Abstract
Background
Component design, size, acetabular orientation, patient gender, and activity level have been suggested as factors leading to elevated metal ion concentrations after-on-metal hip resurfacing arthroplasty (MMHRA). The calculation of the contact patch to rim (CPR) distance integrates component size, design, and acetabular orientation and may be a good predictor of elevated metal ion levels.
Questions/purposes
We evaluated the effects and the predictive value of the CPR distance on serum cobalt (CoS) and chromium (CrS) ion levels.
Methods
We retrospectively studied 182 patients with Conserve Plus MMHRAs at a minimum of 12 months after surgery (median, 57 months; range, 12–165 months). CoS and CrS levels were analyzed using inductively-coupled plasma mass spectrometry. Multiple logistic regression was performed to determine which if any of the factors related to serum ion levels.
Results
Patients with CPR distances of 10 mm or less had a 37-fold increased risk of having elevated CoS of 7 μg/L or higher. Similarly, these patients had an 11-fold increased risk of having elevated CrS of 7 μg/L or higher. Sex and University of California Los Angeles activity scores did not influence the postoperative CoS and CrS levels. The negative predictive value for CPR distance less than 10 mm was 99.3% for CoS greater than 7 μg/L and 98.0% for CrS greater than 7 μg/L.
Conclusions
Our observations suggest the CPR distance would be a useful indicator to determine which patients are at risk for elevated ion levels. Patients with CPR distances greater than 10 mm need not be monitored unless they become symptomatic.
Introduction
Metal-on-metal hip resurfacing arthroplasty (MMHRA) is an option for reconstructing hips that potentially offers enhanced wear characteristics, increased hip stability, and conservation of femoral bone [7, 21, 28]. Patients with metal-on-metal hip arthroplasties, however, may have elevated levels of serum cobalt (CoS) and chromium (CrS) ions [8, 27, 35]. Although the long-term effects of exposure to elevated ion levels released from metal-on-metal articulations are unclear, they are thought to be associated with adverse local tissue reactions [4, 36], such as pseudotumors [11, 13, 23, 33] and systemic disorders that result from metal ion toxicity [37, 39]. De Smet et al. [15] found a strong correlation between serum levels of metal ions and femoral wear, concluding that metal ion testing can be used to estimate metal-on-metal bearing wear. Various factors leading to increased wear and metal ion levels may include component design [25], size [16, 24, 25], acetabular orientation [14, 17, 18], female sex [18, 41], and activity level, although this latter has not yet been established [19, 34]. Langton et al. [25] suggested the resultant distance between the center of the contact patch (the area of the femoral head articular surface that makes contact with the acetabular component during any and all functions) and the acetabular rim (contact patch to rim = CPR distance) determined the susceptibility of the hip to edge loading, which in turn leads to increased wear and high ion levels. The CPR distance is a calculation that describes the distance from the point where a theoretical joint reaction force [9] intersects the cup to the acetabular rim for a patient in the standing position, depending on the coverage, size, and orientation of the acetabular component The wear of articulating surfaces in an artificial hip is a three-dimensional (3-D) phenomenon so it seems appropriate that a 3-D construct (the CPR distance) rather than separate 2-D measurements (cup abduction and anteversion) be used to study the effect of cup positioning on metal ion levels, but the results of Langton et al. [25] remain to be confirmed.
In this study, we sought (1) to characterize the central tendency of serum metal ion levels in a series of patients treated with unilateral MMHRA, (2) to determine the effects of patient activity, sex, and CPR distance on serum metal ion concentrations, and (3) to evaluate the predictive value of a low CPR distance for elevated serum metal ion levels.
Patients and Methods
We retrospectively reviewed data from 1067 patients who underwent 1323 hip resurfacings between November 1996 and February 2011. From this cohort, 296 patients were tested periodically since December 2000 for metal ion levels, depending on availability and proximity to our Los Angeles and Chicago clinics. We excluded 97 patients because of the presence of another joint replacement at the time of the blood drawing and 17 patients who had blood drawn less than 12 months after surgery (to exclude the hips that had yet to reach steady-state wear) [8, 20]. Included in the study cohort were 96 patients who were followed prospectively as part of a multicenter clinical trial and 86 patients who were believed to be at risk for adverse local tissue reactions owing to having a small component size, poor acetabular orientation, or high activity levels, as judged by the senior surgeon. For patients with multiple blood draws, the most recent qualifying draw for each patient was used. The mean age of the patients was 51 years (range, 20–77 years). There were 73 women (40%) and 109 men (60%) in the study. The mean followup for the patients’ last blood draws was 70 months (range, 12–165 months).
