Abstract
Background
Bone mineral density (BMD) in the proximal tibia decreases after TKA and is believed to be a factor in implant migration and loosening. Unicompartmental knee arthroplasty (UKA) is a less invasive procedure preserving knee compartments unaffected by degeneration. Finite element studies have suggested UKA may preserve BMD and that implants of differing stiffnesses might differentially affect BMD but these notions have not been clinically confirmed.
Questions/purposes
We therefore asked whether (1) proximal tibial BMD decreases after UKA, and (2) a cemented metal tibial component with a mobile polyethylene (PE) bearing would have greater BMD loss than a cemented PE tibial component.
Methods
We prospectively followed 48 patients who underwent 50 UKAs using one of two implants: one with a cemented metal tibial baseplate and a mobile PE insert (n = 26) and one with a cemented all-PE tibial component (n = 24). In followup we assessed pain and function (Oxford Knee Score, SF-12, The Knee Society Score©) and radiographs. BMD changes were assessed using quantitative CT osteodensitometry performed postoperatively and at 1 and 2 years after the index procedure.
Results
Mean cancellous BMD decreased 1.9% on the medial side and 1.1% on the lateral side. Mean cortical BMD was static, decreasing 0.4% on the medial side and increasing 0.5% on the lateral side. The greatest observed difference between implants for any region was 3.7%. There were no differences in pain or functional outcome scores.
Conclusions
BMD was preserved 2 years after UKA with no major differences seen between implant types.
Introduction
Unicompartmental knee arthroplasty (UKA) remains a popular operation accounting for approximately 10% of knee arthroplasties in New Zealand and Australia [2, 17]. Although numerous authors have reported 10-year survival greater than 90% [3, 19, 29], registry data suggest inferior survival compared with TKA. In the New Zealand joint registry, the 8-year survival of UKA is 89.9% compared with 10-year survival of 95.6% for TKA [17]. After pain, the most common recorded reason for revision is loosening of the tibial component (24%). Of UKAs failing as a result of tibial loosening, 63% were revised in the first 2 years.
Tibial bone mineral density (BMD) decreases after TKA by 5% to 12% at 2 years [1, 16, 28], 26% at 5 years [20], and by as much as 36.4% at 8 years [13]. Although these studies with limited numbers did not correlate BMD loss with clinical failure, some authors believe diminished BMD is a factor in implant migration and loosening [18, 31]. The same failure mechanism has been proposed for UKA [14, 29, 30]. Certain implant characteristics may further influence BMD loss. Lonner et al. [15] reported greater BMD loss around a tibial component with a long central stem compared with a component with a flat tray and four short pegs. UKA preserves more of the native joint and finite element (FE) models suggest these types of implants closely match physiologic stress in the normal knee [5, 10, 24]. This may reduce the effect of stress shielding seen after TKA. Furthermore, BMD loss may be related to the modulus of the implants. Reports of cemented all-polyethylene (PE) acetabular components in the hip [27] show less BMD loss than titanium cementless implants with PE liners [11].
However, the theoretical arguments for preservation of BMD after UKA or the effect of implants of differing material properties have not been clinically confirmed.
We therefore asked whether (1) proximal tibial BMD decreases after UKA, and (2) a cemented metal tibial component with a mobile PE bearing would have greater tibial BMD loss than a cemented PE tibial component.
Patients and Methods
We prospectively followed 48 patients with medial compartment degenerative knee arthritis who underwent 50 medial compartment UKAs between August 2007 and November 2008. Two bilateral procedures were performed, one simultaneously and one sequentially. In 26 knees we used the Oxford® Partial Knee (Biomet, Swindon, UK) performed by one surgeon (TGL) and in 24 the Genesis® Unicompartmental Knee System (Smith & Nephew, Memphis, TN, USA) performed by another (SVH). All patients older than 50 years presenting with medial compartment osteoarthritis, correctable varus deformity less than 10°, fixed flexion deformity less than 10°, preoperative flexion greater than 100°, and intact cruciate ligaments were included. We excluded patients with inflammatory arthritis, those younger than 50 years, those who had previous failed UKA or prior high tibial osteotomy, or who refused consent. During the study period, we performed a total of 51 UKAs. One patient refused to participate based on the travel distance required for followup. No patients were lost to followup. All patients were followed for 2 years. Approval was obtained from the Central Regional Ethics Committee. This was reapproved annually until the conclusion of this study. Written informed consent for participation in the study was obtained from every participant.
With a two-sided 95% CI, we calculated that a sample size of 20 knees in each cohort would have 80% power to detect a 5% difference in BMD between implants. Based on reports of BMD loss in other studies [1, 13, 16], we do not believe BMD loss less than 5% will make a difference to implant survival or function with UKA. Demographic data were similar for the two patient groups (Table 1).
Table 1.
Demographic data of patients
| Variable | Oxford® implant | Genesis® implant | p value |
|---|---|---|---|
| Number of knees | 25 | 24 | |
| Sex (male:female) | 11:14 | 9:15 | 0.64 |
| Mean age at index operation in years (range) | 69 (50–85) | 71 (55–87) | 0.55 |
The femoral and tibial components of the Oxford® implant were manufactured from cast cobalt-chromium-molybdenum (Co-Cr-Mo) alloy with highly polished articular surfaces. The tibial component has a roughened base with a stabilizing keel and is cemented with an onlay technique onto the resected proximal tibia. A mobile bearing made from ultrahigh-molecular-weight PE (UHMWPE) articulates with the femoral and tibial components. The femoral component of the Genesis® also is manufactured from Co-Cr-Mo with a highly polished surface. It articulates with an UHMWPE tibial component that is cemented with an inlay technique directly into the prepared tibial plateau.
All surgery was performed through a standard medial parapatellar approach. An extramedullary guide was used to align the Oxford® tibial cut. A surface guide was used to align the Genesis® with an extramedullary rod to check coronal and sagittal alignment of the implant with a 90°-burr used to prepare the bone. Intramedullary guides were used to align the femoral components. Both surgeons use a technique that resects minimal tibial bone allowing fit of the implant without overcorrecting the lower limb alignment. All components were cemented, the Oxford® with Refobacin® (Biomet, Warsaw, IN, USA) and the Genesis® with Simplex® with tobramycin (Stryker, Warsaw, IN, USA).
