Abstract
Background
Joint function and durability after TKA depends on many factors, but component alignment is particularly important. Although the transepicondylar axis is regarded as the gold standard for rotationally aligning the femoral component, various techniques exist for tibial component rotational alignment. The impact of this variability on joint kinematics and stability is unknown.
Questions/purposes
We determined how rotationally aligning the tibial component to four different axes changes knee stability and passive tibiofemoral kinematics in a knee after TKA.
Methods
Using a custom surgical navigation system and stability device to measure stability and passive tibiofemoral motion, we tested 10 cadaveric knees from five hemicorpses before TKA and then with the tibial component aligned to four axes using a modified tibial tray.
Results
No changes in knee stability or passive kinematics occurred as a result of the four techniques of tibial rotational alignment. TKA produces a ‘looser’ knee over the native condition by increasing mean laxity by 5.2°, decreasing mean maximum stiffness by 4.5 N·m/°, increasing mean anterior femoral translation during passive flexion by 5.4 mm, and increasing mean internal-external tibial rotation during passive flexion by 4.8°. However, no statistically or clinically important differences occurred between the four TKA conditions.
Conclusions
For all tibial rotations, TKA increased laxity, decreased stiffness, and increased tibiofemoral motion during passive flexion but showed little change based on the tibial alignment.
Clinical Relevance
Our observations suggest surgeons who align the tibial component to any of the axes we examined are expected to have results consistent with those who may use a different axis.
Introduction
TKA is commonly and increasingly used to treat the pain, disability, and loss of motion associated with osteoarthritis [29, 30]. Although most patients experience relief of pain and improved function and quality of life [15, 26, 39, 54], various suboptimal outcomes do occur, ranging from mild anterior knee pain to failures requiring revision surgery [5, 38]. Patients with these suboptimal outcomes often report excessive joint stiffness or looseness [8, 43], limited ROM [41], and difficulty with activities of daily living, such as climbing stairs and walking [10].
Joint function after TKA depends on many factors, but component alignment has been identified as particularly critical [5, 27, 37, 38, 43]. Alignment errors compromise the stability of the joint [8, 42, 51], alter tibiofemoral kinematics [35], and result in patella maltracking and pain [14, 35]. As little as 6.2° internal rotation of the tibial component reportedly relates to postoperative pain [5], while just 3° internal rotation of the femoral component increases varus displacement of the knee [42].
The transepicondylar axis (TEA) of the femur generally is regarded as the gold standard axis for establishing the rotational alignment of the femoral component during TKA [13, 35, 40, 49]. This axis is believed to best approximate the flexion-extension axis of the knee [13] and produces a balanced joint and the most normal patellar tracking [35] and minimizes patellofemoral shear forces [35]. Rotational alignments that deviate from this axis have resulted in abnormal varus-valgus joint displacement and patellofemoral kinematics, and an increase in tibiofemoral wear [3, 35].
Unlike the femoral component, a gold standard does not exist for rotational alignment of the tibial component. Currently, many anatomic landmarks are used to align the tibial component, including the projected femoral TEA [1, 2, 20, 24], medial border of the tibial tubercle [17, 18, 22, 47], medial 1/3 of the tibial tubercle [17, 20, 47], PCL attachment [1, 2, 23, 47], transverse axis of the tibia [18, 20, 47], posterior condylar line of the tibia [18, 20, 23], midsulcus of the tibial spine [17], malleolar axis [1, 18], patellar tendon [1, 2, 23, 24], and axis of the second metatarsal [1]. This lack of a gold standard for tibial component alignment, combined with the difficulty in identifying anatomic landmarks during surgery and variations in anatomy between knees, may lead to variations in the surgeons’ ability to locate tibial component alignment axes as large as 44° internal rotation to 46° external rotation [47]. However, it is not currently understood how this variability in tibial rotational alignment impacts the stability or kinematics of the knee after TKA.
We therefore determined how rotationally aligning the tibial component to four different axes changes knee stability and passive tibiofemoral kinematics in a knee after TKA.
Materials and Methods
We performed a series of experiments on five pairs of fresh-frozen cadaveric limbs (five hemicorpses) containing all structures distal to the pelvis using a custom, image-based surgical navigation system that was created at The Ohio State University (Columbus, OH, USA) and included a Polaris® optical tracking system (Northern Digital, Waterloo, Ontario, Canada) that was controlled by LabVIEW™ (National Instruments, Austin, TX, USA) and MATLAB® (Mathworks, Natick, MA, USA) software. This system, along with previous systems created by the senior author (RAS), has been validated and successfully used previously [16, 45, 46, 48]. Specimens with severe osteoarthritis, prior fractures, damaged soft tissues, or other abnormalities were not included. The average age of the specimens was 71.5 years (range, 57–81 years), with eight knees being from male donors and two knees from a female donor.
We performed an a priori power analysis assuming a difference of 6° and an SD of 2.3°, based on the original Knee Society Scoring System© where points are deducted for greater than 6° joint laxity [25] and our previous study of knee stability showing a SD of 2.3° [46]. Accounting for the six pairwise multiple comparisons among the four different tibial rotational alignment axes that would be investigated, we determined at least six specimens would be needed to achieve a power of 0.8 with an 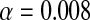 . After initial testing showed the SD associated with some alignment axes was as much as 4°, we performed another power analysis and determined 10 specimens would be needed to determine a difference in joint laxity of at least 6° between test conditions. Before kinematic and stability testing, all specimens were CT scanned using a Philips 64-slice mobile CT system (Philips Healthcare, Andover, MA, USA) to accurately identify the axes used to align the rotation of the femoral and tibial components. Slices were made every 2 mm for the entire length of the limb to ensure adequate observation of anatomic landmarks. The CT data were reconstructed using commercially available software (3D-Doctor; Able Software Corp, Lexington, MA, USA), and we identified the following anatomic landmarks frequently used during TKA: the prominence of the lateral femoral epicondyle [49], the sulcus (or, when absent, the prominence) of the medial femoral epicondyle [49], the most medial border of the tibial plateau [47], the most lateral border of the tibial plateau [47], the PCL attachment on the tibia by identifying the PCL in the posterior condylar notch and selecting the geometric center [2], the medial border of the tibial tubercle [2], and the medial 1/3 of the tibial tubercle [2].