The senior surgeon (HCA) performed all the procedures using the Conserve® Plus prosthesis [2] (Conserve® Plus Total Resurfacing Hip System, Wright Medical Technology, Inc, Arlington, TN, USA) and a posterior approach. The intended positioning of the acetabular component was 42° abduction and 15° anteversion [3]. The Conserve® Plus hip resurfacing device was approved by the FDA.
The patients were followed 6 weeks, 4 months, and then yearly after surgery. Low pelvis AP radiographs and Johnson lateral views were performed at each visit. Pain, walking ability, function, and activity levels were assessed by the University of California, Los Angeles (UCLA) hip scoring system [6].
All the patients’ blood was drawn using a slow-draw technique, and was sent to outside laboratories (Rush University Medical Center Trace Metal Analysis Laboratory, Chicago, IL, USA; Applied Speciation and Consulting, Bothell, WA, USA; Kronos Science Laboratory Services, Phoenix, AZ, USA; London Laboratory Services Group, London, ON, Canada; ARUP Laboratories, Salt Lake City, UT, USA) for analysis. Samples were collected in three 10-mm S-Monovette® syringes (S-Monovette® syringe, Sarstedt, Princeton, NJ, USA) using a Multi-Adapter (Multi-Adapter, Sarstedt) and a butterfly infusion set (Abbott Butterfly® Infusion Set, Abbott®, Abbott Park, IL, USA). The first 10 mm was used to rinse the needle and adapter. The samples were allowed to clot naturally before being centrifuged. Serum samples were stored in acid-washed tubes at −25ºC until they were shipped in dry ice for analysis. All samples were analyzed using inductively coupled plasma mass spectrometry (ICPMS).
Two of us (JPY, AJJ) used Einzel-Bild-Roentgen-Analyse software (EBRA) (EBRA-CUP software, University of Innsbruck, Innsbruck, Austria) on supine AP pelvic radiographs to measure acetabular component abduction and anteversion for all the hips [10, 26]. The intraclass correlation coefficients for interobserver reliability of this method have been reported and are high (0.9) [38]. Using acetabular abduction, anteversion, component size, and coverage angle of the acetabular component, the CPR distance was calculated as previously described [5, 25].
