Abstract
Background
Tibia vara seen in Japanese patients reportedly influences the tibial component alignment when performing TKA. However, it is unclear whether tibia vara affects the component position and size selection.
Questions/purposes
We therefore determined (1) the amount of medial tibial bow, (2) whether the tibia vara influences the aspect ratio of the tibial resected surface in aligning the tibial component with the tibial shaft axis, and (3) whether currently available tibial components fit the shapes of resected proximal tibias in terms of aspect ratio.
Methods
We measured the tibia vara angle (TVA), proximal varus angle (PVA), and the mediolateral and middle AP dimensions of the resected surface using three-dimensional preoperative planning software in 90 knees of 74 female patients with varus osteoarthritis. We determined the correlations of the aspect ratio with TVA or PVA and compared the aspect ratios to those of five prosthesis designs.
Results
The mean TVA and PVA were 0.6° and 2.0°, respectively. The aspect ratio negatively correlated with both TVA and PVA (r = −0.53 and −0.55, respectively). The mean aspect ratio of the resected surface was 1.48 but gradually decreased with increasing AP dimension, whereas four of the five prostheses had a constant aspect ratio.
Conclusions
The aspect ratio of resected tibial surface was inversely correlated to the degree of tibia vara, and currently available prosthesis designs do not fit well to the resected surface in terms of aspect ratio.
Clinical Relevance
The design of a tibial component with a smaller aspect ratio could be developed to obtain better bone coverage in Japanese patients.
Introduction
In the classic alignment method of TKA, the proximal tibia is resected perpendicularly to the tibial shaft axis (TSA; the anatomic longitudinal axis of the tibia) in the coronal plane, and an appropriately sized tibial component is selected so that it covers the resected surface without overhang [23]. This method is reasonable when the femoral component is implanted parallel to the transepicondylar axis (TEA): kinematic studies [4, 7] have demonstrated the tibia rotates around the functional flexion-extension axis located in the distal femur and it coincides with the anatomic TEA [4, 18]. Further, the TSA is perpendicular to the TEA during knee flexion and extension [18, 19]. Several studies of frontal alignment of normal knees [10, 15, 23] have shown the mechanical axis of the lower limb passes through the knee center, and the tibial mechanical axis (TMA; the axis connecting the tibia plateau center and the ankle center) aligns with the TSA [15, 17]. The center of the properly placed tibial component is located at the center of the resected tibial surface and aligns with both the TSA and TMA. A symmetrically shaped tibial component that aligns its center with the TSA covers the maximum area of the resected tibial surface, and the knee after the TKA should mimic physiologic motion as mentioned above [18, 19]. Further, a load passing through the knee should be equally distributed to the medial and lateral compartments because the TMA passes perpendicularly through the center of the prosthetic component [9].
However, Asian people generally have a varus knee alignment [8, 20] and Asian patients with varus knee osteoarthritis (OA) have varying degrees of tibia vara [12, 16, 24]. This anatomic variance has made it difficult to achieve proper frontal alignment and proper component positioning in TKA because the TSA does not align with the TMA and passes through a point lateral to the center of the tibial eminence [12, 14, 20, 24]. Consequently, lateralization of the tibial component is required to accord its center with the TSA, and it often needs downsizing of the component to avoid lateral overhang by the prosthesis. The downsizing results in an uncovered medial edge of the tibia, which can be resected to avoid impingement by the edge. This implantation technique, however, may result in an AP underhang because many of the tibial components of prostheses currently used for Asian patients are designed based on anatomic data from tibias of Western subjects, in whom tibia vara is relatively rare even though varus deformities are common [5, 6, 21]. Therefore, it is unclear whether the aspect ratio (ratio of the mediolateral [ML]/AP length) of currently available tibial components is actually suitable to obtain a proper fitting for Japanese patients with tibia vara.
We therefore determined (1) how much the tibias in Japanese patients actually medially bow, (2) whether the tibia vara influences the aspect ratio of the tibial resected surface in aligning the tibial component with the TSA, and (3) whether currently available tibial components fit the shapes of resected proximal tibias in terms of aspect ratio.
Patients and Methods
The study was performed using CT data from 90 lower limbs in 74 Japanese female patients with primary varus knee OA, scheduled for primary TKAs between January 2010 and March 2012. During the study period, there were only eight male patients undergoing TKA for varus knee deformity. We excluded these eight male patients from the study because we could not compare the anatomic differences between male and female knees due to the small number of males. The mean age at the time of surgery was 73 years (range, 59–86 years). The mean height was 150 cm (133–163 cm). Patients with valgus knee OA, previous knee surgeries, posttraumatic arthritis, and rheumatoid arthritis were excluded from the study. The hospital ethics committee determined the study did not require approval in our country; the patients provided informed consent for participation in the study.
