Abstract
Background
Heterotopic ossification (HO) occurs most commonly after trauma and surgery about the hip and may compromise subsequent function. Currently available animal models describing the cellular progression of HO are based on exogenous osteogenic induction agents and may not reflect the processes following trauma.
Questions/purposes
We therefore sought to characterize the histologic progression of heterotopic bone formation in an animal model that recapitulates the human condition without the addition of exogenous osteogenic material.
Methods
We used a rabbit model that included intramedullary instrumentation of the upper femur and ischemic crush injury of the gluteal muscle. Bilateral surgical induction procedures were performed on 30 animals with the intention of inciting the process of HO; no supplemental osteogenic stimulants were used. Three animals were sacrificed at each of 10 predetermined times between 1 day and 26 weeks postoperatively and the progression of tissue maturation was graded histologically using a five-item scale.
Results
Heterotopic bone reliably formed de novo and consistently followed a pathway of endochondral ossification. Chondroid elements were found in juxtaposition with immature woven bone in all sections that contained mature osseous elements.
Conclusions
These results establish that HO occurs in an animal model mimicking the human condition following surgical trauma about the hip; it is predictable in its histologic progression and follows a pathway of endochondral bone formation.
Clinical Relevance
By showing a consistent pathway of endochondral ossification leading to ectopic bone formation, this study provides a basis for understanding the mechanisms by which HO might be mitigated by interventions.
Introduction
Heterotopic ossification (HO) is the abnormal formation of mature lamellar bone in nonosseous soft tissues [44]. It can be differentiated histologically from dystrophic calcification, such as the pathologic calcification associated with renal failure and hyperparathyroidism, by the presence of trabecular morphologic features characteristic of bone [5]. The causes of HO are numerous and include traumatic [34], neurogenic [9], and genetic origins [17]. Most commonly, HO occurs as a sequel to trauma, especially around the hip, and in association with open reduction and internal fixation of acetabular fractures or after THA [1, 5, 11, 36]. It can cause pain, restrict functional ROM, and ultimately compromise the clinical success of these procedures.
Although often asymptomatic, between 2% and 8% of patients with HO experience compromise in hip function, manifested as pain with decreased ROM or ankylosis of the joint [1, 24, 36]. In contrast, the radiographic prevalence of HO after THA is reportedly as much as 90%, depending on the patient population [3, 11, 36]. Known risk factors for the development of HO include male gender [11], previous HO [8, 11, 24], hypertrophic or posttraumatic arthritis [38], diffuse idiopathic skeletal hyperostosis [7], ankylosing spondylitis [6], and traumatic brain injury [9].
Despite the importance of HO as a clinical entity, its pathophysiologic features are poorly understood. Several studies [20, 22, 25, 42] have characterized the histologic progression of HO, but in each a BMP or bone matrix was added as an induction agent, and the pathway of bone formation varied with the specific exogenous agent. BMP-2 and BMP-4 have been suggested to exclusively induce endochondral ossification [22, 25], whereas other investigators have reported simultaneous endochondral and intramembranous bone formation induced by BMP-2 [20, 42]. Similarly, the study of rare human genetic diseases [12, 17, 18, 40], such as fibrodysplasia ossificans progressiva and progressive osseous heteroplasia, has provided insight into this condition. Yet, extrapolation of the findings from these studies may have only limited applicability to HO as seen after THA. For example, fibrodysplasia ossificans progressiva exhibits characteristics of endochondral ossification whereas progressive osseous heteroplasia is largely characterized by intramembranous ossification [12, 17–19]. Consequently, elucidation of the predominant pathway of HO as observed after surgical trauma in humans is critically dependent on identification of an appropriate animal model in which to study the process.
We previously described an animal model that mimics the human condition of ectopic bone formation after THA by crushing the gluteal muscles and without the use of an exogenous induction agent; the procedure reliably produced HO [39]. However, the progressive cellular process leading to mature HO has been described only in models using exogenous induction agents (eg, BMP or bone matrix). Clinically, HO can occur as free islands of bone in the muscles about the hip or as an outgrowth contiguous with the proximal femur or acetabulum [8]. It is unclear, however, whether the process of HO differs according to its location and whether the process of heterotopic bone formation after surgery about the hip follows a pathway of endochondral or intramembranous ossification, or some variant of the two.
We specifically asked whether a cartilage intermediary would be present before the appearance of heterotopic bone. Secondarily, we asked whether there were any differences in the pattern of histologic progression of ectopic bone formation when contiguous with osseous structures (eg, bony outgrowth from the femoral cortex or periosteum), as compared with ectopic bone that appeared free in the soft tissues (eg, in muscle or synovial tissue surrounding the hip).
Materials and Methods
Thirty 3-kg male New Zealand White rabbits underwent bilateral induction procedures that included femoral reaming with controlled periosteal disruption and intentional gluteal myonecrosis, performed per protocol [39]. No exogenous osteogenic agents were added to the wound. Nine predetermined times were studied. Three animals were randomly assigned to each of nine times for sacrifice at 1, 3, 5, 7, or 10 days or 2, 3, 6, or 26 weeks after surgery. To accommodate anticipated additional studies at times that were thought to be critical for histologic study, one additional animal was assigned to each of the three times at 10 days, 3 weeks, and 6 weeks. Because differentiation of pluripotent mesenchymal stem cells along osteogenic cell lines occurs soon after injury, the times were preferentially distributed early in the postoperative period. All hips were harvested en bloc immediately postmortem for histologic analysis. The current investigation was conducted under a protocol approved by the Institutional Animal Care and Use Committee as a prospective observational study of the histologic characterization and progression of HO in the New Zealand White rabbit after surgery around the hip [39].