. After initial testing showed the SD associated with some alignment axes was as much as 4°, we performed another power analysis and determined 10 specimens would be needed to determine a difference in joint laxity of at least 6° between test conditions. Before kinematic and stability testing, all specimens were CT scanned using a Philips 64-slice mobile CT system (Philips Healthcare, Andover, MA, USA) to accurately identify the axes used to align the rotation of the femoral and tibial components. Slices were made every 2 mm for the entire length of the limb to ensure adequate observation of anatomic landmarks. The CT data were reconstructed using commercially available software (3D-Doctor; Able Software Corp, Lexington, MA, USA), and we identified the following anatomic landmarks frequently used during TKA: the prominence of the lateral femoral epicondyle [49], the sulcus (or, when absent, the prominence) of the medial femoral epicondyle [49], the most medial border of the tibial plateau [47], the most lateral border of the tibial plateau [47], the PCL attachment on the tibia by identifying the PCL in the posterior condylar notch and selecting the geometric center [2], the medial border of the tibial tubercle [2], and the medial 1/3 of the tibial tubercle [2].
The points identified on the CT images then were used to define four axes commonly used to align the tibial component. Axes used in this study were selected based on what was commonly cited and ease of identification from a CT scan. The TEA was defined as the surgical epicondylar axis, the line between the points on the lateral prominence and the medial sulcus (or, when absent, the prominence) of the femoral epicondyles [6], projected onto the tibial plateau when the specimen was in full extension. The transverse axis (TA) was defined as the line between the most medial and lateral points on the tibial plateau. The medial border axis (MBA) was defined as the line between the PCL attachment and the medial border of the tibial tubercle. The medial third axis (MTA) was defined as the line between the PCL attachment and the medial 1/3 of the tibial tubercle.
An experienced orthopaedic surgeon (JFG or MDB) performed a PCL-retaining TKA on each specimen by using implants from the Zimmer® Natural-Knee® II product line (Zimmer Inc, Warsaw, IN, USA). After the knee was exposed, passive optical maker arrays with four reflective spheres were attached to the femur and tibia and anatomic reference frames were established [45]. The greater trochanter, the distal femur, the proximal tibia, and the malleoli were digitized to register the specimen to the CT data using an iterative closest-point algorithm [7].
With the aid of the surgical navigation system, we recorded passive kinematics and stability data for the knee at full extension before and after prosthesis implantation. The femoral component was aligned to the TEA with the aid of the surgical navigation system, while the tibial component was aligned within 1° of the four different axes (TEA, TA, MBA, and MTA). We also used the surgical navigation system to ensure the distal femoral cut and the proximal tibial cut were always within ±1° of neutral varus-valgus rotation and the anterior femoral cut was within ±1° of neutral internal-external rotation. A custom-modified tibial tray with 1° resolution was used to allow for rotation from 25° internal rotation to 25° external rotation and allowed us to test four different alignments on the same specimen. Three trials were recorded for each test condition.
To measure passive kinematics, the skin surrounding the knee was closed with two to three towel clips and the joint was flexed by supporting the foot with an open palm while gently lifting the thigh [45]. The reverse procedure was used to extend the knee. During this motion, the navigation system recorded the position and orientation of the optical reference frame fixed to the femur with respect to the optical reference frame fixed to the tibia. The error associated with the surgical navigation system is minimal, with a linear accuracy of less than 2 mm [44] and a worst-case angular accuracy, in the transverse plane, of approximately 1.25° [48].
To characterize joint stability in the frontal plane, we measured the force-displacement relationship of the knee in the varus-valgus direction using a custom stability device that enabled us to repeatably and accurately apply a ±20-N·m load [48]. While the ideal loads that should be used to assess knee laxity and stiffness during a TKA are unknown, this load was chosen because it ensured we would be able to measure the terminal stiffness of the knee, encompassed a range used to biomechanically evaluate knee stability [9, 31, 32, 36, 51], and was the maximum load that experienced surgeons in this study believed they could use on a patient during TKA. The specimen’s foot was placed in a modified Alvarado boot (Zimmer) [48] while the femur was constrained by a Lane bone clamp held by the surgeon. The load was applied to the limb with an instrumented handle, which included a load cell (Model 31 precision miniature load cell; SENSOTEC, Columbus, OH, USA), while displacement of the limb was measured by the surgical navigation system. The stability device (Fig. 1) was previously validated for intraoperator and interoperator use and showed low mean ± SD moment errors of no greater than −0.11 ± 0.73 N·m [48], ensuring the loads measured by our device are the loads experienced at the knee.
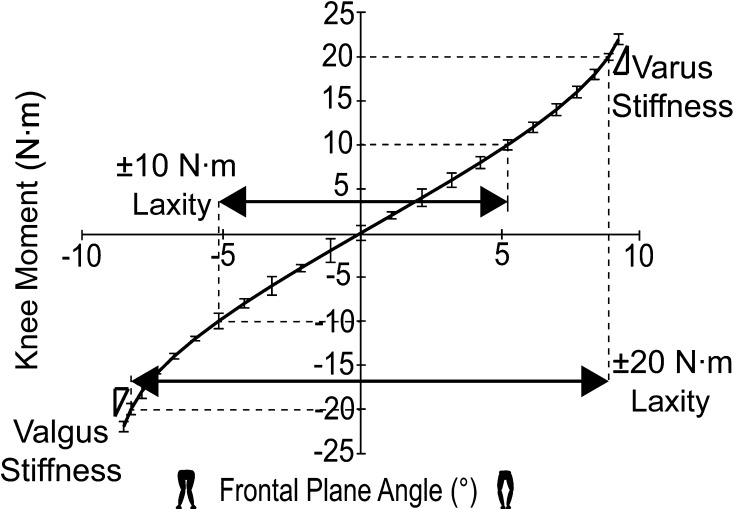
Fig. 1.