In all patients, we obtained multislice CT scans (LightSpeed® VCT; GE Healthcare, Chalfont St Giles, UK) of the hip to the ankle using 1-mm slices with patients in the knee-extended position with the patella facing toward the ceiling. The obtained DICOM data were imported into the three-dimensional (3-D) preoperative TKA planning software (3-D template for TKA; Kyocera Medical Corp, Osaka, Japan). In this software, the operating windows consisted of three multiplanar reformation (MPR) viewers, the frontal, sagittal, and axial planes, and each reformatted image could be simultaneously rotated, cut, and measured in all three operating windows. Digitally reconstructed radiographs (DRRs) could also be displayed in all three directions with this software.
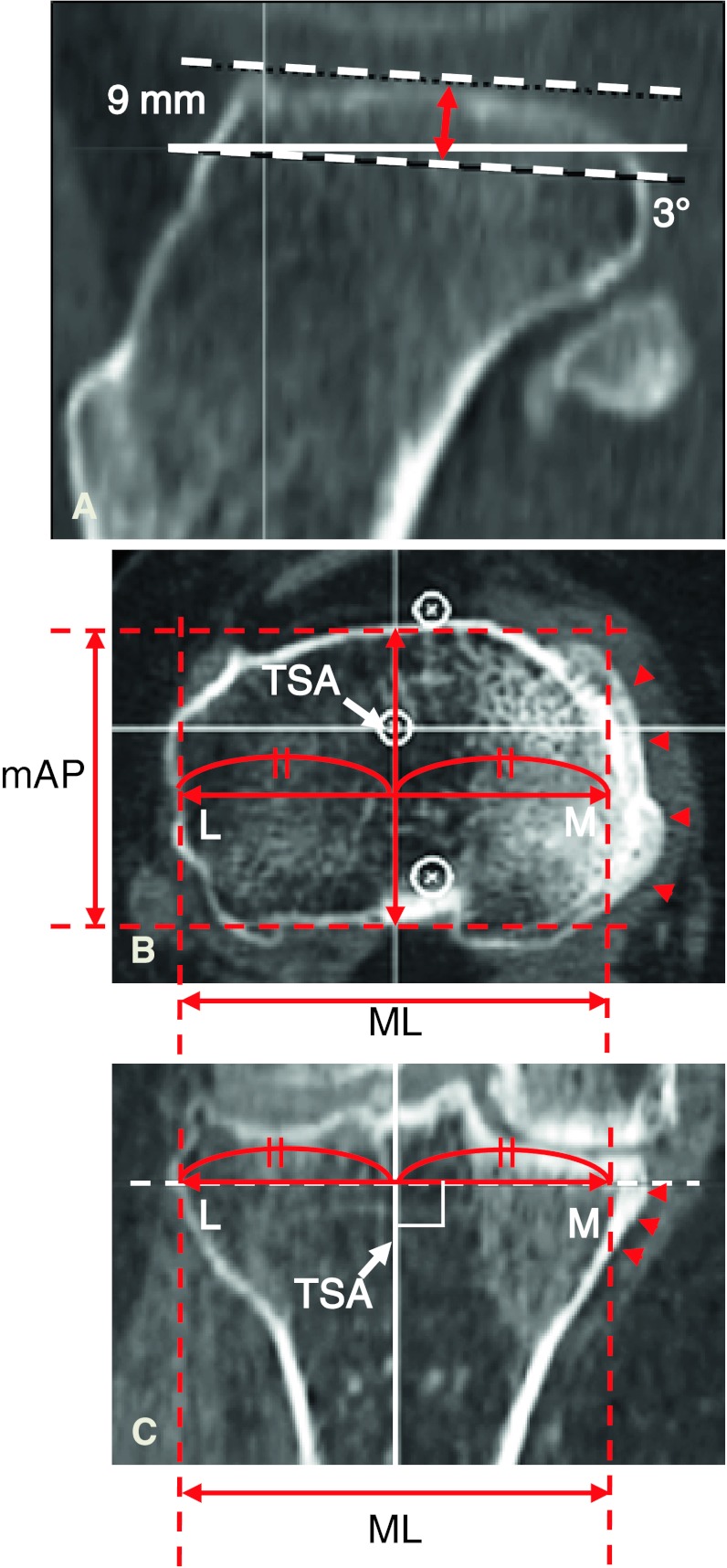
Two investigators (SM, SA) performed all radiographic assessments and measured the resected surface of the proximal tibia using the preoperative planning software. In the radiographic assessment, the severity of the OA according to the Kellgren-Lawrence classification [11] was Grade 3 in 49 knees and Grade 4 in the remaining 41 knees. The hip-knee-ankle angle (measured from full-length, weightbearing AP radiographs) averaged 12° of varus alignment (range, 1°–27°). To determine the orientation of the tibia in a 3-D coordinate system, two axes were defined. First, the TSA was defined as a line connecting the centers of the proximal 1/3 and distal 1/3 of the tibia, confirmed in both coronal and lateral DRR views. Then, the tibial AP axis was drawn as a line connecting the middle of the PCL and the medial border of the patellar tendon at its attachment to the tibial tubercle [1, 2]. After setting each reference point for these two axes, the tibia was verticalized along the TSA and frontalized along the tibial AP axis. Next, the TMA was drawn so that it passed from the center of the tibial eminence to the center of the talar dome. To assess tibia vara, the tibia vara angle (TVA; the angle between the TSA and TMA) was measured (Fig. 1). To assess the medial bowing in the proximal tibia, the proximal vara angle (PVA) was defined as the angle between the TSA and the line connecting the center of the tibial eminence and the center of the proximal 1/3 of the tibia (Fig. 1). Finally, virtual resection of the proximal tibia was performed with the software to evaluate the aspect ratio of the resected tibial surface. The proximal