. Various surgical techniques [9, 11, 16, 17, 19], medications [5, 15, 22], and postoperative protocols [2, 4, 8, 12] to reduce bleeding have been proposed. Various postoperative approaches including cold compresses [6], elastic bandage support [7], and compressive dressings [1] also have been reported for reduction of bleeding, soft tissue edema, and hemarthroses.
One of the compressive dressings is the so-called “Robert Jones bandage.” According to Brodell et al. [1], the bandage was described by Sir John Charnley as “Three layers of wool and three layers of domette bandage. The layers are put on gently but firmly and the whole bandage extends some six inches above and below the joint and attains a thickness of about two inches.” The dressing apparently was proposed to reduce bleeding and minimize edema in traumatized tissue [1]. Using monitored intramuscular compartmental pressure in patients after TKA, Brodell et al. [1] showed such a compression dressing could generate and maintain external compression to the soft tissues over the limb for at least 24 hours. The compression was between 40 to 50 mm Hg pressure at application; pressure decreased to 2 to 10 mm Hg within 48 hours. The reported advantages of the Robert Jones bandage are reduced pain and swelling after surgery [2, 6, 21]. In an animal model, application of a compression bandage reduced postfracture swelling in the tissue [13]. However, if a compressive dressing is applied with an inappropriate technique, an overly tight dressing will obliterate blood flow to subcutaneous tissue and cause tissue ischemia [14]. The complications associated with a compression dressing include peroneal nerve palsy [10] and discomfort from the cumbersome, thick dressing [7]. We have observed pressure ulcers, bruises, and blisters from too-tight dressings, and dried blood from surgical wound bleeding in the cotton; it also renders examination of the wound impossible.
This Robert Jones dressing was modified by Brodell et al. [1], as the elastic layer was pulled quite snugly with more tension distally than proximally in an effort to promote venous drainage. The entire limb was bandaged. The modified Robert Jones bandage (MRJB) is one of the mechanical compression bandages that has been advocated to reduce bleeding in the joint by a tamponade effect [1]. However, whether the potential advantages of the MRJB outweigh the disadvantages is unclear.
We therefore compared (1) postoperative bleeding and units of transfused blood, (2) level of postoperative pain, (3) swelling, and (4) complications in patients who had the MRJB and the conventional dressing applied after primary TKA.
Material and Methods
We conducted a prospective randomized controlled trial, performed by one surgeon (PP) from November 2010 to July 2011. All 60 patients (60 knees), 50 years or older, who were diagnosed with primary osteoarthritis of the knee undergoing unilateral primary TKAs who met our criteria agreed to participate in the study and thus were included. We excluded nine patients: five with bilateral TKAs because of difficulty with blood loss evaluation in each leg, two with vascular compromise of the affected limb, one with a skin problem, and one who we judged could not understand the study or would have difficulty cooperating. Two strata were created for an equivalent distribution of subjects. One set of prenumbered, sealed envelopes was prepared for each stratum, and subjects were assigned to the group specified in the envelope. Thirty patients were randomized to the MRJB group (Group 1), and another 30 were randomized to the conventional dressing group (Group 2). The study was approved by the institutional review board before initiation of the study (Registry #MTU-EC-OT-0-062/54). All patients provided written informed consent before participation in the study.
The sample size was calculated based on the measured postoperative blood loss. We assumed an alpha error of 0.05 and applied an allocation ratio of 1. A sample size of 54 participants with a dropout rate of 10% (six participants) was calculated to provide 80% power to detect a difference of 150 mL in the postoperative blood loss, which we considered clinically relevant. A total of 30 patients in Group 1 and 30 in Group 2 completed the study. The demographic characteristics of the patients in both groups were similar (Table 1).
Table 1.