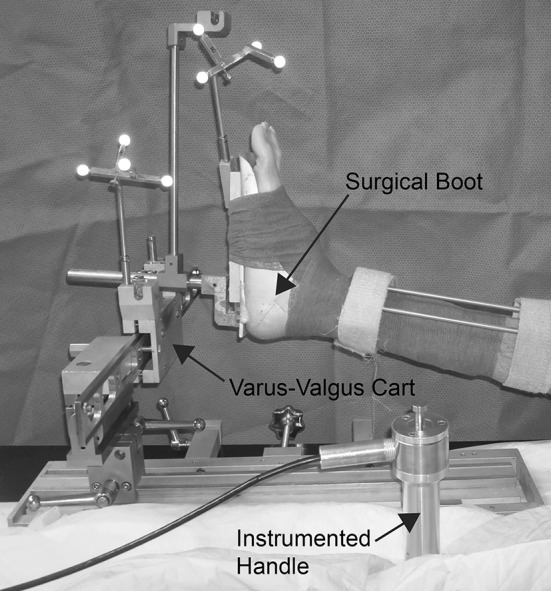
A custom-built stability device was used during testing. The specimen’s foot was placed in a modified Alvarado boot and then placed in the device. The end of the instrumented handle was placed in the varus-valgus cart and a force was applied to the limb while a surgical navigation system tracked the motion of the tibia, femur, boot, and cart.
Similar to Markolf et al. [32], we defined laxity as the amount of motion in degrees that occurred under a given load and stiffness as the slope between two points on the force-displacement curve. Varus-valgus knee stability was analyzed by determining the stiffness at ±20 N·m and the laxity occurring under ±10 N·m and ±20 N·m varus-valgus loads (Fig. 2). We examined three characteristics of passive knee kinematics as a function of knee flexion: varus-valgus rotation at discrete flexion angles (5°, 10°, 15°, 20°, 25°, 30°, 60°, 90°, and 105°), maximum anterior translation of the femur on the tibia [16], and internal-external rotation of the tibia between 5° and 105° flexion [45].
Fig. 2.
This sample stability curve depicts ±10-N·m laxity, ±20-N·m laxity, and varus and valgus terminal stiffness under a 20-N·m load.
We performed repeated-measures ANOVA analyses using Minitab (State College, PA, USA) to determine whether a TKA and different tibial component rotational alignments had an effect on knee stability and kinematics in our 10 specimens. Even though the 10 knees came from five donors, each specimen was analyzed separately and acted as its own control because we saw left-to-right differences in laxity and stiffness in the native condition as large as 44% and 79%, respectively, which are similar to differences reported by other researchers who have noted left-to-right differences in the stability as large as 35% for healthy knees [31]. We performed an additional general linear model ANOVA to investigate the effect of rotational alignment and knee specimen number to confirm whether having two knees from the same donor influenced our results. The specimen number and knee treatment (native knee, TEA, TA, MBA, or MTA) were the independent variables while stiffness, laxity, anterior femoral translation, and internal-external tibial rotation were the dependent variables. Knee flexion angle also was treated as an independent variable when we analyzed varus-valgus rotation during passive flexion as the dependent variable. When we found p ≤ 0.05 between two test conditions, Tukey’s test was used to determine the difference between the average measurements.
Results
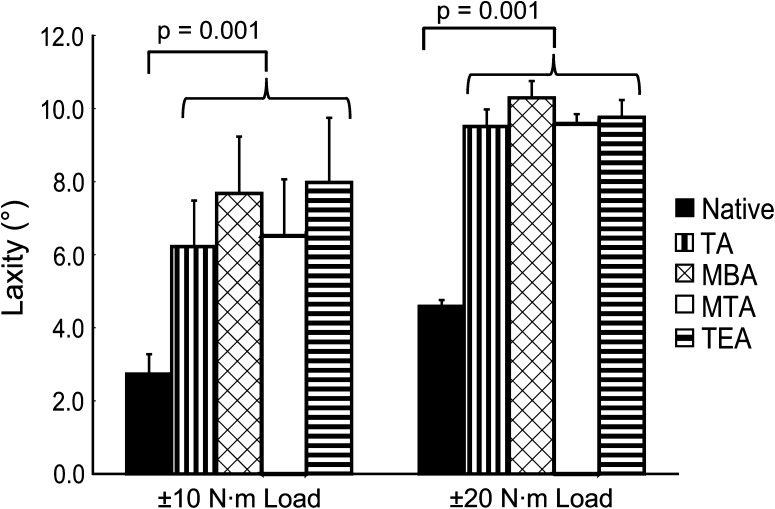
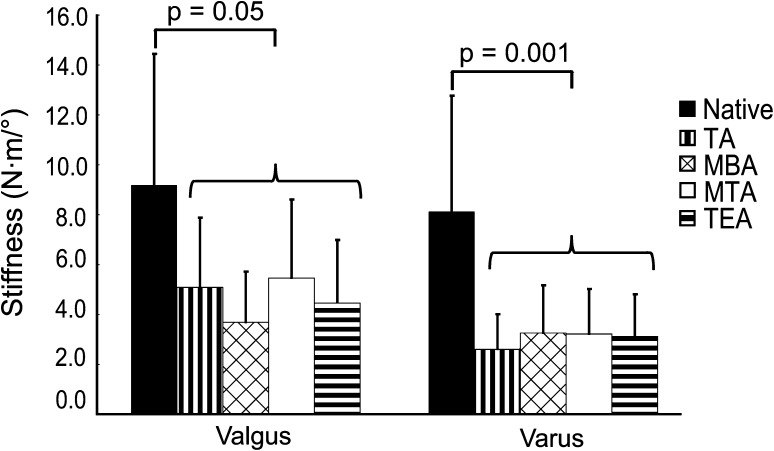
We found that tibial rotational alignment had no effect on ±10 N·m laxity (p = 0.06), ±20 N·m laxity (p = 0.08), 20 N·m varus stiffness (p = 0.55), and 20 N·m valgus stiffness (p = 0.26). However, TKA produces a softer knee by increasing laxity (Fig. 3) and decreasing stiffness (Fig. 4). For ±10- and ±20-N·m loads, TKA increased (p = 0.001 for both) the average amounts of laxity over the native knee (Table 1) by 4.36° and 5.20°, respectively. Average varus (p = 0.001) and valgus (p = 0.05) stiffnesses decreased (Table 1) after TKA by 5.06 N·m/° and 4.50 N·m/°, respectively. We found that specimen number did have an effect on ±20 N·m laxity (p < 0.001), where one specimen showed a larger amount of laxity relative to all other specimens, even the contralateral limb of the same donor. The ±20 N·m laxity measurements for the remaining nine specimens were within 6° of each other, which we judged clinically unimportant based on the Knee Society Scoring System©.