tibia was cut perpendicular to the TSA 9 mm below the lateral tibial plateau with a 3° posterior slope (Fig. 2A). We measured the ML and AP dimensions of the proximal resected surface when the tibial component was set so that its center aligned with the TSA (Fig. 2B–C). That is, ½ of the ML dimension was defined as the distance from the lateral edge of the tibial resected surface to the TSA. The middle AP dimension of the tibia was then measured as the length of a line segment passing through the TSA. The aspect ratio (the ML dimension divided by the AP dimension) was calculated as described previously [6]. We also obtained the ML and middle AP measurements data for five currently available tibial prosthesis designs: (1) Bi-Surface® Total Knee System (Kyocera Medical Corp); (2) Deltafit (Scorpio®; Stryker Orthopaedics, Mahwah, NJ, USA); (3) NexGen® (Zimmer, Inc, Warsaw, IN, USA); (4) PROFIX® Total Knee System (Smith & Nephew, Inc, Memphis, TN, USA); and (5) Vanguard® Complete Knee System (Biomet, Inc, Warsaw, IN, USA). All these products have an ML symmetrical design. To observe the feasibility of these components for Japanese patients, the relationship between the aspect ratio and the middle AP dimension of our patients was analyzed and compared to those of the five tibial prosthesis designs.
Fig. 1.
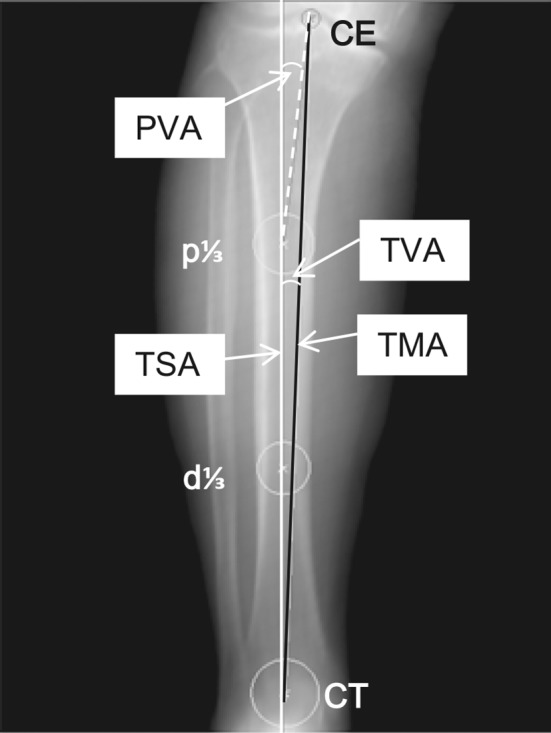
A coronal view of a DRR image used for measurement with the 3-D preoperative planning software is shown. The TSA is the line connecting p1/3 and d1/3. The TMA is the line connecting CE and CT. The TVA is the angle between TSA and TMA. The PVA is the angle between the TSA and the line connecting CE and p1/3. p1/3 = center of the proximal 1/3 of the tibia; d1/3 = center of the distal 1/3 of the tibia; CE = center of the tibial eminence; CT = center of the talar dome.
Fig. 2A–C.
Tibial resection and measurements using the 3-D preoperative planning software are shown. (A) The proximal tibia was resected 9 mm below the lateral tibial plateau with a 3° posterior slope. (B) An axial MPR image of the resected surface is depicted. (C) A coronal MPR image of the resected tibia is shown. Half of the ML dimension is measured as the length from the lateral edge of the resected surface to the TSA. The middle AP (mAP) dimension is the length of the line segment passing through the TSA. Arrowheads indicate the medial edge of the tibial condyle to be resected. M = medial; L = lateral.