Mobilization with weightbearing as tolerated and ROM to comfort were started Day 1 postoperatively. Supervised physiotherapy was started as an inpatient Day 1 with twice daily sessions focusing on quadriceps strengthening, gait rehabilitation, and active knee flexion and extension. This continued in the community with weekly supervision after discharge. Two crutches were used as required with weaning as the patient felt comfortable.
Patients underwent clinical assessment before surgery and then at 6 weeks, 1 year, and 2 years after the index procedure using the Oxford Knee Score (OKS), SF-12, and The Knee Society Score©. Weightbearing AP and lateral radiographs were taken postoperatively and at 1 year for the Oxford® and 1 and 2 years for the Genesis®. At 2-year followup, complete data were available for 48 knees (24 Oxford® and 24 Genesis®). One knee with the Oxford® implant was revised to a TKA for progression of arthritis in the lateral compartment after 18 months. One patient missed her 1-year followup but did have 2-year followup so was included for the 2-year analysis.
Two of us (BIR, JTM) independently assessed radiographs and CT sagittal and coronal scout images for tibial lucency or migration. We defined a clinically relevant lucency as progressive lucent areas greater than 2 mm. This has a reported kappa value for interobserver error for TKA of 0.781 [22]. Subsidence was defined as angulation or subsidence greater than 2 mm from the postoperative position with implant position defined according to criteria summarized by Sarmah et al. [23].
BMD was assessed using quantitative CT-assisted osteodensitometry. CT scans were performed within 7 days after surgery to establish baseline BMD and again at 1 and 2 years after the index procedure. We used quantitative CT to measure BMD because the method is accurate and reproducible about the hip and the knee [12, 21, 25]. Hounsfield units (HUs) obtained from conventional CT scans correlate with BMD values obtained from dual-energy x-ray absorptiometry [26]. Metal artifact can create an apparent increase in BMD of 4% to 7% [34] although this is minimal 5 mm from the implant in axial images. Our baseline scans were performed after surgery so the same error will be present in all subsequent scans. All CT scans were performed in a standard scanner (Siemens Somatom Plus; Siemens, Erlangen, Germany) with a standardized protocol (140 kV, 206 mAs, 150 mm × 150-mm field). Patients were positioned with a standardized foot holder to control for rotation. Coronal and sagittal scout views were performed to align the tibial component in the scanner. This was followed by sequential axial scans with a 2-mm slice thickness beginning at the level of the tibial implant and progressing distally in 5-mm increments (Fig. 1). A phantom core of hydroxyapatite with a known density (800 mg CaHA/mL) was scanned with each patient to allow radiographic density in HU to be converted to BMD. The most proximal slice that included the tibial implant was assigned to be Slice 0. The next consecutive six slices distally were assigned to be Slice 1 to Slice 6. Only Slices 1 to 6 were analyzed. Each slice was divided into halves. This was achieved by creating a line connecting the posterior border of the tibia to the posterior border of the fibula (Fig. 2). Three perpendicular lines then were drawn; the first was adjacent to the medial border of the tibia, the second was adjacent to the lateral border of the tibia, and the third was midway between the previous two. Each slice of each scan then was analyzed using a unique software tool (CAPPA postOP; CAS Innovations, Erlangen, Germany). Cortical and cancellous bone was assessed separately for each half of each slice applying a threshold of 800 HU to define the corticocancellous border. All bone within the cortical margin was assessed as cancellous bone. Cement and prosthesis in the cancellous region were excluded using a threshold of greater than 1000 HU. All images were checked visually.
Fig. 1.
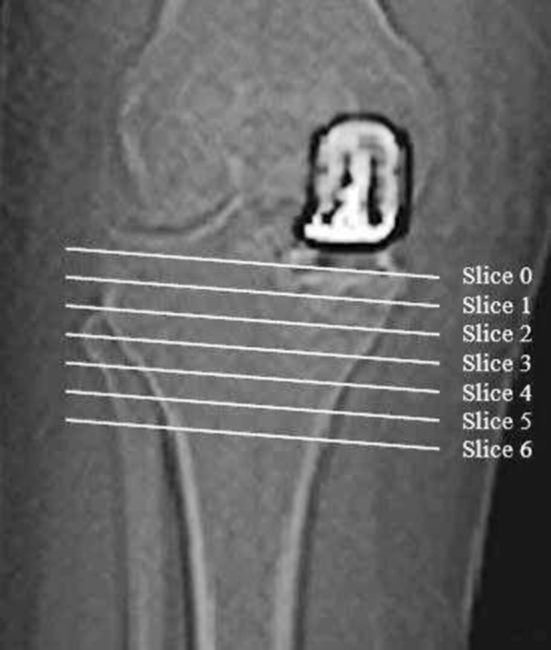
A coronal view of the knee with the Genesis® implant in situ is shown. The lines indicate the six axial slices immediately distal to the tibial implant used for analysis.
Fig. 2.
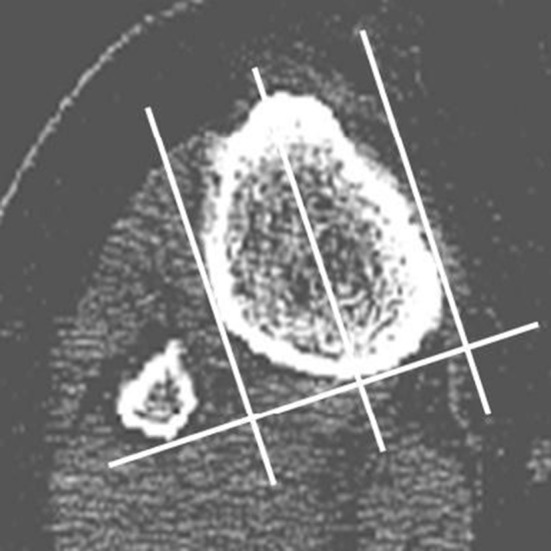
An axial view of the proximal tibia and fibula at Slice 5 with the tibia divided into medial and lateral halves for analysis is shown.