Fig. 3.
Mean values are shown for ±10- and ±20-N·m varus-valgus laxity in full extension for the native knee and all four rotational alignments. The error bars represent one SD. A difference exists between the native condition and any of the four axes, but there is no difference in laxity based on rotational alignment alone. TA = transverse axis; MBA = medial border axis; MTA = medial third axis; TEA = transepicondylar axis.
Fig. 4.
Mean values are shown for varus and valgus stiffness at ±20-N·m load for the native knee and all four rotational alignments. The error bars represent one SD. A difference exists between the native condition and any of the four axes, but there is no difference in stiffness based on rotational alignment alone. TA = transverse axis; MBA = medial border axis; MTA = medial third axis; TEA = transepicondylar axis.
Table 1.
Stability measures for native and TKA knees
| Measurement | Native knees | TKA knees |
|---|---|---|
| ±10-N·m laxity (°) | 2.74 ± 0.54 | 7.10 ± 1.53 |
| ±20-N·m laxity (°) | 4.59 ± 0.17 | 9.79 ± 0.42 |
| Stiffness at 20-N·m valgus (N·m/°) | 9.18 ± 5.27 | 4.68 ± 2.63 |
| Stiffness at 20-N·m varus (N·m/°) | 8.12 ± 4.65 | 3.06 ± 1.70 |
Values are expressed as mean ± SD.
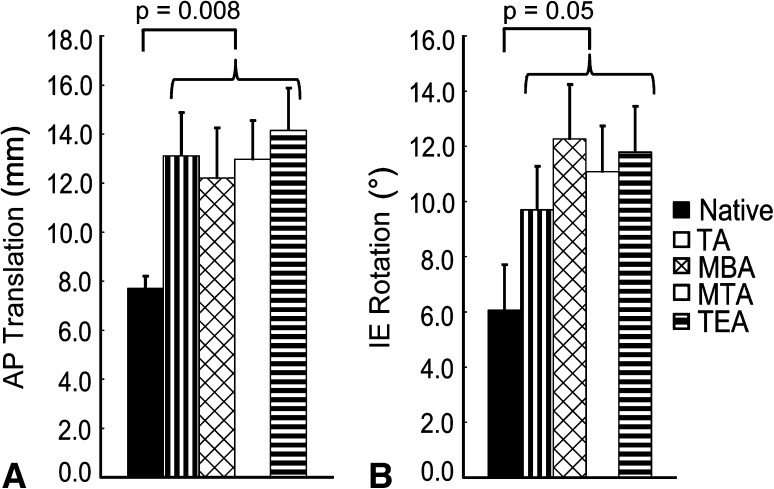
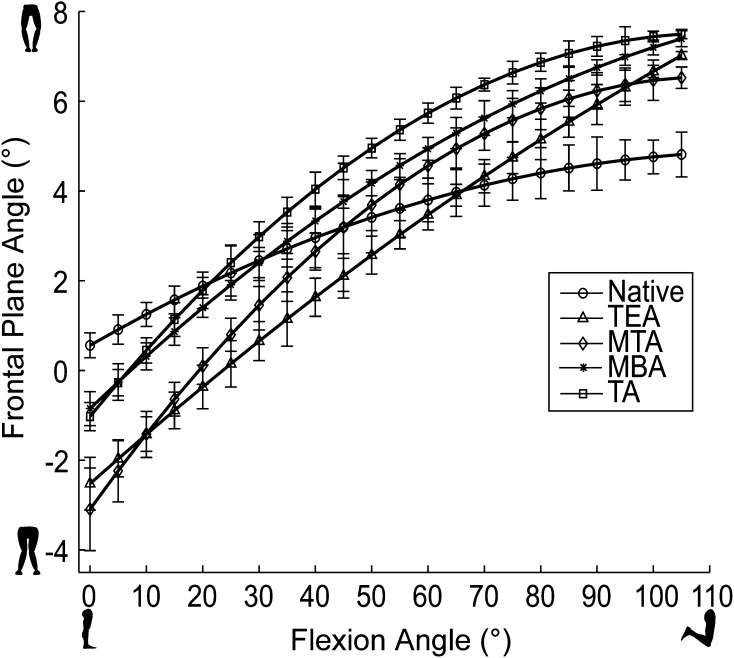
We found that tibial rotational alignment had no effect on anterior translation of the femur (p = 0.51) or internal-external rotation of the tibia (p = 0.98) during passive flexion (Fig. 5), but we did observe some differences (p = 0.001) in varus-valgus position during early flexion (< 15°). Similar to the stability variables, we found that TKA produces a looser knee when compared with the native condition during passive flexion (Table 2) by increasing the average anterior translation of the femur (p = 0.008) from 7.73 mm to 13.11 mm and the average internal-external tibial rotation (p = 0.05) from 5.96° to 10.82°. Varus-valgus position during passive flexion was the only variable to be affected by tibial rotational alignment (Fig. 6). At 5° flexion, aligning to the MBA or TA caused the knee to be in greater valgus (p = 0.001) by 3.20° compared to the native condition. However, there was no statistical difference in varus-valgus position at 5° flexion between the MBA and the TA (p = 0.54). Aligning to the TEA or MTA caused an even larger average valgus increase (p = 0.001) at 5° flexion over the native knee of 4.74°. Once again, there was no statistical difference in varus-valgus position at 5° flexion between the TEA and the MTA (p = 0.77). At 10° flexion, the differences in varus-valgus motion disappeared between the different alignments, but on average, the TKA knee showed 1.96° increase (p = 0.001) in valgus motion over the native condition. By 15° flexion, varus-valgus differences between the native knee and the TKA conditions ceased to exist (p = 0.24).