After the induction of general anesthesia, the rabbits were placed in the prone position and each hindquarter was shaved, prepared, and draped in a sterile fashion. The surgical approach was performed sequentially on each side in identical fashion. A 2.5-cm skin incision was made over the greater trochanter and dissection was carried down sharply to bone through the gluteus maximus in line with its fibers. A pneumatic drill with a 2.5-mm bit was used to enter the femoral medullary canal in the region of the greater trochanter (replicating the human condition of THA), which then was reamed manually with incrementally larger wire brushes to a 4.0-mm diameter. The scrapings produced by the reaming process were left in situ in the surgical wound; no effort was made to clear the debris by irrigation. Although some blood and debris may have been lost as part of the procedure, no efforts were made to drain, irrigate, perform débridement, or remove blood or debris from the wound. Rather, a specific attempt was made to maintain as much debris as naturally possible in the wound by using a watertight running fascial closure. Before closure, ischemic injury to the gluteus medius and minimus muscles was intentionally induced by application of a Kelly clamp for 3 minutes; tissue hypoxia, created by clamping the gluteal musculature, enhances heterotopic bone formation [27]. Muscle ischemia is an inherent consequence of surgery around the hip, particularly with the use of extensile approaches or with revision procedures, both of which are risk factors for HO [1, 5, 11, 36]. The wound then was closed in layers, and a postoperative radiograph was obtained to confirm the absence of iatrogenic femoral fracture. Reproducibility of the procedure was obtained by the consistent supervision of the senior author (VDP) throughout the experimental protocol.
Postoperatively, analgesia was administered subcutaneously using 0.03 mg/kg buprenorphine, twice a day for 3 days. No antiinflammatory medications were administered. Rabbits were permitted to eat, ambulate, and bear weight ad libitum. AP view radiographs of the pelvis to include both hips were obtained routinely at monthly intervals until the time of animal sacrifice. Film quality, which predated digital imaging, was not sufficient to afford quantitative analysis; accordingly, radiographs were used only as a qualitative tool to follow patterns and progression of ectopic bone formation with time.
Thirty rabbits were sacrificed at the nine specified times using 35 mg/kg subcutaneously administered ketamine and 5 mg/kg subcutaneously administered xylazine as sedation, followed by 100 mg/kg intravenously administered pentobarbital to subsequently induce death. Three tissue samples were obtained from each of 60 hips in 30 rabbits; samples included a section of the greater trochanter and femoral head and two sections of the surrounding musculature lateral and superior to the greater trochanter. Ten hips were excluded because of technical problems involving either tissue fixation or staining. Tissue blocks were embedded in paraffin and sectioned (7 μm) after fixation with 10% neutral buffered formalin and decalcification (Cal-EX® Decalcifying Solution, Fisher Scientific, Pittsburgh, PA, USA). Three sequential sections of each sample were stained with hematoxylin, eosin, and Masson’s trichrome dye (collagen) for specific histologic study. A total of 150 tissue samples, three from each of 50 hips, were evaluated.
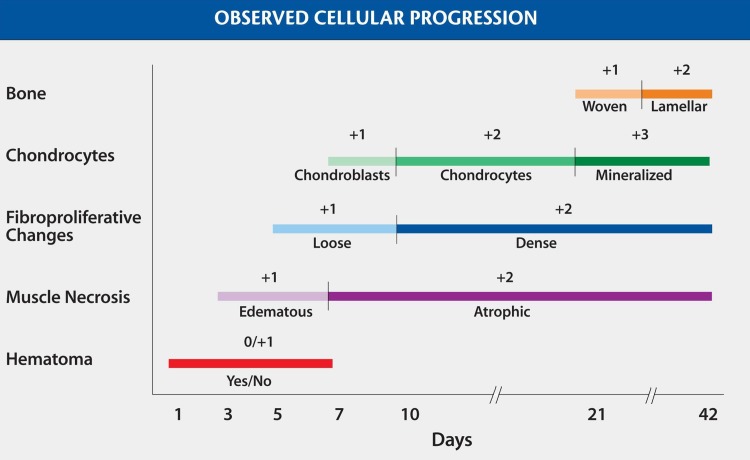
Three of the authors (OT, ACS, CG) analyzed all sections under the direction of an anatomic pathologist (RJC), and the cellular progression of HO was qualitatively examined and described. Endochondral and intramembranous pathways of bone formation were defined histologically by the respective presence (endochondral) or absence (intramembranous) of chondrocytes in direct continuity with the ectopic bone. Once a reproducible time line of cellular progression was established following repeated observation of a consistent qualitative histologic pattern, a grading system was developed as a tool to assess interobserver reliability. The histologic grading scheme focused specifically on assessment of cellular progression and the maturity of HO. Five characteristics of the local tissue environment were evaluated and assigned a numerical score (aggregate absolute range, 0–10 points): (1) the presence or absence of hematoma (0–1 point), (2) the progression of muscle necrosis (1–2 points), (3) the maturation of fibrous stroma (1–2 points), (4) the appearance and maturation of chondrocytes (1–3 points), and (5) the maturation of heterotopic bone (1–2 points). To evaluate the reproducibility and interobserver reliability of the grading system, each specimen was examined microscopically three times, on three separate occasions, in random order by the three independent observers who were blinded to the identity and time frame of each sample. Interobserver agreement of the grading system was evaluated using Fleiss’ weighted kappa statistics, with grading scores closer to 1.0 indicating more reliable agreement. Overall interobserver reliability was graded at 0.95 based on Fleiss’ weighted kappa statistics (Table 1). Observers most reproducibly scored the presence and maturation of chondrocytes (score of 0.93), fibrous stroma (score of 0.90), and ectopic bone (score of 0.89). Interobserver agreement was less reliable for identifying the presence of hematoma (score of 0.58) and characterizing the progression of muscle necrosis (score of 0.60).