Summarized data of the patients
| Parameter | Group 1, MRJB (n = 30) | Group 2, conventional dressing (n = 30) | p value |
|---|---|---|---|
| Age (years) | 69.20 ± 10.70* | 70.23 ± 8.60* | 0.682 |
| Sex (F:M) | 25:5 | 25:5 | |
| Weight (kg) | 63.83 ± 13.43* | 67.20 ± 14.67* | 0.228† |
| Height (cm) | 155.27 ± 5.74* | 158.00 ± 6.81* | 0.098 |
| BMI (kg/m2) | 26.40 ± 5.00* | 26.80 ± 4.90* | 0.487† |
| Hemoglobin (g/dL) | 12.03 ± 1.09* | 11.76 ± 1.37* | 0.395 |
| Hematocrit (%) | 35.80 ± 3.13* | 34.95 ± 3.66* | 0.333† |
| Platelets (× 105) | 2.62 ± 0.75* | 2.56 ± 0.56* | 0.742 |
| Surgically treated limb (R:L) | 16:14 | 14:16 | |
| Operative time (minutes) | 120.07 ± 16.32* | 121.90 ± 15.20* | 0.898† |
| Intraoperative blood loss (mL) | 52.67 ± 49.82* | 39.90 ± 36.58* | 0.353† |
| Hospital stay (days) | 4.70 ± 0.92* | 5.43 ± 3.15* | 0.225 |
| Knee ROM at discharge | 1°–94° | 1°–97° |
* Values are expressed as the mean and SD; †Mann-Whitney U test used when data were not normally distributed; MRJB = modified Robert Jones bandage.
The patients in both groups underwent TKAs using the same anesthetic method, surgeon (PP), and surgical technique, including 750 mg IV tranexamic acid administration 10 minutes before deflating the tourniquet and 3 hours after the operation. A tourniquet was inflated before the skin incision and deflated after the dressing was finished. One Privac® drain (Primed Halberstadt Medizintechnik GmbH, Halberstadt, Germany) was inserted into the joint and removed 48 hours after surgery. The mean estimated intraoperative blood loss was 53 mL in Group 1 and 40 mL in Group 2.
After wound closure, the patients were randomized by computer to either the MRJB or the conventional wound dressing group with use of sequentially numbered, sealed envelopes. In the MRJB group, the sterile gauze pads were placed over the wound, followed by Webril™ padding (Covidien, Mansfield, MA, USA); thick cotton wool was applied from a roll, and each layer overlapped the previous one by ½ at each turn. Webril™ pads and cotton wool together provided a thickness of approximately 2 inches. The final elastic bandage layer was pulled snug, with more tension applied distally than proximally to promote venous drainage (Fig. 1). The MRJB was left in place for 24 hours postoperatively and then changed to the conventional dressing. For the conventional wound dressing group, sterile gauze pads were placed over the wound, followed by self-adhesive, nonwoven fabric tape without any compression (Fig. 2). No blood thinning agent was used in any of the patients, but ankle pumping exercises during the early postoperative period and ambulation on the second postoperative day were used as mechanical prophylaxis for deep vein thrombosis.
Fig. 1.
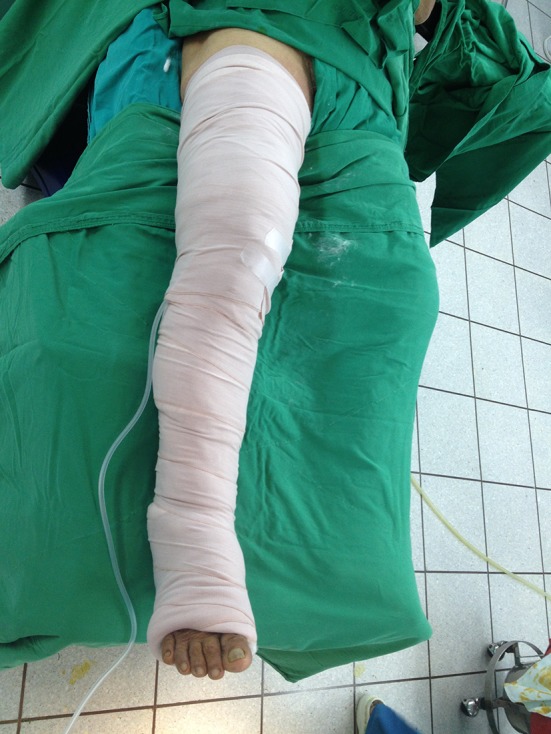
A MRJB is shown on a patient.