To determine how much the tibias in female Japanese patients actually medially bow, the tibia bowing angle and the proximal bowing angle were summarized as means, SDs, and ranges. Linear regression and correlation analyses were performed to determine whether the tibia vara influenced the aspect ratio of the tibial resected surface. Finally, to determine whether currently available tibial components fit the shapes of resected proximal tibias in terms of aspect ratio, the patients’ aspect ratio data were compared with those of five prostheses available in Japan. Linear regression and correlation analyses were performed on an electronic spreadsheet (Excel® 2010; Microsoft Corp, Redmond, WA, USA). Correlations were evaluated using the statistical software (Statcel® 3; OMS Ltd, Saitama, Japan) for the Pearson correlation coefficient test.
Results
The majority (> 83%) of Japanese knees scheduled for TKA had tibia vara. The mean TVA was 0.6° (SD, 0.6°; range, −1.0° to 2.1°) and the mean PVA was 2.0° (SD, 1.5°; range, −1.4° to 6.8°) (Table 1). Seventy-five tibias from 90 knees had positive TVA values, 12 had negative values, and only three had TVAs of 0°, in which the TSA completely aligned with the TMA (Fig. 3). Concerning PVA, 79 tibias had positive values, seven had values of 0°, and four had negative values (Fig. 4).
Table 1.
Summary of the measurements
| Parameter | Mean | SD | Range |
|---|---|---|---|
| Tibia vara angle (°) | 0.6 | 0.6 | −1.0 to 2.1 |
| Proximal varus angle (°) | 2 | 1.5 | −1.4 to 6.8 |
| Mediolateral dimension (mm) | 66.2 | 4.7 | 54.8–78.0 |
| Middle AP dimension (mm) | 44.9 | 2.3 | 40.1–50.3 |
| Aspect ratio | 1.48 | 0.1 | 1.23–1.77 |
Fig. 3.
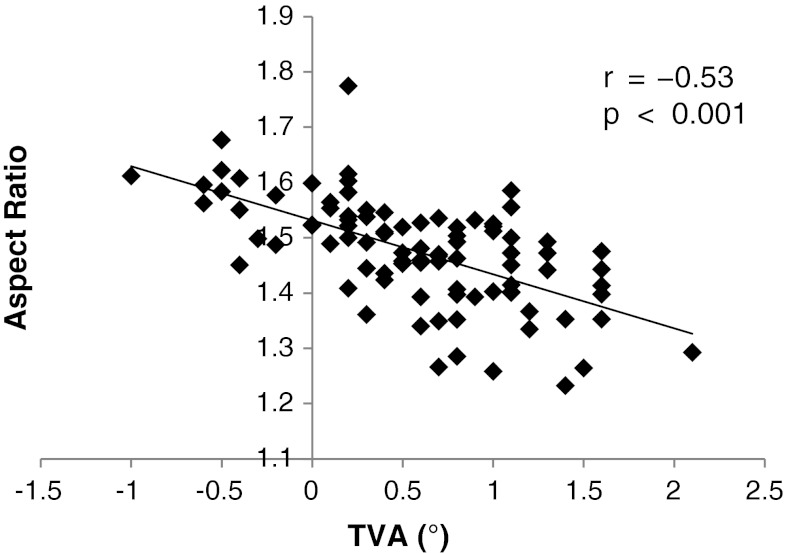
A graph shows there is a strong negative correlation between the aspect ratio and the TVA (r = −0.53, p < 0.001) in the 90 knees.
Fig. 4.
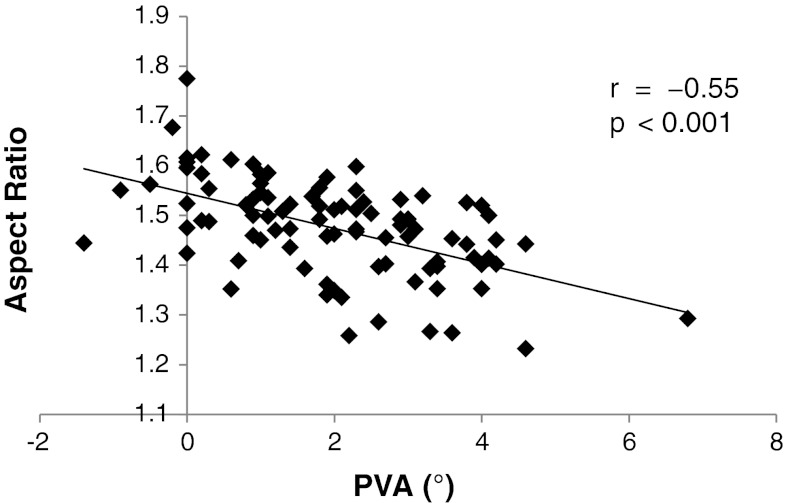
A graph shows there is a strong negative correlation between the aspect ratio and the PVA (r = −0.55, p < 0.001) in the 90 knees.