A multiple logistic regression analysis was performed to identify important relationships between high metal ion levels (defined by a concentration of 7 μg/L or greater [29]) and independent variables: sex, UCLA activity score, and low CPR distance of 10 mm or less [25] (Fig. 1). Acetabular component abduction, anteversion, and size were not included in this model to avoid multicollinearity between CPR distance and the aforementioned three variables. Odds ratios were computed for each independent variable and used in conjunction with the associated p values as a measure of strength of correlation. Sensitivity, specificity, positive predictive value, and negative predictive value of a CPR distance less than 10 mm were computed for high CoS and high CrS. All statistical analyses were completed using Stata® version 6 (Stata®, College Station, TX, USA).
Fig. 1A–B.
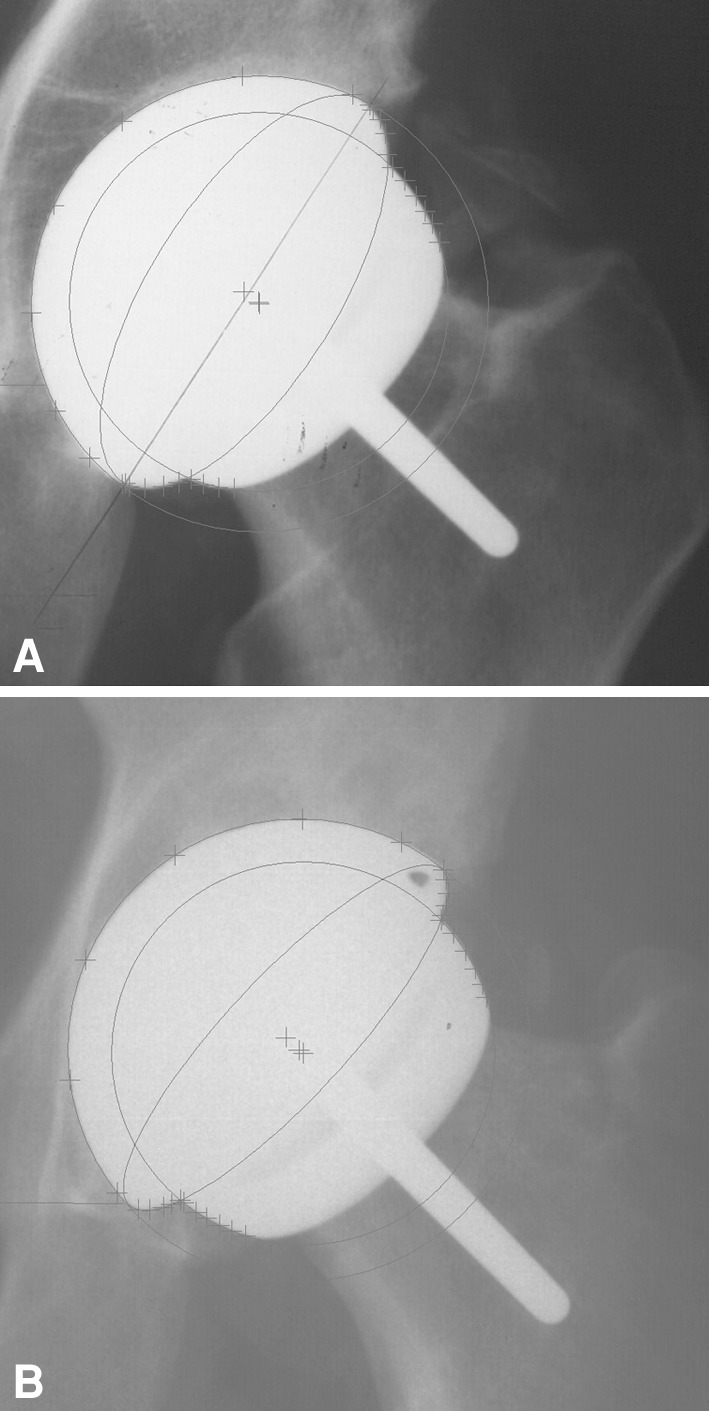
AP radiographs show the cup orientation of two hips reconstructed with 58-mm acetabular components. (A) The CPR distance was 8.0 mm, cup abduction angle 58°, and anteversion 27°. The patient’s CoS was 2.4 μg/L and CrS 3.1 μg/L. (B) The CPR distance was 15.8 mm, cup abduction angle 47°, and anteversion 16°. The patient’s CoS was 0.68 μg/L and CrS 0.91 μg/L.
Results
The median CoS level for the entire cohort was 1.13 μg/L (range, 0.15–175.30 μg/L), and the median CrS level was 1.49 μg/L (range, 0.06–88.70 μg/L) (Table 1).
Table 1.
Measurements of central tendency for the 182 patients
| Variable | Central tendency | Range |
|---|---|---|
| Serum cobalt (μg/L) | 1.13** | 0.15 to 175.30 |
| Serum chromium (μg/L) | 1.49** | 0.06 to 88.70 |
| Femoral component size (mm) | 47.1* | 38 to 56 |
| Acetabular component abduction (°) | 44.7* | 16.2 to 65.7 |
| Acetabular component anteversion (°) | 19.4* | 3.2 to 40.7 |
| CPR distance (mm) | 13.8* | 3.2 to 22.1 |
| UCLA activity score | 7.5* | 3 to 10 |
* mean, **median.
We found associations between low CPR values and CoS and CrS levels. There was a 37-fold increase in the risk of CoS being greater than 7 μg/L (p = 0.005) and an 11-fold increase in the risk of CrS being greater than 7 μg/L (p = 0.003) when CPR distance was 10 mm or less. No associations were shown for sex and UCLA activity scores (Table 2).
Table 2.
Results of multiple logistic regression analysis
| Variable | Serum cobalt levels > 7 μg/L | Serum chromium levels > 7 μg/L | ||
|---|---|---|---|---|
| Odds ratio | p value | Odds ratio | p value | |
| Female sex | 0.3965 | 0.413 | 1.818 | 0.519 |
| UCLA activity score | 0.6265 | 0.224 | 0.6116 | 0.069 |
| CPR < 10 mm | 36.9325 | 0.005 | 11.0485 | 0.003 |
UCLA = University of California, Los Angeles; CPR = contact patch to rim.