We addressed the questions using generalized least-squares models with an autoregressive AR(1) error structure in the individual patients. This model was chosen because it allows us to consider the effects of multiple variables on our primary variable (measured change in BMD). We previously used this statistical method in a similar study for TKA [16]. Before analysis, the Durbin-Watson test indicated the need to use this type of model to account for multivariate effect. To address the first question, the model included bone type, scan position, and followup time as covariates. The response was BMD as a proportion of the BMD at baseline. The coefficient for the followup time covariate at 1 year and 2 years was used to estimate the change in BMD through time. To address the second question, the model included the same covariates with the addition of implant type. For both questions, second-order interaction coefficients were calculated to establish if the association between the response (BMD) and one of the predictors also was dependent on the predictors interacting with each other. None of the coefficients was significant in any of the models. Analysis was performed using the nlme library inside the R statistical package (R Foundation for Statistical Computing, Vienna, Austria).
Results
When comparing BMD at baseline with 2 years postoperatively, for both implant groups combined, mean cortical BMD decreased (p = 0.193) 0.4% on the medial side and increased (p = 0.196) 0.5% on the lateral side. Mean cancellous BMD decreased (p = 0.111) 1.9% on the medial side and decreased (p = 0.174) 1.1% on the lateral side. Mean cancellous BMD decreased (p < 0.001) from baseline by 1.5% at 2 years. Mean cortical BMD of the medial and lateral regions combined decreased (p = 0.392) from baseline by 0.4% at 2 years. Cortical (p = 0.067) and cancellous (p = 0.102) BMD loss at 2 years was greater on the medial compared with the lateral side.
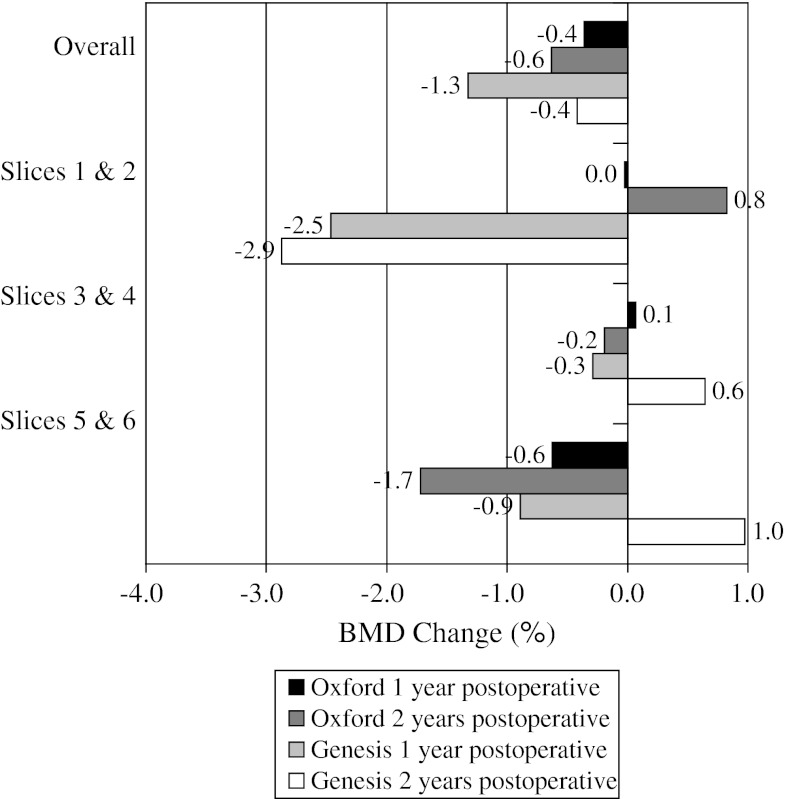
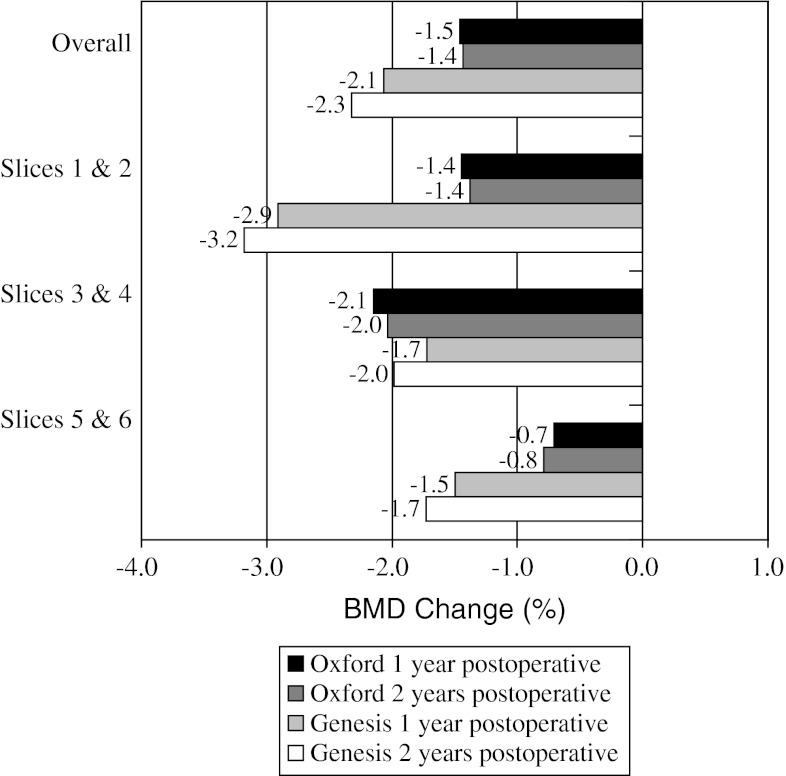
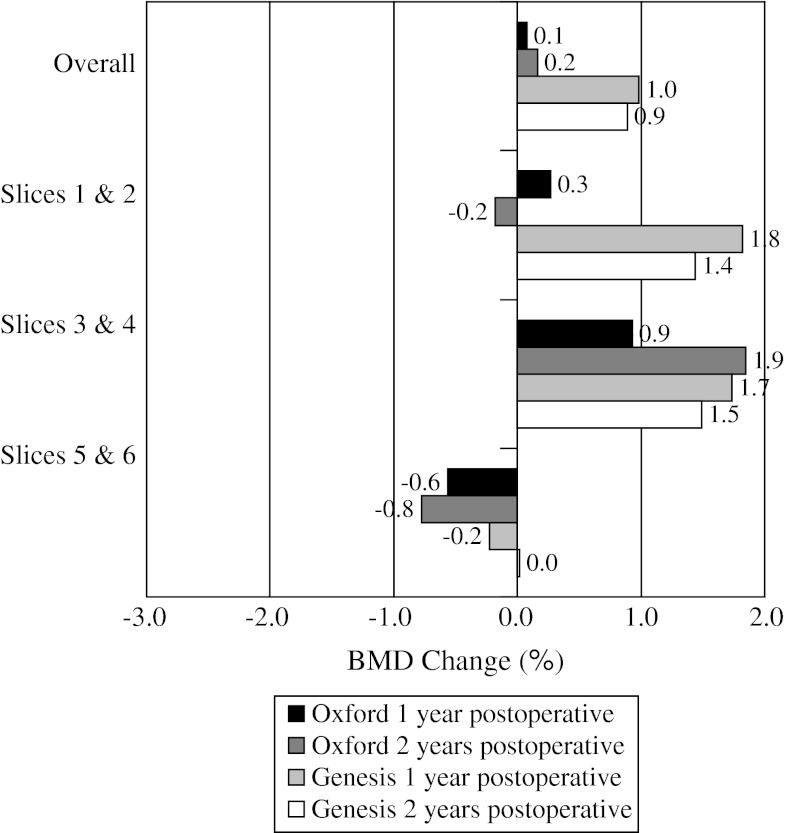
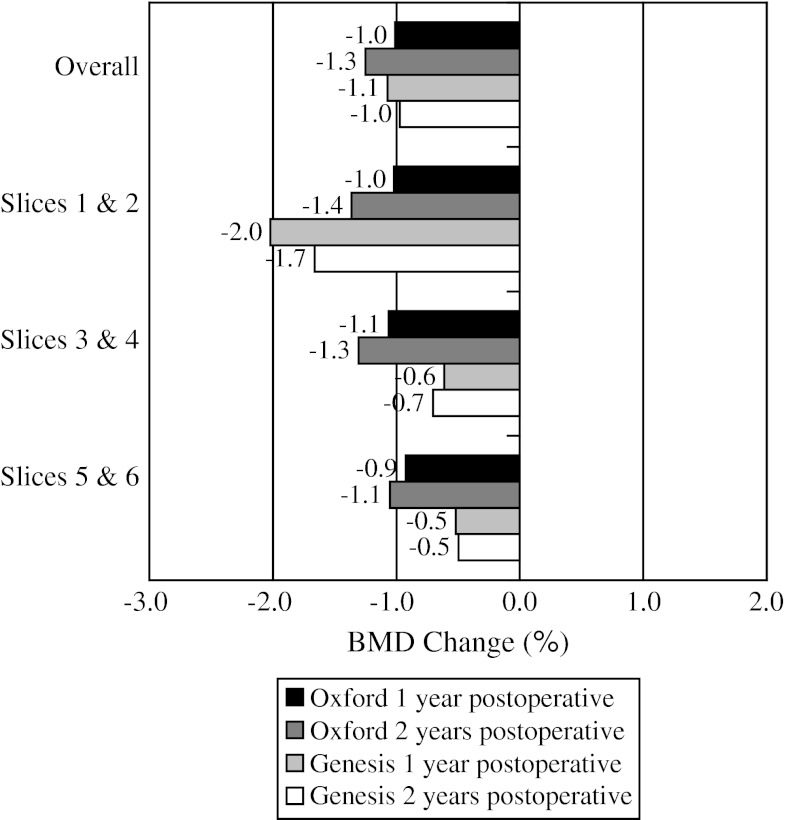
When comparing BMD loss from baseline to 2 years for the individual implants (Table 2), overall medial cortical bone (Fig. 3) for the Oxford® group had greater (p = 0.684) BMD loss of 0.6% compared with loss of 0.4% for the Genesis® group. For medial cancellous bone (Fig. 4), the Genesis® group had greater (p = 0.132) BMD loss of 2.3% compared with 1.4% for the Oxford® group. For lateral cortical bone (Fig. 5), the Genesis® group had a greater (p = 0.101) BMD increase of 0.9% compared with the Oxford® group, which increased by 0.2%. For lateral cancellous bone (Fig. 6) the Oxford® had a greater (p = 0.712) BMD decrease of 1.3% compared with the Genesis® group, which decreased by 1.0%. Slight variations in BMD change were observed for the two implants at 2 years. The greatest observed difference was medial cortical BMD at Slices 1 and 2 where the Oxford® group increased by 0.8%, whereas the Genesis® decreased by 2.9% for a combined difference of 3.7% (p = 0.724). The greatest observed difference between slices occurred with the cortical BMD of the Genesis® with a decrease of 2.9% at Slices 1 and 2 but an increase of 1.0% at Slices 5 and 6 for a combined difference of 3.9% (p = 0.672).
Table 2.