Fig. 2.
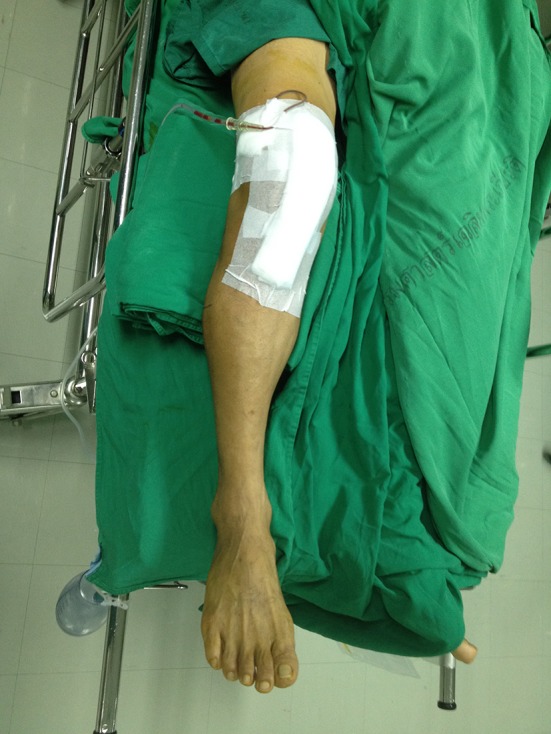
A conventional wound dressing is shown on a patient.
As the primary outcome, postoperative-measured blood loss was recorded in the Privac® drain at 24 and 48 hours. The hematocrits were recorded at 24 and 48 hours postoperatively, and a blood transfusion was indicated when the hematocrit was less than 30%. Secondary outcomes, including a VAS, (ranging from 0 to 10 with 0 being no pain and 10 being the worse imaginable pain) [3] and the amount of knee swelling at 24 and 48 hours postoperatively also were recorded. Knee swelling was measured circumferentially at the thigh (10 cm above the upper pole of the patella) and the leg (10 cm below the lower pole of the patella), by the difference in the circumferences at the preoperative and 24-hour postoperative periods.
The 95% CI of the group differences was calculated for each variable. We used the Kolmogorov-Smirnov goodness of fit test to determine normal distribution and then we performed the two-group comparisons using Student’s t-test for independent samples (with Welch correction when variances were unequal) when data were normally distributed and the Mann-Whitney U test when data were not normally distributed. All statistical analyses were performed using SPSS® software version 17.0 (SPSS Inc, Chicago, IL, USA).
Results
There was no difference (p = 0.438) in total mean drainage between the two groups (Table 2). The total amount of drained blood from Group 1 after 48 hours was 419 mL and from Group 2 was 467 mL (mean difference, 48 mL) (Table 2). At 24 hours after surgery, the MRJB was still in place and the mean blood loss was no different (270 mL in Group 1 and 316 mL in Group 2). The total hematocrit change was similar (p = 0.243) in the two groups: 3.43% in Group 1 and 2.57% in Group 2. There was no difference in terms of the blood transfusion rate at 24 (p = 0.954) and 48 hours (p = 0.643) postoperatively.
Table 2.