Fig. 5A–B.
Mean values are shown for (A) AP translation of the femur and (B) internal-external (IE) rotation of the tibia during passive knee flexion for the native knee and all four rotational alignments. The error bars represent one SD. A difference exists between the native condition and any of the four axes, but there is no difference in these kinematics based on rotational alignment alone. TA = transverse axis; MBA = medial border axis; MTA = medial third axis; TEA = transepicondylar axis.
Table 2.
Passive kinematic measures for native and TKA knees
| Measurement | Native knees | TKA knees |
|---|---|---|
| Anterior translation of femur (mm) | 7.73 ± 0.48 | 13.11 ± 1.78 |
| Internal-external rotation at 5° to 105° (°) | 5.96 ± 1.61 | 10.82 ± 1.69 |
Values are expressed as mean ± SD.
Fig. 6.
Varus-valgus kinematics during passive flexion for a representative specimen is shown. In early flexion, all alignments show more valgus motion than the native condition, but the TA and MBA alignments minimize this. TEA = transepicondylar axis; MTA = medial third axis; MBA = medial border axis; TA = transverse axis.
Discussion
Joint function after TKA is dependent on many factors, but component alignment has been identified as particularly important [5, 27, 37, 38, 43]. While the TEA is generally regarded as the gold standard for rotational alignment of the femoral component, various techniques exist to establish the rotation of the tibial component, and the biomechanical impact of this variability is unknown. We therefore examined varus-valgus laxity and stiffness and tibiofemoral kinematics during passive flexion with the tibial component aligned to four commonly used axes to determine whether a gold standard tibial rotational alignment axis exists.
We note several limitations to our study. First, our findings reflect those obtained by only two experienced arthroplasty surgeons using one particular PCL-retaining TKA system on five pairs of cadaver limbs that did not require any ligament releases. Different surgeons with different specimens using different implants and techniques may yield different results because different implants provide different patterns of stability [21, 53]. Using a PCL-sacrificing implant would most likely result in even more laxity and reduced stiffness since the PCL reportedly provides some varus-valgus stabilization [4]. Second, although all of our specimens came from elderly donors (average age, 71.5 years), the levels of osteoarthritis found in the specimens in our study were estimated to range from none to moderate. Since the majority of our specimens appeared to behave similarly, patients who had undergone TKA, who typically have more severe osteoarthritis and soft tissue contractures, or a larger and more diverse collection of cadaveric specimens with a greater range of anatomic variation may show different results. Third, the manual actuation of our device introduces variability, although we believe it to be small [48]. While the surgeons performing these experiments applied loads quasistatically, ligament response is reportedly dependent on the rate of loading [19]. Fourth, all testing involved passive motion of cadaveric knee specimens and we would expect active load-bearing activities to show different kinematics [28]. Finally, even though all individual knees across all donors appeared to behave similarly, the use of pairs of knees from the same specimen is a potentially serious limitation and should be given more cautious consideration in future work with cadaveric specimens.
Our stability results (laxity and stiffness) for the native and TKA knees are similar to what has been noted by other researchers. One cadaver study of native knees estimated varus-valgus laxities in full extension with applied loads of ±10 and ±20 N·m to be approximately 2° and 4°, respectively, which is comparable to our findings (Table 1) [32]. In patients with severe OA, Siston et al. [46] noted average intraoperative measurements of preimplant and postimplant varus-valgus ROM were 5.9° and 6.5°, respectively, which also overlaps with our cadaver results. However, studies on how varus-valgus laxity changes with TKA have yielded mixed results. Casino et al. [11] found varus-valgus laxity decreased in full extension after TKA, while others found no difference in laxity [42, 46]. We believe these results may differ from ours (laxity increases after TKA) because previous studies used data from osteoarthritic knees, which is known to diminish varus-valgus laxity [9], and did not measure the load applied to the knee. In contrast to laxity, little research exists on the effect of TKA on terminal stiffness of the knee. One cadaver study involving normal knees found the mean terminal varus and valgus stiffness to be 14.0 and 16.5 N·m/°, respectively [32], which is comparable to what we observed for the native knees (Table 1).
Our observations of passive flexion tibiofemoral kinematics of the native and TKA knees also are similar to what has been reported by other researchers. An increase in anterior femoral translation after TKA has been well-documented [12, 16, 45, 55], with Cromie et al. [16] reporting TKA knees showed a mean of 16.1 mm anterior motion, which overlaps with our findings (Table 2). Studies on tibial internal-external rotation after TKA have reported mixed findings, with some researchers noting decreases [45] and others reporting no change [12]. We observed an increase in tibial rotation, but our average value for the native condition is similar to what Siston et al. [45] reported for osteoarthritic knees (4.9° ± 4.1°) and nearly identical to what Victor et al. [52] observed for TKA knees (10.8°).
Given that our mean values of joint laxity for the different alignment axes were within 2°, which is less than the original threshold established by the Knee Society where points are deducted for greater than 6° joint laxity, we are confident in saying the alignment axes do not yield differences in joint laxity that are either statistically different or clinically important. However, published studies on the effect of component rotational alignment on TKA kinematics have reported conflicting results. Similar to our findings, some studies have noted no difference in varus-valgus kinematics in late flexion [45, 50]. However, we observed no change in the translation of the femur during passive kinematics based on tibial component rotation, while others have found that particular alignment to be a key factor in femoral translation during knee flexion [34, 50]. Thompson et al. [50] reported an increase in femoral anterior translation when the tibial component was externally rotated in an Oxford rig simulation. Conversely, Mihalko et al. [34] found internal rotation of the tibia caused the greatest increase in anterior translation during a simulated lunge. Thompson et al. [50] and Mihalko et al. [34] measured translation at the point of contact between the tibia and femoral condyles, which is slightly different from our method that measures the motions of a tibial and a femoral reference frame with origins at the midpoint of the tibial spine and the femoral anterolateral PCL attachment, respectively [45]. However, we believe the disagreement in AP kinematic results is likely because those previous studies simulated different weightbearing activities, whereas we investigated passive flexion-extension kinematics.