Change in BMD at 1 and 2 years postoperatively
| Measurement outcome |
Interval | Region | Slice | Oxford® implant | Genesis® implant | p value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | 95% CI | Mean | SD | 95% CI | |||||||
| Lower | Upper | Lower | Upper | |||||||||
| Change in BMD (%) | 1 year | Medial cortical | 1 & 2 | 0.0 | 6.7 | −2.7 | 2.7 | −2.5 | 7.0 | −5.2 | 0.3 | 0.353 |
| 3 & 4 | 0.1 | 4.8 | −1.8 | 2.0 | −0.3 | 6.7 | −2.9 | 2.3 | 0.403 | |||
| 5 & 6 | −0.6 | 6.5 | −3.2 | 2.0 | −0.9 | 5.1 | −2.9 | 1.1 | 0.403 | |||
| Medial cancellous | 1 & 2 | −1.4 | 2.4 | −2.4 | −0.5 | −2.9 | 2.8 | −4.0 | −1.8 | 0.003 | ||
| 3 & 4 | −2.1 | 2.8 | −3.3 | −1.0 | −1.7 | 1.7 | −2.4 | −1.1 | 0.569 | |||
| 5 & 6 | −0.7 | 2.3 | −1.6 | 0.2 | −1.5 | 1.8 | −2.2 | −0.8 | 0.264 | |||
| Lateral cortical | 1 & 2 | 0.3 | 4.4 | −1.5 | 2.0 | 1.8 | 5.2 | −0.2 | 3.9 | 0.472 | ||
| 3 & 4 | 0.9 | 6.7 | −1.7 | 3.6 | 1.7 | 5.0 | −0.2 | 3.7 | 0.686 | |||
| 5 & 6 | −0.6 | 2.9 | −1.7 | 0.6 | −0.2 | 4.7 | −2.1 | 1.6 | 0.798 | |||
| Lateral cancellous | 1 & 2 | −1.0 | 2.3 | −1.9 | −0.1 | −2.0 | 3.1 | −3.2 | −0.8 | 0.119 | ||
| 3 & 4 | −1.1 | 1.6 | −1.7 | −0.4 | −0.6 | 1.8 | −1.3 | 0.1 | 0.295 | |||
| 5 & 6 | −0.9 | 1.1 | −1.4 | −0.5 | −0.5 | 0.9 | −0.9 | −0.2 | 0.062 | |||
| 2 years | Medial cortical | 1 & 2 | 0.8 | 7.6 | −2.2 | 3.8 | −2.9 | 6.8 | −5.5 | −0.2 | 0.352 | |
| 3 & 4 | −0.2 | 6.1 | −2.6 | 2.3 | 0.6 | 7.3 | −2.2 | 3.5 | 0.403 | |||
| 5 & 6 | −1.7 | 7.7 | −4.8 | 1.4 | 1.0 | 4.1 | −0.6 | 2.6 | 0.403 | |||
| Medial cancellous | 1 & 2 | −1.4 | 3.7 | −2.8 | 0.1 | −3.2 | 3.9 | −4.7 | −1.7 | 0.003 | ||
| 3 & 4 | −2.0 | 3.1 | −3.3 | −0.8 | −2.0 | 2.4 | −2.9 | −1.1 | 0.569 | |||
| 5 & 6 | −0.8 | 2.2 | −1.7 | 0.1 | −1.7 | 1.6 | −2.4 | −1.1 | 0.264 | |||
| Lateral cortical | 1 & 2 | −0.2 | 4.7 | −2.0 | 1.7 | 1.4 | 5.0 | −0.5 | 3.4 | 0.472 | ||
| 3 & 4 | 1.9 | 7.6 | −1.2 | 4.9 | 1.5 | 4.4 | −0.2 | 3.2 | 0.687 | |||
| 5 & 6 | −0.8 | 4.3 | −2.5 | 0.9 | 0.0 | 4.8 | −1.9 | 1.9 | 0.798 | |||
| Lateral cancellous | 1 & 2 | −1.4 | 2.6 | −2.4 | −0.3 | −1.7 | 2.1 | −2.5 | −0.8 | 0.119 | ||
| 3 & 4 | −1.3 | 1.5 | −1.9 | −0.7 | −0.7 | 1.2 | −1.2 | −0.2 | 0.295 | |||
| 5 & 6 | −1.1 | 1.2 | −1.5 | −0.6 | −0.5 | 0.9 | −0.9 | −0.1 | 0.062 | |||
Fig. 3.
The mean BMD change for the medial cortical bone is shown. The greatest observed difference between implants occurred at Slices 1 and 2 and the greatest observed difference between levels occurred at Slices 5 and 6 compared with Slices 1 and 2.
Fig. 4.
The mean BMD change for the medial cancellous bone is shown. Small reductions in BMD were observed at all levels for both implants.
Fig. 5.
The mean BMD change for lateral cortical bone is shown. Slight increases in BMD density were observed proximally.
Fig. 6.
The mean BMD change for the lateral cancellous bone is shown. Slight decreases in BMD were observed at all levels for both implants.
The OKS, SF-12, functional, and Knee Society Scores© all improved at final followup compared with preoperative values (Table 3). No patients had evidence of progressive radiographic lucency or implant subsidence. Other than one patient in the Oxford® group who had progression of lateral compartment osteoarthritis, no revisions or infections occurred.
Table 3.
Functional scores for the implant groups comparing baseline with 2 years postoperatively
| Score | Oxford® implant | Genesis® implant | Combined | p value |
|---|---|---|---|---|
| Knee Society knee score | ||||
| Baseline | 33 | 50 | 41 | 0.246 |
| 2 years postoperatively | 82 | 78 | 80 | |
| Knee Society functional score | ||||
| Baseline | 30 | 55 | 42 | 0.343 |
| 2 years postoperatively | 70 | 78 | 74 | |
| Oxford Knee Score | ||||
| Baseline | 19 | 22 | 21 | 0.772 |
| 2 years postoperatively | 40 | 40 | 40 | |
| SF-12 physical component | ||||
| Baseline | 29 | 31 | 30 | 0.200 |
| 2 years postoperatively | 42 | 46 | 44 | |
| SF-12 mental component | ||||
| Baseline | 50 | 53 | 51 | 0.198 |
| 2 years postoperatively | 52 | 55 | 54 | |
Discussion
Tibial BMD decreases after TKA [1, 13, 15, 16, 18, 20, 28] (Table 4). This has been suggested as a factor contributing to implant migration and loosening in TKA [18, 31] and UKA [14, 29, 30]. Although studies have reported BMD loss ranging from 5% to as much as 36.4% none has been able to correlate this to failure of the implants. However the majority of these studies have followup of 2 years or less and are limited by the small number of patients. Modern implants unlikely would show substantial failure rates within 10 years. It has been suggested that implants preserving metaphyseal bone may result in less BMD loss. Lonner et al. [15] observed that a TKA implant with four short pegs had similar proximal tibial BMD to the control limb 7 years after surgery, whereas BMD was reduced by up to 70% around a stemmed implant. UKA is a less invasive procedure that resurfaces only the affected compartment maintaining the rest of the knee. These implants may transmit more physiologic loads to the proximal tibia [5, 10, 24] because they involve limited bone resection and have no or short stems on the tibial component. In this regard they may not lead to the large reductions in BMD seen about TKA implants. Prospective clinical data supporting this hypothesis are lacking. Furthermore, research in the hip has suggested lower modulus cemented PE implants result in very little BMD loss at 2 years [27] compared with titanium cementless implants that can cause loss of cancellous BMD as much as 40% at 10 years [12]. We therefore assumed that the metal Oxford® tibial component would have a greater stress shielding effect resulting in greater BMD loss compared with the all-PE Genesis® implant. We asked whether (1) proximal tibial BMD decreases after UKA, and (2) a cemented metal tibial component with a mobile PE bearing would have greater BMD loss than a cemented all-PE cemented tibial component.