Blood loss and blood transfusions
| Variable | Group 1, MRJB (n = 30) | Group 2, Conventional dressing (n = 30) | p value |
|---|---|---|---|
| Blood in Hemovac drain | |||
| 24 hours postoperative | 269.83 ± 158.27* | 316.00 ± 174.66* | 0.287 |
| 48 hours postoperative | 149.00 ± 132.94* | 151.00 ± 101.08* | 0.947 |
| Total | 418.83 ± 243.93* | 467.00 ± 233.95* | 0.438 |
| Hematocrit (%) | |||
| Preoperative | 35.80 ± 3.13* | 34.95 ± 3.66* | 0.340 |
| 24 hours postoperative | 31.97 ± 3.15* | 32.70 ± 4.08* | 0.438 |
| 48 hours postoperative | 32.37 ± 2.88* | 32.40 ± 3.29* | 0.966 |
| Total hematocrit change | 3.43 ± 2.62* | 2.57 ± 3.06* | 0.243 |
| Blood transfusion (PRC in unit) | |||
| 24 hours postoperative | 0.17 ± 0.38* | 0.20 ± 0.48* | 0.954† |
| 48 hours postoperative | 0.07 ± 0.25* | 0.10 ± 0.31* | 0.643† |
| Number of patients needing transfusions | 7/30 | 7/30 | 1.0 |
* Values are expressed as the mean and the SD; †Mann-Whitney U test was used when data were not normally distributed; MRJB = Modified Robert Jones bandage; PRC = packed red cells.
Postoperative pain did not differ between the groups at any time (Table 3). There was no difference in mean limb circumference difference at the thigh (p = 0.384) and leg (p = 0.295) postoperatively.
Table 3.
Pain and knee swelling after dressing
| Variable | Group 1, MRJB (n = 30) | Group 2, conventional dressing (n = 30) | p value |
|---|---|---|---|
| VAS (0–10) | |||
| 24 hours postoperative | 3.43 ± 2.21* | 3.80 ± 2.48* | 0.547 |
| 48 hours postoperative | 3.27 ± 2.41* | 3.57 ± 1.89* | 0.460† |
| Knee circumference (cm) | |||
| Preoperative | |||
| Thigh | 44.29 ± 7.54* | 44.39 ± 5.73* | 0.568† |
| Leg | 34.12 ± 3.87* | 34.94 ± 5.78* | 0.944† |
| Postoperative | |||
| Thigh | 45.91 ± 7.21* | 45.41 ± 5.61* | 0.664† |
| Leg | 34.43 ± 4.29* | 35.46 ± 6.00* | 0.612† |
| Difference (postoperative−preoperative) | |||
| Thigh | 1.62 ± 2.51* | 1.02 ± 1.30* | 0.384 |
| Leg | 0.31 ± 1.66* | 0.52 ± 1.05* | 0.295† |
* Values are expressed as the mean and the SD; †Mann-Whitney U test used when data were not normally distributed. MRJB = modified Robert Jones bandage.
Superficial skin complications occurred on the second postoperative day in both groups, with one blister and three bruises occurring in Group 1, and two bruises occurring in Group 2. The mean discharge times for the patients were 4.70 days (Group 1) and 5.43 days (Group 2). There was no tense hemarthrosis, subcutaneous hematoma, peroneal nerve palsy, surgical wound infection, or symptomatic venous thromboembolism in either group until 14 days after surgery.
Discussion
The MRJB is a bulky compression dressing that has had widespread popular use as a postoperative and posttraumatic bandage for more than 60 years. The advantage reportedly was to reduce soft tissue edema and bleeding [1]. However, it is unclear whether the MRJB offers advantages over a conventional dressing in patients who have a TKA. We therefore compared (1) postoperative bleeding and units of transfused blood, (2) level of postoperative pain, (3) swelling, and (4) complications in patients who had the MRJB applied and those who had a conventional dressing applied after primary TKA.
We recognize limitations of this study. First, we did not measure the subbandage pressure in each patient with the MRJB dressing, so the pressures underneath likely differed with each application. However, measurement of subbandage pressure is not practical and would not be used in routine practice. Second, we used a drain to measure blood loss. Blood loss in the vacuum drain may not represent the true blood loss, as previously described [20]. The hidden blood loss in the tissue and joint which was difficult to measure would confound the true blood loss, but blood in the drain would be the best visible loss we can measure.