Table 1.
Fleiss’ weighted kappa statistical analysis for all three raters
| Variable | Fleiss’ weighted kappa | p value | |||
|---|---|---|---|---|---|
| Rater 1 versus Rater 2 | Rater 1 versus Rater 3 | Rater 2 versus Rater 3 | Overall | ||
| Hematoma | 0.57 (0.31–0.83) | 0.70 (0.46–0.94) | 0.44 (0.17–0.72) | 0.58 (0.42–0.73) | 0.3903 |
| Muscle necrosis | 0.70 (0.55–0.84) | 0.44 (0.12–0.77) | 0.44 (0.22–0.66) | 0.60 (0.48–0.71) | 0.1056 |
| Fibrocytes | 0.94 (0.88–0.99) | 0.89 (0.82–0.96) | 0.86 (0.78–0.94) | 0.90 (0.86–0.94) | 0.3257 |
| Chondrocytes | 0.92 (0.86–0.99) | 0.95 (0.90–0.99) | 0.88 (0.81–0.97) | 0.93 (0.90–0.96) | 0.4561 |
| Bone | 0.86 (0.78–0.95) | 0.86 (0.73–0.99) | 0.91 (0.84–0.98) | 0.89 (0.84–0.94) | 0.7104 |
| Total score | 0.94 (0.90–0.98) | 0.95 (0.93–0.97) | 0.93 (0.89–0.97) | 0.95 (0.93–0.96) | 0.6685 |
A repeated measures analysis, conducted with PROC MIXED and SAS 9.1.3 software (SAS Institute Inc, Cary, NC), was used to compare the mean grades of HO between times considering that two hips at each time were from the same rabbit. A Bonferroni correction adjusted for multiple comparisons. The mean score was calculated for each hip at every assigned time based on grades from the three evaluators of 50 suitable hips from 30 rabbits.
Results
The local tissue environment was observed to evolve through a reproducible histologic progression and reliably followed a pathway of endochondral ossification (Fig. 1). Fresh hemorrhages in the interstitial tissue were immediately apparent in tissue samples and disappeared within 1 week of surgery. The skeletal muscle showed signs of fiber degeneration within 72 hours of surgery; edema was present initially, followed by a variation in the diameters of the skeletal muscle fiber, acute fiber necrosis, myophagocytosis, and, in some areas, fiber regeneration. Early fibroblastic proliferation and granulation tissue with loose myxoid extracellular matrix was evident during the first week (Fig. 2), followed with time by more dense accumulation of collagen and organized fibrous connective tissue. Morphologically recognizable chondrocytes appeared in the fibrous stroma during the second week after surgery and reliably preceded the appearance of ectopic bone, which generally was not observed until the third week (Fig. 3). Immature woven bone first appeared from calcified cartilage and evolved into mature lamellar bone with histologic features characteristic of endochondral ossification (Fig. 4). Microvascular invasion, in the form of capillary ingrowth of hypertrophic cartilage, was present and preceded the appearance of calcified cartilage (Fig. 5). There were no instances in which ectopic bone was seen in the absence of chondroid elements or frank chondrocytes. Specifically, even where osseous tissue appeared to arise directly from fibrous tissue or skeletal muscle, there was always an adjacent cartilaginous intermediary consistent with endochondral ossification. In sections in which heterotopic bone was identified, chondrocytes were reliably found in juxtaposition with the ectopic osseous tissue (Fig. 3).
Fig. 1.
A graphic representation of chronologic progression of tissue types as identified in serial specimens with a grading system to assess maturation of HO is shown.
Fig. 2.
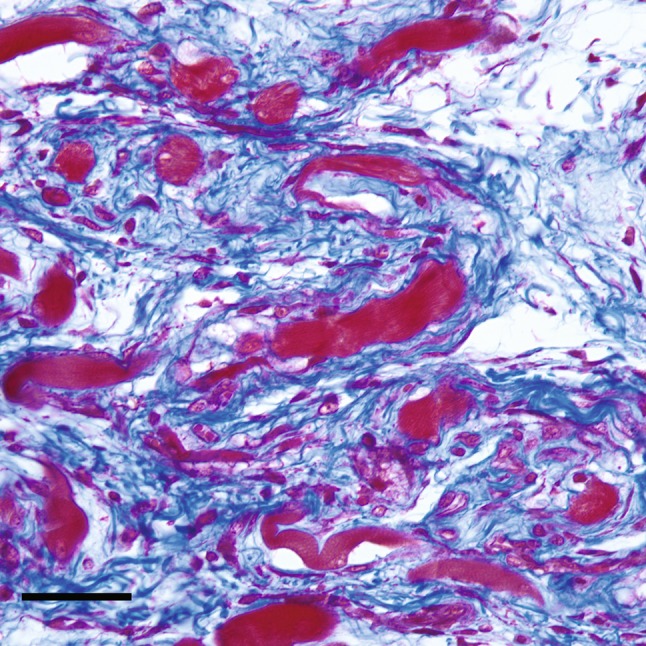
A high-magnification photomicrograph at 10 days after the induction procedure shows loose fibroconnective tissue (collagen fibers stain blue) is admixed with atrophic skeletal muscle fibers (red) (scale bar = 50 μm) (Stain, Masson’s trichrome; original magnification, ×40).