The aspect ratio of the tibial resected surface was associated with the extent of tibia vara. The measurement of the resected surface showed the ML dimension averaged 66.2 mm (range, 54.8–78 mm), the middle AP dimension averaged 44.9 mm (range, 40.1–50.3 mm), and the mean aspect ratio was 1.48 (range, 1.23–1.77) (Table 1). The aspect ratio negatively correlated with the TVA (r = −0.53, p < 0.001) (Fig. 3) and the PVA (r = −0.55, p < 0.001) (Fig. 4).
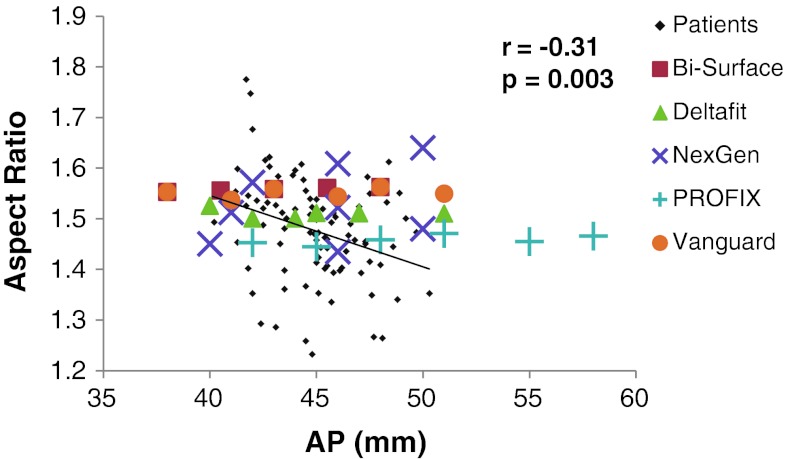
The currently available five tibial components did not fit the shapes of resected proximal tibias in terms of aspect ratio. The aspect ratios of all designs except the PROFIX® Total Knee System were greater than the mean aspect ratio of the tibial resected surface measured in our patients (Table 2). All designs except the NexGen® had almost constant aspect ratios for their AP dimensions, whereas the aspect ratio of the tibial resected surface negatively correlated with middle AP dimension (r = −0.31, p = 0.003) (Fig. 5). In contrast, NexGen® had aspect ratios that increased with the AP dimension, and the various sizes of NexGen® prostheses had two lines with distinct slopes in the aspect ratio (Fig. 5).
Table 2.
Aspect ratios of the five tibial components currently available in Japan
| Prosthesis | Aspect ratio | SD | Range |
|---|---|---|---|
| Bi-Surface® Total Knee System (Kyocera Medical Corp, Osaka, Japan) | 1.56 | 0 | 1.55–1.56 |
| Deltafit (Scorpio®; Stryker Orthopaedics, Mahwah, NJ, USA) | 1.51 | 0.01 | 1.50–1.53 |
| NexGen® (Zimmer, Inc, Warsaw, IN, USA) | 1.53 | 0.09 | 1.43–1.64 |
| PROFIX® Total Knee System (Smith & Nephew, Inc, Memphis, TN, USA) | 1.46 | 0.01 | 1.44–1.47 |
| Vanguard® Complete Knee System (Biomet, Inc, Warsaw, IN, USA) | 1.55 | 0.01 | 1.54–1.56 |
| Current study | 1.48 | 0.10 | 1.23–1.77 |
Fig. 5.
A graph shows the relationship between the aspect ratio and the middle AP dimension in the 90 knees. The aspect ratios of five tibial component designs of various sizes are also plotted. The aspect ratio shows a gradual decline with increasing AP dimension, although the correlation was weak (r = −0.31, p = 0.003). Almost all the aspect ratios of the five different designs available in variable sizes are distributed above the regression line.