In comparing the use of a MRJB for 48 hours with using a cold compression (Aircast® Knee Cryo/Cuff™, DJO™ Global, Vista, CA, USA) during a hospital stay after TKA, Gibbons et al. [5] found more blood loss in the suction tubes of the MRJB group than with the cold compression group (1200 mL versus 720 mL, respectively). However, they reported no differences in the blood transfusion rate, ranges of motion, and pain scores. In a controlled study that compared a wool crepe dressing with cold compressive dressings, Webb et al. [23] also found more blood loss in the suction drainage from the wool crepe dressing (982 mL) than from the cold compression dressing (768 mL). In addition, a higher proportion of patients needed transfusions and narcotic requirements in the compressive wool crepe dressing group. We found that the MJRB tended to reduce blood loss (by 46 mL compared with the conventional dressing) during the first 24 hours; this amount is likely within the range of error of measurement and in any case would not be clinically important. There also was no difference in the amounts of transfused blood in each group. Based on the results, we could not conclude the advantages of reducing blood loss and transfused blood units in clinical practice.
In a study comparing the advantage of the MRJB with the elastic support bandage in patients with knee sprains, Hughes et al. [7] found no differences in the VAS for pain, ranges of motion, and analgesic consumption from the initial period to 3 weeks postoperative. However, they reported an elastic support bandage made the patients feel more comfortable than the MRJB during the first week in the early postinjury period. We found similar levels of pain and swelling in both groups in our study. The complications, including bruises and blisters [7], appeared to occur more in Group 1.
Our observations suggest the use of a MRJB has no effect on total measured postoperative hemorrhage, pain, and knee swelling compared with a conventional wound dressing alone. In usual clinical application, a MRJB may not reach the pressure required for the tamponade effect; therefore, we believe use of a MRJB is unnecessary after TKA.
Acknowledgments
We thank Chumpot Amatyakul MS for help with the statistical analysis.
Footnotes
Each author certifies that he has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Brodell JD, Axon DL, Evarts CM. The Robert Jones bandage. J Bone Joint Surg Br. 1986;68:776–779. doi: 10.1302/0301-620X.68B5.3782244. [DOI] [PubMed] [Google Scholar]
- 2.Charalambides C, Beer M, Melhuish J, Williams RJ, Cobb AG. Bandaging technique after knee replacement. Acta Orthop. 2005;76:89–94. doi: 10.1080/00016470510030382. [DOI] [PubMed] [Google Scholar]
- 3.DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The visual analog scale in the immediate postoperative period: intrasubject variability and correlation with a numeric scale. Anesth Analg. 1998;86:102–106. doi: 10.1097/00000539-199801000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Denis M, Moffet H, Caron F, Ouellet D, Paquet J, Nolet L. Effectiveness of continuous passive motion and conventional physical therapy after total knee arthroplasty: a randomized clinical trial. Phys Ther. 2006;86:174–185. [PubMed] [Google Scholar]
- 5.Gasparini G, Papaleo P, Pola P, Cerciello S, Pola E, Fabbriciani C. Local infusion of norepinephrine reduces blood losses and need of transfusion in total knee arthroplasty. Int Orthop. 2006;30:253–256. doi: 10.1007/s00264-005-0050-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gibbons CE, Solan MC, Ricketts DM, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001;25:250–252. doi: 10.1007/s002640100246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hughes DL, Crosby AC. Treatment of knee sprains: modified Robert Jones or elastic support bandage? J Accid Emerg Med. 1995;12:115–118. doi: 10.1136/emj.12.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson DP. Wound hypoxia: the effect of continuous passive motion. Acta Orthop. 1993;64(suppl 252):34–40. [Google Scholar]