We did note large variability in our results across specimens, and knees from the same donor exhibited right-left differences in laxity and stiffness as large as 44% and 79%, respectively. We suspect the variability is the result of large variations in bony anatomy seen in our specimens. Across all 10 specimens in our study, the most internally and most externally rotated axes were not consistent even when comparing knees from the same donor, and the angle between these two axes ranged from 10.2° to 27.1° (Fig. 7). This large variation among specimens agrees with what other researchers have noted regarding tibial rotational alignment. Akagi et al. [1] found the angle between the TEA and the transmalleolar axis ranged from 8° to 49.4°. Similarly, Matziolis et al. [33] found the angle between the TEA and an axis that aligned to the midpoint point of the tibial tubercle ranged from 0.7° to 43.4°.
Fig. 7A–B.
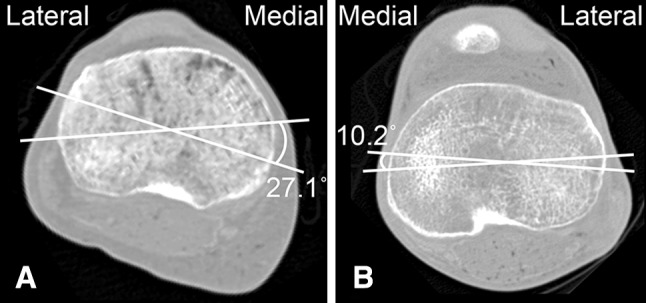
We noted a high degree of variability in specimen anatomy. CT scans of the tibial plateau (most proximal CT slice of the tibia) with the most internal and external axes of two different specimens are shown. (A) For this specimen, the most internal and external axes were the medial border axis and transverse axis, respectively, with 27.1° between the two. (B) For this specimen, the most internal and external axes were the transepicondylar axis and medial third axis, respectively, with a 10.2° angle between the two.
Considering the importance of surgical technique in TKA, our findings suggest surgeons who align the tibial component to any of the axes used in this study may expect to have results consistent with their peers who may be using a different axis. Given the large variability among specimens, this study further suggests there is no gold standard for rotational alignment of the tibial component that can be recommended for use on all patients at this time.
Acknowledgments
We thank Michael Knopp MD, PhD for assistance with the CT images, Zimmer Inc for loans of surgical trays, and Jeff Stanley at Northern Digital, Inc, for technical support with our camera. We also thank the members of The Ohio State University Neuromuscular Biomechanics Laboratory for assistance during testing.
Footnotes
The institution of one or more of the authors has received funding, during the study period, from the Orthopaedic Research and Education Foundation (Rosemont, IL, USA) (RAS), the American Association of Hip and Knee Surgeons (Rosemont, IL, USA) (RAS), and the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Bethesda, MD, USA) (Award Number R01AR056700) (RAS). Each author certifies that he or she, or a member of his or her immediate family, has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA approval status, of any drug or device before clinical use.
Each author certifies that his or her institution approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Akagi M, Mori S, Nishimura S, Nishimura A, Asano T, Hamanishi C. Variability of extraarticular tibial rotation references for total knee arthroplasty. Clin Orthop Relat Res. 2005;436:172–176. doi: 10.1097/01.blo.0000160027.52481.32. [DOI] [PubMed] [Google Scholar]
- 2.Akagi M, Oh M, Nonaka T, Tsujimoto H, Asano T, Hamanishi C. An anteroposterior axis of the tibia for total knee arthroplasty. Clin Orthop Relat Res. 2004;420:213–219. doi: 10.1097/00003086-200403000-00030. [DOI] [PubMed] [Google Scholar]
- 3.Anouchi YS, Whiteside LA, Kaiser AD, Milliano MT. The effects of axial rotational alignment of the femoral component on knee stability and patellar tracking in total knee arthroplasty demonstrated on autopsy specimens. Clin Orthop Relat Res. 1993;287:170–177. [PubMed] [Google Scholar]
- 4.Arima J, Whiteside LA, Martin JW, Miura H, White SE, McCarthy DS. Effect of partial release of the posterior cruciate ligament in total knee arthroplasty. Clin Orthop Relat Res. 1998;353:194–202. doi: 10.1097/00003086-199808000-00022. [DOI] [PubMed] [Google Scholar]
- 5.Barrack RL, Schrader T, Bertot AJ, Wolfe MW, Myers L. Component rotation and anterior knee pain after total knee arthroplasty. Clin Orthop Relat Res. 2001;392:46–55. doi: 10.1097/00003086-200111000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Berger RA, Rubash HE, Seel MJ, Thompson WH, Crossett LS. Determining the rotational alignment of the femoral component in total knee arthroplasty using the epicondylas axis. Clin Orthop Relat Res. 1993;286:40–47. [PubMed] [Google Scholar]
- 7.Besl PJ, McKay ND. A method for registration of 3-D shapes. IEEE Trans Pattern Anal Mach Intell. 1992;14:239–256. doi: 10.1109/34.121791. [DOI] [Google Scholar]
- 8.Bong MR, Di Cesare PE. Stiffness after total knee arthroplasty. J Am Acad Orthop Surg. 2004;12:164–171. doi: 10.5435/00124635-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Brage ME, Draganich LF, Pottenger LA, Curran JJ. Knee laxity in symptomatic osteoarthritis. Clin Orthop Relat Res. 1994;304:184–189. [PubMed] [Google Scholar]
- 10.Byrne JM, Gage WH, Prentice SD. Bilateral lower limb strategies used during a step-up task in individuals who have undergone unilateral total knee arthroplasty. Clin Biomech. (Bristol, Avon) 2002;17:580–585. doi: 10.1016/S0268-0033(02)00061-X. [DOI] [PubMed] [Google Scholar]