Discussion
The principles of the bone-cutting technique, ideal component position, and component designs for TKA have been devised based on normal limb anatomy [10, 15, 23]. Regarding the tibia, to restore more physiologic motion and to obtain even load distribution in knees after TKA, in the frontal alignment, the tibial component is arguably recommended to be set so that its center aligns with the TSA as much as possible. However, in knees with tibia vara, typically seen in Asian patients with varus OA, it often becomes difficult to simultaneously achieve both the ideal component position and sufficient coverage of the resected surface with the tibial component because many prosthetic components currently available are merely designed based on normal anatomy and not taking anatomic variation into account [6]. Therefore, to fit the tibial component in the ideal position, the impact of anatomic variation on component design should also be considered. We therefore determined (1) how much the tibias in Japanese patients actually medially bow, (2) whether the tibia vara influences the aspect ratio of the tibial resected surface in aligning the tibial component with the TSA, and (3) whether currently available tibial components fit the shapes of resected proximal tibias in terms of aspect ratio.
We acknowledge limitations to our study. First, we could not determine the influence of sex because sufficient numbers of male patients could not be gathered. Although patients with varus knee OA undergoing TKA are predominantly women, previous reports [3, 13, 22] have demonstrated several sex differences. Additional studies with large numbers are required to confirm the influence of sex. Second, CT data, which we used for measurements, cannot demonstrate the thickness of remaining cartilage in the lateral tibial plateau. Thickness of the remaining cartilage should affect the thickness of the resected proximal tibia. If the remaining cartilage on the lateral tibial plateau is 2 mm in thickness, the resected tibia including the cartilage could become 11 mm in thickness, when the resection level is determined to be 9 mm below the bony surface of the lateral tibial plateau on CT data. The component with 11 mm in thickness is most frequently used in the clinical setting. We therefore determined the resection level as 9 mm below the bony surface of intact lateral tibial plateau. Third, we accounted for only symmetrically shaped tibial components. Certainly, tibial components with ML asymmetrical shape should provide more preferable fitting. However, only several kinds of components actually have an asymmetrical design, and also to simplify the issue, we performed the measurements with the assumption of fitting a symmetrical tibial component.
Several studies report tibia vara in Asian patients with medial knee OA (Table 3). In a study of 133 Japanese patients with varus knee OA, Nagamine et al. [16] reported an average tibial plateau shift angle (the angle between the central line of the proximal diaphysis and the line from the central point of the plateau to the midpoint of the tibia) of 0.1° (SD, 1.8°; range, −3° to 4°). Ko et al. [12] demonstrated the angle formed an anatomic and mechanical axis after a proximal tibial cut of 1.8° (SD, 1.4°) in Chinese patients scheduled for TKA. Yoo et al. [24] demonstrated the angle formed the TSA and the mechanical axis averaged 0.7° (range, −3° to 4°) in Korean patients. Because the definition and measurement method for TVA differed among these studies, the results also differed. However, all studies including ours demonstrated the tibias of Asian patients with varus knee OA bow medially (positive TVA) in their proximal part (larger PVA than TVA). The mean TVA of 0.6° in our study was similar to that found in Korean patients [24] and smaller than that found in Chinese patients [12]. All of our angular measurements were based on CT and 3-D preoperative planning software while those in the previous reports were based on radiographic measurements. We determined the anatomic orientation of the tibia in 3-D. Therefore, the accuracy of the angular measurement would be higher and our data may be representative of female Japanese patients undergoing TKA. Although the absolute values of the TVA mostly appear to be small, tibia vara has some impact on the tibial component frontal alignment or the component setting [14, 24]. Yoo et al. [24] noted, when a tibial component with a medially offset stem was used, the impingement of the tip of the stem to the medial cortex in the proximal tibia occurred in 6.5% of Korean patients. However, the relative position of the tibial component center to the TSA may not have been considered in selection of the component size in these studies.
Table 3.
Comparison of the TVA in the literature
| Study | Nationality | Knees | TVA | PVA |
|---|---|---|---|---|
| Oswald et al. [17] | Swiss | Normal | 0.1° ± 1.1° | NM |
| Nagamine et al. [16]* | Japanese | Osteoarthritic | 0.1° ± 1.5° (−3° to 7°) | NM |
| Ko et al. [12]* | Chinese | Osteoarthritic | 1.8° ± 1.4° (0°–9°) | NM |
| Yoo et al. [24] | Korean | Osteoarthritic | 0.7° ± 0.6° (−3° to 4°) | NM |
| Current study | Japanese | Osteoarthritic | 0.6° ± 0.6° (−1° to 2.1°) | 2° ± 1.5° (−1.4° to 6.8°) |
* Definition of the TVA is different from that of this study; data are expressed as mean ± SD, with range in parentheses; TVA = tibia vara angle; PVA = proximal varus angle; NM = not measured.