Table 4.
Comparison of studies reporting changes in BMD around knee implants
| Study | Design | Number of knees | Implant | BMD measure | Followup | Results |
|---|---|---|---|---|---|---|
| Levitz et al. [13] | Prospective cohort | 31 | Cemented TKA with central stem | DPA and DEXA | 1 year in 31 patients, 7 years in 8 patients | Equal BMD at 1 year but 36.4% decrease by 8 years (p < 0.05) |
| Regnér et al. [20] | Prospective randomized | 38 | HA-coated versus fiber metal-backed cementless TKA | TEXA | 5 years | Greater BMD decrease in HA-coated TKA (36%) compared with fiber metal (15%) TKA (p = 0.02) |
| Lonner et al. [15] | Prospective cohort | 12 | Tibial component with short pegs versus long central stem cemented TKA | DEXA | 7 years | Greater BMD decrease (20%–70%) with long central stem compared with contralateral tibia (p = 0.004) than short pegs (7% loss to 27% gain) |
| Soininvaara et al. [28] | Prospective cohort | 69 | Cemented TKA with central stem | DEXA | 1 year | Medial BMD decreased 6.6% (p < 0.001), lateral BMD increased 0.4% (p = 0.2) |
| Petersen et al. [18] | Prospective randomized | 16 | HA-coated versus cast mesh cementless TKA with central stem | DEXA | 2 years | BMD decreased by 4.3% in HA-coated group and increased by 6.1% in cast mesh group (p = 0.003) |
| Abu-Rajab et al. [1] | Matched cohort | 38 | Cemented versus cementless rotating-platform TKA | DEXA | 2 years | Tibial BMD decreased up to 8.4%; no significant difference between implants |
| Munro et al. [16] | Prospective randomized | 54 | Rotating-platform versus fixed cemented TKA | qCT | 2 years | Proximal tibial cancellous BMD decreased by 12.6% (p < 0.001) and cortical by 3.6% (p < 0.001); no significant difference between implants |
| Current study | Prospective cohort | 50 | Metal-backed tibia, mobile-bearing versus all-polyethylene cemented UKA | qCT | 2 years | Mean cancellous BMD decreased 1.9% on the medial side (p = 0.111) and decreased 1.1% on the lateral side (p = 0.174); mean cortical BMD was static, decreasing 0.4% on the medial side (p = 0.193) and increasing 0.5% on the lateral side (p = 0.196); the greatest observed difference between implants for any region was 3.7% (p = 0.724) |
Results are reported for changes in tibial BMD; BMD = bone mineral density; UKA = unilateral knee arthroplasty; DPA = dual photon absorptiometry; DEXA = dual-energy x-ray absorptiometry; TEXA = triple-energy x-ray absorptiometry; qCT = quantitative CT; HA = hydroxyapatite.
This study has several limitations. First, we did not randomize patients. UKA is a highly technical procedure with a long learning curve [4, 8]. Both surgeons have experience with their preferred prosthesis so we chose to leave each arm under the direction of the respective surgeon and do not believe a learning curve would influence the findings. Second, there may be bias introduced by design variables other than the tibial component material. The femoral components are different geometry but both should transmit force to the articulation in a similar way. The tibial components are implanted with different techniques The Genesis® inlay technique means the implant is entirely in contact with cancellous bone. The Oxford® onlay technique means the implant is at least partly positioned on cortical bone. This could selectively load the cortical bone creating a stress shielding effect similar to that observed in the acetabulum [12] although the very thin cortical bone in the proximal tibia is not necessarily analogous to the pelvis. Third, we did not obtain long-leg alignment views to determine the true mechanical axis. Both surgeons use a technique to balance soft tissue without correcting the alignment to neutral. Clinical assessment of alignment was included in the Knee Society Score® but numbers were too small to correlate to BMD change. Varus alignment after UKA reportedly does not adversely affect radiologic outcome, clinical scores, or medium-term survival [7] and apparently recurs with time [33]. Hvid et al. [9] showed altered alignment after TKA caused a decrease in BMD in the postoperatively unloaded side. We compared medial and lateral BMD and observed slightly greater BMD loss on the medial side, although the indication for UKA in our series was minimal deformity with little change in the postoperative alignment. Fourth, our study was only powered to detect a 5% difference in BMD and may be underpowered to detect a smaller change than this. As mentioned earlier several studies [1, 13, 16] have not been able to correlate implant migration and failure with BMD loss well above 5% (Table 4). We do not believe a smaller loss of BMD would result in migration and loosening of UKA components. Fifth, BMD is known to decrease with aging with a generalized loss of 0.5% to 1% per year [32]. This may account entirely for the changes we have observed after surgery.
We measured small (< 3% and statistically insignificant) reductions in cancellous BMD except in two of the regions compared. Similarly, comparisons between implants show small (< 3.9%) and insignificant differences across regions. Our data are consistent with FE models. Gillies et al. [5] designed a three-dimensional FE model that compared two cemented all-PE implants, one with a keel and one without. Both were modeled using an inlay technique. Using a stress remodeling algorithm, they found proximal tibial bone had stabilized by 48 months. The keel design had predicted BMD loss of 1% to 6%, whereas the flat no keel design had predicted BMD gain of 4% to 10%. Overall there was a very small amount of resorption, which the authors believed was unlikely to be clinically important. A less complex three-dimensional FE model was designed by Sawatari et al. [24]. They analyzed a metal baseplate UKA and varied the degree of varus-valgus angulation. They were able to show inhomogeneous stress transfer with increased stress in the cancellous region medially as the implant moved in valgus. Their model was unable to predict bone remodeling. Klemme et al. [11] conducted followup assessment of 33 UKAs with radiographs and technetium bone scans. They could not correlate the appearance of radiolucency to clinical outcome. They also observed increased technetium uptake in the surgically treated compartment but not in the nonsurgically treated compartment. This was interpreted as evidence of ongoing bone remodeling, but again this was not associated with failure.