Fig. 3.
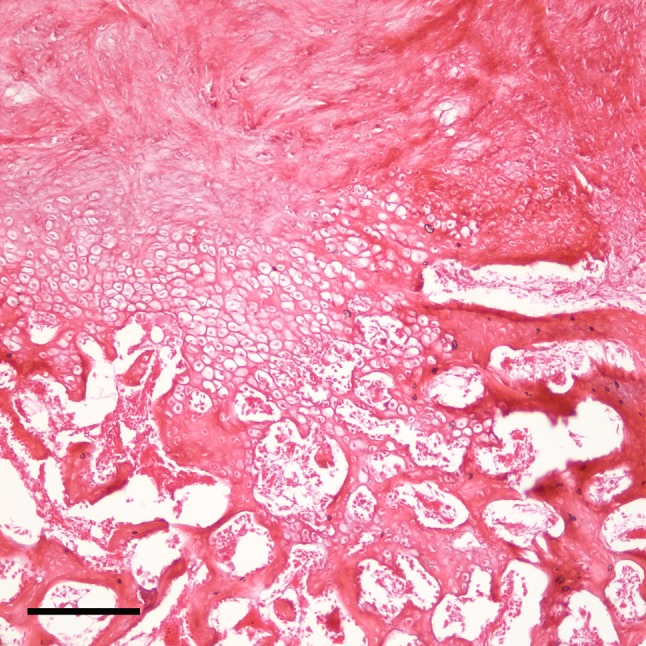
An intermediate-magnification photomicrograph shows skeletal muscle 3 weeks after the induction procedure. Dense fibrous tissue can be seen with collagen deposition in continuity with hypertrophic chondrocytes, calcifying cartilage, and trabecular bone (scale bar = 200 μm) (Stain, hematoxylin and eosin; original magnification, ×10).
Fig. 4.

A high-magnification photomicrograph shows skeletal muscle 4 weeks after the induction procedure. Dense fibrous tissue can be seen with collagen deposition in continuity with palisading chondrocytes, evolving through stages of calcification and ossification reminiscent of the growth plate (scale bar = 100 μm) (Stain, hematoxylin and eosin; original magnification, ×20).
Fig. 5.

An intermediate-magnification photomicrograph shows skeletal muscle 12 weeks after the induction procedure. Typical endochondral ossification is apparent, with capillary ingrowth of cartilaginous matrix around hypertrophic chondrocytes and ongoing matrix mineralization (scale bar = 100 μm) (Stain, hematoxylin and eosin; original magnification, ×20).
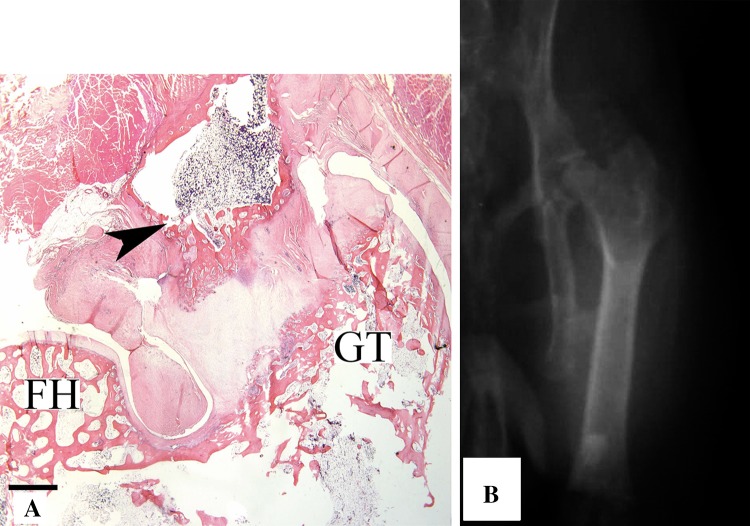
Likewise, there were no differences in the histologic pattern of progression of HO whether the ectopic bone was contiguous with the femur or was an unattached island arising directly from the soft tissue. Heterotopic bone that arose directly from the femur was derived via endochondral ossification that originated from a periosteal proliferation of chondrocytes (Fig. 6A). In the muscle or fibrous tissues, ectopic bone passed through a chondroid intermediary that showed basophilic staining and an architectural pattern that was consistent with cartilage tissue. No osseous differentiation of any type was seen in the absence of chondroid tissue that was undergoing either hypertrophic change or mineralization. Periodic radiographs, obtained at each time of animal sacrifice, showed the typical pattern of heterotopic bone formation. HO was most prevalent in the region of the abductor musculature immediately proximal to the access point in the greater trochanter and was less commonly contiguous with the cephalad surface of the greater trochanter or femoral neck (Fig. 6B).
Fig. 6A–B.