We found the mean aspect ratio of the resected surface was 1.48 (SD, 0.1; range, 1.27–1.77), which is smaller than that found in other studies demonstrating the common characteristics of proximal tibial morphology: 1.54 in Japanese subjects (Uehara et al. [22]), 1.56 in Korean subjects (Kawak et al. [13]), and 1.51 in Chinese subjects (Cheng et al. [3]) (Table 4). Furthermore, the aspect ratio negatively correlated with both TVA and PVA. These findings suggest, in the knee with tibia vara, sufficient coverage is not achieved even if a component designed based on Asian knee morphology was used, and the more the tibia is medially bowed, the narrower is the ML component required to align the component center with the TSA. As a result of ML downsizing, although medial underhang occurs, this may provide a beneficial effect in terms of the ligament balancing to increase the medial gap by removing the uncovered medial edge of the tibial plateau.
Table 4.
Morphometry of the proximal tibia in the literature
| Study | Nationality | Knee | Mediolateral (mm) | AP (mm) | Aspect ratio | |||
|---|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |||
| Uehara et al. [22] | Japanese | Osteoarthritic | 83.0 ± 6.2 | 71.7 ± 4.0 | 53.8 ± 6.6 | 46.6 ± 3.6 | 1.54 | 1.54 |
| Kwak et al. [13] | Korean | Normal | 76.1 ± 4.0 | 67.6 ± 3.1 | 48.2 ± 3.3 | 43.2 ± 2.3 | 1.58 | 1.56 |
| Cheng et al. [3] | Chinese | Normal | 76.4 ± 2.8 | 68.8 ± 4.6 | 51.3 ± 2.0 | 45.7 ± 1.9 | 1.49 ± 0.6 | 1.51 ± 0.6 |
| Current study | Japanese | Osteoarthritic | 66.9 ± 5.6 | 45.3 ± 2.7 | 1.48 ± 0.1 | |||
Data are expressed as mean ± SD.
In the tibia with medial bowing, the five currently available tibial components did not fit the tibial resected surface when the tibial component was lateralized and downsized so that the component center was aligned with the TSA. AP underhang seems to occur when fitting these components to Japanese patients in the ideal position, especially in large knees. In normal Asian subjects, Kawak et al. [13] and Cheng et al. [3] demonstrated a progressive decrease in the aspect ratio with an increase in AP dimension of the resected surface of the normal proximal tibia. On the other hand, in a study of Japanese patients with varus knee OA, Uehara et al. [22] reported the opposite results, in that smaller knees had a longer AP dimension. Although the differences between the studies could be explained by the morphologic alteration resulting from OA, none of these studies took into account whether tibia vara could influence the aspect ratio on devising a proper component design.
In conclusion, we found the majority of Japanese patients scheduled for TKA had tibia vara and the aspect ratio of resected tibial surface was inversely correlated to the degree of tibia vara. In addition, the currently available five tibial components did not fit well to the resected tibial surface in terms of aspect ratio. Our data provide potentially important information for designing tibial components to achieve better bone coverage in Japanese patients.
Acknowledgments
The authors thank E. Matsuki, laboratory assistant, for her assistance in patient data collection.
Footnotes
Each author certifies that he or she, or a member of his or her immediate family, has no funding or commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved or waived approval for the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Akagi M, Mori S, Nishimura S, Nishimura A, Asano T, Hamanishi C. Variability of extraarticular tibial rotation references for total knee arthroplasty. Clin Orthop Relat Res. 2005;436:172–176. doi: 10.1097/01.blo.0000160027.52481.32. [DOI] [PubMed] [Google Scholar]
- 2.Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C. An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res. 2004;420:213–219. doi: 10.1097/00003086-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Cheng FB, Ji XF, Lai Y, Feng JC, Zheng WX, Sun YF, Fu YW, Li YQ. Three dimensional morphometry of the knee to design the total knee arthroplasty for Chinese population. Knee. 2009;16:341–347. doi: 10.1016/j.knee.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 4.Churchill DL, Incavo SJ, Johnson CC, Beynnon BD. The transepicondylar axis approximates the optimum flexion axis of the knee. Clin Orthop Relat Res. 1998;356:111–118. doi: 10.1097/00003086-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Hicks CA, Noble P, Tullos H. The anatomy of the tibial intramedullary canal. Clin Orthop Relat Res. 1995;321:111–116. [PubMed] [Google Scholar]