Although we expected that the more compliant all-PE implant would more evenly distribute stress to the proximal tibia and thus might result in less BMD the Genesis® group had slightly greater BMD loss. The greatest difference we found for any cortical or cancellous region was approximately 4%, however the majority of values was much closer. This may reflect the different proportion of female patients each group. After accounting for the failed implant in a female patient 14 of 25 (56%) Oxford® prostheses were implanted in females against 15 of 24 (63%) Genesis® implants. There was no indication of a functional difference between groups (Table 3). Biomechanically it is possible that the onlay technique used for the Oxford® implant more evenly distributes stress to the proximal tibia, although it is difficult to perceive that the Genesis® implant would stress shield. It is entirely possible that our results reflect a type II error. Regardless, the difference was so small that we believe an important clinical difference would be apparent based on BMD in the short term.
Radiographic assessment of the Oxford® implant showed the occurrence of radiolucent lines at the bone-implant interfaces. At 5 years followup, Gulati et al. [6] reported a 30% rate of complete and 32% rate of partial radiolucent lines around the tibial component. The lucencies did not correlate to implant failure, pain, or functional scores. We observed no radiolucency at last followup although our followup was shorter. Our observations suggest implant type does not influence BMD at 2 years postimplantation.
We conclude that proximal tibial BMD generally is preserved 2 years after UKA and that there is no difference in early BMD change between these two implant designs.
Acknowledgments
We thank Rocco P. Pitto MD, PhD, FRACS at the University of Auckland for assistance with the quantitative CT osteodensitometry analysis and Richard Feltham MBChB, FRANZCR, at Taranaki Base Hospital for assistance with CT scanning protocols and reports for the National Radiation Laboratory.
Footnotes
The institution of one or more of the authors (SVH, TGL) has received, during the study period, funding from the Taranaki Medical Foundation.
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
This work was performed at the Taranaki Base Hospital, New Plymouth, New Zealand.
References
- 1.Abu-Rajab RB, Watson WS, Walker B, Roberts J, Gallacher SJ, Meek RM. Peri-prosthetic bone mineral density after total knee arthroplasty: cemented versus cementless fixation. J Bone Joint Surg Br. 2006;88:606–613. doi: 10.1302/0301-620X.88B5.16893. [DOI] [PubMed] [Google Scholar]
- 2.Annual Report. Adelaide, Australia: Australian Orthopaedic Association; 2010. [Google Scholar]
- 3.Berger RA, Meneghini RM, Jacobs JJ, Sheinkop MB, Valle Della CJ, Rosenberg AG, Galante JO. Results of unicompartmental knee arthroplasty at a minimum of ten years of follow-up. J Bone Joint Surg Am. 2005;87:999–1006. doi: 10.2106/JBJS.C.00568. [DOI] [PubMed] [Google Scholar]
- 4.Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg. 2008;16:9–18. doi: 10.5435/00124635-200801000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Gillies RM, Hogg MC, Kohan L, Cordingley RL. Adaptive bone remodelling of all polyethylene unicompartmental tibial bearings. ANZ J Surg. 2007;77:69–72. doi: 10.1111/j.1445-2197.2006.03980.x. [DOI] [PubMed] [Google Scholar]
- 6.Gulati A, Chau R, Pandit HG, Gray H, Price AJ, Dodd CA, Murray DW. The incidence of physiological radiolucency following Oxford unicompartmental knee replacement and its relationship to outcome. J Bone Joint Surg Br. 2009;91:896–902. doi: 10.1302/0301-620X.91B7.21914. [DOI] [PubMed] [Google Scholar]
- 7.Gulati A, Pandit H, Jenkins C, Chau R, Dodd CA, Murray DW. The effect of leg alignment on the outcome of unicompartmental knee replacement. J Bone Joint Surg Br. 2009;91:469–474. doi: 10.1302/0301-620X.91B4.22105. [DOI] [PubMed] [Google Scholar]
- 8.Hamilton WG, Ammeen D, Engh CA, Jr, Engh GA. Learning curve with minimally invasive unicompartmental knee arthroplasty. J Arthroplasty. 2010;25:735–740. doi: 10.1016/j.arth.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 9.Hvid I, Bentzen SM, Jørgensen J. Remodeling of the tibial plateau after knee replacement: CT bone densitometry. Acta Orthop Scand. 1988;59:567–573. doi: 10.3109/17453678809148787. [DOI] [PubMed] [Google Scholar]
- 10.Iesaka K, Tsumura H, Sonoda H, Sawatari T, Takasita M, Torisu T. The effects of tibial component inclination on bone stress after unicompartmental knee arthroplasty. J Biomech. 2002;35:969–974. doi: 10.1016/S0021-9290(01)00244-5. [DOI] [PubMed] [Google Scholar]
- 11.Klemme WR, Galvin EG, Petersen SA. Unicompartmental knee arthroplasty: sequential radiographic and scintigraphic imaging with an average five-year follow-up. Clin Orthop Relat Res. 1994;301:233–238. [PubMed] [Google Scholar]
- 12.Kress AM, Schmidt R, Vogel T, Nowak TE, Forst R, Mueller LA. Quantitative computed tomography-assisted osteodensitometry of the pelvis after press-fit cup fixation: a prospective ten-year follow-up. J Bone Joint Surg Am. 2011;93:1152–1157. doi: 10.2106/JBJS.J.01097. [DOI] [PubMed] [Google Scholar]
- 13.Levitz CL, Lotke PA, Karp JS. Long-term changes in bone mineral density following total knee replacement. Clin Orthop Relat Res. 1995;321:68–72. [PubMed] [Google Scholar]