- 11.Casino D, Martelli S, Zaffagnini S, Lopomo N, Iacono F, Bignozzi S, Visani A, Marcacci M. Knee stablility before and after total and unicondylar knee replacement: in vivo kinematic evaluation utilizing navigation. J Orthop Res. 2009;27:202–207. doi: 10.1002/jor.20746. [DOI] [PubMed] [Google Scholar]
- 12.Casino D, Zaffagnini S, Martelli S, Lopomo N, Bignozzi S, Iacono F, Russo A, Marcacci M. Intraoperative evaluation of total knee replacement: kinematic assessment with a navigation system. Knee Surg Sports Traumatol Arthrosc. 2009;17:369–373. doi: 10.1007/s00167-008-0699-3. [DOI] [PubMed] [Google Scholar]
- 13.Churchill DL, Incavo SJ, Johnson CC, Beynnon BD. The transepicondylar axis approximates the optimal flexion axis of the knee. Clin Orthop Relat Res. 1998;356:111–118. doi: 10.1097/00003086-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Clayton ML, Thirupathi R. Pattellar complication after total condylar arthroplasty. Clin Orthop Relat Res. 1982;170:152–155. [PubMed] [Google Scholar]
- 15.Colizza WA, Insall JN, Scuderi GR. The posterior stabilized total knee prosthesis: Assessment of polyethylene damage and osteolysis after a ten-year-minimum follow-up. J Bone Joint Surg Am. 1995;77:1713–1720. doi: 10.2106/00004623-199511000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Cromie MJ, Siston RA, Giori NJ, Delp SL. Posterior cruciate ligament removal contributes to abnormal knee motion during posterior stabilized total knee arthroplasty. J Orthop Res. 2008;26:1494–1499. doi: 10.1002/jor.20664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dalury DF. Observations of the proximal tibia in total knee arthroplasty. Clin Orthop Relat Res. 2001;389:150–155. doi: 10.1097/00003086-200108000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Eckhoff DG, Metzger RG, Vandewalle MV. Malrotation associated with implant alignment technique in total knee arthroplasty. Clin Orthop Relat Res. 1995;321:28–31. [PubMed] [Google Scholar]
- 19.Fu FH, Harner CD, Johnson DL, Miller MD, Woo SL. Biomechanics of knee ligaments: basic concepts and clinical application. J Bone Joint Surg Am. 1993;75:1716–1727. [PubMed] [Google Scholar]
- 20.Graw BP, Harris AH, Tripuraneni KR, Giori NJ. Rotational references for total knee arthoplasty tibial components change with level of resection. Clin Orthop Relat Res. 2010;468:2734–2738. doi: 10.1007/s11999-010-1330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haider H, Walker PS. Measurements of constraint of total knee replacement. J Biomech. 2005;38:341–348. doi: 10.1016/j.jbiomech.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 22.Huddleston JI, Scott RD, Wimberley DW. Determination of neutral tibial rotational alignment in rotating platform TKA. Clin Orthop Relat Res. 2005;440:101–106. doi: 10.1097/01.blo.0000185448.43622.77. [DOI] [PubMed] [Google Scholar]
- 23.Ikeuchi M, Yamanaka N, Okanoue Y, Ueta E, Tani T. Determining the rotational alignment of the tibial component at total knee replacement: a comparison of two techniques. J Bone Joint Surg Br. 2007;89:45–49. doi: 10.1302/0301-620X.89B1.17728. [DOI] [PubMed] [Google Scholar]
- 24.Incavo SJ, Coughlin KM, Pappas C, Beynnon BD. Anatomic rotational relationships of the proximal tibia, distal femur, and patella: implications for rotational alignment in total knee arthroplasty. J Arthroplasty. 2003;18:643–648. doi: 10.1016/S0883-5403(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 25.Insall JN, Dorr LD, Scott RD, Scott WN. Rationale of the Knee Society clinical rating system. Clin Orthop Relat Res. 1989;248:13–14. [PubMed] [Google Scholar]
- 26.Insall JN, Hood RW, Flawn LB, Sullivan DJ. The total condylar knee prosthesis in gonarthrosis: a five to nine-year follow-up of the first one hundred consecutive replacements. J Bone Joint Surg Am. 1983;65:619–628. [PubMed] [Google Scholar]
- 27.Insall JN, Kelly M. The total condylar prosthesis. Clin Orthop Relat Res. 1986;205:43–48. [PubMed] [Google Scholar]
- 28.Johal P, Williams A, Wragg P, Hunt D, Gedroyc W. Tibio-femoral movement in the living knee: a study of weight bearing and non-weight bearing knee kinematics using ‘intrventional’ MRI. J Biomech. 2005;38:269–276. doi: 10.1016/j.jbiomech.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Kurtz S, Lau E, Ong K, Zhao K, Kelly M, Bozic KJ. Future young patient demand for primary and revision joint replacement: national projections from 2010 to 2030. Clin Orthop Relat Res. 2009;467:2606–2612. doi: 10.1007/s11999-009-0834-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurtz S, Mowat F, Ong K, Chang N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–1497. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 31.Markolf KL, Graff-Radford A, Amstutz HC. In vivo knee stability: a quantitative assessment using an instrumented clinical testing apparatus. J Bone Joint Surg Am. 1978;60:664–674. [PubMed] [Google Scholar]
- 32.Markolf KL, Mench JS, Amstutz HC. Stiffness and laxity of the knee: the contributions of the supporting structures. A quantitative in vitro study. J Bone Joint Surg Am. 1976;58:583–594. [PubMed] [Google Scholar]
- 33.Matziolis G, Pfitzner T, Thiele K, Matziolis D, Perka C. Influence of the position of the fibular head after implantation of a total knee prosthesis on femorotibial rotation. Orthopedics. 2011;34:e610–e614. doi: 10.3928/01477447-20110627-24. [DOI] [PubMed] [Google Scholar]