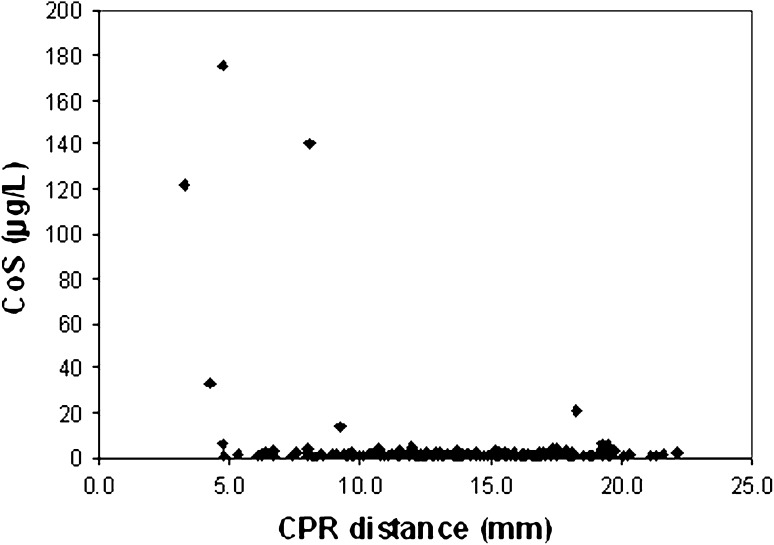
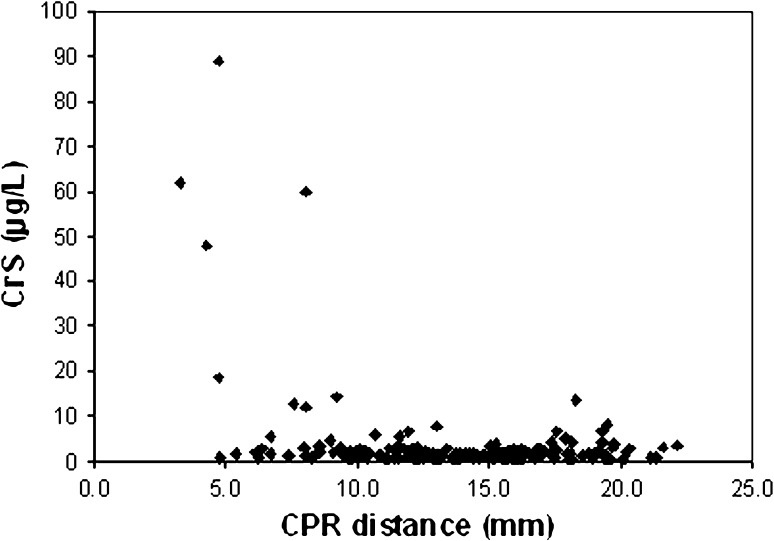
A CPR distance less than 10 mm had a sensitivity of 83.3% and a specificity of 83.5% in predicting a CoS greater than 7 μg/L. Similarly, a CPR distance less than 10 mm had a sensitivity of 72.7% and a specificity of 84.8% in predicting a CrS greater than 7 μg/L. The positive predictive value for CPR distance less than 10 mm was 14.7% for CoS greater than 7 μg/L and 23.5% for CrS greater than 7 μg/L. However, the negative predictive value for CPR distance less than 10 mm was 99.3% for CoS greater than 7 μg/L (Fig. 2) and 98.0% for CrS greater than 7 μg/L (Fig. 3).
Fig. 2.
The scatter plot shows the relationship between CPR distance and CoS ion concentrations. One of 148 patients with a CPR distance greater than 10 mm had CoS ion concentrations greater than 7 μg/L. Twenty-nine of 34 patients with a CPR distance less than 10 mm had CoS ion concentrations less than 7 μg/L.
Fig. 3.
This scatter plot shows the relationship between CPR distance and CrS ion concentrations. Three of 148 patients with a CPR distance greater than 10 mm had CrS ion concentrations greater than 7 μg/L. Twenty-six of 34 patients with a CPR distance less than 10 mm had CrS ion concentrations less than 7 μg/L.
Discussion
MMHRA is associated with an increase in blood metal ion concentration compared with preoperative levels and the British government has issued a medical device alert stating that metal ion levels in excess of 7 μg/L indicate a potential for soft tissue reaction [29]. Component design, size, acetabular orientation, patient sex, and activity level are likely factors contributing to the wear of metal-on-metal bearings. In this study, we characterized the central tendency of serum metal ion levels in a series of patients treated with unilateral MMHRA, and we evaluated the effects and the predictive value of the CPR distance (computed from component design, size, and acetabular orientation) on CoS and CrS ion levels.