- 6.Hitt K, Shurman JR, 2nd, Greene K, McCarthy J, Moskal J, Hoeman T, Mont MA. Anthropometric measurements of the human knee: correlation to the sizing of current knee arthroplasty systems. J Bone Joint Surg Am. 2003;85(suppl 4):115–122. [PubMed] [Google Scholar]
- 7.Hollister AM, Jatana S, Singh AK, Sullivan WW, Lupichuk AG. The axes of rotation of the knee. Clin Orthop Relat Res. 1993;290:259–268. [PubMed] [Google Scholar]
- 8.Hovinga KR, Lerner AL. Anatomic variations between Japanese and Caucasian populations in the healthy young adult knee joint. J Orthop Res. 2009;27:1191–1196. doi: 10.1002/jor.20858. [DOI] [PubMed] [Google Scholar]
- 9.Hsu HP, Garg A, Walker PS, Spector M, Ewald FC. Effect of knee component alignment on tibial load distribution with clinical correlation. Clin Orthop Relat Res. 1989;248:135–144. [PubMed] [Google Scholar]
- 10.Hsu RW, Himeno S, Coventry MB, Chao EY. Normal axial alignment of the lower extremity and load-bearing distribution at the knee. Clin Orthop Relat Res. 1990;255:215–227. [PubMed] [Google Scholar]
- 11.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ko PS, Tio MK, Ban CM, Mak YK, Ip FK, Lam JJ. Radiologic analysis of the tibial intramedullary canal in Chinese varus knees: implications in total knee arthroplasty. J Arthroplasty. 2001;16:212–215. doi: 10.1054/arth.2001.20908. [DOI] [PubMed] [Google Scholar]
- 13.Kwak DS, Surendran S, Pengatteeri YH, Park SE, Choi KN, Gopinathan P, Han SH, Han CW. Morphometry of the proximal tibia to design the tibial component of total knee arthroplasty for the Korean population. Knee. 2007;14:295–300. doi: 10.1016/j.knee.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Matsuda S, Mizu-uchi H, Miura H, Nagamine R, Urabe K, Iwamoto Y. Tibial shaft axis does not always serve as a correct coronal landmark in total knee arthroplasty for varus knees. J Arthroplasty. 2003;18:56–62. doi: 10.1054/arth.2003.50002. [DOI] [PubMed] [Google Scholar]
- 15.Moreland JR, Bassett LW, Hanker GJ. Radiographic analysis of the axial alignment of the lower extremity. J Bone Joint Surg Am. 1987;69:745–749. [PubMed] [Google Scholar]
- 16.Nagamine R, Miura H, Bravo CV, Urabe K, Matsuda S, Miyanishi K, Hirata G, Iwamoto Y. Anatomic variations should be considered in total knee arthroplasty. J Orthop Sci. 2000;5:232–237. doi: 10.1007/s007760050157. [DOI] [PubMed] [Google Scholar]
- 17.Oswald MH, Jakob RP, Schneider E, Hoogewoud HM. Radiological analysis of normal axial alignment of femur and tibia in view of total knee arthroplasty. J Arthroplasty. 1993;8:419–426. doi: 10.1016/S0883-5403(06)80042-2. [DOI] [PubMed] [Google Scholar]
- 18.Stiehl JB, Abbott BD. Morphology of the transepicondylar axis and its application in primary and revision total knee arthroplasty. J Arthroplasty. 1995;10:785–789. doi: 10.1016/S0883-5403(05)80075-0. [DOI] [PubMed] [Google Scholar]
- 19.Stiehl JB, Cherveny PM. Femoral rotational alignment using the tibial shaft axis in total knee arthroplasty. Clin Orthop Relat Res. 1996;331:47–55. doi: 10.1097/00003086-199610000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Tang Q, Zhou Y, Yang D, Xu H, Liu Q. The offset of the tibial shaft from the tibial plateau in Chinese people. J Bone Joint Surg Am. 2010;92:1981–1987. doi: 10.2106/JBJS.I.00969. [DOI] [PubMed] [Google Scholar]
- 21.Teter KE, Bregman D, Colwell CW., Jr Accuracy of intramedullary versus extramedullary tibial alignment cutting systems in total knee arthroplasty. Clin Orthop Relat Res. 1995;321:106–110. [PubMed] [Google Scholar]
- 22.Uehara K, Kadoya Y, Kobayashi A, Ohashi H, Yamano Y. Anthropometry of the proximal tibia to design a total knee prosthesis for the Japanese population. J Arthroplasty. 2002;17:1028–1032. doi: 10.1054/arth.2002.35790. [DOI] [PubMed] [Google Scholar]
- 23.Vail TP, Lang JE. Surgical techniques and instrumentation in total knee arthroplasty. In: Scott WN, editor. Surgery of the Knee. 4. New York, NY: Churchill-Livingstone; 2006. pp. 1455–1521. [Google Scholar]
- 24.Yoo JH, Kang YG, Chang CB, Seong SC, Kim TK. The relationship of the medially-offset stem of the tibial component to the medial tibial cortex in total knee replacements in Korean patients. J Bone Joint Surg Br. 2008;90:31–36. doi: 10.1302/0301-620X.90B1.19605. [DOI] [PubMed] [Google Scholar]