- 14.Lindstrand A, Stenström A, Ryd L, Toksvig-Larsen S. The introduction period of unicompartmental knee arthroplasty is critical: a clinical, clinical multicentered, and radiostereometric study of 251 Duracon unicompartmental knee arthroplasties. J Arthroplasty. 2000;15:608–616. doi: 10.1054/arth.2000.6619. [DOI] [PubMed] [Google Scholar]
- 15.Lonner JH, Klotz M, Levitz C, Lotke PA. Changes in bone density after cemented total knee arthroplasty: influence of stem design. J Arthroplasty. 2001;16:107–111. doi: 10.1054/arth.2001.16486. [DOI] [PubMed] [Google Scholar]
- 16.Munro JT, Pandit S, Walker CG, Clatworthy M, Pitto RP. Loss of tibial bone density in patients with rotating- or fixed-platform TKA. Clin Orthop Relat Res. 2010;468:775–781. doi: 10.1007/s11999-009-0794-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eleven Year Report. Christchurch, New Zealand: New Zealand Orthopaedic Association; 2010. [Google Scholar]
- 18.Petersen MM, Gehrchen PM, Østgaard SE, Nielsen PK, Lund B. Effect of hydroxyapatite-coated tibial components on changes in bone mineral density of the proximal tibia after uncemented total knee arthroplasty: a prospective randomized study using dual-energy x-ray absorptiometry. J Arthroplasty. 2005;20:516–520. doi: 10.1016/j.arth.2004.09.041. [DOI] [PubMed] [Google Scholar]
- 19.Price A, Waite J, Svard U. Long-term clinical results of the medial Oxford unicompartmental knee arthroplasty. Clin Orthop Relat Res. 2005;435:171–180. doi: 10.1097/00003086-200506000-00024. [DOI] [PubMed] [Google Scholar]
- 20.Regnér LR, Carlsson LV, Kärrholm JN, Hansson TH, Herberts PG, Swanpalmer J. Bone mineral and migratory patterns in uncemented total knee arthroplasties: a randomized 5-year follow-up study of 38 knees. Acta Orthop Scand. 1999;70:603–608. doi: 10.3109/17453679908997850. [DOI] [PubMed] [Google Scholar]
- 21.Reilly K, Munro J, Pandit S, Kress A, Walker C, Pitto RP. Inter-observer validation study of quantitative CT-osteodensitometry in total knee arthroplasty. Arch Orthop Trauma Surg. 2007;127:729–731. doi: 10.1007/s00402-007-0351-6. [DOI] [PubMed] [Google Scholar]
- 22.Sadoghi P, Leithner A, Weber P, Friesenbichler J, Gruber G, Kastner N, Pohlmann K, Jansson V, Wegener B. Radiolucent lines in low-contact-stress mobile-bearing total knee arthroplasty: a blinded and matched case control study. BMC Musculoskelet Disord. 2011;12:142. doi: 10.1186/1471-2474-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sarmah SS, Patel S, Hossain FS, Haddad FS. The radiological assessment of total and unicompartmental knee replacements. J Bone Joint Surg Br. 2012;94:1321–1329. doi: 10.1302/0301-620X.94B10.29411. [DOI] [PubMed] [Google Scholar]
- 24.Sawatari T, Tsumura H, Iesaka K, Furushiro Y, Torisu T. Three-dimensional finite element analysis of unicompartmental knee arthroplasty: the influence of tibial component inclination. J Orthop Res. 2005;23:549–554. doi: 10.1016/j.orthres.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt R, Pitto RP, Kress A, Ehremann C, Nowak TE, Reulbach U, Forst R, Müller L. Inter- and intraobserver assessment of periacetabular osteodensitometry after cemented and uncemented total hip arthroplasty using computed tomography. Arch Orthop Trauma Surg. 2005;125:291–297. doi: 10.1007/s00402-005-0812-8. [DOI] [PubMed] [Google Scholar]
- 26.Schreiber JJ, Anderson PA, Rosas HG, Buchholz AL, Au AG. Hounsfield units for assessing bone mineral density and strength: a tool for osteoporosis management. J Bone Joint Surg Am. 2011;93:1057–1063. doi: 10.2106/JBJS.J.00160. [DOI] [PubMed] [Google Scholar]
- 27.Shetty NR, Hamer AJ, Kerry RM, Stockley I, Eastell R, Wilkinson JM. Bone remodelling around a cemented polyethylene cup: a longitudinal densitometry study. J Bone Joint Surg Br. 2006;88:455–459. doi: 10.1302/0301-620X.88B4.16786. [DOI] [PubMed] [Google Scholar]
- 28.Soininvaara TA, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM, Kröger HP. Periprosthetic tibial bone mineral density changes after total knee arthroplasty: one-year follow-up study of 69 patients. Acta Orthop Scand. 2004;75:600–605. doi: 10.1080/00016470410001493. [DOI] [PubMed] [Google Scholar]
- 29.Squire MW, Callaghan JJ, Goetz DD, Sullivan PM, Johnston RC. Unicompartmental knee replacement: a minimum 15 year followup study. Clin Orthop Relat Res. 1999;367:61–72. doi: 10.1097/00003086-199910000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Stewart HD, Newton G. Long-term results of the Manchester knee: surface arthroplasty of the tibiofemoral joint. Clin Orthop Relat Res. 1992;278:138–146. [PubMed] [Google Scholar]
- 31.Therbo M, Petersen MM, Varmarken JE, Olsen CA, Lund B. Influence of pre-operative bone mineral content of the proximal tibia on revision rate after uncemented knee arthroplasty. J Bone Joint Surg Br. 2003;85:975–979. doi: 10.1302/0301-620X.85B7.13882. [DOI] [PubMed] [Google Scholar]
- 32.Warming L, Hassager C, Christiansen C. Changes in bone mineral density with age in men and women: a longitudinal study. Osteoporos Int. 2002;13:105–112. doi: 10.1007/s001980200001. [DOI] [PubMed] [Google Scholar]
- 33.Weale AE, Murray DW, Baines J, Newman JH. Radiological changes five years after unicompartmental knee replacement. J Bone Joint Surg Br. 2000;82:996–1000. doi: 10.1302/0301-620X.82B7.10466. [DOI] [PubMed] [Google Scholar]
- 34.Zannoni C, Viceconti M, Pierotti L, Cappello A. Analysis of titanium induced CT artifacts in the development of biomechanical finite element models. Med Eng Phys. 1998;20:653–659. doi: 10.1016/S1350-4533(98)00076-9. [DOI] [PubMed] [Google Scholar]