We recognize limitations of our study. First the sample of subjects enrolled in the study is likely to have yielded median CoS and CrS values greater than those that could be expected from a randomly selected sample because 86 patients (47%) had blood drawn based on the assumption that they may be at risk for high ion levels. Second, the time between surgery and the last blood draw varied greatly among the subjects and could have influenced the results of our study. However, we found no relationship between the metal ion levels and the length of time to the latest postoperative blood draw (Co: Spearman’s rho = 0.0364, p = 0.6254; Cr: Spearman’s rho = 0.1233, p = 0.0960) and there is evidence in the literature that metal ion levels do not increase with time after the wear-in period of the bearing is completed [8, 20]. Third, the CPR distance is a calculation aiming to detect the hips at risk for edge loading. In case of insufficient abduction or anteversion, the computed CPR distance will be high but the hip will be at risk of lateral or anterior impingement, a possible alternate scenario for an abnormal wear situation. This may have been the reason why one of our patients (the only patient with a false negative for CoS and CrS) had a CPR distance of 18 mm, CoS of 21 μg/L, and CrS of 13 μg/L. In addition, CPR distance calculation does not take into consideration the possible effect of the clearance between femoral and acetabular components. A small clearance is likely to increase the area of the contact patch between components, therefore, reducing the margin of safety before edge-loading wear conditions occur [40]. For this reason, the results of our study might not be transferable to other hip resurfacing designs featuring different clearance parameters.
The median Cos and CrS ion concentrations in our patients are at the low end of the range of levels previously reported for hip resurfacing devices approved by the FDA [1, 22, 31, 32, 42] (Table 3).
Table 3.
Summary of published metal ion studies
| Study | Implant | Number of patients | Followup (months) | Median CoS (range) | Median CrS (range) | Detection method |
|---|---|---|---|---|---|---|
| Witzleb et al. [42] | BHR | 23 | 24 | 4.28 μg/L (range not reported) |
5.12 μg/L (range not reported) |
GF-AAS |
| Kim et al. [22] | Conserve Plus | 97 | 24 | 1.08 μg/L (0.44–7.13) |
1.64 μg/L (0.47–10.95) |
ICPMS |
| Allan et al. [1] | Cormet | 16 | 36 | 2.08 μg/L (range not reported) |
3.55 μg/L (range not reported) |
ICPMS |
| Moroni et al. [31] | BHR | 20 | 24 | 0.75 μg/L (0.08–8.96) |
1.73 μg/L (0.69–7.24) |
GF-AAS |
| Moroni et al. [32] | BHR | 20 | 60 | 0.72 μg/L (0.30–13.50) |
1.63 μg/L (0.49–10.67) |
GF-AAS |
| Current study | Conserve Plus | 182 | 12–165 | 1.13 μg/L (0.15– 175.30) |
1.49 μg/L (0.06–88.70) |
ICPMS |
BHR = Birmingham Hip Replacement; GF-AAS = graphite furnace atomic absorption spectrometry; ICPMS = inductively coupled plasma mass spectrometry.
Our findings also show a clear association between low CPR distance and high ion levels. Langton et al. [25] reported that hips with a low CPR distance (< 10 mm) are at risk for accelerated wear as they found an inverse relationship between metal ion concentrations and CPR distance. However, these results were obtained from a series of patients with two different devices implanted, and their regression model did not include adjustments for sex or activity. The results of our multivariate regression model did confirm the value of their conclusions, this time applied to the Conserve® Plus prosthesis.
Accelerated wear for patients with a low CPR distance is thought to be attributable to an increased susceptibility to edge loading and subluxation. Hip replacements that show optimal wear characteristics are dependent on the proper generation of a fluid-film layer. In edge-loaded components, the articular contact area between the head and the cup is closer to the rim of the cup, disrupting the fluid-film layer and increasing undesired stress forces, leading to an increase in wear [30]. CPR distance is determined by the acetabular orientation angles, which may explain the relationship between increased wear and acetabular orientation, and the coverage arc, which shows a major negative correlation with metal ion concentrations [14, 17, 24]. To achieve an optimal acetabular component positioning for the Conserve® Plus, abduction should be 45º and anteversion should be 15º, with approximately 10º of freedom. This safe zone, however, is device-specific and would be inappropriate for other designs, especially those with lower coverage in smaller sizes. We believe that acetabular loosening, increased wear, and adverse local tissue reactions can be prevented by proper acetabular component orientation while using the instruments and technique as described [3]. Intraoperative radiographs can be used to ensure proper placement and orientation of the socket, which can be easily removed if necessary, cleaned of soft tissue debris, and reinserted with the correct orientation.