(A) A low-magnification photomicrograph shows skeletal muscle 12 weeks after the induction procedure. The orientation provides observation of the head of the femur (FH) and greater trochanter (GT). A mass of heterotopic bone (arrowhead) contiguous with the femur, including marrow elements arising from hypertrophic chondrocytes in a subperiosteal location can be seen (scale bar = 1 mm) (Stain, hematoxylin and eosin; original magnification, ×1). (B) An AP radiograph of the left hip in a rabbit obtained 6 months after surgery shows HO around the hip in characteristic location.
We found changes in the histologic grading between Weeks 1 and 2, Weeks 2 and 3, and Week 1 and 6 months, reflecting most rapid histologic progression during the early periods after injury (Table 2).
Table 2.
Repeated measures analysis
| Comparison | Difference of means | CI adjusted | p value | Alpha |
|---|---|---|---|---|
| Week 1 versus Week 2 | −4.770 | −6.1147 to −3.392 | 0.0000 | 0.01 |
| Week 2 versus Week 3 | −1.873 | −3.510 to −0.237 | 0.0039 | 0.01 |
| Week 3 versus Month 3 | −1.038 | −2.962 to 0.887 | 0.1417 | 0.01 |
| Month 3 versus Month 6 | −0.689 | −2.596 to 1.217 | 0.3177 | 0.01 |
| Week 1 versus Month 6 | −8.370 | −9.937 to −6.803 | 0.0000 | 0.01 |
Discussion
HO is a poorly understood process that can compromise the success of an otherwise technically well-performed THA. Management options often are limited to surgical excision for patients in whom clinically meaningful substantial heterotopic bone has already developed. Despite complete resection, the offending bone has a strong propensity to recur in the absence of adjunctive measures. Although contemporary methods of prophylaxis, including radiation therapy and NSAIDS, eliminate meaningful HO for most patients, they are not without inherent risks [2, 21, 23, 28, 33, 38, 48]. The ideal strategy remains to prevent the initial formation of heterotopic bone through better understanding of the pathophysiology by which ossification occurs in the soft tissues after injury. For this reason, we sought to characterize the cellular progression of heterotopic bone formation in an animal model that most closely mimics the human condition without the addition of exogenous bone stimulants.
We acknowledge certain limitations to our study. First, although we used a model that closely recapitulated the human condition without addition of exogenous osteogenic agents, a limitation of any animal model is the risk of introducing unanticipated variables and species-related differences. The principal differentiating characteristic known about the rabbit regarding HO is its predilection to form bone and heal fractures rapidly. We believe that this characteristic is a positive attribute contributing to a model with a high prevalence of HO, but it may introduce factors that otherwise are unknown to the investigators. Similarly, although we have no indication regarding how much heterotopic bone would be clinically meaningful and restrict ROM in the rabbit, the consistent occurrence of HO provides a reliable model in which to study this phenomenon. The radiographs in this study were obtained with a first-generation x-ray machine that was used before availability of digital radiographic imaging for small animals; as such, they are not reliable for quantitative HO assessment. However, the pattern of HO occurring in the soft tissues about the hip and adjacent to the greater trochanter is similar to that commonly observed in humans after total hip replacement. The model also shares some characteristics of intramedullary nailing, specifically entry of the medullary canal and escape of osteogenic cells into the surrounding soft tissues, but the magnitude of muscle injury and proximity to the hip capsule more closely resemble THA. Although it has been suggested that the limited popularity of this model is related to the complex nature of the surgical procedure [15], we believe that its simplicity of experimental design and lack of an exogenous osteogenic agent provide a more direct approach to investigate the underlying native mechanism of HO in humans. Finally, the emphasis of this study was on observational characterization of histologic progression of the process of heterotopic bone formation through primary or secondary pathways of ossification; as such, we acknowledge the absence of definitive cellular identification by specific immunohistochemical staining.
We found histologic progression of HO to be consistent and to occur predictably through a pathway of endochondral ossification. Much of the current knowledge regarding heterotopic bone growth [16, 20, 22, 25, 42, 47] has been gleaned from studies of nonphysiologic animal models that have induced heterotopic bone formation through the addition of exogenous agents, such as BMP-2, BMP-4, and bone matrix. Among these studies, conflicting observations have been made regarding the mechanisms of bone formation that are dependent on the particular exogenous agent that is used to induce the ectopic bone. For this reason, we believe our findings may resolve this conflict as our animal model produces heterotopic bone without the confounding addition of exogenous induction agents. Nevertheless, the observational nature of the data in this study allows only speculation regarding the mechanism of conversion of woven to lamellar bone, but we believe that typical processes of remodeling with successive osteoclastic resorption and osteoblastic formation would occur in response to the physiologic stimulus of muscle loading and weightbearing. In this model, ectopic bone, whether contiguous with the femur or free in the soft tissues, developed exclusively through endochondral ossification. We are not able to comment, however, on whether these anatomically distinct processes resulted from stimulation of the same stem cell line. It is believed that HO occurs as a result of the misguided and inappropriate differentiation of stem cells, secondary to the effects of local and systemic factors [8, 14, 26, 41]. It is believed that bone marrow-derived or muscle-derived pluripotent mesenchymal progenitor cells in the local environment rapidly differentiate into osteogenic precursors after the inciting event, with the maximal response occurring within 48 hours of stimulus [26, 35, 45]. Mesenchymal stem cell populations likely also play a role in the process of HO [14], but our work does not provide insight regarding whether damaged muscle or endogenous marrow elements are the more likely site of origin of these cells. Some investigators have suggested that damaged muscle is a more likely source of cells integral to the formation of ectopic bone [46], and such is consistent with the recent increase in HO observed in residual limbs following war-related blast amputations [34]. Moreover, mesenchymal progenitor cells derived from traumatized muscle exhibit gene expression profiles characteristic of osteoblasts [13, 14]. This hypothesis is corroborated by the observation that local limited field radiation before THA is effective in preventing HO [31], suggesting that the critical stem cell is present locally before the surgical insult. Once such cellular differentiation is committed (long before radiographic evidence of ectopic bone is evident), all methods of preventing HO are largely ineffective [10, 35, 43]. This is the basis for the contemporary rationale for initiation of prophylaxis for HO within 5 days of surgery on the hip [29, 30, 37].