- 9.Kumar N, Saleh J, Gardiner E, Devadoss VG, Howell FR. Plugging the intramedullary canal of the femur in total knee arthroplasty: reduction in postoperative blood loss. J Arthroplasty. 2000;15:947–949. doi: 10.1054/arth.2000.8592. [DOI] [PubMed] [Google Scholar]
- 10.Idusuyi OB, Morrey BF. Peroneal nerve palsy after total knee arthroplasty: assessment of predisposing and prognostic factors. J Bone Joint Surg Am. 1996;78:177–184. doi: 10.2106/00004623-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 11.Lotke PA, Faralli VJ, Orenstein EM, Ecker ML. Blood loss after total knee replacement: effects of tourniquet release and continuous passive motion. J Bone Joint Surg Am. 1991;73:1037–1040. [PubMed] [Google Scholar]
- 12.Ma T, Khan RJ, Carey Smith R, Nivbrant B, Wood DJ. Effect of flexion/extension splintage post total knee arthroplasty on blood loss and range of motion: a randomised controlled trial. Knee. 2008;15:15–19. doi: 10.1016/j.knee.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Matsen FA, 3rd, Krugmire RB., Jr The effect of externally applied pressure on post-fracture swelling. J Bone Joint Surg Am. 1974;56:1586–1591. [PubMed] [Google Scholar]
- 14.Ogata K, Whiteside LA. Effects of external compression on blood flow to muscle and skin. Clin Orthop Relat Res. 1982;168:105–107. [PubMed] [Google Scholar]
- 15.Ortega-Andreu M, Pérez-Chrzanowska H, Figueredo R, Gómez-Barrena E. Blood loss control with two doses of tranexamic acid in a multimodal protocol for total knee arthroplasty. Open Orthop J. 2011;5:44–48. doi: 10.2174/1874325001105010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parker MJ, Livingstone V, Clifton R, McKee A. Closed suction surgical wound drainage after orthopaedic surgery. Cochrane Database Syst Rev. 2007;3:CD001825. doi: 10.1002/14651858.CD001825.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parker MJ, Roberts CP, Hay D. Closed suction drainage for hip and knee arthroplasty: a meta-analysis. J Bone Joint Surg Am. 2004;86:1146–1152. doi: 10.1302/0301-620X.86B8.14839. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini VD. DVT prophylaxis: better living through chemistry: affirms. Orthopedics. 2010;7;33:642. [DOI] [PubMed]
- 19.Rama KR, Apsingi S, Poovali S, Jetti A. Timing of tourniquet release in knee arthroplasty: meta-analysis of randomized controlled trials. J Bone Joint Surg Am. 2007;89:699–705. doi: 10.2106/JBJS.F.00497. [DOI] [PubMed] [Google Scholar]
- 20.Sehat KR, Evans R, Newman JR. How much blood is really lost in total knee arthroplasty? Correct blood loss management should take hidden loss into account. Knee. 2000;7:151–155. doi: 10.1016/S0968-0160(00)00047-8. [DOI] [PubMed] [Google Scholar]
- 21.Yu GV, Schubert EK, Khoury WE. The Jones compression bandage: review and clinical application. J Am Podiatr Med Assoc. 2002;92:221–231. doi: 10.7547/87507315-92-4-221. [DOI] [PubMed] [Google Scholar]
- 22.Vendittoli PA, Makinen P, Drolet P, Lavigne M, Fallaha M, Guertin MC, Varin F. A multimodal analgesia protocol for total knee arthroplasty: a randomized, controlled study. J Bone Joint Surg Am. 2006;88:282–289. doi: 10.2106/JBJS.E.00173. [DOI] [PubMed] [Google Scholar]
- 23.Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopedics. 1998;21:59–61. doi: 10.3928/0147-7447-19980101-14. [DOI] [PubMed] [Google Scholar]
- 24.Zenios M, Wykes P, Johnson DS, Clayson AD, Kay P. The use of knee splints after total knee replacements. Knee. 2002;225–228. [DOI] [PubMed]