We found no relationship between activity level and metal ion concentrations, and this is consistent with previous studies [12, 14, 19, 34, 41]. It is possible, however, that malpositioned components may be more prone to a compound effect of frequency, duration, and type of activity that is not sufficiently described by the UCLA activity score.
We found no relationship between metal ion concentrations and female sex. This result is in contrast to two previous studies [32, 41] in which female sex was identified as a risk factor for elevated ion levels after hip resurfacing surgery.
An important result from our study is the predictive value of CPR distance on CoS and CrS levels. The low positive predictive value indicates that a low CPR value will not necessarily translate into elevated ion levels. However, the high negative predictive value (99.3% for CoS and 98.0% for CrS) is important to the clinician because it means that patients with a CPR value greater than 10 mm have less than 1% chance to have high CoS levels and 2% chance to have high CrS levels.
Our results confirmed that patients with a low CPR distance are at risk for accelerated wear. Metal ion levels should be monitored on a yearly basis in patients with a CPR that is less than 10 mm, and metal artifact reduction sequence MR or ultrasound images may be indicated to identify periprosthetic solid or fluid masses. However, based on the results of this study, we advocate the use of CPR distance calculation as a first step to evaluate the possibility of edge-loading and abnormal wear of the bearing. If the CPR distance is greater than 10 mm, no additional study is needed unless the patient is symptomatic. We suggest that studies similar to ours be done in large centers performing hip resurfacing with different designs to establish device-specific parameters of proper acetabular component orientation.
Acknowldgments
We thank Patricia Campbell PhD for her contribution to the design of the serum metal ion analysis protocol that was used in this article.
Footnotes
One of the authors (HCA) certifies that he has or may receive payments or benefits, during the study period, an amount of $ ($10,000–$100,000), from Wright Medical Technology Inc, Arlington, TN, USA).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
This work was performed at the Joint Replacement Institute at St. Vincent Medical Center, Los Angeles, CA, USA.
References
- 1.Allan DG, Trammell R, Dyrstad B, Barnhart B, Milbrandt JC. Serum cobalt and chromium elevations following hip resurfacing with the Cormet 2000 device. J Surg Orthop Adv. 2007;16:12–18. [PubMed] [Google Scholar]
- 2.Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed] [Google Scholar]
- 3.Amstutz HC, Beaulé PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: surgical technique. J Bone Joint Surg Am. 2006;88(suppl 1):234–249. doi: 10.2106/JBJS.F.00273. [DOI] [PubMed] [Google Scholar]
- 4.Amstutz HC, Le Duff MJ, Campbell PA, Wisk LE, Takamura KM. Complications after metal-on-metal hip resurfacing arthroplasty. Orthop Clin North Am. 2011;42:207–230. doi: 10.1016/j.ocl.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Amstutz HC, Le Duff MJ, Johnson AJ. Socket position determines hip resurfacing 10-year survivorship. Clin Orthop Relat Res. 2012 Apr 18. [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 6.Amstutz HC, Thomas BJ, Jinnah R, Kim W, Grogan T, Yale C. Treatment of primary osteoarthritis of the hip: a comparison of total joint and surface replacement arthroplasty. J Bone Joint Surg Am. 1984;66:228–241. [PubMed] [Google Scholar]
- 7.Anissian HL, Stark A, Gustafson A, Good V, Clarke IC. Metal-on-metal bearing in hip prosthesis generates 100-fold less wear debris than metal-on-polyethylene. Acta Orthop. Scand. 1999;70:578–582. doi: 10.3109/17453679908997845. [DOI] [PubMed] [Google Scholar]
- 8.Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop Relat Res. 2005;438:177–181. doi: 10.1097/01.blo.0000166901.84323.5d. [DOI] [PubMed] [Google Scholar]
- 9.Bergmann G, Graichen F, Rohlmann A. Hip joint loading during walking and running, measured in two patients. J Biomech. 1993;26:969–990. doi: 10.1016/0021-9290(93)90058-M. [DOI] [PubMed] [Google Scholar]
- 10.Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B. Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87:762–769. doi: 10.1302/0301-620X.87B6.14745. [DOI] [PubMed] [Google Scholar]
- 11.Boardman DR, Middleton FR, Kavanagh TG. A benign psoas mass following metal-on-metal resurfacing of the hip. J Bone Joint Surg Br. 2006;88:402–404. doi: 10.1302/0301-620X.88B3.16748. [DOI] [PubMed] [Google Scholar]