Our observations suggest that the cellular genesis of heterotopic bone is a byproduct of endochondral ossification, regardless of its location about the hip. Although the molecular determination of unwanted ectopic bone remains to be elucidated, future treatment options will aim to redirect this misguided osteogenic process on a more constructive clinical trajectory such as in treatment of fracture nonunions and facilitating surgical fusions.
Our findings suggest that HO, after the surgical trauma of THA in a physiologic animal model, without an exogenous induction agent, progresses via a pathway of endochondral bone formation. This observation was consistent with nearly 3 decades of clinical experience with external beam radiation, which selectively attenuates endochondral ossification over membranous bone formation in preventing HO after THA [4, 31, 32]. Similarly, our findings provided a basis for the action of NSAIDS: in the laboratory, these compounds inhibit the effect of BMPs to induce pluripotent mesenchymal stem cells to differentiate to bone via an endochondral pathway [40].
Acknowledgments
We thank Nishant Merchant MD and Brian Duggan MD for assistance with the study and Senior Editor Dori Kelly MA for assistance with the manuscript.
Footnotes
The institution of one or more of the authors (VDP) has received, during the study period, funding from The Hip Society, The United States Department of Defense, Agency for Healthcare Research and Quality, and Orthopaedic Trauma Association. One of the authors certifies that he (VDP) or a member of his immediate family has or may receive payments or benefits, during the study period, an amount of USD 100,001–USD 1,000,000 from DePuy Orthopaedics, Inc (Warsaw, IN).
All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research editors and board members are on file with the publication and can be viewed on request.
Clinical Orthopaedics and Related Research neither advocates nor endorses the use of any treatment, drug, or device. Readers are encouraged to always seek additional information, including FDA-approval status, of any drug or device prior to clinical use.
Each author certifies that his or her institution approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
This work was performed at the Departments of Orthopaedics and Pathology, University of Maryland School of Medicine, Baltimore, MD, USA.
References
- 1.Ahrengart L. Periarticular heterotopic ossification after total hip arthroplasty: risk factors and consequences. Clin Orthop Relat Res. 1991;263:49–58. [PubMed] [Google Scholar]
- 2.Amstutz HC, Fowble VA, Schmalzried TP, Dorey FJ. Short-course indomethacin prevents heterotopic ossification in a high-risk population following total hip arthroplasty. J Arthroplasty. 1997;12:126–132. doi: 10.1016/S0883-5403(97)90058-9. [DOI] [PubMed] [Google Scholar]
- 3.Ayers DC, Evarts CM, Parkinson JR. The prevention of heterotopic ossification in high-risk patients by low-dose radiation therapy after total hip arthroplasty. J Bone Joint Surg Am. 1986;68:1423–1430. [PubMed] [Google Scholar]
- 4.Ayers DC, Pellegrini VD, Jr, Evarts CM. Prevention of heterotopic ossification in high-risk patients by radiation therapy. Clin Orthop Relat Res. 1991;263:87–93. [PubMed] [Google Scholar]
- 5.Balboni TA, Gobezie R, Mamon HJ. Heterotopic ossification: pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys. 2006;65:1289–1299. doi: 10.1016/j.ijrobp.2006.03.053. [DOI] [PubMed] [Google Scholar]
- 6.Bisla RS, Ranawat CS, Inglis AE. Total hip replacement in patients with ankylosing spondylitis with involvement of the hip. J Bone Joint Surg Am. 1976;58:233–238. [PubMed] [Google Scholar]
- 7.Blasingame JP, Resnick D, Coutts RD, Danzig LA. Extensive spinal osteophytosis as a risk factor for heterotopic bone formation after total hip arthroplasty. Clin Orthop Relat Res. 1981;161:191–197. [PubMed] [Google Scholar]
- 8.Brooker AF, Bowerman JW, Robinson RA, Riley LH., Jr Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed] [Google Scholar]
- 9.Cipriano CA, Pill SG, Keenan MA. Heterotopic ossification following traumatic brain injury and spinal cord injury. J Am Acad Orthop Surg. 2009;17:689–697. doi: 10.5435/00124635-200911000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Craven PL, Urist MR. Osteogenesis by radioisotope labelled cell populations in implants of bone matrix under the influence of ionizing radiation. Clin Orthop Relat Res. 1971;76:231–243. doi: 10.1097/00003086-197105000-00030. [DOI] [PubMed] [Google Scholar]
- 11.DeLee J, Ferrari A, Charnley J. Ectopic bone formation following low friction arthroplasty of the hip. Clin Orthop Relat Res. 1976;121:53–59. [PubMed] [Google Scholar]