- 34.Mihalko WM, Conner DJ, Benner R, Williams JL. How does TKA kinematics vary with transverse plane alignment changes in a contemporary implant? Clin Orthop Relat Res. 2012;470:186–192. doi: 10.1007/s11999-011-2145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller MC, Berger RA, Petrella AJ, Karmas A, Rubash HE. Optimizing femoral component rotation in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:38–45. doi: 10.1097/00003086-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Mills OS, Hull ML. Rotational flexibility of the human knee due to varus/valgus and axial moments in vivo. J Biomech. 1991;24:673–690. doi: 10.1016/0021-9290(91)90332-H. [DOI] [PubMed] [Google Scholar]
- 37.Moreland JR. Mechanisms of failure in total knee arthroplasty. Clin Orthop Relat Res. 1988;226:49–64. [PubMed] [Google Scholar]
- 38.Mulhall KJ, Ghomrawi HM, Scully S, Callaghan JJ, Saleh KJ. Current etiologies and modes of failure in total knee arthroplasty revision. Clin Orthop Relat Res. 2006;446:45–50. doi: 10.1097/01.blo.0000214421.21712.62. [DOI] [PubMed] [Google Scholar]
- 39.Noble PC, Gordon MJ, Weiss JM, Reddix RN, Conditt MA, Mathis KB. Does total knee replacement restore normal knee function? Clin Orthop Relat Res. 2005;431:157–165. doi: 10.1097/01.blo.0000150130.03519.fb. [DOI] [PubMed] [Google Scholar]
- 40.Olcott CW, Scott RD. The Ranawat Award: Femoral component rotation during total knee arthroplasty. Clin Orthop Relat Res. 1999;367:39–42. doi: 10.1097/00003086-199910000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Ritter MA, Lutgring JD, Davis KE, Berend ME. The effect of postoperative range of motion on funtional activities after posterior cruciate-retaining total knee arthroplasty. J Bone Joint Surg Am. 2008;90:777–784. doi: 10.2106/JBJS.F.01022. [DOI] [PubMed] [Google Scholar]
- 42.Romero J, Duronio JF, Sohrabi A, Alexander N, MacWilliams BA, Jones LC, Hungerford DS. Varus and valgus flexion laxity of total knee alignment methods in loaded cadaveric knees. Clin Orthop Relat Res. 2002;394:243–253. doi: 10.1097/00003086-200201000-00029. [DOI] [PubMed] [Google Scholar]
- 43.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM. Insall Award Paper: Why are total knee arthroplasties failing today? Clin Orthop Relat Res. 2002;404:7–13. doi: 10.1097/00003086-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Siston RA, Daub AC, Giori NJ, Goodman SB, Delp SL. Evaluation of methods that locate the center of the ankle for computer-assisted total knee arthroplasty. Clin Orthop Relat Res. 2005;439:129–135. doi: 10.1097/01.blo.0000170873.88306.56. [DOI] [PubMed] [Google Scholar]
- 45.Siston RA, Giori NJ, Goodman SB, Delp SL. Intraoperative passive knee kinematics of osteoarthritic knees before and after total knee arthroplasty. J Orthop Res. 2006;24:1607–1614. doi: 10.1002/jor.20163. [DOI] [PubMed] [Google Scholar]
- 46.Siston RA, Goodman SB, Delp SL, Giori NJ. Coronal plane stability before and after total knee arthroplasty. Clin Orthop Relat Res. 2007;463:43–49. doi: 10.1097/BLO.0b013e318137a182. [DOI] [PubMed] [Google Scholar]
- 47.Siston RA, Goodman SB, Patel JJ, Delp SL, Giori NJ. The high variability of tibial rotational alignment in total knee arthroplasty. Clin Orthop Relat Res. 2006;452:65–69. doi: 10.1097/01.blo.0000229335.36900.a0. [DOI] [PubMed] [Google Scholar]
- 48.Siston RA, Maack TL, Hutter EE, Beal MD, Chaudhari AMW. Design and cadaveric validation of a novel device to quantify knee stability during total knee arthroplasty. J Biomech Eng. 2012;134:115001. Available at: http://dx.doi.org/10.1115/1.4007822. Accessed January 17, 2013. [DOI] [PMC free article] [PubMed]
- 49.Siston RA, Patel JJ, Goodman SB, Delp SL, Giori NJ. The variability of femoral rotational alignment in total knee arthroplasty. J Bone Joint Surg Am. 2005;87:2276–2280. doi: 10.2106/JBJS.D.02945. [DOI] [PubMed] [Google Scholar]
- 50.Thompson JA, Hast MW, Granger JF, Piazza SJ, Siston RA. Biomechanical effects of total knee arthroplasty component malrotation: a computational simulation. J Orthop Res. 2011;29:969–975. doi: 10.1002/jor.21344. [DOI] [PubMed] [Google Scholar]
- 51.Van Damme G, Defoort K, Ducoulombier Y, Van Glabbeek F, Bellemans J, Victor J. What should the surgeon aim for when performing computer-assisted total knee arthroplasty? J Bone Joint Surg Am. 2005;87(suppl 2):52–58. doi: 10.2106/JBJS.E.00447. [DOI] [PubMed] [Google Scholar]
- 52.Victor J, Banks S, Bellemans J. Kinematics of posterior cruciate ligament-retaining and -substituting total knee arthroplasty: a prospective randomised outcome study. J Bone Joint Surg Br. 2005;87:646–655. doi: 10.1302/0301-620X.87B5.15602. [DOI] [PubMed] [Google Scholar]
- 53.Victor J, Mueller JK, Komistek RD, Sharma A, Nadaud MC, Bellemans J. In vivo kinematics after a cruciate-substituting TKA. Clin Orthop Relat Res. 2010;468:807–814. doi: 10.1007/s11999-009-1072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vince KG, Insall JN, Kelly MA. The total condylar prosthesis: 10- to 12-year results of a cemented knee replacement. J Bone Joint Surg Br. 1989;71:792–797. doi: 10.1302/0301-620X.71B5.2584249. [DOI] [PubMed] [Google Scholar]
- 55.Yue B, Varadarajan KM, Moynihan AL, Liu F, Rubash HE, Li G. Kinematics of medial osteoarthritic knees before and after posterior cruciate ligament retaining total knee arthroplasty. J Orthop Res. 2011;29:40–46. doi: 10.1002/jor.21203. [DOI] [PubMed] [Google Scholar]