- 12.De Haan R, Campbell P, Reid S, Skipor AK, De Smet K. Metal ion levels in a triathlete with a metal-on-metal resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2007;89:538–541. doi: 10.1302/0301-620X.89B4.18563. [DOI] [PubMed] [Google Scholar]
- 13.De Haan R, Campbell PA, Su EP, De Smet KA. Revision of metal-on-metal resurfacing arthroplasty of the hip: the influence of malpositioning of the components. J Bone Joint Surg Br. 2008;90:1158–1163. doi: 10.1302/0301-620X.90B9.19891. [DOI] [PubMed] [Google Scholar]
- 14.De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–1297. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 15.De Smet K, De Haan R, Calistri A, Campbell PA, Ebramzadeh E, Pattyn C, Gill HS. Metal ion measurement as a diagnostic tool to identify problems with metal-on-metal hip resurfacing. J Bone Joint Surg Am. 2008;90(suppl 4):202–208. doi: 10.2106/JBJS.H.00672. [DOI] [PubMed] [Google Scholar]
- 16.Desy NM, Bergeron SG, Petit A, Huk OL, Antoniou J. Surgical variables influence metal ion levels after hip resurfacing. Clin Orthop Relat Res. 2011;469:1635–1641. doi: 10.1007/s11999-010-1636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hart AJ, Buddhdev P, Winship P, Faria N, Powell JJ, Skinner JA. Cup inclination angle of greater than 50 degrees increases whole blood concentrations of cobalt and chromium ions after metal-on-metal hip resurfacing. Hip Int. 2008;18:212–219. doi: 10.1177/112070000801800304. [DOI] [PubMed] [Google Scholar]
- 18.Hart AJ, Skinner JA, Henckel J, Sampson B, Gordon F. Insufficient acetabular version increases blood metal ion levels after metal-on-metal hip resurfacing. Clin Orthop Relat Res. 2011;469:2590–2597. doi: 10.1007/s11999-011-1930-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heisel C, Silva M, Skipor AK, Jacobs JJ, Schmalzried TP. The relationship between activity and ions in patients with metal-on-metal bearing hip prostheses. J Bone Joint Surg Am. 2005;87:781–787. doi: 10.2106/JBJS.D.01820. [DOI] [PubMed] [Google Scholar]
- 20.Heisel C, Streich N, Krachler M, Jakubowitz E, Kretzer JP. Characterization of the running-in period in total hip resurfacing arthroplasty: an in vivo and in vitro metal ion analysis. J Bone Joint Surg Am. 2008;90(suppl 3):125–133. doi: 10.2106/JBJS.H.00437. [DOI] [PubMed] [Google Scholar]
- 21.Hing CB, Back DL, Bailey M, Young DA, Dalziel RE, Shimmin AJ. The results of primary Birmingham hip resurfacings at a mean of five years: an independent prospective review of the first 230 hips. J Bone Joint Surg Br. 2007;89:1431–1438. doi: 10.1302/0301-620X.89B11.19336. [DOI] [PubMed] [Google Scholar]
- 22.Kim PR, Beaulé PE, Dunbar M, Lee JK, Birkett N, Turner MC, Yenugadhati N, Armstrong V, Krewski D. Cobalt and chromium levels in blood and urine following hip resurfacing arthroplasty with the Conserve Plus implant. J Bone Joint Surg Am. 2011;93(suppl 2):107–117. doi: 10.2106/JBJS.J.01721. [DOI] [PubMed] [Google Scholar]
- 23.Kwon YM, Ostlere SJ, McLardy-Smith P, Athanasou NA, Gill HS, Murray DW. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty. 2011;26:511–518. doi: 10.1016/j.arth.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Langton DJ, Jameson SS, Joyce TJ, Webb J, Nargol AV. The effect of component size and orientation on the concentrations of metal ions after resurfacing arthroplasty of the hip. J Bone Joint Surg Br. 2008;90:1143–1151. doi: 10.1302/0301-620X.90B9.20785. [DOI] [PubMed] [Google Scholar]
- 25.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AV. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and Birmingham Hip Resurfacing arthroplasties. J Bone Joint Surg Br. 2009;91:1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 26.Langton DJ, Sprowson AP, Mahadeva D, Bhatnagar S, Holland JP, Nargol AV. Cup anteversion in hip resurfacing: validation of EBRA and the presentation of a simple clinical grading system. J Arthroplasty. 2010;25:607–613. doi: 10.1016/j.arth.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 27.MacDonald SJ, McCalden RW, Chess DG, Bourne RB, Rorabeck CH, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;406:282–296. doi: 10.1097/00003086-200301000-00039. [DOI] [PubMed] [Google Scholar]
- 28.McMinn D, Treacy R, Lin K, Pynsent P. Metal on metal surface replacement of the hip: experience of the McMinn prosthesis. Clin Orthop Relat Res. 1996;329(suppl):S89–S98. doi: 10.1097/00003086-199608001-00009. [DOI] [PubMed] [Google Scholar]
- 29.MHRA. Medical Device Alert: All metal-on-metal (MoM) hip replacements (MDA/2012/008). http://www.mhra.gov.uk/home/groups/dts-bs/documents/medicaldevicealert/con143787.pdf. Accessed September 18, 2012.