- 12.Gannon FH, Valentine BA, Shore EM, Zasloff MA, Kaplan FS. Acute lymphocytic infiltration in an extremely early lesion of fibrodysplasia ossificans progressiva. Clin Orthop Relat Res. 1998;346:19–25. doi: 10.1097/00003086-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Jackson WM, Aragon AB, Bulken-Hoover JD, Nesti LJ, Tuan RS. Putative heterotopic ossification progenitor cells derived from traumatized muscle. J Orthop Res. 2009;27:1645–1651. doi: 10.1002/jor.20924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ji Y, Christopherson GT, Kluk MW, Amrani O, Jackson WM, Nesti LJ. Heterotopic ossification following musculoskeletal trauma: modeling stem and progenitor cells in their microenvironment. In: Rhim JS, Krenner R, editors. Human Cell Transformation: Role of Stem Cells and the Microenvironment. New York, NY: Springer Science + Business Media, LLC.; 2011. pp. 39–50. [DOI] [PubMed] [Google Scholar]
- 15.Kan L, Kessler JA. Animal models of typical heterotopic ossification. J Biomed Biotechnol. 2011;2011:309287. doi: 10.1155/2011/309287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kantorowitz DA, Miller GJ, Ferrara JA, Ibbott GS, Fisher R, Ahrens CR. Preoperative versus postoperative irradiation in the prophylaxis of heterotopic bone formation in rats. Int J Radiat Oncol Biol Phys. 1990;19:1431–1438. doi: 10.1016/0360-3016(90)90355-N. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan FS, Hahn GV, Zasloff MA. Heterotopic ossification: two rare forms and what they can teach us. J Am Acad Orthop Surg. 1994;2:288–296. doi: 10.5435/00124635-199409000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Kaplan FS, Shore EM. Progressive osseous heteroplasia. J Bone Miner Res. 2000;15:2084–2094. doi: 10.1359/jbmr.2000.15.11.2084. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan FS, Tabas JA, Gannon FH, Finkel G, Hahn GV, Zasloff MA. The histopathology of fibrodysplasia ossificans progressiva: an endochondral process. J Bone Joint Surg Am. 1993;75:220–230. doi: 10.2106/00004623-199302000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Kawai M, Bessho K, Maruyama H, Miyazaki J, Yamamoto T. Human BMP-2 gene transfer using transcutaneous in vivo electroporation induced both intramembranous and endochondral ossification. Anat Rec A Discov Mol Cell Evol Biol. 2005;287:1264–1271. doi: 10.1002/ar.a.20245. [DOI] [PubMed] [Google Scholar]
- 21.Kjaersgaard-Andersen P, Nafei A, Teichert G, Kristensen O, Schmidt SA, Keller J, Lucht U. Indomethacin for prevention of heterotopic ossification: a randomized controlled study in 41 hip arthroplasties. Acta Orthop Scand. 1993;64:639–642. doi: 10.3109/17453679308994587. [DOI] [PubMed] [Google Scholar]
- 22.Kotajima S, Kishimoto KN, Watanuki M, Hatori M, Kokubun S. Gene expression analysis of ectopic bone formation induced by electroporatic gene transfer of BMP4. Ups J Med Sci. 2006;111:231–241. doi: 10.3109/2000-1967-044. [DOI] [PubMed] [Google Scholar]
- 23.Lo TC, Healy WL, Covall DJ, Dotter WE, Pfeifer BA, Torgerson WR, Wasilewski SA. Heterotopic bone formation after hip surgery: prevention with single-dose postoperative hip irradiation. Radiology. 1988;168:851–854. doi: 10.1148/radiology.168.3.3136510. [DOI] [PubMed] [Google Scholar]
- 24.Lowell JD. Heterotopic ossification in revision arthroplasty. In: Turner RH, Scheller AD Jr, editors. Revision Total Hip Arthroplasty. New York, NY: Grune and Stratton; 1982. pp. 359–378. [Google Scholar]
- 25.Nakagawa T, Sugiyama T, Shimizu K, Murata T, Narita M, Nakamura S, Tagawa T. Characterization of the development of ectopic chondroid/bone matrix and chondrogenic/osteogenic cells during osteoinduction by rhBMP-2: a histochemical and ultrastructural study. Oral Dis. 2003;9:255–263. doi: 10.1034/j.1601-0825.2003.02912.x. [DOI] [PubMed] [Google Scholar]
- 26.Naraghi FF, DeCoster TA, Moneim MS, Miller RA, Rivero D. Heterotopic ossification. Orthopedics. 1996;19:145–151. doi: 10.3928/0147-7447-19960201-10. [DOI] [PubMed] [Google Scholar]
- 27.Olmsted-Davis E, Gannon FH, Ozen M, Ittmann MM, Gugala Z, Hipp JA, Moran KM, Fouletier-Dilling CM, Schumara-Martin S, Lindsey RW, Heggeness MH, Brenner MK, Davis AR. Hypoxic adipocytes pattern early heterotopic bone formation. Am J Pathol. 2007;170:620–632. doi: 10.2353/ajpath.2007.060692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parkinson JR, Evarts CM, Hubbard LF. Radiation therapy in the prevention of heterotopic ossification after total hip arthroplasty. Hip. 1982;211–227. [PubMed]