- 30.Morlock MM, Bishop N, Zustin J, Hahn M, Ruther W, Amling M. Modes of implant failure after hip resurfacing: morphological and wear analysis of 267 retrieval specimens. J Bone Joint Surg Am. 2008;90(suppl 3):89–95. doi: 10.2106/JBJS.H.00621. [DOI] [PubMed] [Google Scholar]
- 31.Moroni A, Savarino L, Cadossi M, Baldini N, Giannini S. Does ion release differ between hip resurfacing and metal-on-metal THA? Clin Orthop Relat Res. 2008;466:700–707. doi: 10.1007/s11999-007-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moroni A, Savarino L, Hoque M, Cadossi M, Baldini N. Do ion levels in hip resurfacing differ from metal-on-metal THA at midterm? Clin Orthop Relat Res. 2011;469:180–187. doi: 10.1007/s11999-010-1424-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CL, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;90:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 34.Pattyn CA, Lauwagie SN, Verdonk RC. Whole blood metal ion concentrations in correlation with activity level in three different metal-on-metal bearings. J Arthroplasty. 2011;26:58–64. doi: 10.1016/j.arth.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Savarino L, Granchi D, Ciapetti G, Cenni E, Nardi Pantoli A, Rotini R, Veronesi CA, Baldini N, Giunti A. Ion release in patients with metal-on-metal hip bearings in total joint replacement: a comparison with metal-on-polyethylene bearings. J Biomed Mater Res. 2002;63:467–474. doi: 10.1002/jbm.10299. [DOI] [PubMed] [Google Scholar]
- 36.Schmalzried TP. Metal-metal bearing surfaces in hip arthroplasty. Orthopedics. 2009;32: pii: orthosupersite.com/view.asp?rID = 42831. doi: 10.3928/01477447-20090728-06. [DOI] [PubMed]
- 37.Steens W, von Foerster G, Katzer A. Severe cobalt poisoning with loss of sight after ceramic-metal pairing in a hip: a case report. Acta Orthop. 2006;77:830–832. doi: 10.1080/17453670610013079. [DOI] [PubMed] [Google Scholar]
- 38.Tiberi JV, Pulos N, Kertzner M, Schmalzried TP. A more reliable method to assess acetabular component position. Clin Orthop Relat Res. 2012;470:471–476. doi: 10.1007/s11999-011-2006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tower SS. Arthroprosthetic cobaltism: neurological and cardiac manifestations in two patients with metal-on-metal arthroplasty. A case report. J Bone Joint Surg Am. 2010;92:2847–2851. doi: 10.2106/JBJS.J.00125. [DOI] [PubMed] [Google Scholar]
- 40.Underwood RJ, Zografos A, Sayles RS, Hart A, Cann P. Edge loading in metal-on-metal hips: low clearance is a new risk factor. Proc Inst Mech Eng H. 2012;226:217–226. doi: 10.1177/0954411911431397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vendittoli PA, Mottard S, Roy AG, Dupont C, Lavigne M. Chromium and cobalt ion release following the Durom high carbon content, forged metal-on-metal surface replacement of the hip. J Bone Joint Surg Br. 2007;89:441–448. doi: 10.1302/0301-620X.89B4.18054. [DOI] [PubMed] [Google Scholar]
- 42.Witzleb WC, Ziegler J, Krummenauer F, Neumeister V, Guenther KP. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop. 2006;77:697–705. doi: 10.1080/17453670610012863. [DOI] [PubMed] [Google Scholar]