- 29.Pellegrini VD., Jr Radiation prophylaxis of heterotopic ossification. Int J Radiat Oncol Biol Phys. 1994;30:743–744. doi: 10.1016/0360-3016(92)90967-M. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrini VD, Jr, Evarts CM. Radiation prophylaxis of heterotopic bone formation following total hip arthroplasty: current status. Semin Arthroplasty. 1992;3:156–166. [PubMed] [Google Scholar]
- 31.Pellegrini VD, Jr, Gregoritch SJ. Preoperative irradiation for prevention of heterotopic ossification following total hip arthroplasty. J Bone Joint Surg Am. 1996;78:870–881. doi: 10.2106/00004623-199606000-00010. [DOI] [PubMed] [Google Scholar]
- 32.Pellegrini VD, Jr, Konski AA, Gastel JA, Rubin P, Evarts CM. Prevention of heterotopic ossification with irradiation after total hip arthroplasty: radiation therapy with a single dose of eight hundred centigray administered to a limited field. J Bone Joint Surg Am. 1992;74:186–200. [PubMed] [Google Scholar]
- 33.Persson PE, Sodemann B, Nilsson OS. Preventive effects of ibuprofen on periarticular heterotopic ossification after total hip arthroplasty: a randomized double-blind prospective study of treatment time. Acta Orthop Scand. 1998;69:111–115. doi: 10.3109/17453679809117608. [DOI] [PubMed] [Google Scholar]
- 34.Potter BK, Burns TC, Lacap AP, Granville RR, Gajewski DA. Heterotopic ossification following traumatic and combat-related amputations: prevalence, risk factors, and preliminary results of excision. J Bone Joint Surg Am. 2007;89:476–486. doi: 10.2106/JBJS.F.00412. [DOI] [PubMed] [Google Scholar]
- 35.Puzas JE, Miller MD, Rosier RN. Pathologic bone formation. Clin Orthop Relat Res. 1989;245:269–281. [PubMed] [Google Scholar]
- 36.Ritter MA, Vaughan RB. Ectopic ossification after total hip arthroplasty: predisposing factors, frequency, and effect on results. J Bone Joint Surg Am. 1977;59:345–351. [PubMed] [Google Scholar]
- 37.Rumi MN, Deol GS, Bergandi JA, Singapuri KP, Pellegrini VD., Jr Optimal timing of preoperative radiation for prophylaxis against heterotopic ossification: a rabbit hip model. J Bone Joint Surg Am. 2005;87:366–373. doi: 10.2106/JBJS.C.00974. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer JR, Myers MA, Rosier RN, Puzas JE. Heterotopic ossification: clinical and cellular aspects. Calcif Tissue Int. 1991;49:208–215. doi: 10.1007/BF02556120. [DOI] [PubMed] [Google Scholar]
- 39.Schneider DJ, Moulton MJ, Singapuri K, Chinchilli V, Deol GS, Krenitsky G, Pellegrini VD., Jr The Frank Stinchfield Award: inhibition of heterotopic ossification with radiation therapy in an animal model. Clin Orthop Relat Res. 1998;355:35–46. doi: 10.1097/00003086-199810000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Shafritz AB, Shore EM, Gannon FH, Zasloff MA, Taub R, Muenke M, Kaplan FS. Overexpression of an osteogenic morphogen in fibrodysplasia ossificans progressiva. N Engl J Med. 1996;335:555–561. doi: 10.1056/NEJM199608223350804. [DOI] [PubMed] [Google Scholar]
- 41.Spencer RF. The effect of head injury on fracture healing: a quantitative assessment. J Bone Joint Surg Br. 1987;69:525–528. doi: 10.1302/0301-620X.69B4.3611151. [DOI] [PubMed] [Google Scholar]
- 42.Stoeger T, Proetzel G, Welzel H, Papadimitriou A, Dony C, Balling R, Hofmann C. In situ gene expression analysis during BMP2-induced ectopic bone formation in mice shows simultaneous endochondral and intramembranous ossification. Growth Factors. 2002;20:197–210. doi: 10.1080/0897719021000069579. [DOI] [PubMed] [Google Scholar]
- 43.Sylvester JE, Greenberg P, Selch MT, Thomas BJ, Amstutz H. The use of postoperative irradiation for the prevention of heterotopic bone formation after total hip replacement. Int J Radiat Oncol Biol Phys. 1988;14:471–476. doi: 10.1016/0360-3016(88)90262-3. [DOI] [PubMed] [Google Scholar]
- 44.Thomas BJ. Heterotopic bone formation after total hip arthroplasty. Orthop Clin North Am. 1992;23:347–358. [PubMed] [Google Scholar]
- 45.Tonna EA, Cronkite EP. Autoradiographic studies of cell proliferation in the periosteum of intact and fractured femora of mice utilizing DNA labeling with H3-thymidine. Proc Soc Exp Biol Med. 1961;107:719–721. doi: 10.3181/00379727-107-26733. [DOI] [PubMed] [Google Scholar]
- 46.Toom A, Suutre S, Märtson A, Haviko T, Selstam G, Arend A. Lack of a central role for osteoprogenitor cells from the femoral canal in heterotopic ossification of the hip: an experimental study in a rat model. J Bone Joint Surg Br. 2010;92:298–303. doi: 10.1302/0301-620X.92B2.22630. [DOI] [PubMed] [Google Scholar]
- 47.Urist MR. Bone: formation by autoinduction. Science. 1965;150:893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- 48.Vielpeau C, Joubert JM, Hulet C. Naproxen in the prevention of heterotopic ossification after total hip replacement. Clin Orthop Relat Res. 1999;369:279–288. doi: 10.1097/00003086-199912000-00029. [DOI] [PubMed] [Google Scholar]